Abstract
An important consideration for antichlamydial vaccine development is the induction of cross-serovar protection, since multiple serovars (D to L) of Chlamydia trachomatis cause genital infections. We have shown previously that vaccination with C. trachomatis-derived recombinant chlamydial protease-like activity factor (rCPAF) induced significant earlier resolution of Chlamydia muridarum infection and reduced oviduct pathology. However, the vaccinated mice continued to shed chlamydiae for up to 2 weeks after challenge. In this study, C. trachomatis serovar D recombinant proteins, such as recombinant major outer membrane protein (rMOMP), recombinant inclusion membrane protein A (rIncA), and rCPAF were administered intranasally, individually or in combinations, with murine interleukin-12 (IL-12) as an adjuvant, and cross-species immunity against intravaginal C. muridarum infection was examined. Immunization with rCPAF plus IL-12 (rCPAF+IL-12), compared to immunization with rIncA+IL-12 or rMOMP+IL-12, induced the greatest antigen-specific gamma interferon production from purified CD4+ T cells and concurrently enhanced serum antibody production. All (100%) the animals vaccinated with rCPAF+IL-12 alone or in any combination completely resolved the infection by day 18 after challenge compared to animals vaccinated with rIncA+IL-12 (50%), rMOMP+IL-12 (33%), or phosphate-buffered saline (mock vaccinated; 0%). Moreover, oviduct pathology in mice vaccinated by any regimen that included rCPAF, but not rMOMP+IL-12 or rIncA+IL-12 alone, was markedly reduced compared to mock-immunized animals. The addition of rMOMP and/or rIncA did not significantly enhance the rCPAF+IL-12-induced effect on bacterial clearance or oviduct pathology. These results suggest a greater conservation of protective linear antigenic epitopes within CPAF than MOMP or IncA across the examined serovars and the need to identify other highly conserved antigens for use with rCPAF in a multisubunit recombinant vaccine.
Chlamydia trachomatis is an obligate intracellular gram-negative bacterium that is the leading cause of bacterial sexually transmitted disease worldwide (4, 21). The majority of genital chlamydial infections are initially asymptomatic and untreated, despite the availability of effective antimicrobial therapy, and may lead to severe complications, such as pelvic inflammatory disease, ectopic pregnancy, and infertility (4, 19, 21, 38, 39). Additionally, the incidence rates of genital chlamydial infections have increased over the last decade, indicating the need for timely development of an efficacious chlamydial vaccine (4).
Multiple serovars (D to L) of the organism cause genital infections in humans (4, 21). Therefore, an ideal antichlamydial vaccine should induce cross-serovar immunity. We have recently demonstrated that immunization with recombinant chlamydial protease-like activity factor (rCPAF) from C. trachomatis (serovar L2) plus interleukin-12 (IL-12) or CpG deoxynucleotides (CpG) induces cross-species protection, as indicated by the significant earlier resolution of Chlamydia muridarum genital infection and the reduced development of upper reproductive pathology (7, 24). However, the CPAF-vaccinated animals shed chlamydiae for up to 2 weeks after challenge, albeit for a significantly shorter time than that for the mice vaccinated with phosphate-buffered saline (PBS) (mock vaccinated), and did not display detectable resistance to infection. Since CPAF is secreted into the host cytosol during the later stages of the chlamydial developmental cycle (40), we reasoned that the addition of other candidate antigens expressed at earlier times in the cycle would enhance the vaccination regimen towards further reducing the duration of chlamydial shedding in rCPAF-vaccinated animals. To this end, the chlamydial major outer membrane protein (MOMP) is accessible to the host immune system when abundantly expressed on the surface of the extracellular infective phase (elementary body [EB]), but not after entry into the phagosome where there is relative seclusion from the host immune system by a sturdy inclusion membrane (12). During the metabolically active phase (reticulate body), Chlamydia synthesizes the inclusion membrane proteins (e.g., inclusion membrane protein A [IncA]) that localize to the inclusion membrane (13). Therefore, it would appear that vaccination with select individual chlamydial proteins would elicit an immune response against different aspects of the organism's developmental cycle; thus, a targeted combinatorial vaccination approach may induce optimal protective immunity.
An important consideration for inducing cross-serovar protection is that even subtle variations in the amino acid sequence of a protein across chlamydial serovars might significantly affect the secondary and tertiary protein conformation. Therefore, there is a greater likelihood of conservation of protective linear epitopes compared to conformational epitopes. To this end, T cells recognize only linear antigenic epitopes, whereas B cells can directly recognize conformational epitopes, suggesting that the induction of T-cell-mediated immunity, but not B-cell-mediated immunity, may be suitable for generating cross-serovar protection. This is also corroborated by the fact that T helper 1 (Th1)-type CD4+ T cells, which recognize only linear epitopes, are required for optimal resolution of genital chlamydial infection (4, 16, 21, 37). The screening of such conserved protective linear T-cell epitopes can be accomplished using recombinant candidate antigens generated by heterologous (e.g., Escherichia coli [used in this study]) expression vector systems (6, 14).
In this study, we examined the protective efficacy of recombinant proteins from C. trachomatis serovar D (a common cause of chlamydial sexually transmitted disease in humans), recombinant MOMP (rMOMP), recombinant IncA (rIncA), or recombinant chlamydial protease-like activity factor (rCPAF), against vaginal C. muridarum challenge. Female BALB/c animals were intranasally (i.n.) immunized with the proteins, individually or in combinations, with murine recombinant IL-12, a well-established mucosal Th1 adjuvant (1-3), and evaluated for the induction of cross-species protection against C. muridarum challenge. Animals vaccinated with rCPAF plus IL-12 (rCPAF+IL-12) alone exhibited significantly accelerated resolution of genital infection and minimal development of oviduct pathology. However, there was no significant additive or synergistic effect of the other evaluated antigen(s) to the cross-species protective immunity induced by rCPAF+IL-12 vaccination alone.
MATERIALS AND METHODS
Recombinant proteins.
