Abstract
Enteropathogenic Escherichia coli (EPEC) infection triggers the release of ATP from host intestinal cells, and the ATP is broken down to ADP, AMP, and adenosine in the lumen of the intestine. Ecto-5′-nucleotidase (CD73) is the main enzyme responsible for the conversion of 5′-AMP to adenosine, which triggers fluid secretion from host intestinal cells and also has growth-promoting effects on EPEC bacteria. In a recent study, we examined the role of the host enzyme CD73 in EPEC infection by testing the effect of ecto-5′-nucleotidase inhibitors. Zinc was a less potent inhibitor of ecto-5′-nucleotidase in vitro than the nucleotide analog α,β-methylene-ADP, but in vivo, zinc was much more efficacious in preventing EPEC-induced fluid secretion in rabbit ileal loops than α,β-methylene-ADP. This discrepancy between the in vitro and in vivo potencies of the two inhibitors prompted us to search for potential targets of zinc other than ecto-5′-nucleotidase. Zinc, at concentrations that produced little or no inhibition of EPEC growth, caused a decrease in the expression of EPEC protein virulence factors, such as bundle-forming pilus (BFP), EPEC secreted protein A, and other EPEC secreted proteins, and reduced EPEC adherence to cells in tissue culture. The effects of zinc were not mimicked by other transition metals, such as manganese, iron, copper, or nickel, and the effects were not reversed by an excess of iron. Quantitative real-time PCR showed that zinc reduced the abundance of the RNAs encoded by the bfp gene, by the plasmid-encoded regulator (per) gene, by the locus for the enterocyte effacement (LEE)-encoded regulator (ler) gene, and by several of the esp genes. In vivo, zinc reduced EPEC-induced fluid secretion into ligated rabbit ileal loops, decreased the adherence of EPEC to rabbit ileum, and reduced histopathological damage such as villus blunting. Some of the beneficial effects of zinc on EPEC infection appear to be due to the action of the metal on EPEC bacteria as well as on the host.
Enteropathogenic Escherichia coli (EPEC) infection is a common cause of watery diarrhea in children in developing countries. The mechanism by which EPEC triggers watery diarrhea is obscure, since unlike several other types of diarrhea-producing E. coli strains, EPEC produces no toxins. We recently theorized that the release of adenine nucleotides from the host intestinal cells, followed by the breakdown to adenosine, could trigger watery diarrhea by the activation of adenosine receptors in the intestine (8).
Our interest in zinc was prompted by studies of ecto-5′-nucleotidase, a key extracellular enzyme which catalyzes the hydrolysis of extracellular 5′-AMP to adenosine. Zinc and the nucleotide analog α,β-methylene-ADP are the classical inhibitors of this enzyme that were tested in vitro and in vivo in a recent study by one of our laboratories (9). Although α,β-methylene-ADP is about eightfold more potent than zinc acetate in the inhibition of ecto-5′-nucleotidase, in vivo, zinc acetate was superior to α,β-methylene-ADP in its ability to block EPEC infection-induced fluid accumulation in rabbit ileal loops. Zinc treatment also decreased EPEC adherence to intestinal villi, an effect not explained by the known roles of ecto-5′-nucleotidase. We initiated the present study to try to determine if zinc had molecular targets other than ecto-5′-nucleotidase that could account for its biological activity, and we were surprised to find that zinc had effects on EPEC bacteria as well as on the host intestinal cells.
MATERIALS AND METHODS
Bacterial strains used.
The bacterial strains used are listed in Table 1. Bacteria were grown overnight in LB broth at 37°C with shaking at 300 rpm and then subcultured into Dulbecco's modified Eagle medium (DMEM). The DMEM used in this report refers to DMEM-F12 medium supplemented with 18 mM NaHCO3 and 25 mM HEPES, pH 7.4, but without serum or antibiotics.
TABLE 1.
Bacterial strains used in this study
| Strain | Serotype | Relevant genotype | Characteristic(s) | Reference(s) |
|---|---|---|---|---|
| Wild-type EPEC strains | ||||
| E2348/69 | O127:H6 | 21 | ||
| B171-8 | O11:NM | 13 | ||
| E851/71 | O142:H6 | 21 | ||
| JCP88 | O119:B14 | 27, 28 | ||
| Mutants and derivative of E2348/69 | ||||
| JPN15 | EAF−; plasmid-cured derivative | 22 | ||
| JPN15(pJLM161) | perABC restored on plasmid JLM161 | 23 | ||
| SE796 | Δler | 12 | ||
| OG127 | Δper | 14, 15 | ||
| Mutant of B171-8 | ||||
| B171-Fma | Mutation in bfpF | Hyperadherent; overproduces BFP | 4 |
Materials.
The following reagents were obtained from Sigma-Aldrich Chemicals: adenosine, zinc acetate, NiCl2, MnCl2, and CuSO4. FeSO4 was from MP Biomedicals, Irvine, CA.
Cell culture.
HeLa cells and T84 colon cancer cells were grown in DMEM-F12 medium supplemented with 7.5% fetal bovine serum (Gibco/Invitrogen, Grand Island, NY), 18 mM NaHCO3, 20 μg/ml vancomycin, and 15 μg/ml gentamicin, as described previously (6).
Adherence assay.
Quantitative adherence assays were performed in duplicate or triplicate wells of HeLa cells grown in collagen-coated 24-well plates. HeLa cells were used at 90% confluence; cells were rinsed and changed into 0.5 ml of antibiotic-free DMEM per well and then infected with EPEC at a multiplicity of infection of 100:1. EPEC bacteria were grown overnight in LB broth and then subcultured for 2 h in DMEM to induce production of bundle-forming pili (BFP) and other virulence factors. After 2 h of infection to allow EPEC adherence, the monolayers were washed twice with sterile phosphate-buffered saline to remove unbound bacteria, and then the monolayers were solubilized in 1% Triton X-100 detergent in water. The Triton extracts were subjected to serial dilution and spread on LB agar plates, and the number of colonies were counted. Adherence was calculated as a percentage of the input inoculum.
ATP release assay.
ATP release was measured into the supernatant medium of T84 cells, using an inhibitor-regenerator system as described previously (6, 7), and the multiplicity of infection was again 100:1. ATP was measured using a luciferase kit (Roche Molecular Biochemicals, Indianapolis, IN). As a technical control, we tested whether a range of 0.1 to 1 mM zinc would interfere with the detection of ATP by using the luciferase method and found that it did not.
Analysis of EPEC protein secretion and EPEC secreted proteins.
To assess the effect of zinc on the EPEC secreted proteins (Esp), we subcultured EPEC strains for 5 h in serum-free DMEM and collected supernatants by centrifugation and sterile filtration. Ten milliliters of supernatant was frozen and then concentrated by lyophilization overnight. The lyophilized material was redissolved in 1.25 ml of water, resulting in an eightfold concentration. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed, and then the gels were subjected to silver staining, using a Sterling Silver stain kit (National Diagnostics, Atlanta, GA), and the total protein was measured by the method of Bradford (5), using a Bio-Rad protein assay kit.
