Abstract
Inkoo virus (INKV), a member of the California serogroup orthobunyaviruses, is circulating widely in northern Europe. Although the virus was discovered over 40 years ago, the disease associations and immune responses in human infection are poorly characterized. We first developed an immunofluorescence assay (IFA) for the detection of INKV antibodies in humans, and then we studied a panel of 1,292 sera in patients with a febrile illness in Finland. We found four acute (immunoglobulin M [IgM] positive) INKV infections and an IgG seroprevalence of 51.3%. The data indicate that the infection has become more common than it was in the 1960s, especially in southern Finland. Two distinct IgG IFA fluorescence patterns were observed: a granular pattern in sera from patients with acute INKV infection and a diffuse pattern in those with long-standing immunity. Further analysis with a panel of INKV-positive sera (n = 18; verified by neutralization assay) of protein-specific responses, using immunoprecipitation and IFA based on baculovirus-expressed INK N, Gn, and Gc proteins, demonstrated a strong IgG response predominantly towards N protein in the acute phase. In contrast, in patients with long-standing immunity, the Gc response was more prominent and the N response was weaker. In conclusion, a diagnostic IgG IFA pattern distinguishing between acute infection and long-standing immunity was observed. N protein seems to be the optimal antigen for the serodiagnosis of acute infection, and the Gc protein could be appropriate for the serosurveillance of INKV.
California serogroup viruses belong to the genus Orthobunyavirus in the family Bunyaviridae and include 12 antigenically related mosquito-borne viruses. La Crosse virus and Tahyna virus (TAHV) are considered the most significant human pathogens within the serogroup. La Crosse virus, especially, is an important cause of encephalitis and meningitis in children in the United States (19). Most of the known California serogroup viruses are commonly found in North America; three are known to circulate in Europe. TAHV causes a febrile illness in children, and the infection is common in central Europe (1). Snowshoe hare virus (SSHV), originally discovered in North America (6), is found in Russia (7), and Inkoo virus (INKV) is widespread in most countries in northern Europe.
INKV was first isolated from mosquitoes in Finland in 1964 (4), and its principal vector is Aedes communis. INKV infection is common in Finland, with a 24% human seroprevalence documented in the 1960s (2). Phylogenetic studies indicate that the closest relative to INKV is Jamestown Canyon virus, found in the United States (8, 28). Disease associations of INKV have been poorly characterized, but some anecdotal cases from Finland suggest it as a causative agent of central nervous system (CNS) symptoms in children and young adults.
Like to other viruses in the family Bunyaviridae, California serogroup viruses have a tripartite, negative-sense, single-stranded RNA genome. The INKV L segment codes for the viral RNA polymerase, the M segment for Gn (30 to 32 kDa), Gc (125 kDa), and NSm proteins (18 kDa), and the S segment for N (26 to 27 kDa) and NSs proteins (11 to 13 kDa) (9).
Studies with monoclonal antibodies against La Crosse virus suggest that N protein is the dominant antigen in the IgM response (12). Gc protein is the major target of neutralizing antibodies and acts as a ligand for cellular receptors on erythrocytes. Gc is therefore involved in the hemagglutination inhibition (12, 14, 17) and also in viral entry, playing a critical role in the fusion between the viral envelope and the cellular membrane (23, 25). Gc undergoes a pH-dependent conformational change associated with cell-to-cell and virus-to-cell fusion (10, 11, 15, 22). Gn protein is poorly immunogenic (12, 18), but it is required for targeting Gc to Golgi apparatus from the endoplasmic reticulum (5). Furthermore, a role in cell-to-cell fusion cannot be ruled out (15, 24) and Gn is also thought to be the protein responsible for viral attachment in the mosquito midgut (20). Gc contains virus-specific epitopes that could be used in the typing of California serogroup viruses, whereas N protein seems to be more conserved (12, 13).
The human immune response to INKV infection has not been well characterized. In this work, we first developed immunoglobulin G (IgG) and IgM immunofluorescence assays (IFAs) to study the current seroprevalence and to detect acute disease in humans. We observed a distinct fluorescence pattern in the IgG IFA analysis of sera from patients with acute INKV infection that was different from those with long-standing immunity. To study this phenomenon, we analyzed the protein-specific responses towards the INKV structural proteins in the acute infection and long-standing immunity by using baculovirus-expressed INKV N, Gn, and Gc proteins as antigen or by immunoprecipitation of native viral proteins.
MATERIALS AND METHODS
Viruses and cells.
The prototype INKV strain KN3641, which is the original isolate from Finland (4), and the prototype strains of TAHV (Bardos strain 92) and SSHV (SSH-1) were used.
Vero E6 cells were grown in minimum essential medium at 37°C in a 5% CO2 incubator, and Spodoptera frugiperda cells (Sf-9 cells) were grown in Sf-900 medium (Gibco, Paisley, United Kingdom) at 27°C. Both media were supplemented with 10% fetal calf serum (Gibco) and penicillin-streptomycin.
