Abstract
Serum-complement-mediated bactericidal antibody (SBA) remains the serologic hallmark of protection against meningococcal disease, despite experimental and epidemiologic data that SBA may underestimate immunity. We measured bactericidal activity against three strains of Neisseria meningitidis group B in sera from 48 healthy adults and in whole blood from 15 subjects. Blood was anticoagulated with lepirudin, a specific thrombin inhibitor not known to activate complement. Depending on the test strain, protective SBA titers of ≥1:4 were present in only 8 to 15% of the subjects, whereas bactericidal activity was present in 40 to 87% of subjects according to the blood assay. Among SBA-negative subjects, blood from 23 to 42% gave a decrease of ≥2 log10 CFU/ml after 1 h of incubation, and blood from 36 to 83% gave a decrease of ≥1 log10 after 2 h. For most blood samples, bactericidal antibodies primarily were directed against noncapsular antigens, since activity was not inhibited by group B polysaccharide. For some SBA-negative subjects, white cells were not needed, since similar respective bactericidal activities were observed in blood and plasma. Bactericidal activity by whole blood of SBA-negative subjects can be rapid (<1 h) and effective (≥2 log10) and, among all subjects, was four- to sixfold more prevalent than a positive SBA. Thus, while an SBA titer of ≥1:4 predicts protection against meningococcal disease, a titer of <1:4 is poorly predictive of susceptibility. More sensitive assays than SBA are needed to assess protective meningococcal immunity, or we risk underestimating the extent of immunity in the population and the effectiveness of new meningococcal vaccines.
Studies by Goldschneider et al. in the 1960s provided compelling data that a serum bactericidal antibody (SBA) titer of 1:4 or greater predicts protection against developing meningococcal disease (reviewed in reference 14). Additional evidence for protective immunity comes from studies demonstrating passive protection by bactericidal antibody in animal models of meningococcal disease (36) and a correlation between the ability of humans to mount SBA responses to vaccination with clinical evidence of meningococcal vaccine effectiveness (15, 16, 25, 35). The importance of SBA in protection also is underscored by clinical observations of greatly increased rates of meningococcal disease in persons with deficiencies in terminal complement components (10, 11, 30), whose sera cannot support bacteriolysis.
The ability of SBA to confer protection against meningococcal disease is now widely accepted (3, 4) and, for purposes of licensure of new meningococcal vaccines, regulatory agencies generally accept SBA as evidence tantamount to vaccine effectiveness (5). What is more controversial is whether persons with serum bactericidal titers of <1:4 also can be protected against developing meningococcal disease (28). For example, a whole-blood assay that measures both serum and opsonophagocytic bactericidal activity against Neisseria meningitidis is reported to be positive in many persons whose SBA titers are <1:4 (12, 17, 18). These data, together with recent epidemiologic data (38), suggest that the SBA results may grossly underestimate the proportion of the population naturally immune from developing meningococcal disease.
For the measurement of whole-blood bactericidal activity, blood needs to be anticoagulated. Up to now, investigators have used either citrate (19) or heparin (12, 17-19, 26). However, the choice of anticoagulant can be a critical factor influencing the ability of N. meningitidis to survive in human blood (19), and both citrate and heparin are known to interact with critical steps in the inflammatory network (34), including complement activation. For example, heparin is known to bind with at least 13 different proteins in the complement cascade (32), and heparin can have a direct inhibitory effect on the killing of certain bacteria by human serum (7). Thus, one cannot exclude the possibility that the previously reported bactericidal data from testing whole blood were confounded by the effects of the anticoagulant used on complement activation. The primary purpose of the present study was to investigate the basis of naturally acquired meningococcal group B immunity in healthy adults living in the San Francisco Bay area, using an assay performed with blood anticoagulated with the highly specific recombinant protein thrombin inhibitor lepirudin (Refludan; Berlex), which is reported not to activate complement (34). Secondary objectives were to define the kinetics of killing of N. meningitidis in the whole-blood assay and the reproducibility of the results and to determine whether naturally acquired antibodies that confer protection in the human blood assay are directed against capsular or noncapsular antigens, since there are few, if any, data addressing these questions in the previously published studies.
MATERIALS AND METHODS
Study subjects.