Immunoblot analysis.
Expression levels of the BFP and EspA were examined by immunoblotting. Since BFP remains attached to the EPEC bacterial cell, whole-cell EPEC bacterial cell suspensions were solubilized in SDS sample buffer with 1% β-mercaptoethanol. For EspA, the concentrated supernatants were analyzed. Both antibodies used were kind gifts of Michael Donnenberg, University of Maryland, Baltimore, MD. Rabbit polyclonal antibody against BFP was raised against purified bundlin, which is the product of bfpA and the main structural subunit of BFP, and was purified by using a protein G column; this antibody was used at a dilution of 1:25,000. Rabbit polyclonal antibody against EspA was also immunopurified and was used at a final dilution of 1:15,000. A secondary antibody was goat anti-rabbit antibody conjugated to peroxidase, used at a dilution of 1:3000, and blots were developed by chemiluminescence as described previously (7), using Lumiglo reagents from Kierkegard and Perry (Gaithersburg, MD). The densities of bands on the x-ray film were quantitated using a scanning densitometer with an ImageOne program (Bio-Rad, Hercules, CA).
Analysis of RNA expression by reverse transcription and qRT-PCR.
EPEC strains were subcultured at a dilution of 1:100 into DMEM plus various concentrations of zinc or other metals for 5 h. Bacteria (0.5 ml) were collected and lysed by incubation with 40 μg of lysozyme for 5 min, and then RNA was isolated using RNeasy RNA purification kits (QIAGEN, Inc., Valencia, CA). RNA was subjected to reverse transcription using Invitrogen Superscript III reverse transcriptase; 5 μl of purified RNA was used per 50 μl of reaction. For most genes, random DNA hexanucleotide primers at 3 μM were used as reverse transcription primers, but for the low-abundance genes per and ler, gene-specific primers at 0.2 μM were used. Reverse transcription reaction was carried out at 55°C for 1 h. Copy DNA from reverse transcription was diluted 100-fold in RNase-free water, and then 2.5 μl was subjected to quantitative real-time (qRT)-PCR (final dilution of 1:1,000), using oligonucleotide primers for the genes of interest or for rrsB (16S rRNA) as the normalizing gene, following the methods of Leverton and Kaper (20). The oligonucleotide primers used for bfpA, espA, eae, tir, ler, and rrsB were those reported by Leverton and Kaper (20). The oligonucleotides used for per were 5′-GGGACATGGAAATTGTCGGAATCG-3′ and 5′-TGCATTTCATTGAGGTTCGCAGT-3′, and those for espB were 5′-GCTCTGATTGGTG GTGCTAT-3′ and 5′-CCTGCCTTCT GTGCTAATTC-3′, which were designed by using Primer Quest software (Integrated DNA Technologies, which was also where oligonucleotides were purchased).
PCR was carried out using a MyiQ single-color qRT-PCR machine from Bio-Rad (Hercules, CA), using SYBR green dye to monitor the amplification. Relative expression was calculated by the ΔΔCT (the “Livak”) method as described previously (1), where CT is the number of cycles to threshold. SYBR green PCR reagents were from Bio-Rad, and to reduce the cost, the PCR volume was reduced to 25 μl. PCR was performed using a two-step protocol, with an annealing temperature of 58.7°C and denaturation at 95°C for 30 s for each cycle (i.e., with no extension step) for 35 cycles. Thermal melt-curve analysis was performed at the end of the PCR amplification and showed a single sharp peak for each of the genes analyzed.
EPEC infection in the rabbit ileal loop model.
For details of the surgical procedures used to create the ileal loops, see the supplemental material. Experimental infection of the rabbits was performed at the Boedeker laboratory (Baltimore, MD, and then New Mexico) and at the Crane laboratory (Buffalo, NY). Animal use was approved by the institutional animal care and use committees at all of the sites.
Data analysis.
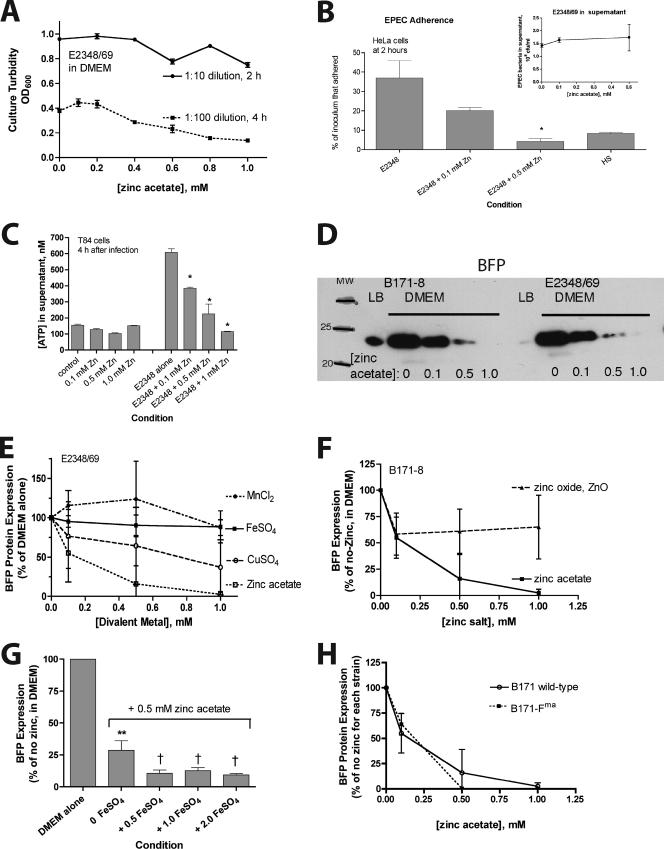
Error bars shown in the graphs are standard deviations. The statistical analysis was analysis of variance, using InStat 3.0 software (GraphPad Software, San Diego, CA), with the Tukey-Kramer posttest for multiple comparisons. Zinc was active in inhibiting the expression of many virulence factors at concentrations of 0.2 to 0.5 mM. To avoid excessive clutter on graphs, asterisks indicating statistical significance are not included in every instance where zinc had a significant effect. For example, zinc at concentrations of ≥0.1 mM produced significant inhibition of BFP (Fig. 1E, F, and G), but these instances are not flagged by asterisks.
FIG. 1.