Patient samples and antisera.
The samples were patient sera from routine diagnostics at the Department of Virology, Helsinki University Central Hospital Laboratory, collected during June through August in 2001 through 2004 (the reference number of the institutional review board permit is 119/E0/05). The patient groups studied had either febrile illnesses due to a clinically suspected Puumala hantavirus infection (n = 1,292) or CNS symptoms with suspected viral etiology (n = 1,402). The panel of patients with suspected hantavirus infection was used for the seroprevalence studies, and a subset of the panel was used for testing the sensitivity and specificity of the IFA. A panel of nine patients with acute INKV infection and nine patients with old immunity to INKV was collected from both patient panels and used in further analyses. Mouse hyperimmune ascites fluid (MHAF) against INKV was characterized previously (4).
INKV IFA.
Vero E6 cells were grown in 75-cm2 cell culture flasks (Greiner Bio-One, Frickenhausen, Germany) and infected with INKV when confluent. The cell monolayer was washed with phosphate-buffered saline (PBS) prior to inoculation with 400 μl of 500 to 1,000 PFU per ml. The flask was incubated at 37°C for 1 h, and 20 ml of medium was added. When the cytopathic effect (CPE) appeared after 2 days, cells were harvested with trypsin-EDTA, washed three times with PBS, and resuspended in 7 ml. To each well in the slides (Immuno-Cell, Mechelen, Belgium), 20 μl of cell suspension was added and slides were air dried and fixed with ice-cold acetone for 7 min. Slides were stained with diluted human sera and MHAF. In INKV IgG and IgM tests, the serum dilutions were incubated for 30 min and 4 h, respectively, at 37°C and washed with cold PBS. Cells were stained with fluorescein isothiocyanate-conjugated anti-human IgG or IgM (Jackson ImmunoResearch Laboratories, Baltimore, MD) for 30 min at 37°C, washed with PBS, and examined with a fluorescence microscope. A positive signal from 30% of cells (70% of cells are negative) with a specific fluorescence pattern was required for a positive result.
Virus neutralization assay.
Neutralization test was performed in 96-well plates using Vero E6 cells. INKV, TAHV, and SSHV (20 μl of 500 to 1,000 PFU per ml) were incubated with serially twofold diluted serum samples (starting from 1:20) in a one-to-one ratio at 37°C for 1 h. Cells were inoculated with the suspensions and observed after 2 days to detect CPE. The neutralization titer was defined as the final dilution completely inhibiting the CPE.
Plasmids with INKV N, Gn, and Gc protein coding regions.
RNA extracted from ultracentrifuged INKV cell culture supernatant was used as a template to generate the PCR products of the coding regions of the N, Gn, and Gc proteins. RNA was extracted with the QIAGEN viral blood extraction kit (QIAGEN, Hilden, Germany). Expand reverse transcriptase mix (10 μl of RNA template, 1.67 μM of each primer [incubated for 10 min at 65°C], 5.7 μl expand buffer, 2.9 μl dichlorodiphenyltrichloroethane [100 mM], 2.9 μl deoxynucleoside triphosphate [10 mM], 0.7 μl RNase inhibitor, and 1.4 μl Expand reverse transcriptase [50 U/μl; added and incubated for 1 h at 43°C] [Roche Diagnostics, Mannheim, Germany]) was used to synthesize the first-strand cDNA, together with specific PCR primers. The following primers, containing suitable restriction sites (underlined), were used in reverse transcription and PCR: for the N protein, T TTA GAT CTG AAT TCT GAT ATG GGA GAT TTG GT (Bgl II) and TT TGG TAC CTG CTC TTG TTT AAG GCA GC (KpnI); for the Gn protein, TT TGG TAC CGC ACA TTA CAC TGG CTG CTC (KpnI) and TTT GGA TCC ATT CCA AAT ATG GAA GTC CTA AAC (BamHI); and for the Gc protein, TT TCC ATG GCT AAT CAC ATA TTT ACT TAT (NcoI) and TT TGG TAC CAA TCA TCT TAT TTT CAC CTC CT (KpnI).
Each PCR product was digested, gel purified, and ligated overnight at 15°C (1 μl T4 DNA ligase [400 U/μl], 1 μl T4 DNA ligase buffer [10×; New England Biolabs, Ipswich, MA], 6 μl insert, and 2 μl vector) into pAcYML1 vector (kindly provided by Johan Peränen, Institute of Biotechnology, University of Helsinki; plasmid construct was modified from that in reference 21. Constructs were transformed into Escherichia coli strain DH5 α (Invitrogen, Paisley, United Kingdom) by heat shock at 42°C for 45 s. Bacteria were suspended in Luria broth, grown for an hour in a shaker, and plated on a Luria broth-ampicillin (250 μg/ml) plate. Plasmids were purified using the QIAprep Spin Miniprep kit (QIAGEN) and restricted to confirm the right insert before being grown on a larger scale.