We obtained serum samples for the measurement of bactericidal activity from 48 healthy adults ranging in age from 21 to 57 years (mean ± standard deviation, 33 ± 11). The samples were obtained between 2002 and 2007 from participants enrolled in a group C meningococcal conjugate vaccine trial (40) or in a study of naturally acquired meningococcal immunity. Of the 48 subjects, 31 (65%) were female. The percentages of white, Hispanic, Asian, and others were 73, 4, 15, and 10, respectively. A convenience sample of 15 subjects (all employees of the hospital or research institute) agreed to provide whole blood on two to four occasions for additional studies of meningococcal immunity. They ranged in age from 21 to 57 years (mean ± standard deviation, 36 ± 13). The guidelines of the Institutional Review Board of Children's Hospital & Research Center at Oakland were strictly adhered to by using an approved protocol, and all subjects provided informed, written consent.
N. meningitidis strains.
We used three group B strains as test organisms for the measurement of bactericidal activity. NZ98/254 (B:4;P1.7-2,4) is lineage 3, sequence type (ST) 42, and is representative of the strain that caused a recent epidemic in New Zealand (2, 9). Strain 8047 (B:2b:P1.5-1,2-2) was isolated from a patient with meningococcal disease in the United States and is ST 8 and cluster A4 lineage, as described previously (6). Strain BZ232 (B:NT;P1.5-2,2-2) is ST 38 and electrophoretic type 76 and was isolated from a patient in The Netherlands.
Growth of bacteria.
Frozen aliquots of bacteria were subcultured overnight at 37°C in 5% CO2 on chocolate agar (Remel, Rancho Cordova, CA). Individual colonies were picked and added to 7 ml of Mueller-Hinton broth (BD, Franklin Lakes, NJ) supplemented with 0.25% (wt/vol) glucose and 0.02 mM cytidine-5′-monophospho-N-acetylneuraminic acid (Sigma, St. Louis, MO). The bacteria were grown at 37°C in a sealed glass tube for approximately 2 h (from an A620 of ∼0.10 to ∼0.6). The culture was transferred and diluted to a final volume of 50 ml in Dulbecco's buffer containing Ca2+ and Mg2+ (designated Dulbecco's ++; Mediatech, Herndon, VA) with added 1% bovine serum albumin (radioimmunoassay grade; Sigma). The bacterial suspension was centrifuged for 10 min at a relative centrifugal force of 2,908, and the bacteria were resuspended in 7 ml of the same buffer.
Serum bactericidal assay.
For serum bactericidal assays, all test sera were heated for 30 min at 56°C to inactivate complement. The complement source was serum from a healthy adult with no detectable bactericidal activity and normal hemolytic complement activity. The final bactericidal reaction mixture contained 20% (vol/vol) complement, serial twofold dilutions of human serum diluted in Dulbecco's ++ buffer and approximately 12 μl of Dulbecco's ++ buffer containing 300 to 400 CFU of N. meningitidis. After 60 min of incubation at 37°C, aliquots from the microtiter plates (Nunc, Rochester, NY) were plated onto chocolate agar (Remel). The plates were incubated at 37°C in 5% CO2, and the following day the numbers of CFU per milliliter were determined. The lowest serum dilution tested was 1:4. The bactericidal titer was defined as the serum dilution resulting in a 50% decrease in CFU per milliliter compared to the CFU per milliliter of the control at time zero. Typically, bacteria incubated with the negative control antibody and complement showed a 150 to 200% increase in CFU per milliliter during the 60 min of incubation.
Whole-blood bactericidal assay.
To avoid the effects of heparin or chelation on complement activation in the whole-blood bactericidal assay, we used recombinant hirudin (lepirudin) as the anticoagulant (final concentration of 27.8 μg/ml, which excludes the weight of mannitol) (34). Hirudin is a specific thrombin inhibitor that is reported not to effect complement activation, since its effect is limited to the final step of coagulation (34). Blood was drawn using a syringe containing the anticoagulant and was assayed for bactericidal activity within 2 h. All experiments were performed in replicate sterile glass tubes containing 1 ml of anticoagulated blood (six tubes per subject). One set of duplicates contained blood alone; a second set contained blood and group B meningococcal polysaccharide (50 μg/ml); and a third set contained, as a negative control group, group A meningococcal polysaccharide (50 μg/ml). The meningococcal polysaccharides were the gifts of Novartis Vaccines, Siena, Italy. Twenty microliters of buffer containing approximately 2,000 CFU of the target strain was added to each vial, and the tubes were capped and gently mixed and incubated at 37°C on a rocking shaker. Aliquots were removed at 0, 1, and 2 h and, for some experiments, also at 3 or 4 h. Volumes of 100, 10, and 10 μl of a 1:10 dilution were plated onto chocolate agar plates and incubated overnight at 37°C in 4% CO2 to ascertain the numbers of CFU per milliliter. For the first four donors, we also measured hemolytic complement activity in plasma or whole blood obtained at times 0, 1, 2, and 3 h (EZ Complement CH50 test; Diamedix Corp., Miami, FL). There was no evidence of significant complement consumption (mean decrease in CH50 [reciprocal dilution of total serum complement that lyses 50% of cells] at 3 h compared to that at time zero, 3.5%; range, 2 to 7%).