Effect of zinc on EPEC growth, adherence, and BFP expression as analyzed at the protein level. EPEC strains were grown overnight in LB broth medium and then subcultured into serum-free DMEM with or without zinc acetate added to the concentration indicated. (A) The effect of zinc added to DMEM is shown for dilutions of 1:10 for 2 h and of 1:100 for 4 h. For the measurement of adherence and the analysis of BFP expression, a 2-h subculture was used (B and D to H). OD600, optical density at 600 nm. (B) Effect of zinc on EPEC adherence to HeLa cells compared to the nonpathogenic nonadherent E. coli strain HS. The inset shows the lack of inhibition of bacterial CFU recovered from the supernatant medium. (C) Effect of zinc on EPEC-induced ATP release from T84 cells. EPEC was allowed to adhere to T84 cells for 35 min in the absence of zinc, and then medium was changed to include the ATP regenerating system, and zinc was added. Zinc significantly inhibited EPEC-induced ATP release at all concentrations tested (P < 0.05). (D) Effect of zinc on BFP expression. Strains were subcultured for 2 h in LB medium (noninducing conditions) or DMEM (an inducing condition) plus various concentrations of zinc. Whole-cell bacterial suspensions were collected in SDS sample buffer and then subjected to electrophoresis followed by Western immunoblotting with an antibody against bundlin, the main structural subunit of BFP. In panels D to H, equal volumes of solubilized bacteria were loaded in each lane, since there is little or no effect of zinc on growth with a 2-h exposure. (E to H) Effect of zinc on BFP expression as analyzed by Western blotting with quantitation by densitometry scanning. Each line shown is the result (mean ± standard deviation) of at least two separate experiments. To allow comparison of different blots, band density is expressed as a percentage of the no-zinc control for each experiment. In panels E, F, and H, zinc at concentrations of 0.1 mM and higher resulted in statistically significant inhibition of BFP expression levels. (G) Symbols: **, statistically significant inhibition of BFP by zinc alone; †, statistically significant greater inhibition by FeSO4 plus zinc compared to that of zinc alone. (H) Strain B171-Fma overexpresses BFP, but when expressed as a percentage of the no-zinc condition for that mutant strain, the potency of zinc in the inhibition of BFP is the same as that for the wild type.
RESULTS
As stated in the introduction above, the rationale for testing the effect of zinc in EPEC infection was our hypothesis that zinc could block EPEC bacterium-induced fluid secretion via the inhibition of ecto-5′-nucleotidase, a host cell enzyme (9). Zinc acetate at 1 mM did inhibit EPEC bacterium-induced fluid accumulation in the model of rabbit intestinal loop infection (see Fig. 6), but this was accompanied by a significant decrease in EPEC bacterial adherence to the intestinal epithelium, an effect which was not explained by effects on ecto-5′-nucleotidase. Therefore, we expanded our study to determine if zinc had effects on EPEC bacteria that could account for zinc's effects on EPEC adherence. We found that zinc did have prominent effects on EPEC bacteria, including inhibition of expression of several EPEC virulence factors, such as BFP and EspA. We expanded our study by examining the effects of zinc on other gene transcripts by using real-time PCR and by testing the roles of the transcription factors per and ler in zinc's effects.
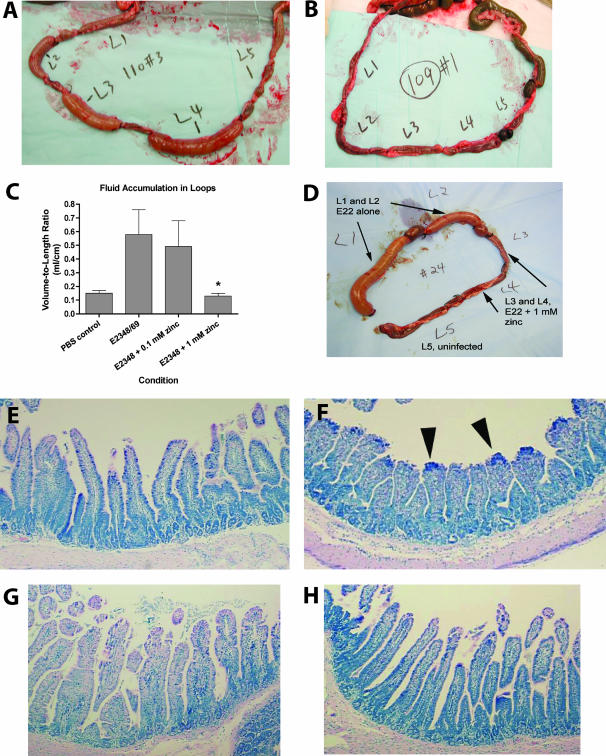
FIG. 6.
Effects of zinc on EPEC-induced fluid secretion, intestinal adherence, EPEC bacterial numbers, and histologic damage in the rabbit ileal loop model of infection. Ten-centimeter ileal loops were infected with 109 CFU of EPEC E2348/69, and intestinal loops and loop fluid samples were recovered 18 to 20 h later. (A and B) Gross-view photographs of the EPEC-infected loops in the absence (A) or presence (B) of 1 mM zinc acetate, added once at the time of infection. Loop L5 in each rabbit was the uninfected control. (C) Volume-to-length ratios of fluid accumulation in intestinal loops. The asterisk indicates a significant decrease compared to the value for the no-zinc condition. (D) Gross-view photograph of ileal loops infected with 108 CFU per loop of rabbit EPEC strain E22, showing that the protective effects of zinc were confined to the loops actually treated with zinc, with no cross-protection of neighboring untreated loops. (E to H) Effect of zinc on histological damage induced by human EPEC E2348/69 infection; ileal tissues were fixed, stained with hematoxylin and eosin stain, and photographed at a magnification of ×100. (E) Uninfected control ileum, showing long slender villi; (F) ileum infected with EPEC E2348/69 for 20 h, showing EPEC adherence primarily at the villus tips (dense blue staining, indicated by arrowheads), with villus blunting and crypt hyperplasia; (G) ileum infected with E2348/69 plus 0.1 mM zinc acetate, showing less villus blunting. EPEC bacteria are visible in the intestinal lumen (top of photograph), but fewer are adhering to the villi; (H) ileum infected with E2348/69 plus 1 mM zinc acetate, showing normal villus height, normal crypt depth, and no visible adherent EPEC.
Figure 1 shows the effects of zinc on EPEC growth and bacterial adherence to cultured cells and on BFP, as analyzed at the protein level. The effects of zinc are also contrasted with those of other transition metals. Figure 1A shows the effect of zinc on EPEC growth in DMEM. At short incubation times, zinc had little or no inhibitory effect on EPEC growth (less than 10% inhibition of growth at 1 mM zinc). With longer periods of subculture, low zinc concentrations (0.05 to 0.2 mM zinc acetate) still had no inhibitory effect on EPEC growth, while higher concentrations inhibited growth by up to 60%. Figure 1B shows that 0.5 mM zinc reduced the adherence of EPEC strain E2348/69 to HeLa cells to levels that were lower than those of the nonpathogenic, nonadherent E. coli strain HS. While zinc decreased EPEC adherence to the monolayer, it had no effect on the numbers of EPEC bacteria recovered from the supernatant medium (Fig. 1B, inset). The effects of zinc on EPEC adherence to T84 cells were similar to that seen with HeLa cells. Visual adherence assays showed that 0.1 to 0.3 mM zinc also abolished the localized adherence pattern and converted the adherence pattern to one of diffuse adherence (photographs not shown). Since the localized adherence phenotype is strongly dependent on BFP, this was a clue that BFP might be affected by zinc.
