Abstract
Brucella is a facultative intracellular pathogen of various mammals and the etiological agent of brucellosis. We recently demonstrated that dendritic cells (DCs), which are critical components of adaptive immunity, are highly susceptible to Brucella infection. Furthermore, Brucella prevented the infected DCs from engaging in maturation processes and impaired their capacity to present antigen to naive T cells and to secrete interleukin-12 (IL-12). The lipopolysaccharide (LPS) phenotype is largely associated with the virulence of Brucella. Depending on whether they express the O-side chain of LPS or not, the bacteria display a smooth or rough phenotype. Rough Brucella mutants are attenuated and induce a potent protective T-cell-dependent immune response. Due to the essential role of DCs in the initiation of T-cell-dependent adaptive immune responses, it seemed pertinent to study the interaction between rough Brucella strains and human DCs. In the present paper, we report that, in contrast to smooth bacteria, infection of DCs with rough mutants of Brucella suis or Brucella abortus leads to both phenotypic and functional maturation of infected cells. Rough mutant-infected DCs then acquire the capacity to produce IL-12 and to stimulate naive CD4+ T lymphocytes. Experiments with rough and smooth purified LPS of Brucella supported the hypothesis of an indirect involvement of the O-side chain. These results provide new data concerning the role of LPS in Brucella virulence strategy and illuminate phenomena contributing to immune protection conferred by rough vaccine strains.
Members of the Brucella genus are small gram-negative bacteria. As facultative intracellular pathogens, they induce diseases in a wide range of mammals, such as ruminants, humans, and marine mammals. Brucellosis is one of the five most common bacterial zoonoses in the world (33) and the most frequent bacterial anthropozoonosis (23), with more than 500,000 new cases annually (37). Infections of domestic animals are particularly feared due to systematic abortions of gravid females and chronic orchitis of males. Human infections occur through inhalation of aerosols or consumption of infected food. Also known as Malta fever, human brucellosis consists of acute infection, characterized by undulant fever and asthenia, which develops in 30% of infected patients into a chronic disease with erratic recurrent fevers and localized infections such as endocarditis, encephalitis, and spondylitis. Following invasion of the lymphoid system, the bacteria develop within mononuclear phagocytes, and infected cells may participate in the dissemination of the bacteria in specific locations of the body. A Th2-specific immune response has been reported in chronic brucellosis patients (22, 40). In mice, which are not natural hosts for Brucella and display a certain resistance to infection, the protection is conferred by a Th1-oriented immune response depending on the Th1-specific cytokines gamma interferon (IFN-γ) and interleukin-12 (IL-12) (45, 54, 55). Therefore, the ability of Brucella strains to chronically infect their human hosts seems to be related to their ability to avoid the establishment of a protective Th1-specific response (8, 22, 40, 52). The classical cellular models for in vitro experiments are human or murine macrophages in which brucellae are able to multiply up to several-thousandfold (26, 31). The role of macrophages is mainly restricted to the innate phase of the immune response, so macrophage models provide only a few direct data about the adaptive immune response to Brucella. For this reason, we have developed an experimental model of Brucella interaction with human dendritic cells (DCs). DCs have a common ontogeny with macrophages and have been revealed in the last 10 years as the crucial cell population defining the initial point of a specific immune response. DCs not only select antigen-specific T cells but also determine their subsequent function and orientation (Th1/Th2 effector T cells or regulatory T cells) and consequently determine the final efficiency of the adaptive response.
We have initially observed that human DCs are highly permissive host cells for Brucella (4) and could constitute a preferential niche for bacterial proliferation. These results raised the question of the impact of DC infection on the initiation of an adaptive response. Recently we have reported that Brucella strains avoid the maturation of DCs and their secretion of IL-12 through an Omp25-dependent mechanism regulating tumor necrosis factor alpha (TNF-α) secretion (5). The subsequent antigen presentation to naive T cells was therefore significantly altered. These phenomena could be directly related to the immune status of chronically infected hosts.
Historically, the first parameter associated with the virulence strategy of Brucella has been the smooth phenotype of virulent strains, as demonstrated in 1938 by the attenuation of Brucella abortus rough strain 45/20 (47). The rough phenotype is due to the absence of the external O-side chain of lipopolysaccharide (LPS). This O-side chain protects Brucella from bactericidal peptides (20, 21, 35) and complement-mediated lysis (17) and is implicated in the absence of intracellular fusion between Brucella-containing phagosomes and lysosomes (39). Moreover, spontaneous or genetically constructed rough mutants confer protection against Brucella reinfection in vivo and no longer display residual virulence. For this reason the rough vaccine strain RB51 has supplanted the traditional smooth strain S19 in several parts of the world (36). Our previous results demonstrating Brucella avoidance of DC maturation and impairment of T-cell stimulation (5) prompted us to analyze the relationship between rough Brucella strains and DCs, in order to determine to what extent the lack of the O-side chain could influence the induction of a protective immune response by rough mutants.
MATERIALS AND METHODS
Bacteria.
Bacterial strains mentioned in this study are listed in Table 1 and in our previous publications (4, 28). The bacteria were transformed with pBBR1-KGFPcons (6). The constitutive green fluorescent protein expression allowed rapid monitoring of phagocytosis and intracellular proliferation by fluorescence microscopy or flow cytometry (4). The green fluorescent protein-expressing Escherichia coli S17.1 D3 (50) was a generous gift from A. Givaudan, INRA UMR 1133, Montpellier, France.
TABLE 1.