Plasma bactericidal assay.
For experiments using plasma, the anticoagulated blood was centrifuged for 10 min at 3,405 × g, and the plasma was centrifuged a second time to ensure that all of the cells were removed, which was verified by microscopy. To test bactericidal activity, we used undiluted plasma that was stored at −70°C to preserve the internal complement. The plasma bactericidal assay was performed in a manner similar to that of the serum bactericidal assay, except that 60 μl of undiluted plasma was mixed with 6 μl of bacteria suspended in Dulbecco's ++ buffer (approximately 500 CFU). The microtiter plates were incubated on a rotating shaker at 37°C, and aliquots were removed at 0, 1, and 2 h and were cultured on chocolate agar plates to ascertain the number of CFU per milliliter.
Statistical analyses.
The proportion of sera with serum bactericidal titers of ≥1:4 (considered a protective titer when measured with human complement [5, 13]) was computed along with the respective 95% confidence intervals according to the method of Newcombe (27) and using a website calculator (http://faculty.vassar.edu/lowry/prop1.html). Bactericidal activity in the whole-blood assay was defined as either a decrease of ≥1 log10 in the number of CFU per milliliter after 2 h of incubation compared to that of the respective CFU per milliliter at time zero or, for a more rigorous definition, a decrease of ≥2 log10 in the number of CFU per milliliter after 1 h of incubation.
RESULTS
SBAs.
Three of 48 sera (6%) were bactericidal at a serum dilution of 1:4 or greater against all three strains. There were no other sera that killed more than one strain. Overall, the number of sera with bactericidal titers of ≥1:4 were 7 (15%) against strain 8047, 6 (13%) against strain NZ98/254, and 4 (8%) against strain BZ232. The upper limits of the 95% confidence intervals are 27, 25, and 20%, respectively.
Whole-blood bacterial killing assay.
We assayed bactericidal activity in whole blood from 15 of the 48 subjects. Of the 15, the SBA titers were ≥1:4 in one subject against all three strains, in another subject only against strain NZ98/254, and in two other subjects only against strain 8047. Sera from the remaining 11 subjects had SBA titers of <1:4 against all three strains.
Reproducibility of the whole-blood assay.
Blood samples from each of the 15 donors were assayed for bactericidal activity against each of the three strains in at least two experiments 1 to 4 weeks apart (total of 90 independent data points on different samples). The respective results in the second experiment in relation to those observed in the first experiment are summarized in Table 1. In general, there was excellent concordance between the respective results. For example, after 2 h of incubation, there were 18 samples for which we observed a decrease of ≥2 log10 CFU/ml of a strain compared to the respective CFU per milliliter at time zero; 7 samples for which we observed a decrease between 1 and 1.9 log10; and 20 samples for which we observed no decrease or a decrease of <1 log10. With only one exception, we obtained identical respective results when a second sample of blood from the same donor was retested 1 to 4 weeks later. The one donor with a discordant result showed a decrease of 0.8 log10 CFU/ml in blood in the first experiment and 1.2 log10 in the second experiment.
TABLE 1.