Figure 1C shows that zinc, when added to the medium after EPEC had already adhered to the host cell monolayer, was still able to abolish EPEC-induced ATP release from T84 cells in culture. ATP release is a complex and late form of EPEC infection-induced damage to host cells that requires intimate adherence, type III secretion, and delivery of bacterial effectors to the host cell.
Figure 1D shows the results of immunoblotting analysis of BFP expression in two EPEC strains. Figure 1D shows that the expression of BFP, which is greatly increased when EPEC strains are grown in DMEM, was reduced to undetectable levels by 0.5 to 1.0 mM zinc in EPEC strains B171-8 and E2348/69. A concentration of 0.5 mM zinc also abolished the ability of EPEC to form clumps or autoaggregates in DMEM, a phenotype dependent on the BFP (34; also data not shown).
Figure 1E shows a comparison of the effects of three other transition metals with that of zinc on BFP expression. Zinc acetate was more potent and more efficacious in the inhibition of BFP than MnCl2, FeSO4, and CuSO4. Ferric iron in the form of Fe2(SO4)3 gave results similar to those of FeSO4 (data not shown). Compared to zinc acetate, zinc oxide was less efficacious in inhibiting BFP (Fig. 1F). We did not test the effects of chromium or cobalt since we felt that these metals would be too toxic to test in vivo in the rabbit ileal loop model, even if they showed activity against EPEC in vitro.
The inhibitory effects of 0.5 mM zinc acetate on BFP expression were not reversed by an excess of iron in the form of FeSO4 (Fig. 1G); in fact, the addition of 2 mM FeSO4 actually inhibited BFP expression even more than 0.5 mM zinc alone. This suggested that zinc is not acting merely as an iron antagonist, for example, by competing with iron uptake.
Figure 1H shows that zinc was able to inhibit BFP expression in the EPEC mutant strain B171-Fma, a hyperadherent mutant strain which overexpresses BFP due to an induced mutation in bfpF (4). B171-Fma contains changes in two consecutive amino acids (Gly139 and Lys140) within the nucleotide-binding domain in BfpF. In Fig. 1H, the BFP inhibition curves were generated by normalizing results to the no-zinc control of each strain.
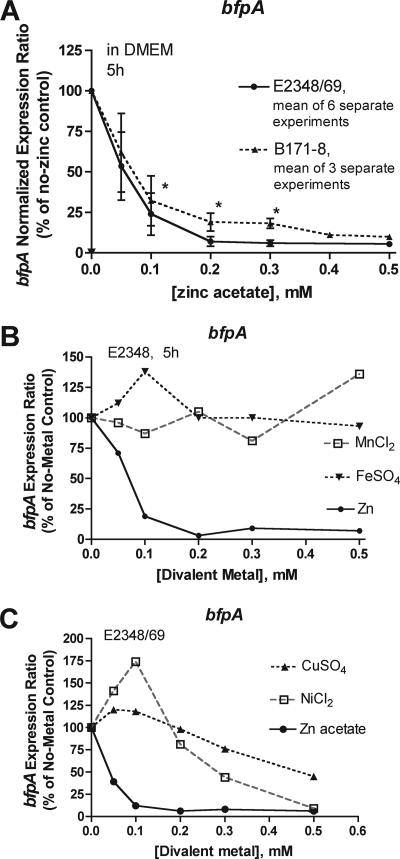
Figure 2 shows the effect of zinc on bfpA RNA levels in EPEC; bfpA encodes bundlin, the main structural subunit of BFP. Figure 2A shows that zinc inhibited bfpA expression in two EPEC strains at concentrations of 0.1 mM and higher. Note that 0.1 to 0.2 mM zinc did not inhibit EPEC growth (Fig. 1A). Figure 2B and C show the effects of other divalent metals on bfpA expression. MnCl2 and FeSO4 did not inhibit bfpA expression at the concentrations tested (Fig. 2B). CuSO4 and NiCl2 inhibited bfpA expression but not as potently as zinc (Fig. 2C). Both CuSO4 and NiCl2 also inhibited bacterial growth at concentrations above 0.2 mM, but this effect was compensated for by the normalization method used.
FIG. 2.
Effect of zinc on the expression of BFP as analyzed by reverse transcription and qRT-PCR. Bacteria were lysed with lysozyme, RNA was extracted and subjected to reverse transcription, and then the cDNA was diluted 1,000-fold. Real-time PCR was done in triplicate wells using oligonucleotide primers and monitored by fluorescence from SYBR green dye as described in Materials and Methods. Unknown gene expression was normalized relative to that of rrsB (16S rRNA). The normalized expression ratio was calculated using the 2(−ΔΔCT) method, and then the ratio was multiplied by 100 to express it as a percentage of the no-zinc control. The growth period of strains was 5 h. (A) Effect of zinc on the expression of bfpA in two EPEC strains. Results are means ± standard deviations for 6 and 3 separate experiments, respectively. Symbols: *, statistically significant inhibition compared to the no-zinc condition by analysis of variance with Tukey-Kramer posttest for multiple comparisons. (B and C) Effects of other divalent metals on the expression of bfpA. CuSO4 and NiCl2 inhibited bacterial growth at concentrations above 0.2 mM, but this is taken into account by the normalization method used.
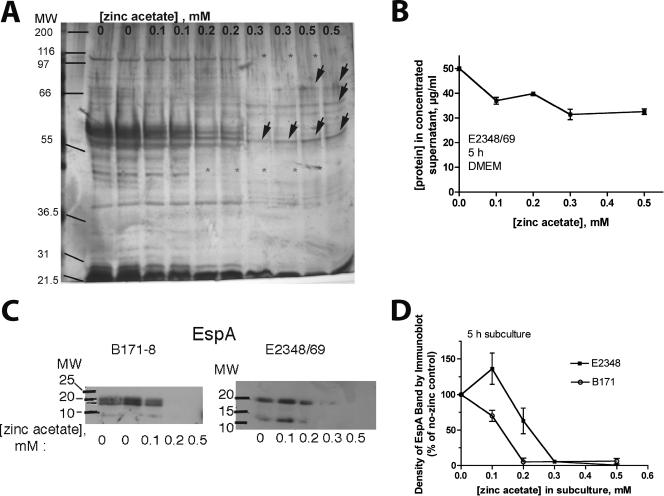
Figure 3 shows the effects of zinc on the production of EPEC secreted proteins, as analyzed at the protein level. Figure 3A and B show the effects of zinc on protein secretion as analyzed by polyacrylamide gel electrophoresis with silver-stained gels and by protein assay. Figure 3A shows that the effects of zinc were not uniform across different secreted proteins. While some protein bands decreased in intensity with zinc, other proteins appeared not to be inhibited by zinc at all and may even have been increased in the presence of 0.3 to 0.5 mM zinc (Fig. 3A). Figure 3B shows the effect of zinc on the total protein concentration in the EPEC supernatants after concentration. Figure 3C and D show the effect of zinc on EspA in EPEC supernatant medium by immunoblot analysis. The expression of EspA of strain B171-8 was more potently inhibited by zinc and was completely undetectable at 0.2 mM zinc compared to that of strain E2348/69, for which a 0.3 mM or higher concentration of zinc was needed to completely suppress the EspA band. Figure 3C also provides a hint that zinc may cause dysregulation of protein processing, because with strain E2348/69, 0.1 mM zinc triggered increased amounts of a ∼12-kDa band recognized by the EspA antibody, apparently a proteolytic fragment of authentic EspA, which has a predicted mass of 20.5 kDa. Figure 3D shows a graph of the densitometry tracings of the EspA bands, with each experiment shown normalized to the no-zinc control.