INKV N, Gn, and Gc protein transfection.
Purified constructs (EndoFree plasmid maxi kit; QIAGEN) were transfected with baculovirus DNA to Sf-9 cells. FuGENE 6 transfection reagent (Roche Diagnostics) was suspended with Sf-900 medium, incubated 10 min, and combined with 250 ng BaculoGold baculovirus DNA (Pharmingen, San Diego, CA) and 2 μg of purified plasmid construct suspension and applied to Sf-9 cells (2 × 106 cells per 25-cm2 flasks) for 2 h at room temperature and an hour at 27°C. After 4 days, the cells were harvested and supernatants were used to infect new insect cells. Acetone-fixed slides were prepared from Sf-9 cells infected for 3 days, and IFA was performed as described above.
Metabolic labeling and immunoprecipitation of native virus and expressed proteins.
Vero E6 cells were infected with INKV as described above. At 2 days postinfection, infected and mock-infected cells were washed with PBS and labeled with 50 μCi/ml L-[35S]methionine (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) for 4 h at 37°C. Cells were lysed with lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.75% Triton X-100, 5 mM EDTA, and protease inhibitor) on ice for 1 hour and pelleted by centrifugation at 15,700 × g for 30 min at 4°C. INKV proteins were immunoprecipitated with 10 μl of serum samples from individuals with acute INKV infection or long-standing immunity or from MHAF overnight at 4°C. After incubation with 20% G-Sepharose (diluted in 50 mM Tris-HCl, 150 mM NaCl) for 2 h at 4°C, pelleted beads were washed four times with lysis buffer and once with 50 mM Tris-HCl plus 150 mM NaCl. Bound proteins were analyzed with sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis under reducing conditions, transferred to membranes, and detected in an X-ray film after 2 weeks of incubation at −70°C. Intensity values of the protein bands were measured by the Bio-Rad Gel Doc documentation system with Quantity One software.
Sf-9 cells expressing INKV N, Gn, and Gc proteins were labeled 1 day postinfection with 50 μCi/ml L-[35S]methionine and cysteine (Amersham Pharmacia Biotech) for 72 h at 27°C. Cells were lysed and pelleted as described above. Proteins were immunoprecipitated with MHAF for 2 h. Otherwise the immunoprecipitation was performed as described above.
RESULTS
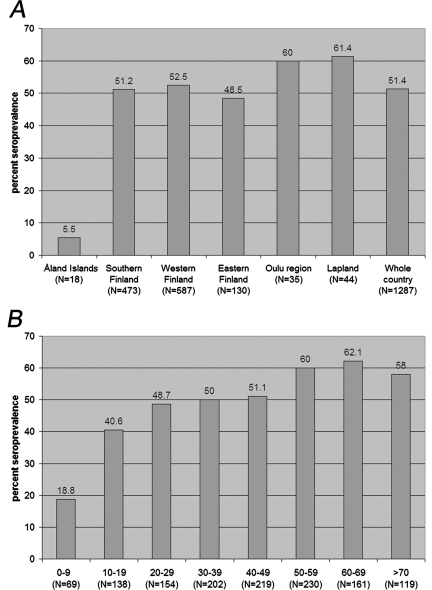
An IFA for the detection of INKV IgG and IgM antibodies was developed based on acetone-fixed, INKV-infected, Vero E6 cells, and it was used to screen for antibodies from a panel of patients (n = 1,292) with acute febrile illnesses and for whom hantavirus infection was suspected. The samples were obtained in the summer, during the mosquito season. Results (Fig. 1A and B) showed that INKV infection is very common in the Finnish population, with an overall seroprevalence of 51% (40% in females and 59% in males). INKV seroprevalence was high throughout the country, excluding the Åland islands, where the sample size was small. The infection is acquired at a young age, since 40% of persons in the group aged 10 to 19 years were already INKV seropositive. We further compared the performance of the IgG IFA with a neutralization test, based on the last serum dilution able to totally inhibit the CPE. Specificity and sensitivity of the IgG IFA were studied with 40 samples with neutralizing antibodies and 54 samples negative by the neutralization test. Taking titer 20 as a cutoff titer for both tests, 35 of 40 sera with neutralizing antibodies were positive by INKV IgG IFA. Of 54 neutralizing-negative sera, 49 were negative by INKV-IgG IFA. Therefore, the sensitivity of the IFA was 87.5% and the specificity was 90.7% when the neutralization test was considered the gold standard.
FIG. 1.
(A) Geographical distribution of INKV IgG seroprevalence in Finland. (B) Seroprevalence in different age groups.