Reproducibility of the whole-blood assaya
| Incubation time (h) | Reduction in bacterial count (log10 CFU/ml) | No. of samples in assay 1 that showed bacterial count reduction | % of samples (95% confidence interval) in assay 2 that showed a bacterial count reduction (log10 CFU/ml) of:
|
||
|---|---|---|---|---|---|
| <1 | 1 to 1.9 | ≥2 | |||
| 2 | ≥2 | 18 | 0 (0-18) | 0 (0-18) | 100 (82-100) |
| 2 | 1-1.9 | 7 | 0 (0-35) | 100 (65-100) | 0 (0-35) |
| 2 | <1 | 20 | 95 (76-99) | 5 (0-24) | 0 (0-16) |
| 1 | ≥2 | 17 | 0 (0-18) | 6 (1-27) | 94 (73-99) |
| 1 | 1-1.9 | 5 | 0 (0-44) | 100 (56-100) | 0 (0-44) |
| 1 | <1 | 23 | 87 (68-95) | 13 (5-32) | 0 (0-14) |
Blood samples from each of the 15 donors were assayed for killing against each of the three strains in at least two sets of experiments (against all three strains, a total of 90 strain-specific data points from replicate assays done on two occasions 1 to 4 weeks apart were used).
When there was discordance between the respective results of two experiments, the assay was repeated a third time and the consensus result was used to assign the value. Overall, the percentages (95% confidence intervals) of subjects whose whole blood gave ≥1 log killing were 40% (20 to 64%) for strain BZ232, 47% (25 to 70%) for strain NZ98/254, and 87% (62 to 96%) for strain 8047. Note that there was no evidence of greater killing of group B strains by whole blood from the six persons previously immunized with capsular group C or with group A, C, Y, and W-135 polysaccharide-based vaccines (33% against BZ323, 33% against NZ98/254, and 83% against strain 8047, compared to 44, 56, and 89%, respectively, for whole blood from the nine persons not previously immunized; P > 0.5).
Examples of whole-blood bactericidal activity of subjects with SBA titers of ≥1:4.
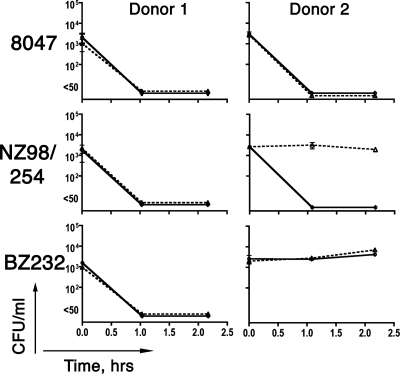
The results from an experiment testing blood of donor 1, whose serum killed all three strains, are shown in Fig. 1 (left). Within 1 h of incubation in blood, the CFU per milliliter of the bacteria from all three strains went from ∼103 at time zero to not detectable (<50 CFU/ml). Whole-blood bactericidal activity was not inhibited by the addition of 50 μg/ml of group B polysaccharide. Thus, despite the killing of all three strains, the bactericidal antibodies in the blood assay appeared to be directed primarily against noncapsular antigens.
FIG. 1.
Bactericidal activity of whole blood from donors with positive serum bactericidal titers. Serum from donor 1 was bactericidal against all three strains (titer of ≥1:4). Serum from donor 2 was bactericidal only against strain NZ98/254. Solid lines, bacteria incubated in blood in the absence of polysaccharide inhibitor. Dashed lines, bacteria incubated in blood containing 50 μg/ml of group B meningococcal polysaccharide. Error bars represent the range of the CFU per milliliter measured in duplicate blood specimens assayed in parallel. No significant inhibition of blood bactericidal activity was observed in samples containing 50 μg per ml of a control, irrelevant group meningococcal polysaccharide (group A; data not shown).
The results of testing the blood of donor 2 also are shown in Fig. 1 (right). The serum from this donor was bactericidal against strain NZ98/254 but not against the other two strains. In the whole-blood assay, we observed a decrease of ≥2 log10 CFU/ml of strain NZ98/254, and the activity was inhibited by the addition of group B polysaccharide (Fig. 1, right) but not by the addition of an irrelevant control polysaccharide (group A; data not shown). Bactericidal activity of blood from donor 2 also was present against strain 8047 but not against strain BZ232. However, in contrast to strain NZ98/254, the bactericidal activity against strain 8047 was not inhibited by group B polysaccharide. Thus, BZ232 was resistant to killing by anticapsular antibodies in the blood of donor 2 that were sufficient to activate bactericidal activity against strain NZ98/254, while absorption of antibodies to group B polysaccharide did not eliminate whole-blood bactericidal activity against strain 8047. The latter observation implies that the blood of donor 2 also contained antibodies against noncapsular antigens that activated bactericidal activity against strain 8047 but not against the other two strains. These data underscore strain variability in susceptibility to different antibodies that can contribute to whole-blood bactericidal antibodies.