The recombinant chlamydial proteins were purified as previously described (24, 35). The sequences of MOMP (NCBI nucleotide accession no. X77364), IncA (GenBank accession no. AF326998), and CPAF (40) from serovar D were used for generation of the recombinant proteins. Briefly, rMOMP and rIncA were cloned into PGEX vectors and expressed with glutathione S-transferase fused to the N terminus of the protein. Since the sequences of CPAF from serovar D and serovar L2 share 99% amino acid identity with each other (11) and 82% identity each with C. muridarum CPAF (11), an existing rCPAF construct generated using sequence from C. trachomatis L2 genome with a six-histidine tag cloned into pBAD vectors was used (7, 23-25, 40). The fusion proteins were expressed in Escherichia coli with l-arabinose (for rCPAF) or isopropyl-β-d-thiogalactopyranoside (for rMOMP and rIncA) as an inducer and extracted by bacterial lysis using sonication in a Triton X-100 lysis buffer. Ni-nitrilotriacetic acid agarose beads (Amersham, NJ) were used for purification of rCPAF, and glutathione-conjugated agarose beads (Pharmacia & Upjohn, MI) were used for purification of rMOMP and rIncA. Each fusion protein was concentrated using Centriplus YM-10 tubes (Millipore, MA), suspended in PBS with proteinase inhibitor cocktail (Roche, CA), aliquoted, and then stored at −20°C. The purity of each protein was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and by Western blotting using antigen-specific murine antibodies (40). As a standard procedure, the endotoxin levels in the purified protein samples were measured using the Limulus amebocyte assay (Sigma-Aldrich, MO) and consistently found to be <1 endotoxin unit (EU)/mg of protein (1 EU = 0.2 ng). Murine recombinant IL-12 was obtained as a generous gift from Wyeth (Cambridge, MA).
Bacteria.
Chlamydia muridarum was grown on confluent HeLa cell monolayers. Cells were lysed using a sonicator (Fisher Scientific, PA), and EBs were purified on Renograffin gradients as described previously (26). Aliquots of bacteria were stored at −70°C in sucrose-phosphate-glutamine (SPG) buffer.
Mice.
Four- to-six week-old, female BALB/c mice were obtained from Charles River Laboratory (Bar Harbor, ME). Mice were housed and bred at the University of Texas at San Antonio and provided food and water ad libitum. Animal care and experimental procedures were performed in compliance with the Institutional Animal Care and Use Committee (IACUC) guidelines.
Intranasal immunization procedure.
Animals were immunized as described previously (7, 23-25). Groups of mice (six mice per group) were anesthetized (3% isofluorane) and immunized i.n. on day 0 with 15 μg rCPAF, 15 μg rMOMP, or 15 μg rIncA alone or with combinations of 15 μg rCPAF plus 15 μg rMOMP, 15 μg rCPAF plus 15 μg rIncA, or 15 μg rCPAF plus 15 μg rMOMP plus 15 μg rIncA, all dissolved in 25 μl of sterile PBS. This was accompanied on days −1, 0, and +1 with 0.5 μg of recombinant murine IL-12 (Wyeth, MA) in PBS containing 1% normal mouse serum. Mice were boosted i.n. with the same doses on days 14 and 28. The dose of rCPAF that was selected (15 μg/mouse) provided robust protection against genital C. muridarum challenge in our studies using BALB/c mice (7, 23-25). Equivalent doses of the other antigens (rMOMP and rIncA) were used for this comparative study. Groups of mice (six mice/group) received 15 μg of the unrelated antigen hen egg lysozyme (HEL) plus 0.5 μg IL-12, 0.5 μg IL-12 alone, or PBS (mock) alone as controls, on days 0, 14, and 28. As previously described, no significant toxicity was observed with the IL-12 treatment regimen (15, 23).
Antigen-specific CD4+ T-cell responses.
Fourteen days after the initial (day 0) i.n. immunization with individual recombinant chlamydial antigens, the spleens were removed from the mice (three mice/group), and splenocytes were layered over a Ficoll density gradient to collect mononuclear cells. CD4+ T-cell populations were isolated using magnetic particles (Stem Cell Technologies, Canada), and the purity was determined to be at least >95% of CD4+ T cells by flow cytometry using an allophycocyanin-conjugated anti-CD4 monoclonal antibody (BD Biosciences, CA). A separate pool of naïve splenocytes was prepared from animals immunized with PBS (mock immunized) and treated with mitomycin C (25 μg/107 cells) for 20 min and used as a source of antigen-presenting cells (APCs) (23). The purified CD4+ T cells (105 cells/well) were cultured in triplicate with APCs (105 cells/well) and stimulated for 72 h in vitro with individual recombinant chlamydial antigens (1 μg/ml) or with the unrelated antigen HEL (1 μg/ml), UV-inactivated C. muridarum (105 IFU/well), or medium alone in culture plates. Supernatants from the culture wells were analyzed for gamma interferon (IFN-γ) and IL-4 production using BD OptELISA kits (BD Pharmingen, NJ) as described previously (7, 23-25). Positive and negative controls were included in all assays.
Detection of antibody and isotype levels by ELISAs.
Ten days after the final (day 28) immunization, sera from the animals were analyzed by enzyme-linked immunosorbent assays (ELISAs) as described previously (7, 23-26). Microtiter plates were coated overnight with rCPAF (5 μg/ml) in sodium bicarbonate buffer (pH 9.5). Serial dilutions of serum were added in triplicate to wells followed by either goat anti-mouse total immunoglobulin, immunoglobulin G1 (IgG1), IgG2a, IgG2b, IgM, or IgA conjugated to alkaline phosphatase (Southern Biotechnology Associates, AL). After the wells were washed, p-nitrophenyl phosphate substrate (Sigma, MO) was added for color development, and the absorbance at 405 nm was measured using a μQuant microplate reader (Biotek Instruments, VT). Reciprocal serum dilutions corresponding to 50% maximal binding were used to obtain titers.
Vaginal C. muridarum challenge and determination of bacterial shedding.
One month following the final (day 28) vaccination, animals were challenged intravaginally (i.vag.) with 105 inclusion-forming units (IFU) of C. muridarum in 5 μl of SPG buffer as described previously (7, 23-25). The estrous cycle of animals was synchronized using two subcutaneous injections of Depo-Provera (Pharmacia Upjohn, MI) on days −10 and −3 before challenge. Vaginal swabs were obtained on the indicated days after challenge, followed by plating of the swab material in triplicate on HeLa cell monolayers grown on culture coverslips. Chlamydial inclusions were detected using an anti-Chlamydia genus-specific murine monoclonal primary antibody and goat anti-mouse IgG secondary antibody conjugated to fluorescein isothiocyanate (FITC) plus Hoechst nuclear stain. The number of inclusions was counted using a Zeiss Axioskop microscope, and results were expressed as the average number of inclusions per animal group.
Gross and histopathology.