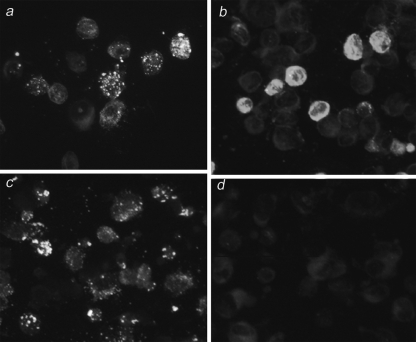
Most of the samples positive by IgG IFA showed a diffuse fluorescence pattern, but a small minority showed a distinct granular fluorescence pattern. We observed that results for individuals with a long-standing immunity (negative by IgM IFA) showed a diffuse fluorescence pattern with IgG IFA (Fig. 2b). In contrast, results for those with a likely acute INKV infection (positive by IgM IFA) showed a granular fluorescence pattern (Fig. 2a). To screen for acute INKV infections, in addition to the serum panel of those with suspected hantavirus infections, we studied a panel of sera from patients with CNS symptoms with suspected viral etiology (n = 1,402). All samples were taken during the mosquito season. Only a few acute infections were found for patients in both panels: four from those with suspected hantavirus infections and five from patients with CNS symptoms.
FIG. 2.
IFA of INKV IgG- and IgM-positive acute and long-standing immunity serum samples using acetone-fixed INKV-infected Vero E6 cells as antigen. IgG IFA results for early (a) and late (b) immune response sera. IgM IFA results for early (c) and late (d) immune response sera.
With a neutralization assay, we verified that the serological response was towards INKV for all samples from patients with acute infection. We selected these nine cases (both IgM and IgG positive) and nine of those with long-standing immunity (IgM negative and IgG positive) for further studies (Table 1). In all individuals studied, the INKV neutralization titer was higher than TAHV or SSHV titers (fourfold in 11 of 15 and less than fourfold 4 in 15). Therefore, we consider the detected antibodies likely to be towards INKV.
TABLE 1.
Results for selected patient sera tested by INKV IFA, protein-specific IgG IFA, and neutralization testa
| Patient no. or specimen type | IFA result
|
Protein-specific IgG IFA titer
|
Neutralization titer
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| IgG titer | IgG pattern
|
IgM result | |||||||
| g | d | N | Gc | INKV | SSHV | TAHV | |||
| 1 | 160 | + | − | Pos | 120 | <20 | 160 | 80 | 80 |
| 2 | 320 | + | − | Pos | 160 | 20 | 320 | <20 | <20 |
| 3 | 40 | + | − | Pos | 20 | <20 | 160 | 20 | 40 |
| 4 | 40 | + | − | Pos | ND | ND | 320 | <10 | <20 |
| 5 | 160 | + | − | Pos | ND | ND | 160 | 20 | 20 |
| 6 | 80 | + | − | Pos | <20 | <20 | 160 | 20 | 20 |
| 7 | 320 | + | − | Pos | 160 | <20 | 160 | 40 | 20 |
| 8 | 320 | + | − | Pos | 160 | 60 | ND | ND | ND |
| 9 | ≥80 | + | − | Pos | 20 | <20 | 640 | ND | ND |
| 10 | 120 | − | + | Neg | 40 | 40 | 160 | 80 | 40 |
| 11 | 120 | − | + | Neg | <20 | 40 | 160 | 80 | 80 |
| 12 | 240 | − | + | Neg | <20 | <20 | 320 | <20 | 20 |
| 13 | 80 | − | + | Neg | <20 | <20 | 200 | 40 | 20 |
| 14 | 120 | − | + | Neg | <20 | 40 | 220 | 160 | 160 |
| 15 | 40 | − | + | Neg | 40 | 80 | 200 | 20 | 20 |
| 16 | 60 | − | + | Neg | 20 | 80 | 200 | 60 | 40 |
| 17 | 160 | − | + | Neg | <20 | 20 | 320 | 80 | 40 |
| 18 | 320 | − | + | Neg | <20 | 160 | 640 | ND | ND |
| MHAF | 640 | − | + | Neg | ND | ND | ≥1,280 | ≥1,280 | ≥1,280 |
Results are from using INKV-infected Vero E6 cells and Sf-9 insect cells expressing individual INKV proteins as antigens and by neutralization test with INKV, SSHV, and TAHV. The N-specific titer difference between acute (patients 1 to 9) and long-standing immune responses (patients 10 to 18) was significant (P was 0.02, t was 2.8, and df was 6.5 by Student's t test for independent samples; the mean ± standard deviation for acute cases was 91.4 ± 74.7, and that for long-standing-immunity cases was 11.1 ± 17.6), but for Gc-specific titers, the difference was not statistically significant (P was 0.06, t was −2.1, df was 11.7, the mean ± SD for acute cases was 11.4 ± 22.7, and that for long-standing-immunity cases was 51.1 ± 50.1). Equal variances were not assumed. +, with indicated pattern; −, without indicate pattern; g, granular; d, diffuse; Neg, negative; Pos, positive; ND, not determined.