FIG. 3.
Effect of zinc on protein secretion and expression of EPEC secreted protein A (EspA). EPEC strains were subcultured in serum-free DMEM for 5 h, and then bacteria were removed by centrifugation and filtration, and 10 ml of the supernatant medium was collected, frozen, lyophilized overnight, and resuspended in 1.25 ml of water, resulting in an eightfold concentration. Secreted protein was analyzed by gel electrophoresis and silver staining (A), quantitated by protein assay (B) or analyzed by Western immunoblotting using antibody against EspA (C and D). Panel A shows silver staining of proteins secreted into culture supernatants, adjusted so that equal amounts of protein (0.6 μg) were loaded per lane. Concentrations of zinc 0.2 mM and higher resulted in the disappearance of several bands (gray asterisks), while other protein bands were spared from inhibition and perhaps even increased in density at these concentrations (arrows). Panel B shows the effect of zinc on the total protein concentration in EPEC supernatant; the supernatant had been concentrated eightfold by lyophilization and resuspension. (C and D) Equal volumes of concentrated supernatant were loaded in each lane. Panel D shows a quantitation of the EspA bands by densitometry. Each line in the graph represents the average of two separate experiments for each EPEC strain. A comparison of panels B and D shows that concentrations of zinc which inhibited total supernatant protein by 20 to 30% inhibited EspA by greater than 90%. MW, molecular weight.
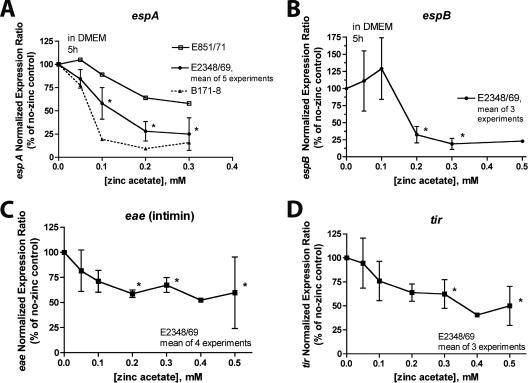
To further investigate the effects of zinc on EPEC virulence factors, we analyzed its effects on RNA transcribed from genes of the locus for enterocyte effacement (LEE). Figure 4 shows that zinc had effects on RNA transcribed from espA (Fig. 4A), espB (Fig. 4B), eae (Fig. 4C), and tir (Fig. 4D). Zinc also inhibited the expression of espF (data not shown). Zinc's effects on eae and tir seemed to be less profound that its effects on the esp gene transcripts.
FIG. 4.
Effect of zinc on the abundance of RNA transcribed from genes in the LEE. EPEC virulence gene expression was analyzed as described in the legend to Fig. 2 and in Materials and Methods. (A) Effect of zinc on espA is shown for E2348/69 (means ± standard deviations from 5 experiments) and for strains E851/71 and B171-8 (single experiment with PCR samples run in triplicate). (B) Effect of zinc on espB. (C) Effect of zinc on eae, encoding intimin. (D) Effect of zinc on tir. Asterisks show statistical significance compared to the value for the no-zinc condition. Note that the inhibitory effect of zinc on eae and tir appeared to be less profound than its effect on the esp gene transcripts.
The tir, cesT, and eae genes are cotranscribed in a single polycistronic message (LEE5), so a similarity in the regulation of the eae and tir genes by zinc would be expected.
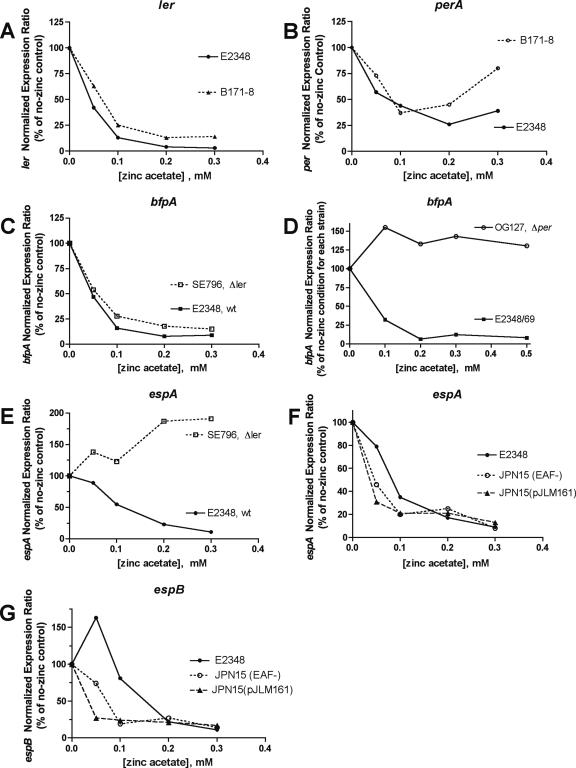
Figure 5 shows experiments performed to try to determine the mechanism by which zinc inhibits the expression of BFP and Esp. Figure 5A shows that zinc inhibits the expression of the LEE-encoded regulator (ler) gene. Figure 5B shows that zinc also inhibits the expression of perA, although not as potently or as completely as it does the expression of ler. The Per proteins activate the transcription of per as well as bfp and ler, so zinc might interfere with Per's ability to activate itself (autoactivation loop). Figure 5C shows that zinc has equal effects on the bfpA gene of the wild-type strain E2348/69 and of the Δler mutant, showing that ler is not required for zinc's inhibition of bfpA. Figure 5D shows the role of per in the inhibitory effect of zinc on bfpA. In the strain with a deletion of perA, perB, and perC (the mutant strain OG127), bfpA RNA was about 1% as abundant as that in the wild-type strain in DMEM in the absence of zinc. However, bfpA expression in strain OG127 was totally insensitive to the inhibition by zinc (Fig. 5D). In the wild-type strain E2348/69, zinc inhibited bfpA expression by nearly 99%, meaning that in the presence of 0.5 mM zinc, the wild type and the Δper mutant had virtually identical levels of bfpA expression. Figure 5D shows that the inhibitory effects of zinc on bfpA require per.
FIG. 5.