Genital tracts were removed from the mice at various indicated time points after challenge, examined for the presence of hydrosalpinx, then fixed in 10% neutral formalin, and embedded into paraffin blocks. Serial horizontal sections (5 μm) were prepared and stained using hematoxylin and eosin. Representative sections were stained and visualized using a Zeiss Axioskop microscope and scored in a blind fashion as described previously (24). Dilatation of oviducts was scored as follows: 0, no significant dilatation; 1, mild dilatation of a single cross section of an oviduct; 2, one to three dilated cross sections of an oviduct; 3, more than three dilated cross sections of an oviduct; 4, confluent pronounced dilatation of the oviduct. Cellular parameters (polymorphonuclear cells [PMNs], mononuclear, and plasma cells) were individually scored as follows: 0, no significant presence of infiltration; 1, presence of infiltration at a single focus; 2, presence of infiltration at two to four foci; 3, presence of infiltration at more than four foci; or 4, confluent infiltration. Results are expressed as means ± standard deviations (SDs) of the scores from all animals in a group. For differences in the degree of oviduct dilatation in different groups, coronal sections were obtained from genital tracts of each animal within all groups. The oviductal regions were examined (left and right, separately) at ×10 magnification, and images were acquired using a Zeiss Axioskop 2 research microscope. Each image was stored in the Axiovision software (1,250 pixels [width] by 1,050 pixels [height]) and printed individually onto paper (8.5 in. by 11 in.) at 600 dots per inch using a laserjet printer (Hewlett Packard, Palo Alto, CA). The diameter of the oviduct and the wall thickness was measured in millimeters and expressed as a ratio of oviduct diameter to corresponding wall thickness. The ratios for individual oviducts, along with the mean ± standard error of the mean, within each group is shown. On the basis of the measurements in naïve animals of comparable age (data not shown), a ratio of ≥20 was determined to indicate dilatation.
Statistical analyses.
All data sets were reported without removing any outliers. For comparison of two groups, the Student t test (for normally distributed values) or the Mann-Whitney rank sum test (for values not distributed normally) was used to compare values of continuous variables. For experiments with more than two groups of animals, analysis of variance (ANOVA) followed by multiple comparison of means was used. To analyze differences in the time required for bacterial clearance, the Kaplan-Meier test was used. Differences between groups were considered statistically significant if P values were <0.05. All data shown are representative of two or three independent experiments conducted under the same conditions, and each experiment shown was analyzed independently.
RESULTS
Purification of recombinant chlamydial proteins.
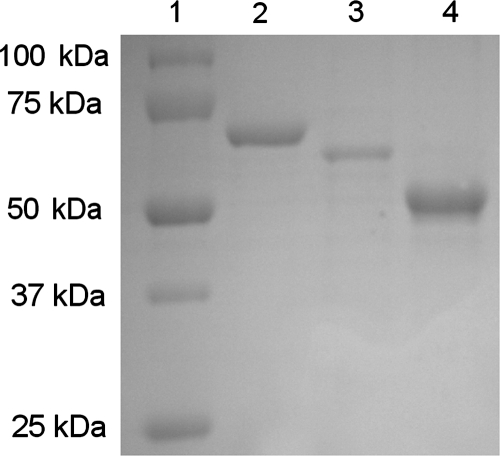
Recombinant proteins rCPAF, rIncA, and rMOMP were cloned and expressed as described previously (24, 35). As shown in Fig. 1, each purified protein exhibited a distinct band (rCPAF, 72-kDa band; rMOMP, 66-kDa band; rIncA, 56-kDa band) after SDS-PAGE and staining with Coomassie blue. The purity of the proteins was further confirmed by Western blotting using antigen-specific monoclonal antibodies (data not shown); the proteins were then divided into aliquots, which was used as the source of recombinant protein for all experiments.
FIG. 1.
SDS-PAGE of the purified recombinant chlamydial proteins. Lane 1, molecular mass standards (in kilodaltons); lane 2, rCPAF (72 kDa); lane 3, rMOMP (66 kDa); lane 4, rIncA (56 kDa). Results are representative of three independent experiments.
Cellular response after i.n. immunization.
Groups of animals were vaccinated individually with rCPAF, rIncA, rMOMP, the unrelated antigen HEL, or PBS (mock vaccinated). Additionally, all groups of animals (with the exception of mock-vaccinated animals) received IL-12 on days −1, 0, and +1. Fourteen days after immunization, purified CD4+ T cells were cultured with mitomycin C-treated APCs and stimulated for 72 h with 1 μg of individual antigen, UV-inactivated C. muridarum (105 IFU/well), or medium alone, and the supernatants were analyzed for IFN-γ and IL-4 production. As shown in Table 1, mice vaccinated with rCPAF exhibited the highest antigen-specific IFN-γ production (1,525 ± 172 pg/ml) compared to mice immunized with rMOMP (228 ± 72 pg/ml) or rIncA (972 ± 128 pg/ml). Additionally, purified CD4+ T cells from C. muridarum-infected animals exhibited the greatest IFN-γ production upon stimulation with rCPAF (295 ± 95 pg/ml), followed by rIncA (270 ± 80 pg/ml) and rMOMP (155 ± 45 pg/ml). As expected, purified CD4+ T cells from C. muridarum-infected animals exhibited high levels of IFN-γ production (1,305 ± 113 pg/ml) and minimal IL-4 production (below the limit of detection) upon in vitro stimulation with UV-inactivated C. muridarum EBs (data not shown). Purified CD4+ T cells from animals immunized with HEL+IL-12, IL-12 alone, or PBS (mock immunized) displayed minimal IFN-γ production upon stimulation with any chlamydial antigen, indicating the specificity of the measured cytokine responses. These results indicate that immunization with rCPAF, rIncA, or rMOMP elicit Th1-type cellular responses, but rCPAF elicits the greatest induction of IFN-γ production among these antigens.
TABLE 1.
Intranasal rCPAF+IL-12 vaccination induces robust cell-mediated immune responsesa
| Immunization | Cellular recall IFN-γ (pg/ml) productionb (mean ± SD) upon in vitro stimulation with:
|
||
|---|---|---|---|
| rCPAF | rMOMP | rIncA | |
| PBS (mock) | <32 | <32 | <32 |
| IL-12 | <32 | <32 | <32 |
| rCPAF+IL-12 | 1,525 ± 172 | 87 ± 34 | 66 ± 24 |
| rMOMP+IL-12 | 64 ± 31 | 228 ± 72 | 96 ± 45 |
| rIncA+IL-12 | 58 ± 26 | 90 ± 32 | 972 ± 128 |
| C. muridarum | 295 ± 95 | 155 ± 45 | 270 ± 80 |
| HEL+IL-12 | 65 ± 30 | 52 ± 21 | 35 ± 13 |
Animals (three mice/group) were immunized i.n. with rCPAF+IL-12, rMOMP+IL-12, rIncA+IL-12, C. muridarum (105 IFU), the unrelated antigen HEL+IL-12, IL-12 alone, or PBS (mock immunized) alone. On day 14, the animals were euthanized, and splenic CD4+ T cells were purified and tested for antigen-specific IFN-γ production by ELISAs.