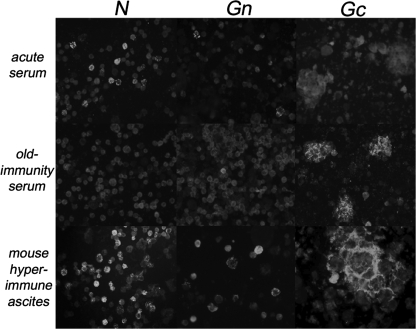
The protein specificity of the antibody response in early and late INKV infection was studied using recombinant INKV N, Gc, and Gn antigens and immunoprecipitation of native viral proteins. This was carried out to elucidate the phenomenon behind the distinct granular and diffuse fluorescence patterns in the IgG IFA. We expressed INKV N, Gn, and Gc proteins in insect cells by using the baculovirus system, and proteins of expected sizes were detected with MHAF immunoprecipitation (Fig. 3). All expressed proteins could also be detected by MHAF in the IFA (Fig. 4).
FIG. 3.
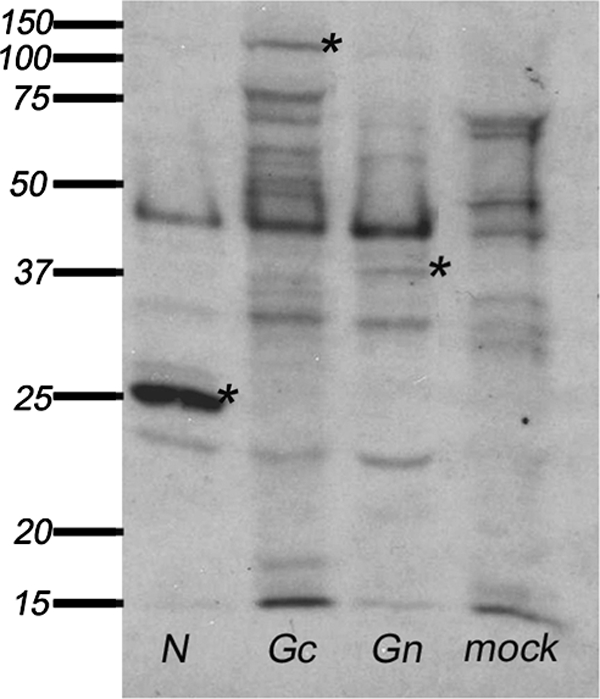
Immunoprecipitation with MHAF of proteins from Sf-9 insect cells infected with baculoviruses expressing INKV N, Gc, or Gn, or from mock-infected cells. Asterisks indicate N, Gc, and Gn proteins.
FIG. 4.
IgG IFA using acetone-fixed Sf-9 cells expressing INKV N, Gn, and Gc proteins by recombinant baculovirus. IFA slides were stained with acute and old immunity human serum samples and hyperimmune mouse ascites fluid.
Although the construct expressing N allows the expression of the NSs from an overlapping reading frame, we could not detect NSs. In immunoblotting, the MHAF was reactive with N but not with the glycoproteins (data not shown), probably due to lack of conformational epitopes in the reducing conditions. Insect cells infected with recombinant baculovirus expressing Gc protein formed cell fusions that could not be seen in cells infected with any other baculovirus constructs (Fig. 4).
INKV protein-specific IgG responses were studied with the selected human serum samples (Table 1) using an IFA based on the baculovirus-expressed proteins as antigens. IgM-positive sera recognized INKV N protein, and occasionally a weak response was observed for cells expressing Gc and Gn (Fig. 4). In contrast, sera from individuals with long-standing immunity (IgG positive and IgM negative) recognized predominantly the Gc protein and showed only a weak or no reaction in N protein- and Gn protein-specific IFA.
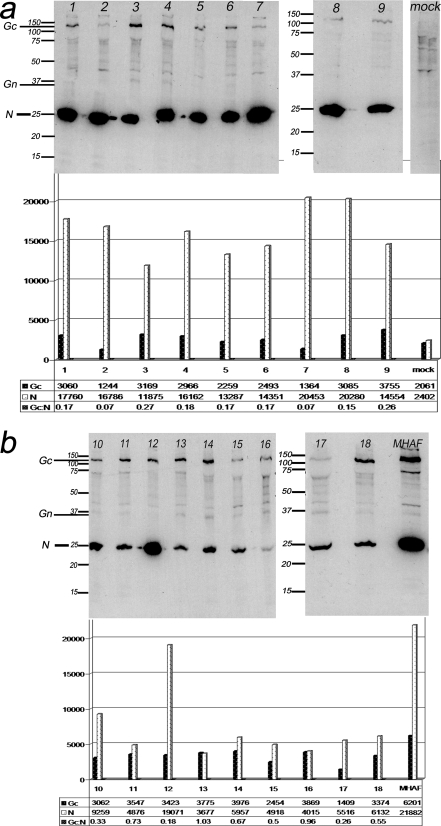
The same panel of sera was studied by immunoprecipitation of native INKV proteins grown in Vero E6 cells. Serum samples from individuals with acute infection (nine of nine) showed a strong IgG response towards N protein (Fig. 5a), whereas the response towards Gc protein was considerably weaker. Some sera from acute cases precipitated Gn as well. Samples from cases with long-standing immunity precipitated both Gc and N (Fig. 5b). The response to N was much weaker in eight out of nine of these sera than in the sera from acute infections. Gn was weakly recognized by most patients with long-standing immunity. The immunoprecipitation results were quantified by densitometry, and the ratio of Gc to N precipitated by the antibodies was considerably lower in the acute-phase sera; statistical analysis (Student's t test for independent samples) showed a significant (P = 0.02; t = 2.6; df = 15.2; the mean ± standard deviation [SD] for acute cases was 0.3 ± 0.2, and that for long-standing-immunity cases was 0.6 ± 0.3) difference between individuals with acute infection and long-standing immunity.