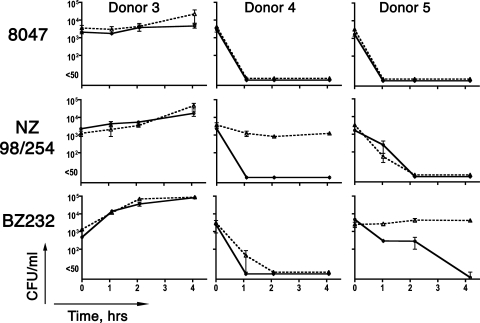
Examples of whole-blood bactericidal activity of subjects with SBA titers of <1:4.
Blood from donor 3 showed no killing against any of the three strains tested (Fig. 2, left), while blood from donors 4 (middle) and 5 (right) was bactericidal against all three strains, although the respective kinetics were different for the different strains. For donor 4, there was a decrease of ≥2 log10 CFU/ml within 1 h of incubation with each of the three strains; for donor 5, it took 1 h for a decrease of ≥2 log10 CFU/ml of strain 8047, 2 h for strain NZ98/254, and 4 h for strain BZ232.
FIG. 2.
Bactericidal activity of whole blood from donors with serum bactericidal titers of <1:4 against all three strains. Symbols are identical to those described in the legend to Fig. 1.
The antibodies responsible for the killing of strain NZ98/254 by blood from donor 4 appeared to be directed against the group B capsule, since killing was inhibited by the addition of group B polysaccharide. However, the addition of the polysaccharide inhibitor had no significant effect on the bactericidal activity of blood against the other two strains.
For purposes of analyses, we defined significant whole-blood bactericidal activity as either a decrease of ≥1 log10 in CFU per milliliter after 2 h of incubation or, for a more rigorous definition, a decrease of ≥2 log10 in the number of CFU per milliliter after 1 h of incubation. The results, stratified for each of these definitions for subjects with SBA titers of <1:4, are summarized in Table 2. Using the less rigorous definition, blood from 10 of the 12 serum-bactericidal-negative subjects (83%) killed strain 8047, 5 of 13 (38%) killed strain NZ98/254, and 5 of 14 (36%) killed strain BZ232. The corresponding numbers and percentages using the second more rigorous definition were 5 of 12 (42%) for strain 8047, 3 of 13 (23%) for strain NZ98/254, and 4 of 14 (29%) for strain BZ232. Nearly all of the whole-blood bactericidal activity could be attributed to antibodies directed at noncapsular antigens, since, with few exceptions, activity was not inhibited by the addition of group B polysaccharide (Table 1).
TABLE 2.
Killing of N. meningitidis group B strains by whole blood from subjects with serum bactericidal titers of <1:4
| Test strain | No. of SBA-negative subjects | No. (%; 95% CI) of blood samples with killing activity according to definition:
|
Killing inhibited by group B polysaccharidec (no. of samples with inhibited activity/no. of samples with killing activity) | |
|---|---|---|---|---|
| 1a | 2b | |||
| 8047 | 12 | 10 (83; 55-95) | 5 (42; 19-68) | 1/10 |
| NZ98/254 | 13 | 5 (38; 18-64) | 3 (23; 8-50) | 2/5 |
| BZ232 | 14 | 5 (36; 16-61) | 4 (29; 12-55) | 1/5 |
Defined as a ≥1 log10 decrease in CFU/milliliter after 2 h of incubation compared to the CFU/milliliter at time zero.
Defined as a ≥2 log10 decrease in CFU/ml after 1 h of incubation compared to the CFU/milliliter at time zero.
Data are for blood samples showing killing as specified by definition 1 and showing a greater than 90% inhibition of killing when the blood contained 50 μg/ml of group B polysaccharide.
Whole-blood bactericidal activity does not necessarily require polymorphonuclear leukocytes.