Results are representative of two independent experiments. The cellular recall IFN-γ production values were <32 for cells from animals immunized by all the immunization regimens shown after the cells were stimulated in vitro with medium.
Humoral response after i.n. immunization.
Groups of animals were vaccinated individually with rCPAF, rIncA, rMOMP, HEL, or PBS (mock vaccinated) or in combinations of rCPAF+rIncA, rCPAF+MOMP, or rCPAF+rIncA+rMOMP on days 0, 14, and 28, respectively. Additionally, all groups of animals (except the mock-vaccinated group) received IL-12 on days −1, 0, +1, 14, and 28. The serum antibody responses against rCPAF, rIncA, or rMOMP were measured 10 days after the last booster immunization. As shown in Table 2, animals vaccinated with rCPAF alone or in combination with any antigen displayed high titers of serum anti-rCPAF total antibody, IgG1, IgG2a, and IgG2b. These antigen-specific antibody and isotype responses were also observed after vaccination with rMOMP or rIncA (Table 2). As expected, animals immunized with HEL+IL-12, IL-12 alone, or PBS (mock immunized) exhibited minimal anti-CPAF, anti-IncA, or anti-MOMP antibodies (Table 2). Additionally, the purity of the antigens and the specificity of immune responses were further confirmed by the minimal levels of cross-reactive antibodies in vaccinated mice. The minimal cross-reactivity in the cellular and humoral immune responses between the glutathione S-transferase-tagged proteins in this study and the previous demonstration that the six-His tag in rCPAF by itself does not induce antigen-specific responses (24) suggest that the different purification tags may not have significantly contributed to or adversely affected the effects of vaccination.
TABLE 2.
Intranasal rCPAF+IL-12 vaccination induces robust humoral immune responsesa
| Immunization | Antibody titerb
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum anti-CPAF antibody
|
Serum anti-MOMP antibody
|
Serum anti-IncA antibody
|
||||||||||
| Total Abc | IgG1 | IgG2a | IgG2b | Total Ab | IgG1 | IgG2a | IgG2b | Total Ab | IgG1 | IgG2a | IgG2b | |
| PBS (mock) | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| IL-12 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| rCPAF+IL-12 | 6,180 ± 452 | 3,602 ± 888 | 5,261 ± 319 | 1,928 ± 655 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| rMOMP+IL-12 | <100 | <100 | <100 | <100 | 3,802 ± 160 | 3,740 ± 764 | 1,915 ± 185 | 2,775 ± 648 | <100 | <100 | <100 | <100 |
| rIncA+IL-12 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | 3,647 ± 115 | 1,411 ± 351 | 3,380 ± 286 | 1,387 ± 324 |
| rCPAF+rMOMP+IL-12 | 4,140 ± 348 | 3,085 ± 838 | 5,462 ± 585 | 1,462 ± 280 | 4,693 ± 172 | 3,774 ± 211 | 2,013 ± 243 | 2,706 ± 332 | <100 | <100 | <100 | <100 |
| rCPAF+rIncA+IL-12 | 5,015 ± 957 | 3,914 ± 921 | 5,410 ± 317 | 2,560 ± 541 | <100 | <100 | <100 | <100 | 3,155 ± 198 | 1,776 ± 373 | 3,297 ± 201 | 1,353 ± 166 |
| rCPAF+rMOMP+rIncA+IL-12 | 4,323 ± 635 | 3,461 ± 610 | 4,898 ± 736 | 1,901 ± 495 | 4,697 ± 138 | 3,873 ± 341 | 2,176 ± 180 | 3,059 ± 461 | 3,877 ± 78 | 1,534 ± 152 | 2,921 ± 86 | 1,529 ± 230 |
| HEL+IL-12 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
Animals (six mice/group) were immunized i.n. with rCPAF+IL-12, rMOMP+IL-12, rIncA+IL-12, C. muridarum (105 IFU), HEL+IL-12, IL-12, or PBS (mock immunized). Animals were bled 10 days after the final booster immunization, and sera were analyzed for anti-CPAF antibody, anti-MOMP antibody, and anti-IncA antibody levels by ELISAs using microtiter plates coated with the corresponding antigens.
Results are expressed as means ± SDs of reciprocal serum dilutions corresponding to 50% maximal binding. Results are representative of two independent experiments.
Total Ab, total antibody.
Chlamydial clearance after genital challenge in vaccinated animals.
Groups of animals were vaccinated individually with rCPAF, rIncA, and rMOMP or in combinations of rCPAF+rIncA, rCPAF+rMOMP, and rCPAF+rIncA+rMOMP or vaccinated with HEL and PBS (mock vaccinated) on days 0, 14 and 28, respectively. Additionally, all groups of animals (except the mock-vaccinated group) received IL-12 on days −1, 0, +1, 14, and 28. The efficacy of the different vaccination regimens against genital C. muridarum challenge was examined by monitoring vaginal chlamydial shedding at 3-day intervals after challenge. As shown in Table 3, vaccination with rCPAF+IL-12 induced resolution of infection in 33% of the mice as early as day 12, in 67% of the mice by day 15, and in all mice (100%) by day 18 after challenge. In comparison, the majority of rMOMP- or rIncA-vaccinated animals were still shedding Chlamydia at day 18 after challenge (67% or 50%, respectively). The majority (83% each) of the rMOMP- or rIncA-vaccinated animals displayed resolution of infection at day 24, with 100% mice exhibiting resolution on day 27 after challenge. Furthermore, the addition of rMOMP, rIncA, or both to the rCPAF+IL-12 vaccination regimen did not significantly enhance the kinetics of bacterial resolution compared to vaccination with rCPAF+IL-12 alone, with 100% of animals in each group resolving the infection by day 18 after chlamydial challenge. Animals immunized with PBS (mock immunized) or the unrelated antigen (HEL+IL-12) began to resolve the infection between days 21 and 30 after challenge. As previously shown (24), resolution of infection in animals treated with IL-12 alone was comparable to animals treated with PBS (mock immunized). These results demonstrate the following. (i) Vaccination with rMOMP, rIncA, or rCPAF all enhance chlamydial clearance. (ii) The efficacy of rCPAF in enhancing the chlamydial clearance was greater than those of other antigens examined. (iii) rMOMP and/or rIncA contributed minimally to the rCPAF+IL-12 regimen.
TABLE 3.