FIG. 5.
Immunoprecipitation of proteins from INKV-infected Vero E6 cells with (a) human sera (lanes 1 to 9), representing acute INKV infection (IgM positive and IgG positive) and an INKV IgG-negative serum (mock). (b) Human sera (lanes 10 to 18) from patients with long-standing INKV immunity (IgM negative and IgG positive) and a MHAF. Intensity of the N and Gc proteins bands are trace quantity values (intensity/millimeter). Statistical analysis for the Gc/N ratio between acute and long-standing immunity (Student's t test for independent samples) (the mean ± SD for long-standing-immunity cases was 0.6 ± 0.3, and that for acute cases was 0.3 ± 0.2) showed that the difference observed between the two groups was significant. P = 0.02; t = 2.6; df = 15.2 (equal variances not assumed).
DISCUSSION
Although INKV was isolated decades ago from mosquitoes in Finland, little is known about its natural cycle, disease associations, and antibody kinetics or the true incidence of clinical INKV infection. Most studies of INKV were performed soon after its isolation in the 1960s, and INKV infection was found to be common in the human population in Finland at that time. A comparison of the panels collected 40 years apart showed that INKV infection has become more common in central and southern Finland. In the 1960s, INKV seroprevalence in the whole country was 24% (2); the current seroprevalence had doubled to 50%. In northern Finland, high seroprevalence (61%) prevails. Our panel is geographically representative, although the studied population may not represent the entire population and may not be directly comparable to the panel of the 1960s.
Because of the lack of detailed knowledge on the natural reservoirs and the abundance of the INKV vectors, it is difficult to speculate the reason for the increased seroprevalence in southern Finland. Furthermore, it is possible that other European members of California serogroup viruses circulate in Finland and contribute to the INKV seroprevalence. Our results were based on an IFA, and the previous data were based on a neutralization assay, which may explain some of the differences observed. The IFA is more cross-reactive between viruses in the California serogroup, and some evidence suggests the presence of TAHV and SSHV in Finland (3; our unpublished data). However, results from the neutralization tests show that the majority of the IgG IFA INKV-positive sera represented true INKV infections.
We found few cases with an acute INKV infection from the screened patient serum panels. Although INKV infections seem common in Finland according to the seroprevalence, the virus is rarely diagnosed as a disease requiring hospitalization. From the screened 1,402 cases with a suspected viral CNS infection, only five had acute INKV infections. This result indicates that the infection is often mild, asymptomatic, or causes symptoms other than CNS disease. This indication is supported by the detection of four acute INKV infections among patients with suspected hantavirus infections, which usually are acute generalized infections accompanied by high fever. The age of the acute patients ranged from 8 to 55 years. Detailed symptoms of the acute INKV patients are not known. Moreover, the age distribution of the seroprevalence suggests that most infections occur in children, whose febrile infections not involving CNS may have been underrepresented in our serum panels.
We detected distinct differences in the IgG IFA fluorescence patterns between the early and late antibody responses. Granular fluorescence seemed to be diagnostic for acute infection, while all of the cases with long-standing INKV immunity showed diffuse fluorescence patterns. Based on our protein-specific IFA and results of the immunoprecipitation analysis, the ratio of antibody responses to N versus Gc protein is the likely determinant of the different fluorescence pattern. Only acute-phase, IgM-positive serum samples, for which N protein was strongly precipitated in native virus immunoprecipitation and baculovirus-expressed INKV N was recognized with the IgG IFA, gave a clear granular fluorescence pattern in the IgG IFA. On the other hand, sera from individuals with long-standing immunity, for which INKV Gc was predominantly recognized with the IgG IFA, showed a diffuse IgG IFA fluorescence pattern. The ratio of N-to-Gc response, calculated from the intensity of the immunoprecipitation bands, was significantly different between these two groups. The response towards Gn was weak in both acute and long-standing immunity samples.