Brucella strains used in the study
| Strain | Phenotypes | Characteristic(s) and/or source |
|---|---|---|
| B. suis 1330 | Smooth, virulent | WT Brucella suis (ATCC 23444) |
| B. suis manB mutant | Rough, attenuated | B. suis manB::mTn5Km2 mini-Tn5 insertion mutant of B. suis 1330 invalidated on phosphomannose gene, kanamycin resistant |
| B. abortus 2308 | Smooth, virulent | WT Brucella abortus, laboratory collection (CITA, Zaragoza, Spain) |
| B. abortus 45/20 | Rough, attenuated | Vaccine strain of Brucella abortus, laboratory collection (CITA, Zaragoza, Spain) |
Antibodies and reagents.
All antibodies used for DC phenotypic analysis were purchased from BD Pharmingen, San Diego, CA, except for mouse anti-CCR7 (R&D Systems) and anti-HLA ABC (Beckman-Coulter). LPS from E. coli O55:B5 was purchased from Sigma-Aldrich. LPS from B. abortus 2308 (smooth) and B. abortus 45/20 (rough) was a generous gift from I. Moriyón (University of Navarra, Pamplona, Spain) (1, 21).
DC preparation.
Immature DCs were prepared from peripheral blood circulating monocytes obtained by centrifugation on Ficoll-Hypaque (Sigma, Lyon, France) of buffy coat from healthy donors provided by the EFS (Etablissement Français du Sang). CD14+ monocytes were purified by magnetic positive separation (Miltenyi Biotec, Paris, France) and then differentiated for 5 days in complete medium (RPMI 1640, 10% fetal calf serum, 50 μM β-mercaptoethanol, 500 U/ml of IL-4, and 1,000 U/ml of granulocyte-macrophage colony-stimulating factor [both cytokines from Immunotools]) (4).
Infection experiments.
Immature DCs were harvested, resuspended in RPMI plus 10% fetal calf serum, and infected for 1 h at 37°C with bacterial concentrations corresponding to a CFU/DC ratio of 5:1. The cells were then washed in phosphate-buffered saline (Invitrogen) and reincubated in fresh medium supplemented with 50 μg/ml gentamicin in order to kill remaining extracellular bacteria (25).
Maturation analysis.
At 48 h postinfection (p.i.), DCs were labeled with mouse anti-human monoclonal antibodies followed by a phycoerythrin-conjugated goat anti-mouse polyclonal antibody (BD Pharmingen) and analyzed on a FACSCalibur cytometer (Becton Dickinson, San Jose, CA).
Cytokine measurement.
For cytokine measurement, supernatants were collected and concentrations of TNF-α and IL-12 p70 were measured with the OptEIA human enzyme-linked immunosorbent assay set (BD Pharmingen) or quantified by flow cytometry using the CBA Flex set (BD Biosciences).
Antigenic presentation to naive human T lymphocytes.
Human naive CD4+ T cells were prepared using the EasySep human naive CD4+ T-cell enrichment kit (Stem Cell Technologies), according to the manufacturer's instructions. Naive T cells (CD3+ CD4+ CD45RA+) were stained intracellularly at 37°C in RPMI with 1 μM 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE; Sigma-Aldrich), washed extensively in medium, and plated in a 96-well culture plate (105 cells per well). Infected DCs (24 h p.i.) were added at the required concentration so that DC/T-cell ratios ranged from 0 to 0.1. Five days later, the cells were stained with a mouse anti-human CD3 antibody (UCHT1; BD Pharmingen) followed by an Alexa 647 F(ab′)2 fragment of goat anti-mouse immunoglobulin G (IgG) (Molecular Probes, United Kingdom). Analysis was performed by flow cytometry using a FACSCalibur cytometer to detect the decrease of CFSE fluorescence intensity resulting from cellular divisions.
Statistical analysis.
Wilcoxon rank tests or paired Student t tests (in the case of normal distribution) were applied to determine statistical differences, using SigmaStat software.
RESULTS
Induction of human DC maturation by rough Brucella strains.
To determine if the dissimilar immunogenic properties of rough and smooth Brucella strains could originate from differential interactions with DCs, we first analyzed the entry of infected human DCs into maturation processes.
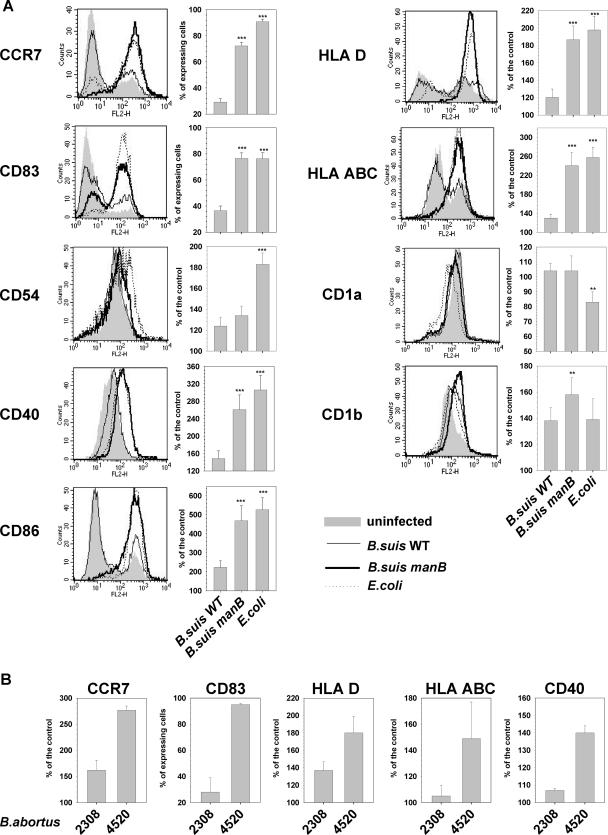
Figure 1A analyzes DC maturation after 48 h of infection with smooth Brucella suis wild type (WT), rough B. suis manB mutant, or E. coli as a positive control, since these bacteria have been reported to induce strong maturation of DCs (48). In contrast to the infection with smooth B. suis WT, infection with the rough B. suis manB mutant induced an up-regulation of maturation marker expression on the DC surface. The expression levels of both costimulation (CCR7, CD83, CD40, and CD86) and antigen presentation (HLA-D and HLA-ABC) molecules were comparable following the infection of DCs with rough Brucella or E. coli. For these six markers, the up-regulation was statistically significant compared to WT-infected DCs (P < 0.001 for each). Nevertheless, the maturation state resulting from infection with the B. suis manB mutant did not seem identical to that induced by E. coli. Indeed, DCs infected with rough Brucella did not show any modulation of the adhesion molecule CD54 (also named ICAM-1) in contrast to E. coli-infected DCs. Moreover, the expression of CD1a was down-modulated neither on DCs infected with the rough mutant nor on DCs infected with the smooth strain, in contrast to the decrease observed with the control E. coli strain. Finally, infection with the rough B. suis manB mutant induced a slight but significant up-regulation of CD1b expression compared to B. suis WT (P < 0.01), whereas fully mature E. coli-infected DCs did not display such an increase in CD1b expression. Altogether these results showed that, in contrast to the virulent smooth strain, the rough attenuated manB strain was able to induce a powerful expression of maturation markers on the surface of infected DCs. Similar results were obtained when the rough vaccine strain B. abortus 45/20 was compared to the smooth and virulent parental strain B. abortus 2308 (Fig. 1B).