Percentage of immunized animals shedding Chlamydia after genital challengea
| Immunization | % Mice shedding Chlamydia from the vagina on the indicated day after i.vag. challenge
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | |
| PBS (mock) | 100 | 100 | 100 | 100 | 100 | 100 | 50 | 50 | 33 | 0 |
| rCPAF+IL-12 | 100 | 100 | 100 | 67 | 33 | 0 | 0 | 0 | 0 | 0 |
| rMOMP+IL-12 | 100 | 100 | 100 | 100 | 100 | 67 | 50 | 17 | 0 | 0 |
| rIncA+IL-12 | 100 | 100 | 100 | 100 | 83 | 50 | 33 | 17 | 0 | 0 |
| rCPAF+rMOMP+IL-12 | 100 | 100 | 100 | 67 | 50 | 0 | 0 | 0 | 0 | 0 |
| rCPAF+rIncA+IL-12 | 100 | 100 | 100 | 50 | 17 | 0 | 0 | 0 | 0 | 0 |
| rCPAF+rMOMP+rIncA+IL-12 | 100 | 100 | 100 | 83 | 67 | 0 | 0 | 0 | 0 | 0 |
| HEL+IL-12 | 100 | 100 | 100 | 100 | 100 | 100 | 67 | 50 | 33 | 0 |
Animals (six mice/group) were immunized with three doses of rCPAF+IL-12, rMOMP+IL-12, rIncA+IL-12, rCPAF+rMOMP+IL-12, rCPAF+rIncA+IL-12, rCPAF+rMOMP+rIncA+IL-12, HEL+IL-12, or PBS (mock immunized). One month after the final booster vaccination, mice were challenged i.vag. with 105 IFU of C. muridarum. Chlamydial shedding was measured at the indicated days following challenge. The time to resolution differed significantly between the experimental groups (P = 0.0002 by the Kaplan-Meier test). Specifically, rCPAF+IL-12, rCPAF+rMOMP+IL-12, rCPAF+rIncA+IL-12, and rCPAF+rMOMP+rIncA+IL-12, each demonstrated significantly earlier resolution, but not rMOMP+IL-12 or rIncA+IL-12, compared to PBS (mock) or HEL+IL-12 (p < 0.05). Results are representative of two independent experiments.
Chlamydia-induced upper genital tract pathology in vaccinated animals.
The major problem with genital chlamydial infections in humans is the development of inflammatory complications in the upper genital tract (4, 21). Likewise, mice infected i.vag. with C. muridarum develop typical complications in the upper genital tract, such as hydrosalpinx and oviduct dilatation (21). The effect of the vaccination regimen on the development of upper genital tract pathology was examined on day 80 after challenge. As shown in Table 4, rCPAF+IL-12 vaccination prevented the development of hydrosalpinx on day 80 after chlamydial challenge in the majority of the animals (0% bilateral, 33% unilateral), which was significantly lower than animals immunized with PBS (mock immunized) (83% bilateral). An intermediate degree of protection, not significantly different from that of either the group vaccinated with PBS (mock vaccinated) or rCPAF+IL-12, against oviduct pathology was observed in the group vaccinated with rMOMP+IL-12 (33% bilateral, 33% unilateral) or rIncA+IL-12 (50% bilateral, 17% unilateral). Furthermore, the effects of rCPAF+rMOMP+IL-12 (0% bilateral, 17% unilateral), rCPAF+rIncA+IL-12 (0% bilateral, 0% unilateral), and rCPAF+rMOMP+rIncA+IL-12 (17% bilateral, 17% unilateral) were not significantly different from that of vaccination with rCPAF+IL-12 alone (0% bilateral, 33% unilateral). As expected, the incidence of hydrosalpinx in animals vaccinated with HEL+IL-12 (67% bilateral, 17% unilateral) was comparable to that of animals immunized with PBS (mock immunized).
TABLE 4.
rCPAF+IL-12 immunization reduces the development of oviduct pathologya
| Immunization | % Mice developing hydrosalpinx
|
Oviduct dilatation score (mean ± SD) | Cellular infiltration score (mean ± SD)
|
|||
|---|---|---|---|---|---|---|
| Bilateral | Unilateral | PMNs | Mononuclear cells | Plasma cells | ||
| PBS (mock) | 83 | 0 | 2.41 ± 0.23 | 1.28 ± 0.22 | 2.7 ± 0.26 | 1.58 ± 0.20 |
| rCPAF+IL-12 | 0 | 33 | 0.66 ± 0.21 | 0.66 ± 0.17 | 0.79 ± 0.24 | 0.45 ± 0.07 |
| rMOMP+IL-12 | 33 | 33 | 1.18 ± 0.23 | 1.12 ± 0.22 | 1.62 ± 0.49 | 1.00 ± 0.20 |
| rIncA+IL-12 | 50 | 17 | 1.25 ± 0.21 | 0.79 ± 0.15 | 1.5 ± 0.34 | 0.70 ± 0.10 |
| rCPAF+rMOMP+IL-12 | 0 | 17 | 0.5 ± 0.13 | 0.75 ± 0.25 | 0.83 ± 0.15 | 0.41 ± 0.13 |
| rCPAF+rIncA+IL-12 | 0 | 0 | 0.5 ± 0.12 | 0.62 ± 0.14 | 0.65 ± 0.10 | 0.45 ± 0.11 |
| rCPAF+rMOMP+rIncA+IL-12 | 17 | 17 | 0.67 ± 0.30 | 0.75 ± 0.11 | 0.75 ± 0.17 | 0.55 ± 0.12 |
| HEL+IL-12 | 67 | 17 | 2.16 ± 0.33 | 1.41 ± 0.22 | 2.66 ± 0.27 | 1.75 ± 0.18 |
Animals (six mice/group) were immunized with three doses of rCPAF+IL-12, rMOMP+IL-12, rIncA+IL-12, rCPAF+rMOMP+IL-12, rCPAF+rIncA+IL-12, rCPAF+rMOMP+rIncA+IL-12, HEL+IL-12, or PBS (mock immunized). One month after the final booster vaccination, mice were challenged i.vag. with 105 IFU of C. muridarum. At day 80 following C. muridarum challenge, animals were euthanized, and tissues were collected for further analysis. The development of bilateral and unilateral hydrosalpinx was studied as a measure of gross pathology. Quantitative histopathological scoring of oviduct dilatation and cellular infiltration into the genital tracts following chlamydial challenge was also performed. Groups of animals vaccinated with rCPAF+IL-12, rCPAF+rMOMP+IL-12, rCPAF+rIncA+IL-12, or rCPAF+rMOMP+rIncA+IL-12 each displayed significantly reduced incidence of oviduct dilatation, but not groups vaccinated with rMOMP+IL-12 or rIncA+IL-12, compared to groups vaccinated with PBS (mock vaccinated) or HEL+IL-12 (P < 0.05 by ANOVA). Results are representative of two independent experiments.