Studies with La Crosse virus have shown that monoclonal antibodies raised against N protein were mainly of IgM class. N protein also seems to be the major target for the early antibody response in other California serogroup virus infections, although this has not been shown for human infections (12). A strong early human anti-N response and granular IgG IFA fluorescence pattern at the acute phase of infection has also been observed with another member of Bunyaviridae family, Puumala virus (27). There is evidence that the granular fluorescence pattern in IFA within the Bunyaviridae family is caused by N protein packed in inclusion bodies (26, 27), which is dependent on the trimerization and oligomerization of the N protein (16).
Interestingly, when the INKV proteins were expressed in insect cells, the Gc construct caused cell fusion. Fusions were visible both in intact cell culture and after several rounds of washing and suspending in IFA (Fig. 4). The relatively high expression level of N in insect cells and its capability of recognizing IgM antibodies (data not shown) demonstrates its potential to be used as antigen in IgM tests. Notably, N protein cross-reacts among all California serogroup viruses. Our data suggest that Gc protein could be useful for seroprevalence studies, and as shown earlier, it would also be the appropriate antigen for typing the California serogroup viruses (12).
The immunological mechanism behind the weakening of the N response is not clear, but it could be related to unsuccessful maturation of the anti-N response. A possible change in IgG subclass composition during the infection could also explain the weaker response, but this explanation requires further studies. However, the differences observed cannot be explained due to the involvement of heterologous California serogroup viruses, because all the selected samples were confirmed to have antibodies specific for INKV by neutralization.
In summary, we detected high (51%) INKV human seroprevalence and found acute INKV infections in Finland by using IFA. We also observed a specific fluorescence pattern, distinguishing acute and past immunity, by using the IgG IFA. This difference was shown to be due to a different protein specificity of the IgG response, and it could be a universal phenomenon among orthobunyavirus infections. By expressing different INKV proteins, we introduced candidate recombinant antigens for serological assays. N protein appeared to be a useful tool to detect acute INKV infections, whereas Gc could be suited for serosurveys. The phenomenon behind the weakening of the initially strong anti-N protein IgG response remains to be elucidated.
Acknowledgments
We thank Leena Kostamovaara, Tytti Manni, and Pirjo Sarjakivi for technical assistance.
This work was supported by grants from Finnish Konkordia Fund, Laboratoriolääketieteen edistämissäätiö, Hospital District of Helsinki, Uusimaa EVO TYH 4211 and TYH 6215, and the National Technology Agency.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Bardos, V., M. Medek, V. Kania, and Z. Hubalek. 1975. Isolation of Tahyna virus from the blood of sick children. Acta Virol. 19:447. [PubMed] [Google Scholar]
- 2.Brummer-Korvenkontio, M. 1973. Arboviruses in Finland. V. Serological survey of antibodies against Inkoo virus (California group) in human, cow, reindeer, and wildlife sera. Am. J. Trop. Med. Hyg. 22:654-661. [DOI] [PubMed] [Google Scholar]
- 3.Brummer-Korvenkontio, M. 1974. Bunyamwera arbovirus supergroup in Finland, A study on Inkoo and Batai viruses. Commentationes Biologicae. 76:1-52. [Google Scholar]
- 4.Brummer-Korvenkontio, M., P. Saikku, P. Korhonen, I. Ulmanen, T. Reunala, and J. Karvonen. 1973. Arboviruses in Finland. IV. Isolation and characterization of Inkoo virus, a Finnish representative of the California group. Am. J. Trop. Med. Hyg. 22:404-413. [PubMed] [Google Scholar]
- 5.Bupp, K., K. Stillmock, and F. Gonzalez-Scarano. 1996. Analysis of the intracellular transport properties of recombinant La Crosse virus glycoproteins. Virology 220:485-490. [DOI] [PubMed] [Google Scholar]
- 6.Burgdorfer, W., V. F. Newhouse, and L. A. Thomas. 1961. Isolation of California encephalitis virus from the blood of a snowshoe hare (Lepus americanus) in western Montana. Am. J. Hyg. 73:344-349. [DOI] [PubMed] [Google Scholar]
- 7.Butenko, A. M., E. A. Vladimirtseva, S. D. Lvov, C. H. Calisher, and N. Karabatsos. 1991. California serogroup viruses from mosquitoes collected in the USSR. Am. J. Trop. Med. Hyg. 45:366-370. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, W. P., and C. Huang. 1999. Sequence comparisons of medium RNA segment among 15 California serogroup viruses. Virus Res. 61:137-144. [DOI] [PubMed] [Google Scholar]