FIG. 1.
Analysis of DC maturation in response to infection with rough or smooth Brucella suis. (A) Immature human DCs were infected with B. suis WT, B. suis manB mutant, or E. coli and stained at 48 h p.i. for maturation marker expression. For each surface molecule studied, a cytometry analysis histogram from one representative experiment is presented on the left, and the compilation histogram on the right includes results (means ± standard errors of the means) from 21 independent experiments performed on cells from 21 distinct donors (**, P < 0.01, and ***, P < 0.001, versus B. suis WT-infected DCs, computed by Wilcoxon rank tests for CCR7 and CD83 or by paired Student t tests for other markers). (B) Immature DCs were infected with B. abortus 2308 and B. abortus 45/20 and stained at 48 h p.i. for maturation marker expression. Histograms include results from three independent experiments performed with cells from three distinct donors.
TNF-α secretion by human DCs infected with rough strains.
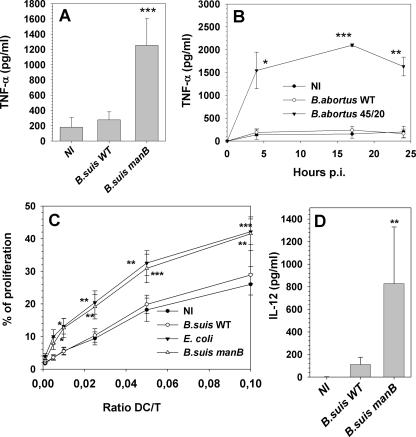
In a previous work (5) we established that the lack of DC maturation during infection with smooth virulent B. suis was related to the absence of TNF-α secretion. Indeed this cytokine is absolutely essential for DC commitment to maturation processes (43, 51). As rough strains of Brucella are potent inducers of DC maturation, we measured TNF-α secretion during infection of human DCs. As expected, infection with virulent smooth WT B. suis (Fig. 2A) or B. abortus (Fig. 2B) did not induce a higher secretion of TNF-α than that measured in uninfected immature DCs. By contrast, infection with the manB mutant of B. suis led to a statistically significant secretion of TNF-α at 24 h p.i. (P < 0.001) which is approximately 1,000 pg/ml above the basal level measured with uninfected or WT-infected DCs (Fig. 2A). Kinetic analysis of TNF-α secretion by DCs infected with smooth B. abortus WT and rough mutant 45/20 confirmed this result: smooth Brucella did not induce any TNF-α secretion but rough mutants did (Fig. 2B). Additionally, it demonstrated that TNF-α secretion induced by rough Brucella appeared early after the onset of infection and peaked around 17 h p.i.
FIG. 2.
TNF-α secretion, induction of naive T-cell proliferation, and IL-12 secretion by human DCs infected with smooth and rough Brucella strains. (A, B, and D) Immature human DCs were infected with B. suis WT and B. suis manB mutant (A and D) or with B. abortus WT and B. abortus 45/20 (B). Supernatants were collected at 24 h p.i. (A and D) or at different times p.i. (B) and assayed for TNF-α concentrations (A and B) or IL-12 concentrations (D). Results are means ± standard errors of the means of 15 (A and D) or 3 (B) independent experiments performed on cells from distinct donors. (C) At 24 h p.i. DCs not infected (NI) or infected with B. suis WT, B. suis manB mutant, or E. coli were tested for their abilities to stimulate allogeneic naive CD4+ T-lymphocyte proliferation. Coculture of infected DCs and T cells was performed at different DC/T-cell ratios for 5 days before determination of lymphoproliferation percentages by CFSE analysis. Results are means ± standard errors of the means of nine independent experiments performed on cells from nine distinct donors. Statistical differences versus Brucella WT-infected DCs are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001, all computed by paired Student t tests).
Stimulation of naive T lymphocytes by human DCs infected with smooth or rough Brucella strains.