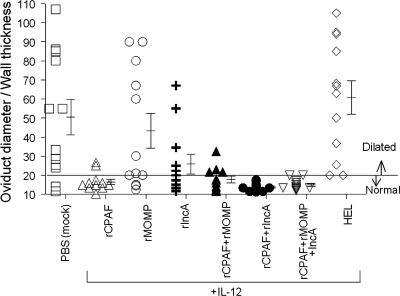
The incidence of oviduct dilatation and cellular infiltration was also scored microscopically on day 80 after challenge. As shown in Table 4, animals immunized with PBS (mock immunized) displayed a high degree of oviduct dilatation (2.41 ± 0.23) on day 80 after chlamydial challenge. Animals vaccinated with rCPAF+IL-12 displayed significantly reduced dilatation of oviducts (0.66 ± 0.21) compared to animals immunized with PBS (mock immunized). Animals vaccinated with the combinations rMOMP+IL-12 (1.18 ± 0.23) and rIncA+IL-12 (1.25 ± 0.21) also displayed reductions in oviduct dilatation compared to animals immunized with PBS (mock immunized), but not as great a reduction as those for the CPAF-immunized animals. Animals vaccinated with the combinations rCPAF+rMOMP+IL-12 (0.5 ± 0.13), rCPAF+rIncA+IL-12 (0.5 ± 0.12), and rCPAF+rMOMP+rIncA+IL-12 (0.67 ± 0.3) displayed reduced oviduct dilatation that was not statistically different from that of animals vaccinated with rCPAF+IL-12 alone (0.66 ± 0.21). The degree of oviduct dilatation was also measured on day 80 after challenge. As shown in Fig. 2, animals vaccinated with rCPAF+IL-12 (16.48 ± 1.36) displayed significantly reduced (P < 0.05) dilatation of oviducts compared to control animals immunized with PBS (mock immunized) (50.40 ± 9.33) or HEL+IL-12 (60.74 ± 8.77) on day 80 after chlamydial challenge. In contrast, animals vaccinated with rMOMP+IL-12 (43.33 ± 9.08) or rIncA+IL-12 (25.86 ± 5.13) displayed oviduct dilatation ratios that were lower than, but not significantly different from, the ratios of the control animals vaccinated with either PBS (mock) or HEL+IL-12. The lowest oviduct dilatation scores were exhibited by rCPAF+IL-12-vaccinated animals, but the levels were not significantly different from those of rMOMP+IL-12- and rIncA+IL-12-vaccinated animals. Animals vaccinated with the combinations rCPAF+rMOMP+IL-12 (17.79 ± 1.77), rCPAF+rIncA+IL-12 (13.72 ± 0.58), and rCPAF+rMOMP+rIncA+IL-12 (14.96 ± 0.79) displayed reduced oviduct dilatation that was not statistically different from animals vaccinated with rCPAF+IL-12 alone (16.48 ± 1.36). The degree of oviduct dilatation of animals vaccinated with HEL+IL-12 (60.74 ± 8.77) was similar to that of animals vaccinated with PBS (mock vaccinated) (50.40 ± 9.33).
FIG. 2.
Degree of oviduct dilatation after vaccination and chlamydial challenge. Animals (six mice/group) were immunized with three doses of rCPAF+IL-12, rMOMP+IL-12, rIncA+IL-12, rCPAF+rMOMP+IL-12, rCPAF+rIncA+IL-12, rCPAF+rMOMP+rIncA+IL-12, HEL+IL-12, and PBS (mock immunized). One month after the final booster vaccination, mice were challenged i.vag. with 105 IFU of C. muridarum. At day 80 following challenge, animals were euthanized and their genital tracts were processed and stained with hematoxylin and eosin. The degree of oviduct dilatation was measured and expressed as the ratio of oviduct diameter to wall thickness for each mouse and as the mean ratio ± standard error for each group. The oviduct dilatation ratio differed significantly between the experimental groups (P < 0.001 by ANOVA). rCPAF+IL-12, rCPAF+rMOMP+IL-12, rCPAF+rIncA+IL-12, and rCPAF+rMOMP+rIncA+IL-12 each demonstrated significantly reduced dilatation compared to PBS (mock immunized) (P < 0.05).
The infiltration of PMNs, mononuclear cells, and plasma cells was also examined and found to be reduced in animals vaccinated with rCPAF+IL-12 alone or in combination with the other antigens, with significant reductions in mononuclear and plasma cell frequencies (Table 4). Animals vaccinated with rMOMP+IL-12 or rIncA+IL-12 alone displayed intermediate, albeit not significantly different, numbers of all cell types examined compared to the numbers of cell types in animals immunized with rCPAF+IL-12 and PBS (mock immunized) on day 80 after challenge. Collectively, these results demonstrate the following. (i) Vaccination with rMOMP, rIncA, or rCPAF reduces the development of oviduct pathology. (ii) rCPAF had higher efficacy in reducing oviduct pathology than the other examined antigens did. (iii) rMOMP and/or rIncA contributed minimally to the effects of rCPAF+IL-12 regimen towards reduction of oviduct pathology.
DISCUSSION
The presence of multiple serovars of Chlamydia trachomatis that cause genital infections suggests the need to identify vaccine candidates that provide cross-serovar protection. We have demonstrated that rCPAF+IL-12 vaccination enhances chlamydial clearance and reduces the development of oviduct pathology (23-25) but does not induce complete resistance to infection. In this study, we used a recombinant multisubunit vaccination approach with three defined chlamydial antigens, including rMOMP, rIncA, and rCPAF from C. trachomatis serovar D, individually or in combinations, with IL-12 as an adjuvant, and studied the cross-species protective efficacy against vaginal C. muridarum challenge.
After i.n. immunization, all the respective antigens induced robust CD4+ Th1-type antigen-specific cellular responses, indicated by high levels of IFN-γ but minimal IL-4 production. However, rCPAF immunization induced the greatest IFN-γ, followed by vaccination with rIncA and rMOMP, respectively. Additionally, while each chlamydial antigen tested induced high levels of serum antibody, rCPAF, followed by rIncA, induced greater levels of IgG2a than those of IgG1, whereas rMOMP induced greater levels of IgG1 than those of IgG2a. These results suggest that rCPAF, rIncA, and rMOMP may have various degrees of potency as Th1 immunogens. In this regard, Th1 responses have been shown to be highly important for protective immunity against primary genital chlamydial infection (5, 29-31). Specifically, mice depleted of CD4+ T cells or those deficient in major histocompatibility complex MHC class II production displayed an inability to resolve genital chlamydial infection (22, 25). Additionally, mice deficient in IFN-γ production also displayed inabilities to optimally resolve and prevent dissemination of chlamydial infections (8, 17, 18, 29, 31, 32). Conversely, Th1 responses from immune cells or from chronically infected cells, have been thought to cause collateral tissue damage, leading to the sequelae of chlamydial infections (4). Therefore, the rapid induction of an optimal Th1 response leading to early chlamydial clearance and subsequent early exit of immune cells from genital tissues seems to be important for both inducing bacterial clearance and reducing upper genital pathology.