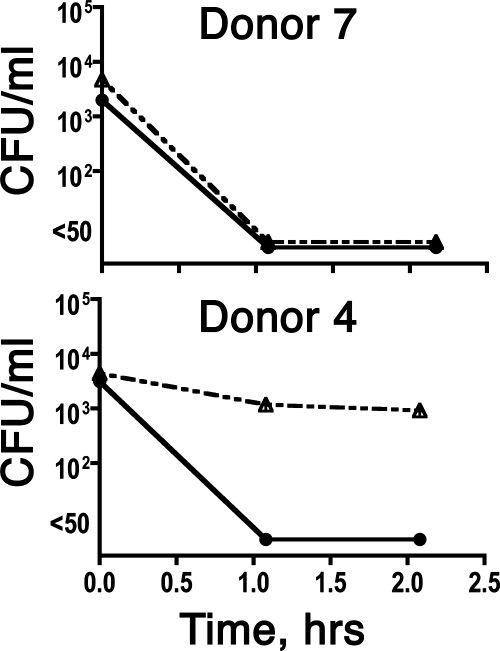
Stored plasma samples that had been prepared at the time of the whole-blood bactericidal assays were available for 10 of the 15 subjects. If the respective serum sample had a bactericidal titer of ≥1:4, the plasma samples alone efficiently killed the bacteria, with sterile cultures achieved by 1 h of incubation (≥2 log10 decrease in CFU per milliliter compared to that at time zero). Conversely, for subjects with SBA titers of <1:4, if the respective blood sample was negative for killing (<1 log10 decrease in CFU per milliliter), the respective plasma sample also was negative. Among subjects with serum bactericidal titers of <1:4, there were four whose whole blood gave a decrease of ≥2 log10 CFU/ml and whose corresponding plasma alone gave a decrease of ≥2 log10 (an example is donor 7 against strain BZ232) (Fig. 3, top). In contrast, there also were two other SBA-negative subjects whose whole blood gave a decrease of ≥2 log10 CFU/ml but whose corresponding plasma samples alone gave no decrease or <1 log10 decrease (see, for example, donor 4 against BZ232) (Fig. 3, bottom). Thus, the presence of leukocytes was required for whole-blood bactericidal activity for some, but not all, subjects with serum bactericidal titers of <1:4.
FIG. 3.
Bactericidal activity of plasma against N. meningitidis group B, strain BZ232. Both donors had serum bactericidal titers of <1:4. Solid lines and closed circles, bacteria incubated with whole blood. Dashed lines with open triangles, bacteria incubated in 91% plasma that had been stored frozen to preserve internal complement activity.
DISCUSSION
As described in the introduction, compelling data going back to the studies of Goldschneider et al. indicate that complement-mediated SBAs confer protection against developing meningococcal disease (13). However, recent seroepidemiologic data are inconsistent with the hypothesis that SBAs are the primary mechanism responsible for the observed age-related acquisition of meningococcal immunity. For example, the annual incidence of group B meningococcal disease in England is reported to be ∼75 per 100,000 infants 6 to 12 months of age, which decreases to ∼10 per 100,000 in the age group 4 to 5 years and <3 per 100,000 in the age group 10 to 12 years (38). However, the prevalence of SBA titers of ≥1:4 is less than 10% in all three age groups, and it is only by age 19 years that the prevalence increases to 50% (38). This increase in SBA prevalence coincides with an increase in asymptomatic group B colonization during the teenage years (8, 23). Thus, many teenagers with SBA titers of <1:4 are exposed to potentially pathogenic N. meningitidis strains (21, 23), yet few develop disease (39). The Goldschneider study showing that persons with SBA titers of ≥1:4 were protected from developing meningococcal disease also described SBA-negative recruits who became colonized with the epidemic group C strain: the attack rate was high (5/13; 39%), but not everyone developed disease (13). Taken together, the data imply the existence of mechanisms other than SBA to explain the observed age-related increase in protective meningococcal immunity.
Given the recent seroepidemiologic data, our hypothesis in the present study was that some, but not all, adults with SBA titers of <1:4 are protected against developing meningococcal disease. Our finding that whole blood from some SBA-negative subjects killed >99% of the bacteria within 1 h of incubation supports our hypothesis. However, it is impossible to be certain that such persons would be entirely safe from developing meningococcal disease if they actually were exposed.