Analysis of the expression of surface molecules related to DC maturation is restricted to a phenotypic but not functional description of the infection impact on DC physiology. Therefore, we studied the interaction between infected DCs and naive T cells, in order to determine whether DC maturation resulting from infection with rough strains could allow an effective antigen presentation. Interaction of mature DCs with naive T lymphocytes usually leads to the induction of T-cell proliferation, so we tested the stimulatory properties of DCs infected with rough Brucella. To perform these experiments, immature DCs were infected with smooth B. suis WT, the rough B. suis manB mutant, or E. coli as a positive control for antigen presentation activity of fully mature DCs. At 24 h p.i., DCs were put in contact with allogeneic human naive CD45RA+ CD4+ T cells stained intracellularly with CFSE and plated at different DC/T-cell ratios varying from 0.1 to 0.005 (i.e., from 10 T cells per DC to 200 T cells per DC). T-lymphocyte proliferation was evaluated 5 days later by flow cytometry analysis, as shown in Fig. 2C. E. coli-infected DCs clearly showed a higher capacity to induce the response of naive T cells than noninfected DCs (closed triangles versus closed circles). As previously demonstrated (5), DCs infected with virulent B. suis were unable to provide a more efficient stimulation of T-cell proliferation than immature noninfected DCs. By contrast, DCs infected with the rough manB mutant acquired the capacity to induce powerful proliferation of naive T cells, quantitatively similar to that observed with E. coli-infected cells (open triangles versus closed triangles) and noticeably improved compared to smooth B. suis WT (open circles versus open triangles). This result confirmed that rough Brucella strains not only induced the up-regulation of maturation markers on the DC surface but were also able to stimulate an efficient antigen presentation activity within these cells.
Secretion of IL-12 by Brucella-infected human DCs.
When mature DCs present antigens to naive T cells and stimulate their proliferation, they also influence the subsequent polarization of the adaptive immune response towards a Th1 or a Th2 profile, through differential cytokine secretion. IL-12 is a key cytokine secreted by DCs and drives the establishment of a Th1 response. Figure 2D presents IL-12 secretion by human DCs infected with B. suis WT or the B. suis manB mutant. The rough strain induced a significantly higher secretion of IL-12 than did smooth B. suis (P = 0.01, n = 15): it was on the average 200-fold greater for each individual experiment.
Induction of DC maturation by rough and smooth Brucella LPS.
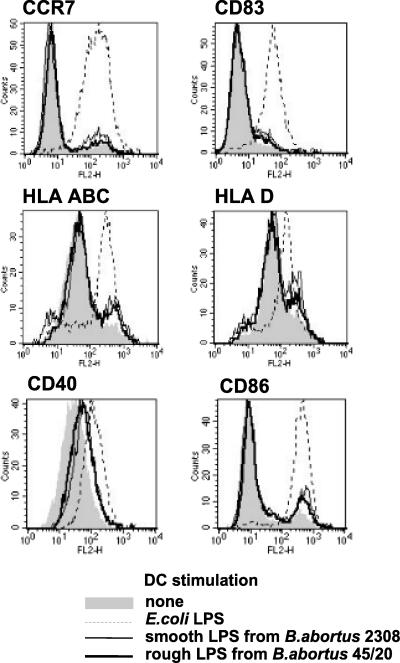
To determine whether different capacities to induce DC maturation and antigen-presenting activities of smooth and rough Brucella strains were attributable to LPS alone, human immature DCs were stimulated with rough LPS from B. abortus 45/20 or smooth LPS from B. abortus 2308. After 48 h of stimulation, modulation of maturation marker expression was analyzed by flow cytometry (Fig. 3). At equal concentrations, E. coli LPS induced a potent up-regulation of maturation marker expression, whereas both smooth and rough Brucella LPS appeared far less active for all considered markers. The comparison of the stimulatory activities of rough and smooth Brucella LPS showed no detectable difference: (i) the percentages of LPS-treated DCs expressing CCR7 and CD83 remained low and equivalent, and (ii) the slight up-regulation of the antigen-presenting molecules HLA-ABC and HLA-D and of the costimulation molecule CD86 were equivalent for each type of LPS. The modulation of CD40 was more pronounced but still similar with rough and smooth Brucella LPS.
FIG. 3.
Analysis of DC maturation in response to stimulation with rough or smooth LPS from B. abortus. Human immature DCs were stimulated for 48 h with 200 ng of E. coli smooth LPS, 200 ng smooth LPS from B. abortus 2308, or 100 ng of rough LPS from B. abortus 45/20 or were not stimulated. The concentration of smooth LPS was doubled to take into account the absence of O-side chain on rough LPS (28). Cytometry analysis histograms of maturation marker expression are from one experiment which is representative of three independent experiments.
DISCUSSION
We have recently established that, unlike most intracellular bacteria, Brucella strains invade human DCs, grow extensively within them (4), and do not induce their maturation (5). To determine whether the protective immunological properties of rough strains could be explained by a particular interaction with DCs, we analyzed the impact of the infection with rough mutants on human DC physiology.
We observed that, in contrast to infection with the WT smooth strain, infection with rough Brucella leads to a powerful up-regulation of maturation marker expression at the surface of infected DCs. The potent acquisition of CCR7 by infected cells after contact with rough Brucella implies that DCs become able to translocate into the lymph node, a prerequisite to encounter antigen-specific T lymphocytes (41). Rough Brucella strains also have the capacity to induce the expression of costimulatory molecules involved in T-cell/DC interaction within the lymph node, especially CD83 and CD40, which are crucial for T-cell activation.
To stimulate antigen-specific T cells, peptidic antigens derived from the degradation of pathogen proteins are loaded onto major histocompatibility complex class II (MHC-II) and MHC-I to be recognized by CD4+ or CD8+ T cells, respectively. Infection with rough Brucella leads to a powerful expression of these two antigen-presenting molecules, in contrast to infection with smooth strains. It suggests that, following contact with rough Brucella, infected DCs are able to induce a CD4+ as well as a CD8+ T-cell response. This is in agreement with several reports establishing that the protection conferred by the rough vaccine strain RB51 is exclusively provided by a T-cell response (30) and that CD8+ T cells play a major role in protection against brucellosis (2).