In this regard, vaccination with rCPAF+IL-12 resulted in significantly accelerated resolution of genital chlamydial infection, as well as reduction in the incidence of hydrosalpinx, oviduct dilatation, and cellular infiltration. These results are in agreement with our recent studies (7, 23-25) demonstrating the protective efficacy of rCPAF against genital chlamydial infections. rIncA and rMOMP individually induced lower, albeit not significantly different, levels of protective immunity than the level induced by rCPAF, and the levels of protective immunity correlated with the levels of Th1-type response induced by each of these regimens. Given that CPAF is secreted only in later phases of the chlamydial developmental cycle (∼12 to 18 h after the initial infection), it is interesting that immune responses against this antigen provided better protection than the early phase structural antigens, rMOMP and rIncA. The ability of CPAF to be actively secreted into the host cytosol may influence antigen presentation and subsequent activation of cell-mediated responses (9-11, 40-42) during the infection. In this regard, we have demonstrated that rCPAF+IL-12-induced immunity was highly dependent upon antigen presentation via the major histocompatibility complex class II pathway (25), antigen-specific CD4+ T cells (23), and endogenous IFN-γ production (24).
The incorporation of rMOMP, rIncA, or both into the rCPAF+IL-12 treatment regimen resulted in levels of protection comparable to the level of protection shown by vaccination with rCPAF+IL-12 alone. The failure to elicit additive or synergistic protective immunity with these combination regimens suggests multiple possibilities including the following. (i) The C. trachomatis serovar D proteins used in this study exhibit different degrees of amino acid identity (MOMP, 82%; IncA, 52%; and CPAF, 82%) with the respective proteins from C. muridarum. While IncA inherently has low amino acid identity, MOMP and CPAF share comparable levels of sequence identity. However, the differential induction of cross-serovar protection in this study suggests that more protective linear epitopes within CPAF are conserved than within MOMP. (ii) rCPAF displays immunogenic dominance compared to rMOMP or rIncA. To this end, Sharma et al. (33-35) have demonstrated that CPAF was a dominant immunogen in Chlamydia-seropositive humans compared to a wide repertoire of other chlamydial proteins. (iii) The usage of progesterone treatment before challenge, an important component in the murine model, may suppress immune responses and render the genital tract more susceptible to infection (4), making it difficult to assess the precise effects of vaccination on early infection clearance. Finally, (iv) the reduction of oviduct pathology in most animals vaccinated with rCPAF+IL-12 may mask the additive effects of combinatorial approaches upon upper genital pathology.
Based on the results from this study, certain possibilities that cannot be excluded are as follows. (i) The conformational epitopes within MOMP or IncA may induce cross-protection. This issue may be addressed using proteins purified from the bacterium and refolded to native configuration, as has been demonstrated for chlamydial MOMP (28). However, the refolding of proteins for vaccines may be tedious compared to the ease of mass-producing recombinant proteins and per se does not lend itself well to the identification of conserved linear antigenic epitopes. In this context, (ii) a direct comparison of the most protective forms of the various antigens (such as refolded MOMP), may yield results different from this study. Finally, (iii) it is also possible that usage of rMOMP or rIncA from serovar D may provide better immune protection against challenge with the same serovar or other human serovars. However, a vaccination study with recombinant human serovar proteins in direct protection against human serovar chlamydial infection in mice has constraints, including the strict host tropism linked to differential IFN-γ sensitivity of Chlamydia (27) and therefore, the limited infectivity of human serovars in mice. This issue may possibly be overcome by using humanized mouse models of infection (20, 36). Such mice have been used to study other infectious agents, including human immunodeficiency virus and Epstein-Barr virus, as well as toxic shock syndrome toxin 1 (20, 36).
In summary, we have shown that i.n. vaccination with rCPAF+IL-12 accelerated clearance of C. muridarum after i.vag. challenge, reduced the development of oviduct pathology, and induced strong cross-species protection, all to a much greater extent than identical doses of rMOMP+IL-12 or rIncA+IL-12, when all antigens were derived from C. trachomatis serovar D. However, the addition of rMOMP and/or rIncA to the rCPAF+IL-12 regimen did not contribute significantly to protective immunity against C. muridarum, suggesting the need to further optimize the source and doses of rMOMP and rIncA or evaluate other antigens for use with rCPAF towards inducing robust cross-serovar protective immunity against genital chlamydial infections.