Previously published studies using the whole-blood bactericidal assay indicated that there was an age-related acquisition of the ability of blood to kill N. meningitidis (18) and that whole blood from a substantial proportion of adults with SBA titers of <1:4 was bactericidal against N. meningitidis group B strains (12, 18, 19). The results of the present study extend these observations: depending on the strain tested, we observed whole-blood bactericidal activity (≥1 log10) to be approximately four- to sixfold more prevalent than the respective prevalence of SBA titers of ≥1:4. Further, the kinetics of whole-blood bactericidal activity of SBA-negative adults can be rapid (<1 h) and highly effective (decrease of >2 log10 CFU/ml), and the results were reproducible when measured in blood samples obtained from the same donor in different weeks. Our results were from assays of blood anticoagulated with lepirudin, which, unlike heparin, is reported not to activate complement (34). Taking the previously published and present data together, there is a strong scientific rationale to conclude that an SBA titer of ≥1:4 is sufficient to confer protection against meningococcal disease, but it is not required.
The ability of whole blood from donors with serum bactericidal titers of <1:4 to be bactericidal for N. meningitidis can be explained by two possible antibody-mediated mechanisms: the presence of nonbactericidal opsonic antibodies (1, 20, 29, 30) and the presence of complement-mediated bactericidal antibodies below the threshold of detection of the serum bactericidal assay. It also is possible that innate immunity, such as more effective alternative complement activation, contributed to bactericidal activity in the whole-blood bactericidal assay. Our data are consistent with all three explanations, since in some subjects with SBA titers of <1:4 bactericidal activity was present in 91% plasma, which could have resulted from the high concentrations of antibody and/or complement present compared to those of the assay used for measuring SBA. In other subjects, bactericidal activity was observed with the whole-blood assay but was greatly decreased or not observed at all in the 91% plasma bactericidal assay, which implies that white cells also were needed.
Based on the inhibition of whole-blood bactericidal activity by group B polysaccharide, the naturally acquired bactericidal antibody in the majority of the blood samples appears to be directed against noncapsular antigens. This conclusion is the opposite of that reported by Toropainen et al., based on their studies of passive protective activity of absorbed human serum in the infant rat group B meningococcal bacteremia model (37). The most likely explanation for the discrepant results is the different assays used, one using an animal model and the other an ex vivo human model. Two recent studies reported that factor H (fH), an important down-regulatory molecule in the complement cascade, binds specifically to N. meningitidis cells and enhances resistance of the organism to serum bacteriolysis (22, 33). Binding of fH by meningococci appears to be specific for human, and not rat, fH (31). Thus, in the absence of binding of fH in the infant rat model, the organisms are more susceptible to clearance by antibody and complement, and the presence of low concentrations of group B anticapsular antibodies may be capable of eradicating bloodstream infection. However, these antibody concentrations may not be sufficient for bactericidal activity of N. meningitidis in human blood, in which the bacteria have fH bound to their surface. To understand the actual mechanisms, further studies are needed.
In previous studies, the whole-blood bactericidal assay was used to measure antibody responses of children (26) and adults (12) to meningococcal vaccination. However, we agree with the conclusions of Findlow et al. (12) that despite the apparent utility of the resulting data, the whole-blood bactericidal assay is primarily a research tool and is unlikely to be realistic for the measurement of responses to vaccines in large clinical trials, since fresh blood is required and the assay cannot be performed on stored samples. What is needed, therefore, is a reproducible assay that distinguishes between protective and nonprotective sera with bactericidal titers of <1:4. Given the contribution of white cells to the bactericidal activity of whole blood of some donors, the assay likely will require direct measurement of opsonic antibodies (24, 41) or the prediction of opsonic activity (for example, the ability of serum antibody to activate C3b deposition on the bacterial surface, performed in the absence of leukocytes) (42, 43). By relying on SBA results alone, we are underestimating the extent of meningococcal immunity in the population as well as the potential effectiveness of new meningococcal vaccines.
Acknowledgments
This work was supported by Public Health Service grants RO1 AI46464 and R21 AI061533 (to D.G.) from the National Institute of Allergy and Infectious Diseases, NIH. The assays were performed in a facility funded by Research Facilities Improvement Program grant number CO6 RR-16226 from the National Center for Research Resources, NIH. The serum samples and blood specimens were obtained in part during studies conducted in the Pediatric Clinical Research Center at Children's Hospital & Research Center at Oakland, which is supported by NIH grant M01-RR01271.