Mechanism of action of zinc on EPEC virulence gene expression: role of per and ler. Expression of the indicated genes was examined by reverse transcription-PCR and qRT-PCR, as described in the legend to Fig. 2 and Materials and Methods. EPEC strains and mutants were grown in DMEM for 5 h. (A and B) Effect of zinc on ler and per in wild-type EPEC strains E2348/69 and B171-8. (C) Comparison of the ability of zinc to inhibit bfpA in the Δler mutant and the wild-type (wt) strain. (D) Effect of zinc on bfpA in the Δper mutant strain OG127 compared to that in the wild-type strain E2348/69. (E) Comparison of the ability of zinc to inhibit espA in the Δler mutant and the wild type. (F and G) Comparison of the ability of zinc to inhibit espA and espB expression in the wild-type strain E2348/69, in strain JPN15 lacking the EAF plasmid, and in JPN15(pJLM161), with perABC restored on a plasmid.
Figure 5E shows that the inhibitory effect of zinc on espA is totally abolished in the Δler mutant; in fact, in the Δler mutant, zinc paradoxically increased espA expression by almost twofold.
Figure 5F shows, in contrast, that per was not necessary for zinc's effects on espA. In Fig. 5F, espA inhibition by zinc was not abolished in strain JPN15 (which lacks per carried on the EAF plasmid); instead, zinc appeared slightly more potent in the inhibition of espA expression in the JPN15 strain. Figure 5E to G show that zinc inhibition of espA and espB is dependent on ler but independent of per.
Figure 6 shows the effects of zinc on EPEC infection in the rabbit ileal loop model. Figure 6A, B, and D show the gross appearance of EPEC-infected rabbit ileal loops with and without zinc added once to the loop at the time of infection. Rabbits were euthanized, and intestinal loops were collected at 19 to 20 h after infection. In loops inoculated with EPEC E2348/69 alone (Fig. 6A), the ileal loops were tensely distended with fluid, like sausages. In loops which received 1 mM zinc along with the E2348/69 bacteria, the loops remained flaccid (Fig. 6B). Fluid accumulation in the loops, expressed as the volume-to-length ratio, is shown in Fig. 6C. One millimolar zinc completely blocked EPEC infection-induced fluid secretion into the loops (Fig. 6C; P < 0.05 compared to the value for EPEC alone). Interestingly, 0.1 and 1 mM zinc did not reduce the number of EPEC bacteria recovered from the loop fluid, expressed as CFU per loop (Table 2). The lack of inhibition of EPEC bacterial numbers by zinc accords with the in vitro results (Fig. 1B and inset) and suggests that zinc is not acting simply by inhibiting EPEC growth. Figure 6E shows the histology of healthy rabbit ileum from control (uninfected) loops, while Fig. 6F shows the damage caused by EPEC infection without zinc. Figure 6G and H show the histology of loops infected with bacterial strain E2348/69 plus 0.1 mM zinc and E2348 plus 1 mM zinc, respectively. Ileal tissues were subjected to quantitative histological analysis by a blinded observer, and the EPEC-induced damage score included the villus-to-crypt ratio (a measure of the degree of villus blunting) and the percentage of enteroadherence (the percentage of the villus surface covered by EPEC bacteria). Both concentrations of zinc tested significantly reduced the percentage of enteroadherence by EPEC and protected against villus blunting (increased villus-to-crypt ratio) compared to that of E2348/69 infection without zinc (Table 2).
TABLE 2.
Effects of zinc on bacterial adherence and histopathology in rabbit ileal loops infected with EPEC strain E2348/69
| Infection condition | Histopathologya
|
|||
|---|---|---|---|---|
| Ileal volume-to-length ratio (ml/cm) | Villus-to-crypt ratio | % Enteroadherence | CFU of EPEC recovered from loop fluid (CFU/loop) | |
| E2348/69 without zinc | 0.58 | 2.6 | 5.3 | 3 × 1010 |
| E2348/69 plus 0.1 mM zinc acetate | 0.48 | 2.6 | 1.8* | 1.3 × 1010 |
| E2348/69 plus 1 mM zinc | 0.13* | 3.8* | 0.58* | 2.1 × 1010 |
| Uninfected (phosphate-buffered saline) | 0.14 | 3.9 | 0 | 0 |
*, P < 0.01 compared to E2348-infected cells with no zinc.
Figure 6D shows the gross appearance of a rabbit loop experiment performed with rabbit EPEC strain E22 with and without 1 mM zinc, demonstrating that the effects of zinc are confined to the actual loops receiving zinc, i.e., there was no cross-protection of neighboring loops. This localized protection suggests that zinc is acting primarily within the lumen of the intestine or in the immediate vicinity and not primarily by systemic absorption.
DISCUSSION
We began investigating the effects of zinc on EPEC infection-induced fluid secretion because of its ability to inhibit ecto-5′-nucleotidase, a host cell enzyme needed for the conversion of 5′-AMP to adenosine, a potent secretagogue in human and animal small and large intestines (9). This theory appeared to be borne out by the ability of zinc to block EPEC infection-induced fluid secretion into rabbit intestinal loops (Fig. 5A to C). However, analysis of the rabbit intestinal tissues also showed significantly reduced EPEC adherence and reduced villus blunting, effects which did not accord with the known roles of ecto-5′-nucleotidase. In addition, zinc was more efficacious in the inhibition of EPEC-induced fluid secretion into intestinal loops than α,β-methylene-ADP (data not shown), even though α,β-methylene-ADP is a more potent inhibitor of ecto-5′-nucleotidase than zinc (9, 26, 33). Both of these findings led us to consider whether zinc might be acting on a target or targets other than ecto-5′-nucleotidase.
Zinc, especially at concentrations of 0.5 to 1 mM and at longer durations of subculture, did inhibit EPEC bacterial growth (Fig. 1A). At lower zinc concentrations, the inhibition of EPEC E2348/69 growth was not seen, however (Fig. 1A). At these lower, non-growth-inhibitory concentrations of zinc, strong inhibition by zinc was observed for the expression levels of BFP and EspA at the protein level (Fig. 1 and 3). When analyzed at the level of RNA transcription, zinc strongly inhibited the abundance of RNA transcribed from bfpA and several of the esp genes, as well as the transcriptional regulators perA and ler themselves (Fig. 2 and 4). The inhibitory effects of zinc were not closely mimicked by other transition metals (ferrous and ferric iron, copper, nickel, or manganese). In addition, the effects of zinc were not reversed by an excess of ferrous iron (Fig. 1G).
While this study was under way, Crowther et al. reported that BfpD, a component of the EPEC BFP, is a zinc-dependent ATPase (10). This initially led us to consider whether BfpD might be a target of zinc, leading to the inhibition of BFP expression at high concentrations of zinc. However, BfpD is not inhibited by zinc, even at concentrations as high as 10 mM (L. J. Crowther, personal communication), so this seems to rule out BfpD as a target for the inhibition of BFP or other virulence factors.