- 9.Elliott, R. M., C. S. Schmaljohn, and M. S. Collett. 1991. Bunyaviridae genome structure and gene expression. Curr. Top. Microbiol. Immunol. 169:91-141. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Scarano, F. 1985. La Crosse virus G1 glycoprotein undergoes a conformational change at the pH of fusion. Virology 140:209-216. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Scarano, F., N. Pobjecky, and N. Nathanson. 1984. La Crosse bunyavirus can mediate pH-dependent fusion from without. Virology 132:222-225. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Scarano, F., R. E. Shope, C. H. Calisher, and N. Nathanson. 1982. Characterization of monoclonal antibodies against the G1 and N proteins of LaCrosse and Tahyna, two California serogroup bunyaviruses. Virology 120:42-53. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Scarano, F., R. E. Shope, C. H. Calisher, and N. Nathanson. 1983. Monoclonal antibodies against the G1 and nucleocapsid proteins of LaCrosse and Tahyna viruses. Prog. Clin. Biol. Res. 123:145-156. [PubMed] [Google Scholar]
- 14.Grady, L. J., S. Srihongse, M. A. Grayson, and R. Deibel. 1983. Monoclonal antibodies against La Crosse virus. J. Gen. Virol. 64:1699-1704. [DOI] [PubMed] [Google Scholar]
- 15.Jacoby, D. R., C. Cooke, I. Prabakaran, J. Boland, N. Nathanson, and F. Gonzalez-Scarano. 1993. Expression of the La Crosse M segment proteins in a recombinant vaccinia expression system mediates pH-dependent cellular fusion. Virology 193:993-996. [DOI] [PubMed] [Google Scholar]
- 16.Kaukinen, P., V. Kumar, K. Tulimäki, P. Engelhardt, A. Vaheri, and A. Plyusnin. 2004. Oligomerization of hantavirus N protein: C-terminal alpha-helices interact to form a shared hydrophobic space. J. Virol. 78:13669-13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kingsford, L., and D. W. Hill. 1983. The effect of proteolytic cleavage of La Crosse virus G1 glycoprotein on antibody neutralization. J. Gen. Virol. 64:2147-2156. [DOI] [PubMed] [Google Scholar]
- 18.Kingsford, L., L. D. Ishizawa, and D. W. Hill. 1983. Biological activities of monoclonal antibodies reactive with antigenic sites mapped on the G1 glycoprotein of La Crosse virus. Virology 129:443-455. [DOI] [PubMed] [Google Scholar]
- 19.LeDuc, J. W. 1987. Epidemiology and ecology of the California serogroup viruses. Am. J. Trop. Med. Hyg. 37:60S-68S. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig, G. V., B. A. Israel, B. M. Christensen, T. M. Yuill, and K. T. Schultz. 1991. Role of La Crosse virus glycoproteins in attachment of virus to host cells. Virology 181:564-571. [DOI] [PubMed] [Google Scholar]
- 21.Matsuura, Y., R. D. Possee, H. A. Overton, and D. H. Bishop. 1987. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J. Gen. Virol. 68:1233-1250. [DOI] [PubMed] [Google Scholar]
- 22.Pekosz, A., and F. Gonzalez-Scarano. 1996. The extracellular domain of La Crosse virus G1 forms oligomers and undergoes pH-dependent conformational changes. Virology 225:243-247. [DOI] [PubMed] [Google Scholar]
- 23.Pekosz, A., C. Griot, N. Nathanson, and F. Gonzalez-Scarano. 1995. Tropism of bunyaviruses: evidence for a G1 glycoprotein-mediated entry pathway common to the California serogroup. Virology 214:339-348. [DOI] [PubMed] [Google Scholar]
- 24.Plassmeyer, M. L., S. S. Soldan, K. M. Stachelek, J. Martin-Garcia, and F. Gonzalez-Scarano. 2005. California serogroup Gc (G1) glycoprotein is the principal determinant of pH-dependent cell fusion and entry. Virology 338:121-132. [DOI] [PubMed] [Google Scholar]
- 25.Plassmeyer, M. L., S. S. Soldan, K. M. Stachelek, S. M. Roth, J. Martin-Garcia, and F. Gonzalez-Scarano. 2007. Mutagenesis of the La Crosse Virus glycoprotein supports a role for Gc (1066-1087) as the fusion peptide. Virology 358:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao, H., S. M. Xia, Z. Y. Chan, G. Song, and R. Yanagihara. 1987. Morphology and morphogenesis of viruses of hemorrhagic fever with renal syndrome. II. Inclusion bodies—ultrastructural markers of hantavirus-infected cells. Intervirology. 27:45-52. [DOI] [PubMed] [Google Scholar]
- 27.Vapalahti, O., H. Kallio-Kokko, A. Närvänen, I. Julkunen, Å Lundkvist, A. Plyusnin, H. Lehvaslaiho, M. Brummer-Korvenkontio, A. Vaheri, and H. Lankinen. 1995. Human B-cell epitopes of Puumala virus nucleocapsid protein, the major antigen in early serological response. J. Med. Virol. 46:293-303. [DOI] [PubMed] [Google Scholar]
- 28.Vapalahti, O., A. Plyusnin, Y. Cheng, T. Manni, M. Brummer-Korvenkontio, and A. Vaheri. 1996. Inkoo and Tahyna, the European California serogroup bunyaviruses: sequence and phylogeny of the S RNA segment. J. Gen. Virol. 77:1769-1774. [DOI] [PubMed] [Google Scholar]