Besides the classical MHC molecules, human DCs express several antigen-presenting molecules from the CD1 family (CD1a, -b, -c, and -d) which allow lipid antigen presentation. CD1s are involved in the induction of adaptive cellular responses and especially in the activation of CD1-restricted CD8+ T cells (through CD1a, CD1b, and CD1c), γ1δ1 T cells (through CD1c), or NKT cells (through CD1d). In this study, we did not observe any modulation of CD1c or CD1d expression (data not shown), and similar CD1a expression was reported for rough and smooth strain-infected DCs. By contrast, rough Brucella infection induced a significant up-regulation of CD1b expression compared to infection with smooth B. suis WT. Such an event could result from the discrepancy of intracellular trafficking between smooth and rough Brucella strains. In contrast to the other CD1 family members, intracellular CD1b is mainly distributed in phagolysosomes (46). Phagosomes containing rough Brucella fuse very efficiently with late endosomes and lysosomes (39, 44), which could lead to their colocalization with CD1b, in contrast to smooth virulent Brucella strains that avoid phagosome/lysosome fusion (10, 11). Colocalization with rough Brucella could allow subsequent CD1b loading by lipid antigens followed by translocation to the plasma membrane. Further investigations of simultaneous intracellular trafficking of Brucella and CD1 family members, together with the analysis of the CD1-restricted T-cell response in brucellosis, will be needed to elucidate these phenomena.
DC maturation induced by rough Brucella infection is similar but not identical to that resulting from E. coli infection: (i) the adhesin ICAM-1 (CD54) is weakly modulated by rough Brucella compared to E. coli, and (ii) E. coli-infected DCs express lower amounts of CD1a and CD1b. These results indicate that rough Brucella and E. coli do not trigger exactly the same maturation processes.
Our analyses of maturation marker expression established that, in contrast to smooth virulent bacteria, rough Brucella strains are able to induce a phenotypic maturation of infected human DCs. TNF-α is a multipotent proinflammatory cytokine fundamental for defense against a variety of intracellular pathogens and is primarily involved in DC maturation (43, 51). The assessment of TNF-α secretion by DCs infected with rough B. suis or B. abortus showed that, in contrast to smooth virulent strains, these rough mutants were able to trigger a potent and early secretion of this cytokine. The control of TNF-α secretion by human host cells implicates the Omp25 protein of Brucella (32), which is expressed at the outer membrane of both smooth and rough Brucella strains (12, 13). The consistent secretion of TNF-α by DCs infected with rough bacteria could be due to the higher stimulatory activity of rough strains (28, 29), which could then exceed the inhibitory activity of Omp25 or trigger activation pathways which escape the Omp25 inhibitory activity. As proposed by previous studies (43, 51), TNF-α secretion by human DCs infected with rough Brucella as well as E. coli turned out to be directly implicated in maturation of these cells, since anti-TNF-α blocking antibodies cause a strong maturation decrease (data not shown).
As expected from maturation analyses, the investigation of naive T-cell stimulation by infected DCs showed that rough Brucella induced the acquisition of a potent antigen presentation activity. The resulting proliferation of naive CD4+ lymphocytes could be superimposed on that induced by E. coli, attesting to its efficiency and suggesting that the induction could have risen to a maximum. Rough mutants of Brucella are thus able to initiate the first phase of a T-cell-dependent adaptive immune response. Furthermore, we have observed that rough Brucella-infected DCs, concomitantly with T-cell stimulation, secrete the cytokine IL-12. DC-derived IL-12 potently stimulates IFN-γ production by activated naive T cells (15). Therefore, our results suggest that, in contrast to smooth strains of Brucella, infection with rough mutants could trigger the early processes leading to the development of a protective Th1-oriented immune response. These conclusions would be in agreement with previous reports claiming the essential role of IL-12 and IFN-γ (and more generally of the Th1 response) for protection against brucellosis (2) and with the T-cell dependence of vaccine protection conferred by rough Brucella strains (30).
In view of the discrepancies in DC responses to infection with rough or smooth Brucella strains, the direct effect of purified rough or smooth LPS molecules on DC maturation was explored. Although the stimulation was performed with an LPS dose corresponding to more than 10-fold the amount carried by bacteria during our infection experiments (28), DCs displayed a very weak modulation of maturation marker expression. Above all, no difference could be determined between their responses to rough or smooth LPS of Brucella abortus. These results are in line with the very low endotoxinic properties of Brucella LPS (24, 27, 42) and with the equivalent stimulation of macrophages by rough and smooth LPS (28). It means that the ability of rough Brucella strains to induce DC maturation is not related to a direct effect of their LPS. The discrepancies between smooth and rough Brucella stimulatory activities agree with a previous hypothesis (28): the absence of the O-side chain could allow the exposure of bacterial surface molecules that should normally be hidden. These unmasked determinants would then be responsible for the stimulation of DCs and for distinct phagocytosis pathways (4, 39).
The precise role of LPS in the induction of anti-Brucella immunity is still unclear (except for its role in anti-LPS T-cell-independent response). Previous works have established, in a murine macrophage model, that purified smooth LPS from B. abortus 2308 could affect antigen presentation by segregating surface MHC-II molecules into megarafts on the macrophage membrane (18, 19, 34). Such a phenomenon could also occur during DC infection and take part in control of DC maturation by smooth bacteria. Since rough LPS of Brucella 45/20 does not display such properties (34), rough strains would then be unable to prevent DC maturation. Nevertheless, the formation of MHC-II-containing megarafts required a high dose of purified LPS (20 μg/ml) and LPS-containing macrodomains have never been observed in macrophages infected with whole bacteria (16, 38). Moreover, it does not prevent up-regulation of MHC-II expression on the cell surface. Consequently, we cannot definitely evaluate the possible involvement of such a process in our observations.