Acknowledgments
This work was supported by National Institutes of Health grant SO6GM008194-24.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arulanandam, B. P., and D. W. Metzger. 1999. Modulation of mucosal and systemic immunity by intranasal interleukin 12 delivery. Vaccine 17:252-260. [DOI] [PubMed] [Google Scholar]
- 3.Arulanandam, B. P., M. O'Toole, and D. W. Metzger. 1999. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J. Infect. Dis. 180:940-949. [DOI] [PubMed] [Google Scholar]
- 4.Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 5:149-161. [DOI] [PubMed] [Google Scholar]
- 5.Cain, T. K., and R. G. Rank. 1995. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 63:1784-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, T. G., and D. Cassidy-Hanley. 2005. Recombinant subunit vaccines: potentials and constraints. Dev. Biol. (Basel) 121:153-163. [PubMed] [Google Scholar]
- 7.Cong, Y., M. Jupelli, M. N. Guentzel, G. Zhong, A. K. Murthy, and B. P. Arulanandam. 2007. Intranasal immunization with chlamydial protease-like activity factor and CpG deoxynucleotides enhances protective immunity against genital Chlamydia muridarum infection. Vaccine 25:3773-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter, T. W., K. H. Ramsey, G. S. Miranpuri, C. E. Poulsen, and G. I. Byrne. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect. Immun. 65:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong, F., M. Pirbhai, Y. Zhong, and G. Zhong. 2004. Cleavage-dependent activation of a Chlamydia-secreted protease. Mol. Microbiol. 52:1487-1494. [DOI] [PubMed] [Google Scholar]
- 10.Dong, F., H. Su, Y. Huang, Y. Zhong, and G. Zhong. 2004. Cleavage of host keratin 8 by a Chlamydia-secreted protease. Infect. Immun. 72:3863-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong, F., Y. Zhong, B. Arulanandam, and G. Zhong. 2005. Production of a proteolytically active protein, chlamydial protease/proteasome-like activity factor, by five different Chlamydia species. Infect. Immun. 73:1868-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields, K. A., and T. Hackstadt. 2002. The chlamydial inclusion: escape from the endocytic pathway. Annu. Rev. Cell Dev. Biol. 18:221-245. [DOI] [PubMed] [Google Scholar]
- 13.Hackstadt, T., M. A. Scidmore-Carlson, E. I. Shaw, and E. R. Fischer. 1999. The Chlamydia trachomatis IncA protein is required for homotypic vesicle fusion. Cell. Microbiol. 1:119-130. [DOI] [PubMed] [Google Scholar]
- 14.Hansson, M., P. A. Nygren, and S. Stahl. 2000. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 32:95-107. [DOI] [PubMed] [Google Scholar]
- 15.Huber, V. C., B. P. Arulanandam, P. M. Arnaboldi, M. K. Elmore, C. E. Sheehan, B. V. Kallakury, and D. W. Metzger. 2003. Delivery of IL-12 intranasally leads to reduced IL-12-mediated toxicity. Int. Immunopharmacol. 3:801-809. [DOI] [PubMed] [Google Scholar]
- 16.Igietseme, J. U., K. H. Ramsey, D. M. Magee, D. M. Williams, T. J. Kincy, and R. G. Rank. 1993. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg. Immunol. 5:317-324. [PubMed] [Google Scholar]
- 17.Ito, J. I., and J. M. Lyons. 1999. Role of gamma interferon in controlling murine chlamydial genital tract infection. Infect. Immun. 67:5518-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson, M., K. Schon, M. Ward, and N. Lycke. 1997. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect. Immun. 65:1032-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly, K. A. 2003. Cellular immunity and Chlamydia genital infection: induction, recruitment, and effector mechanisms. Int. Rev. Immunol. 22:3-41. [DOI] [PubMed] [Google Scholar]
- 20.Melkus, M. W., J. D. Estes, A. Padgett-Thomas, J. Gatlin, P. W. Denton, F. A. Othieno, A. K. Wege, A. T. Haase, and J. V. Garcia. 2006. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 12:1316-1322. [DOI] [PubMed] [Google Scholar]
- 21.Morrison, R. P., and H. D. Caldwell. 2002. Immunity to murine chlamydial genital infection. Infect. Immun. 70:2741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphey, C., A. K. Murthy, P. A. Meier, M. N. Guentzel, G. Zhong, and B. P. Arulanandam. 2006. The protective efficacy of chlamydial protease-like activity factor vaccination is dependent upon CD4+ T cells. Cell. Immunol. 242:110-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murthy, A. K., J. P. Chambers, P. A. Meier, G. Zhong, and B. P. Arulanandam. 2007. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect. Immun. 75:666-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murthy, A. K., Y. Cong, C. Murphey, M. N. Guentzel, T. G. Forsthuber, G. Zhong, and B. P. Arulanandam. 2006. Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele. Infect. Immun. 74:6722-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murthy, A. K., J. Sharma, J. J. Coalson, G. Zhong, and B. P. Arulanandam. 2004. Chlamydia trachomatis pulmonary infection induces greater inflammatory pathology in immunoglobulin A deficient mice. Cell. Immunol. 230:56-64. [DOI] [PubMed] [Google Scholar]
- 27.Nelson, D. E., D. P. Virok, H. Wood, C. Roshick, R. M. Johnson, W. M. Whitmire, D. D. Crane, O. Steele-Mortimer, L. Kari, G. McClarty, and H. D. Caldwell. 2005. Chlamydial IFN-gamma immune evasion is linked to host infection tropism. Proc. Natl. Acad. Sci. USA 102:10658-10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pal, S., E. M. Peterson, and L. M. de la Maza. 2005. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 73:8153-8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 30.Perry, L. L., K. Feilzer, J. L. Portis, and H. D. Caldwell. 1998. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J. Immunol. 160:2905-2914. [PubMed] [Google Scholar]
- 31.Perry, L. L., H. Su, K. Feilzer, R. Messer, S. Hughes, W. Whitmire, and H. D. Caldwell. 1999. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J. Immunol. 162:3541-3548. [PubMed] [Google Scholar]
- 32.Rank, R. G., K. H. Ramsey, E. A. Pack, and D. M. Williams. 1992. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect. Immun. 60:4427-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma, J., A. M. Bosnic, J. M. Piper, and G. Zhong. 2004. Human antibody responses to a Chlamydia-secreted protease factor. Infect. Immun. 72:7164-7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma, J., F. Dong, M. Pirbhai, and G. Zhong. 2005. Inhibition of proteolytic activity of a chlamydial proteasome/protease-like activity factor by antibodies from humans infected with Chlamydia trachomatis. Infect. Immun. 73:4414-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma, J., Y. Zhong, F. Dong, J. M. Piper, G. Wang, and G. Zhong. 2006. Profiling of human antibody responses to Chlamydia trachomatis urogenital tract infection using microplates arrayed with 156 chlamydial fusion proteins. Infect. Immun. 74:1490-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun, Z., P. W. Denton, J. D. Estes, F. A. Othieno, B. L. Wei, A. K. Wege, M. W. Melkus, A. Padgett-Thomas, M. Zupancic, A. T. Haase, and J. V. Garcia. 2007. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204:705-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, S., Y. Fan, R. C. Brunham, and X. Yang. 1999. IFN-gamma knockout mice show Th2-associated delayed-type hypersensitivity and the inflammatory cells fail to localize and control chlamydial infection. Eur. J. Immunol. 29:3782-3792. [DOI] [PubMed] [Google Scholar]
- 38.Westrom, L., R. Joesoef, G. Reynolds, A. Hagdu, and S. E. Thompson. 1992. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex. Transm. Dis. 19:185-192. [PubMed] [Google Scholar]
- 39.Westrom, L., and P. A. Mardh. 1983. Chlamydial salpingitis. Br. Med. Bull. 39:145-150. [DOI] [PubMed] [Google Scholar]
- 40.Zhong, G., P. Fan, H. Ji, F. Dong, and Y. Huang. 2001. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J. Exp. Med. 193:935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong, G., T. Fan, and L. Liu. 1999. Chlamydia inhibits interferon gamma-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. J. Exp. Med. 189:1931-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong, G., L. Liu, T. Fan, P. Fan, and H. Ji. 2000. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon gamma-inducible major histocompatibility complex class I expression in Chlamydia-infected cells. J. Exp. Med. 191:1525-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]