The mechanism of action of zinc on EPEC bacteria clearly involved the per gene and the ler gene. Indeed, there was a clear separation of zinc's inhibitory powers on different virulence factors. Zinc's inhibitory effects on bfpA were totally dependent on per but independent of ler (Fig. 5C and D). Conversely, the inhibition of transcription from the esp genes was totally dependent on ler but independent of per (Fig. 5E to G). Zinc had weaker inhibitory effects on the expression of tir and eae. The LEE5 operon, which carries tir, cesT, and eae, was initially reported to be independent of regulation by ler (12, 23). Later it was shown that LEE5 is regulated by ler but that ler regulation of LEE5 is somewhat more complex and more dependent on environmental factors (17). This may explain why the inhibitory effects of zinc on the expression of tir and eae genes are less complete than zinc's effects on other transcripts (Fig. 4C and D and Fig. 7B).
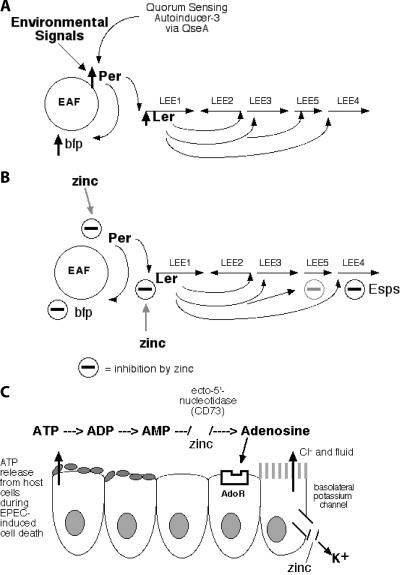
FIG. 7.
Sketch of the effects of zinc on EPEC bacteria and host intestinal secretory pathways. (A) Simplified scheme of regulation of bfp and LEE-carried genes in the absence of zinc. EAF is a large plasmid, whereas the LEE is on the E. coli chromosome. (B) Diagram showing known actions of zinc in EPEC. The effects on zinc on LEE5 appear to be weaker (gray minus sign) than on bfp or on LEE4 carrying the esps gene. (C) Effect of zinc on secretory pathways in host intestinal cells. AdoR, adenosine receptors. Figure 7A is based on experimental findings reported by other authors (23, 30, 31).
The effects of zinc on EPEC bacteria are sufficient to completely explain the decreased adherence of EPEC bacteria to rabbit intestinal epithelium, as seen with HeLa cells (Fig. 1B) and in the rabbit ileal loop infections (Table 2). Zinc strongly inhibits BFP and EspA expression and also inhibits intimin, all of which are important EPEC adhesins (24).
The findings in this study challenge many long-held views about the actions of zinc in infectious diarrhea and about who might benefit from zinc in the prevention or treatment of diarrheal disease. Beneficial effects of zinc supplementation have been observed in treatment and prevention studies of diarrhea in many locations around the world (2, 3, 11, 16, 25, 32). Until recently, these beneficial effects of zinc on the duration and severity of diarrhea were almost always interpreted as the result of correction of a zinc deficiency (11, 35). However, evidence is emerging that the beneficial effects of zinc are not limited to those individuals with a preexisting zinc deficiency. For example, Sazawal et al. found that the protective effects of zinc were about as strong in the entire study population as in those subjects with significant zinc deficiency (plasma zinc levels of <60 μg/dl [29]). Studies with experimental animals challenged with enteric pathogens have also shown protective effects of zinc supplements, even though the animals were well nourished and had normal zinc levels. For example, Kelleher et al. found that zinc provided some added benefit against the challenge by enteropathogenic E. coli (strain E2348/69) in the rhesus monkey compared to that from a probiotic supplement (Lactobacillus reuteri) alone (19); these monkeys were not zinc deficient.
Other cherished beliefs challenged by the results presented here are the ideas that zinc acts by systemic absorption and by bolstering the acquired immune response to pathogens. While some systemic absorption of zinc probably can occur, our results (Fig. 6D) also show that zinc's protective effects can remain localized to the portion of the intestine that actually receives the zinc. This suggests that zinc is acting locally, even within the lumen of the gastrointestinal tract, and that not all its effects require systemic absorption. In addition, the strong protective effects of zinc seen at 20 h of infection seem too early to be attributable either to enhanced humoral or cell-mediated immunity. Last, the relatively high concentrations of zinc needed to see protection in vitro and in vivo have led us to regard zinc as acting more like a drug and less like a micronutrient in its protective effects against EPEC infection.
If zinc is acting by interfering with the pathogen's expression of virulence factors, the effects of zinc may be pathogen specific. In the zinc-diarrhea field trials mentioned above, there was often little diagnostic microbiology performed, and in particular, few attempts were made to diagnose the difficult-to-detect but common pathogens such as rotavirus, enterotoxigenic E. coli, and EPEC. Therefore, field studies of zinc in diarrheal disease did not reveal if there were particular pathogens against which zinc was especially helpful, such as EPEC, or if zinc was beneficial “across the board” for infectious diarrhea in general. Our study raises the possibility that particular pathogens may be more responsive to zinc than others, and identifying other such zinc-susceptible infectious agents should be a goal for future research.
Last, we do not discount the idea that some of the salutary effects of zinc in diarrheal disease are due to effects on host cells (Fig. 7C). Our work suggests that ecto-5′-nucleotidase may be one such target (9). Hoque et al. have also shown that zinc inhibits a basolateral potassium channel which is necessary for the intestinal cell to sustain a chloride secretory response (18), and other new host cell targets of zinc may be identified.
Supplementary Material
Acknowledgments
We thank Michael S. Donnenberg for providing valuable polyclonal antibodies against BFP and EspA and James B. Kaper for providing instructive EPEC mutants. The following graduate students contributed to this research during their rotations through the laboratory: Sandra Small, Stacy Amico, Robert Gerrish, and Jyoti Madhusoodanan.
This work was supported by grants from the NIAID of the National Institutes of Health (R21 066055 and RO1 AI 050652, to J.K.C.).
Editor: F. C. Fang
Footnotes
Published ahead of print on 17 September 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Anonymous. 2005. Real-time PCR applications guide. Bio-Rad Laboratories, Hercules, CA.