Classical LPS activates macrophages and DCs through binding on TLR-4. One study reports that TLR-4 is not implicated in the anti-Brucella response in mice (53), whereas another describes exacerbated brucellosis in TLR-4-knockout mice (9). Nevertheless, the respective effects of DC stimulation by isolated LPS or by living bacteria are clearly distinct (48), even when the bacteria carry a highly active LPS (as E. coli for instance): the whole bacteria probably bind not only to TLR-4 but also to a set of various receptors. A very recent study exploring the mechanisms of mouse resistance to Brucella reports that TLR-4 could play a moderate role, together with TLR-9, in the late phase of the infection. Moreover, this paper confirms the major role of IFN-γ in anti-Brucella resistance and demonstrates the role of nitric oxide production by a subpopulation of DCs (14).
The intracellular bacterium Coxiella burnetii, which is the agent of the zoonotic Q fever, displays features in common with Brucella at physiopathological, epidemiological, and bacteriological levels as well. Coxiella shares with Brucella the rare capacity to proliferate within DCs (together with Francisella, they are the only three bacterial genera having this capacity [3, 7]) and to not induce DC maturation during infection (49). Coxiella rough mutants, which are defective for the O-side chain of LPS, induce full maturation of human DCs and trigger TNF-α and IL-12 secretion (49). Consequently it seems that Coxiella and Brucella interact with DCs in quite similar ways; it could be related to the major role of their LPS within their virulence strategies and could furthermore account for physiopathological similarities.
Finally, the ability of rough Brucella to induce maturation of DCs and then the secretion of Th1-related cytokines and the stimulation of naive T cells could be a critical parameter of the protective Th1 cellular immune response induced by these mutants (2, 30). Considering the irreplaceable role of DCs for both initiation and orientation of adaptive immune responses, our results also suggest that the model of Brucella/DC interaction could be particularly more relevant than the traditional macrophage models for the comprehension of the relationship between Brucella virulence and specific immune responses of infected hosts. It could be a precious tool allowing an improved approach for the initial identification of candidate strains in future vaccine strategies.
Acknowledgments
This work was supported by institutional grants from INSERM.
We thank Viviane Zomosa for attentively correcting and improving the manuscript.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 15 October 2007.
REFERENCES
- 1.Aragon, V., R. Diaz, E. Moreno, and I. Moriyon. 1996. Characterization of Brucella abortus and Brucella melitensis native haptens as outer membrane O-type polysaccharides independent from the smooth lipopolysaccharide. J. Bacteriol. 178:1070-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin, C. L., and R. Goenka. 2006. Host immune responses to the intracellular bacteria Brucella: does the bacteria instruct the host to facilitate chronic infection? Crit. Rev. Immunol. 26:407-442. [DOI] [PubMed] [Google Scholar]
- 3.Ben Nasr, A., J. Haithcoat, J. E. Masterson, J. S. Gunn, T. Eaves-Pyles, and G. R. Klimpel. 2006. Critical role for serum opsonins and complement receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in phagocytosis of Francisella tularensis by human dendritic cells (DC): uptake of Francisella leads to activation of immature DC and intracellular survival of the bacteria. J. Leukoc. Biol. 80:774-786. [DOI] [PubMed] [Google Scholar]
- 4.Billard, E., C. Cazevieille, J. Dornand, and A. Gross. 2005. High susceptibility of human dendritic cells to invasion by the intracellular pathogens Brucella suis, B. abortus, and B. melitensis. Infect. Immun. 73:8418-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billard, E., J. Dornand, and A. Gross. 2007. Brucella suis prevents human dendritic cell maturation and antigen presentation through regulation of tumor necrosis factor alpha secretion. Infect. Immun. 75:4980-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevieille, J. P. Liautard, M. Ramuz, and D. O'Callaghan. 2002. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. USA 99:1544-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosio, C. M., and S. W. Dow. 2005. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 175:6792-6801. [DOI] [PubMed] [Google Scholar]
- 8.Briones, G., N. Inon de Iannino, M. Roset, A. Vigliocco, P. S. Paulo, and R. A. Ugalde. 2001. Brucella abortus cyclic beta-1,2-glucan mutants have reduced virulence in mice and are defective in intracellular replication in HeLa cells. Infect. Immun. 69:4528-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campos, M. A., G. M. S. Rosinha, I. C. Almeida, X. S. Salgueiro, B. W. Jarvis, G. A. Splitter, N. Qureshi, O. Bruna-Romero, R. T. Gazzinelli, and S. C. Oliveira. 2004. Role of Toll-like receptor 4 in induction of cell-mediated immunity and resistance to Brucella abortus infection in mice. Infect. Immun. 72:176-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celli, J., C. de Chastellier, D. M. Franchini, J. Pizarro-Cerda, E. Moreno, and J. P. Gorvel. 2003. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198:545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celli, J. 2006. Surviving inside a macrophage: the many ways of Brucella. Res. Microbiol. 157:93-98. [DOI] [PubMed] [Google Scholar]
- 12.Cloeckaert, A., J. M. Verger, M. Grayon, and N. Vizcaino. 1996. Molecular and immunological characterization of the major outer membrane proteins of Brucella. FEMS Microbiol. Lett. 145:1-8. [DOI] [PubMed] [Google Scholar]