- 2.Baqui, A. H., R. E. Black, S. El Arifeen, M. Yunus, J. Chakraborty, S. Ahmed, and J. P. Vaughan. 2002. Effect of zinc supplementation started during diarrhoea on morbidity and mortality in Bangladeshi children: community randomised trial. BMJ 325:1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhandari, N., R. Bahl, S. Taneja, T. Strand, K. Molbak, R. J. Ulvik, H. Sommerfelt, and M. K. Bhan. 2002. Substantial reduction in severe diarrheal morbidity by daily zinc supplementation in young north Indian children. Pediatrics 109:e86. [DOI] [PubMed] [Google Scholar]
- 4.Bieber, D., S. Ramer, C.-Y. Wu, W. Murray, T. Tobe, R. Fernandez, and G. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein by dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Crane, J., S. Choudhari, T. Naeher, and M. Duffey. 2006. Mutual enhancement of virulence by enterotoxigenic and enteropathogenic Escherichia coli. Infect. Immun. 74:1505-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane, J., T. Naeher, S. Choudhari, and E. Giroux. 2005. Two pathways for ATP release from host cells in enteropathogenic Escherichia coli infection. Am. J. Physiol. Gastrointest. Liver Physiol. 289:G407-G417. [DOI] [PubMed] [Google Scholar]
- 8.Crane, J., R. Olson, H. Jones, and M. Duffey. 2002. Release of ATP during host cell killing by enteropathogenic E. coli and its role as a secretory mediator. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G74-G86. [DOI] [PubMed] [Google Scholar]
- 9.Crane, J., I. Shulgina, and T. Naeher. 2007. Ecto-5′-nucleotidase and intestinal ion secretion by enteropathogenic Escherichia coli. Purinergic Signal. 3:233-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowther, L. J., A. Yamagata, L. Craig, J. A. Tainer, and M. S. Donnenberg. 2005. The ATPase activity of BfpD is greatly enhanced by zinc and allosteric interactions with other Bfp proteins. J. Biol. Chem. 280:24839-24848. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Dutta, P., U. Mitra, A. Datta, S. K. Niyogi, S. Dutta, B. Manna, M. Basak, T. S. Mahapatra, and S. K. Bhattacharya. 2000. Impact of zinc supplementation in malnourished children with acute watery diarrhoea. J. Trop. Pediatr. 46:259-263. [DOI] [PubMed] [Google Scholar]
- 12.Elliott, S., V. Sperandio, J. Giron, S. Shin, J. Mellies, L. Wainwright, S. Jutcheson, T. McDaniel, and J. Kaper. 2000. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 68:6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giron, J., A. Ho, and G. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710-713. [DOI] [PubMed] [Google Scholar]
- 14.Giron, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Duarte, O., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta, D. N., S. K. Mondal, S. Ghosh, K. Rajendran, D. Sur, and B. Manna. 2003. Impact of zinc supplementation on diarrhoeal morbidity in rural children of West Bengal, India. Acta Paediatr. 92:531-536. [PubMed] [Google Scholar]
- 17.Haack, K. R., C. L. Robinson, K. J. Miller, J. W. Fowlkes, and J. L. Mellies. 2003. Interaction of Ler at the LEE5 (tir) operon of enteropathogenic Escherichia coli. Infect. Immun. 71:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoque, K., V. Rajendran, and H. Binder. 2005. Zinc inhibits cAMP-stimulated Cl secretion via basolateral K-channel blockade in rat ileum. Am. J. Physiol. Gastrointest. Liver Physiol. 288:G956-G963. [DOI] [PubMed] [Google Scholar]
- 19.Kelleher, S., I. Casa, N. Carbajal, and B. Lonnerdal. 2002. Supplementation of infant formula with the probiotic Lactobacillus reuteri and zinc: impact on enteric infection and nutrition in infant rhesus monkeys. J. Pediatr. Gastroenterol. Nutr. 35:162-168. [DOI] [PubMed] [Google Scholar]
- 20.Leverton, L., and J. Kaper. 2005. Temporal expression of enteropathogenic Escherichia coli virulence genes in an in vitro model of infection. Infect. Immun. 73:1034-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine, M., D. Nalin, R. Hornick, E. Bergquist, D. Waterman, C. Young, S. Sotman, and B. Rowe. 1978. Escherichia coli strains that cause diarrhea but do not produce heat-labile or heat-stable enterotoxins and are not invasive. Lancet 1:1119-1122. [DOI] [PubMed] [Google Scholar]
- 22.Levine, M., J. Nataro, H. Karch, M. Baldini, J. Kaper, R. Black, M. Clements, and A. O'Brien. 1985. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J. Infect. Dis. 152:550-559. [DOI] [PubMed] [Google Scholar]
- 23.Mellies, J. L., S. J. Elliott, V. Sperandio, M. S. Donnenberg, and J. B. Kaper. 1999. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol. Microbiol. 33:296-306. [DOI] [PubMed] [Google Scholar]
- 24.Nougayrede, J.-P., P. Fernandes, and M. S. Donnenberg. 2001. Adhesion of enteropathogenic Escherichia coli to host cells. Cell. Microbiol. 5:359-372. [DOI] [PubMed] [Google Scholar]
- 25.Penny, M. E., J. M. Peerson, R. M. Marin, A. Duran, C. F. Lanata, B. Lonnerdal, R. E. Black, and K. H. Brown. 1999. Randomized, community-based trial of the effect of zinc supplementation, with and without other micronutrients, on the duration of persistent childhood diarrhea in Lima, Peru. J. Pediatr. 135:208-217. [DOI] [PubMed] [Google Scholar]
- 26.Picher, M., L. Burch, A. Hirsh, J. Spychala, and R. Boucher. 2003. Ecto 5′-nucleotidase and nonspecific alkaline phosphatase. J. Biol. Chem. 278:13468-13479. [DOI] [PubMed] [Google Scholar]
- 27.Rothbaum, R., A. McAdams, R. Giannella, and J. Partin. 1982. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology 83:441-454. [PubMed] [Google Scholar]
- 28.Rothbaum, R., J. Partin, K. Saalfield, and A. McAdams. 1983. An ultrastructural study of enteropathogenic Escherichia coli infection in human infants. Ultrastruct. Pathol. 4:291-304. [DOI] [PubMed] [Google Scholar]
- 29.Sazawal, S., R. Black, M. Bhan, N. Bhandari, A. Sinha, and S. Jalla. 1995. Zinc supplementation in young children with acute diarrhea in India. N. Engl. J. Med. 333:839-844. [DOI] [PubMed] [Google Scholar]
- 30.Sircili, M., M. Walters, L. Trabulsi, and V. Sperandio. 2004. Modulation of enteropathogenic Escherichia coli virulence by quorum sensing. Infect. Immun. 72:2329-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spears, K., A. Roe, and D. Gally. 2006. A comparison of enteropathogenic and enterohaemorraghic Escherichia coli pathogenesis. FEMS Microbiol. Lett. 255:187-202. [DOI] [PubMed] [Google Scholar]
- 32.Strand, T. A., R. K. Chandyo, R. Bahl, P. R. Sharma, R. K. Adhikari, N. Bhandari, R. J. Ulvik, K. Molbak, M. K. Bhan, and H. Sommerfelt. 2002. Effectiveness and efficacy of zinc for the treatment of acute diarrhea in young children. Pediatrics 109:898-903. [DOI] [PubMed] [Google Scholar]
- 33.Synnestvedt, K., G. T. Furuta, K. M. Comerford, N. Louis, J. Karhausen, H. K. Eltzschig, K. R. Hansen, L. F. Thompson, and S. P. Colgan. 2002. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Investig. 110:993-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vuopio-Varkila, J., and G. Schoolnik. 1991. Localized adherence by enteropathogenic Escherichia coli is an inducible phenotype associated with the expression of new outer membrane proteins. J. Exp. Med. 174:1167-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wapnir, R. A. 2000. Zinc deficiency, malnutrition and the gastrointestinal tract. J. Nutr. 130:1388S-1392S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.