- 13.Cloeckaert, A., M. S. Zygmunt, G. Bezard, and G. Dubray. 1996. Purification and antigenic analysis of the major 25-kilodalton outer membrane protein of Brucella abortus. Res. Microbiol. 147:225-235. [DOI] [PubMed] [Google Scholar]
- 14.Copin, R., P. De Baetselier, Y. Carlier, J. J. Letesson, and E. Muraille. 2007. Myd88-dependent activation of B220-CD11b+LY-6C+ dendritic cells during Brucella melitensis infection. J. Immunol. 178:5182-5191. [DOI] [PubMed] [Google Scholar]
- 15.de Jong, E. C., H. H. Smits, and M. L. Kapsenberg. 2005. Dendritic cell-mediated T cell polarization. Springer Semin. Immunopathol. 26:289-307. [DOI] [PubMed] [Google Scholar]
- 16.Delrue, R. M., M. Martinez-Lorenzo, P. Lestrate, I. Danese, V. Bielarz, P. Mertens, X. De Bolle, A. Tibor, J. P. Gorvel, and J. J. Letesson. 2001. Identification of Brucella spp. genes involved in intracellular trafficking. Cell. Microbiol. 3:487-497. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Prada, C. M., M. Nikolich, R. Vemulapalli, N. Sriranganathan, S. M. Boyle, G. G. Schurig, T. L. Hadfield, and D. L. Hoover. 2001. Deletion of wboA enhances activation of the lectin pathway of complement in Brucella abortus and Brucella melitensis. Infect. Immun. 69:4407-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forestier, C., E. Moreno, J. Pizarro-Cerda, and J. P. Gorvel. 1999. Lysosomal accumulation and recycling of lipopolysaccharide to the cell surface of murine macrophages, an in vitro and in vivo study. J. Immunol. 162:6784-6791. [PubMed] [Google Scholar]
- 19.Forestier, C., F. Deleuil, N. Lapaque, E. Moreno, and J. P. Gorvel. 2000. Brucella abortus lipopolysaccharide in murine peritoneal macrophages acts as a down-regulator of T cell activation. J. Immunol. 165:5202-5210. [DOI] [PubMed] [Google Scholar]
- 20.Freer, E., E. Moreno, I. Moriyón, J. Pizarro-Cerdá, A. Weintraub, and J.-P. Gorvel. 1996. Brucella-Salmonella lipopolysaccharide chimeras are less permeable to hydrophobic probes and more sensitive to cationic peptides and EDTA than are their native Brucella sp. counterparts. J. Bacteriol. 178:5867-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freer, E., J. Pizarro-Cerda, A. Weintraub, J. A. Bengoechea, I. Moriyon, K. Hultenby, J. P. Gorvel, and E. Moreno. 1999. The outer membrane of Brucella ovis shows increased permeability to hydrophobic probes and is more susceptible to cationic peptides than are the outer membranes of mutant rough Brucella abortus strains. Infect. Immun. 67:6181-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giambartolomei, G. H., M. V. Delpino, M. E. Cahanovich, J. C. Wallach, P. C. Baldi, C. A. Velikovsky, and C. A. Fossati. 2002. Diminished production of T helper 1 cytokines correlates with T cell unresponsiveness to Brucella cytoplasmic proteins in chronic human brucellosis. J. Infect. Dis. 186:252-259. [DOI] [PubMed] [Google Scholar]
- 23.Godfroid, J., A. Cloeckaert, J. Liautard, S. Kohler, D. Fretin, K. Walravens, B. Garin-Bastuji, and J. Letesson. 2005. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 36:313-326. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein, J., T. Hoffman, C. Frasch, E. F. Lizzio, P. R. Beining, D. Hochstein, Y. L. Lee, R. D. Angus, and B. Golding. 1992. Lipopolysaccharide (LPS) from Brucella abortus is less toxic than that from Escherichia coli, suggesting the possible use of B. abortus or LPS from B. abortus as a carrier in vaccines. Infect. Immun. 60:1385-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross, A., M. Bouaboula, P. Casellas, J. P. Liautard, and J. Dornand. 2003. Subversion and utilization of the host cell cyclic adenosine 5′-monophosphate/protein kinase A pathway by Brucella during macrophage infection. J. Immunol. 170:5607-5614. [DOI] [PubMed] [Google Scholar]
- 26.Gross, A., S. Spiesser, A. Terraza, B. Rouot, E. Caron, and J. Dornand. 1998. Expression and bactericidal activity of nitric oxide synthase in Brucella suis-infected murine macrophages. Infect. Immun. 66:1309-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarvis, B. W., T. H. Harris, N. Qureshi, and G. A. Splitter. 2002. Rough lipopolysaccharide from Brucella abortus and Escherichia coli differentially activates the same mitogen-activated protein kinase signaling pathways for tumor necrosis factor alpha in RAW 264.7 macrophage-like cells. Infect. Immun. 70:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez de Bagues, M. P., A. Gross, A. Terraza, and J. Dornand. 2005. Regulation of the mitogen-activated protein kinases by Brucella spp. expressing a smooth and rough phenotype: relationship to pathogen invasiveness. Infect. Immun. 73:3178-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jimenez de Bagues, M. P., A. Terraza, A. Gross, and J. Dornand. 2004. Different responses of macrophages to smooth and rough Brucella spp.: relationship to virulence. Infect. Immun. 72:2429-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiménez de Bagüés, M. P., P. H. Elzer, S. M. Jones, J. M. Blasco, F. M. Enright, G. G. Schurig, and A. J. Winter. 1994. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect. Immun. 62:4990-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jimenez de Bagues, M. P., S. Dudal, J. Dornand, and A. Gross. 2005. Cellular bioterrorism: how Brucella corrupts macrophage physiology to promote invasion and proliferation. Clin. Immunol. 114:227-238. [DOI] [PubMed] [Google Scholar]
- 32.Jubier-Maurin, V., R.-A. Boigegrain, A. Cloeckaert, A. Gross, M.-T. Alvarez-Martinez, A. Terraza, J. Liautard, S. Kohler, B. Rouot, J. Dornand, and J. P. Liautard. 2001. Major outer membrane protein Omp25 of Brucella suis is involved in inhibition of tumor necrosis factor alpha production during infection of human macrophages. Infect. Immun. 69:4823-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko, J., and G. A. Splitter. 2003. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin. Microbiol. Rev. 16:65-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapaque, N., F. Forquet, C. de Chastellier, Z. Mishal, G. Jolly, E. Moreno, I. Moriyon, J. E. Heuser, H. He, and J. Gorvel. 2006. Characterization of Brucella abortus lipopolysaccharide macrodomains as mega rafts. Cell. Microbiol. 8:197-206. [DOI] [PubMed] [Google Scholar]
- 35.Martinez de Tejada, G., J. Pizarro-Cerda, E. Moreno, and I. Moriyon. 1995. The outer membranes of Brucella spp. are resistant to bactericidal cationic peptides. Infect. Immun. 63:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moriyon, I., M. J. Grillo, D. Monreal, D. Gonzalez, C. Marin, I. Lopez-Goni, R. C. Mainar-Jaime, E. Moreno, and J. M. Blasco. 2004. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet. Res. 35:1-38. [DOI] [PubMed] [Google Scholar]
- 37.Pappas, G., P. Panagopoulou, L. Christou, and N. Akritidis. 2006. Brucella as a biological weapon. Cell. Mol. Life Sci. 63:2229-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pizarro-Cerdá, J., M. Desjardins, E. Moreno, S. Akira, and J. P. Gorvel. 1999. Modulation of endocytosis in nuclear factor IL-6(−/−) macrophages is responsible for a high susceptibility to intracellular bacterial infection. J. Immunol. 162:3519-3526. [PubMed] [Google Scholar]
- 39.Porte, F., A. Naroeni, S. Ouahrani-Bettache, and J. P. Liautard. 2003. Role of the Brucella suis lipopolysaccharide O antigen in phagosomal genesis and in inhibition of phagosome-lysosome fusion in murine macrophages. Infect. Immun. 71:1481-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rafiei, A., S. K. Ardestani, A. Kariminia, A. Keyhani, M. Mohraz, and A. Amirkhani. 2006. Dominant Th1 cytokine production in early onset of human brucellosis followed by switching towards Th2 along prolongation of disease. J. Infect. 53:315-324. [DOI] [PubMed] [Google Scholar]
- 41.Randolph, G. J., G. Sanchez-Schmitz, and V. Angeli. 2005. Factors and signals that govern the migration of dendritic cells via lymphatics: recent advances. Springer Semin. Immunopathol. 26:273-287. [DOI] [PubMed] [Google Scholar]
- 42.Rasool, O., E. Freer, E. Moreno, and C. Jarstrand. 1992. Effect of Brucella abortus lipopolysaccharide on oxidative metabolism and lysozyme release by human neutrophils. Infect. Immun. 60:1699-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritter, U., A. Meissner, J. Ott, and H. Korner. 2003. Analysis of the maturation process of dendritic cells deficient for TNF and lymphotoxin-alpha reveals an essential role for TNF. J. Leukoc. Biol. 74:216-222. [DOI] [PubMed] [Google Scholar]
- 44.Rittig, M. G., A. Kaufmann, A. Robins, B. Shaw, H. Sprenger, D. Gemsa, V. Foulongne, B. Rouot, and J. Dornand. 2003. Smooth and rough lipopolysaccharide phenotypes of Brucella induce different intracellular trafficking and cytokine/chemokine release in human monocytes. J. Leukoc. Biol. 74:1045-1055. [DOI] [PubMed] [Google Scholar]
- 45.Sathiyaseelan, J., R. Goenka, M. Parent, R. M. Benson, E. A. Murphy, D. Fernandesa, A. S. Foulkes, and C. L. Baldwin. 2006. Treatment of Brucella-susceptible mice with IL-12 increases primary and secondary immunity. Cell. Immunol. 243:1-9. [DOI] [PubMed] [Google Scholar]
- 46.Schaible, U. E., and S. H. Kaufmann. 2000. CD1 and CD1-restricted T cells in infections with intracellular bacteria. Trends Microbiol. 8:419-425. [DOI] [PubMed] [Google Scholar]
- 47.Schurig, G. G., N. Sriranganathan, and M. J. Corbel. 2002. Brucellosis vaccines: past, present and future. Vet. Microbiol. 90:479-496. [DOI] [PubMed] [Google Scholar]
- 48.Scott, K., M. Manunta, C. Germain, P. Smith, M. Jones, P. Mitchell, D. Dessi, K. B. Bamford, R. I. Lechler, P. L. Fiori, G. R. Foster, and G. Lombardi. 2005. Qualitatively distinct patterns of cytokines are released by human dendritic cells in response to different pathogens. Immunology 116:245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shannon, J. G., D. Howe, and R. A. Heinzen. 2005. Virulent Coxiella burnetii does not activate human dendritic cells: role of lipopolysaccharide as a shielding molecule. Proc. Natl. Acad. Sci. USA 102:8722-8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sicard, M., K. Brugirard-Ricaud, S. Pagès, A. Lanois, N. E. Boemare, M. Brehélin, and A. Givaudan. 2004. Stages of infection during the tripartite interaction between Xenorhabdus nematophila, its nematode vector, and insect hosts. Appl. Environ. Microbiol. 70:6473-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trevejo, J. M., M. W. Marino, N. Philpott, R. Josien, E. C. Richards, K. B. Elkon, and E. Falck-Pedersen. 2001. TNF-alpha-dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc. Natl. Acad. Sci. USA 98:12162-12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vemulapalli, R., Y. He, S. M. Boyle, N. Sriranganathan, and G. G. Schurig. 2000. Brucella abortus strain RB51 as a vector for heterologous protein expression and induction of specific Th1-type immune responses. Infect. Immun. 68:3290-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss, D. S., K. Takeda, S. Akira, A. Zychlinsky, and E. Moreno. 2005. Myd88, but not Toll-like receptors 4 and 2, is required for efficient clearance of Brucella abortus. Infect. Immun. 73:5137-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhan, Y., and C. Cheers. 1998. Control of IL-12 and IFN-gamma production in response to live or dead bacteria by TNF and other factors. J. Immunol. 161:1447-1453. [PubMed] [Google Scholar]
- 55.Zhan, Y., Z. Liu, and C. Cheers. 1996. Tumor necrosis factor alpha and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect. Immun. 64:2782-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]