We are grateful to Maggie Ching and Ray Chen for expert technical assistance and to Sanjay Ram, University of Massachusetts School of Medicine, and Johan Holst, Norwegian Institute of Public Health, for reviewing the manuscript and providing helpful comments.
Footnotes
Published ahead of print on 3 October 2007.
REFERENCES
- 1.Aase, A., G. Bjune, E. A. Hoiby, E. Rosenqvist, A. K. Pedersen, and T. E. Michaelsen. 1995. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect. Immun. 63:3531-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, M. G., D. R. Martin, C. E. Kieft, and D. Lennon. 2001. A 10-year serogroup B meningococcal disease epidemic in New Zealand: descriptive epidemiology, 1991-2000. J. Paediatr. Child. Health 37:S13-S19. [DOI] [PubMed] [Google Scholar]
- 3.Balmer, P., and R. Borrow. 2004. Serologic correlates of protection for evaluating the response to meningococcal vaccines. Expert Rev. Vaccines 3:77-87. [DOI] [PubMed] [Google Scholar]
- 4.Borrow, R., P. Balmer, and E. Miller. 2005. Meningococcal surrogates of protection-serum bactericidal antibody activity. Vaccine 23:2222-2227. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, R., G. M. Carlone, N. Rosenstein, M. Blake, I. Feavers, D. Martin, W. Zollinger, J. Robbins, I. Aaberge, D. M. Granoff, E. Miller, B. Plikaytis, L. van Alphen, J. Poolman, R. Rappuoli, L. Danzig, J. Hackell, B. Danve, M. Caulfield, S. Lambert, and D. Stephens. 2006. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine 24:5093-5107. [DOI] [PubMed] [Google Scholar]
- 6.Caugant, D. A., L. F. Mocca, C. E. Frasch, L. O. Froholm, W. D. Zollinger, and R. K. Selander. 1987. Genetic structure of Neisseria meningitidis populations in relation to serogroup, serotype, and outer membrane protein pattern. J. Bacteriol. 169:2781-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, T., J. Swanson, J. Wilson, and R. J. Belland. 1995. Heparin protects Opa+ Neisseria gonorrhoeae from the bactericidal action of normal human serum. Infect. Immun. 63:1790-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claus, H., M. C. Maiden, D. J. Wilson, N. D. McCarthy, K. A. Jolley, R. Urwin, F. Hessler, M. Frosch, and U. Vogel. 2005. Genetic analysis of meningococci carried by children and young adults. J. Infect. Dis. 191:1263-1271. [DOI] [PubMed] [Google Scholar]
- 9.Devoy, A. F., K. H. Dyet, and D. R. Martin. 2005. Stability of PorA during a meningococcal disease epidemic. J. Clin. Microbiol. 43:832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa, J. E., and P. Densen. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4:359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fijen, C. A., E. J. Kuijper, A. J. Hannema, A. G. Sjoholm, and J. P. van Putten. 1989. Complement deficiencies in patients over ten years old with meningococcal disease due to uncommon serogroups. Lancet ii:585-588. [DOI] [PubMed] [Google Scholar]
- 12.Findlow, J., S. Taylor, A. Aase, R. Horton, R. Heyderman, J. Southern, N. Andrews, R. Barchha, E. Harrison, A. Lowe, E. Boxer, C. Heaton, P. Balmer, E. Kaczmarski, P. Oster, A. Gorringe, R. Borrow, and E. Miller. 2006. Comparison and correlation of Neisseria meningitidis serogroup B immunologic assay results and human antibody responses following three doses of the Norwegian meningococcal outer membrane vesicle vaccine MenBvac. Infect. Immun. 74:4557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granoff, D. M., L. Harrison, and R. Borrow. Meningoccal vaccines. In S. A. Plotkin, P. Offit, and W. A. Orenstein (ed.), Vaccines, 5th ed., in press. W. B. Saunders Company, Philadelphia, PA.
- 15.Holst, J., B. Feiring, J. E. Fuglesang, E. A. Hoiby, H. Nokleby, I. S. Aaberge, and E. Rosenqvist. 2003. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine 21:734-737. [DOI] [PubMed] [Google Scholar]
- 16.Holst, J., B. Feiring, L. M. Naess, G. Norheim, P. Kristiansen, E. A. Hoiby, K. Bryn, P. Oster, P. Costantino, M. K. Taha, J. M. Alonso, D. A. Caugant, E. Wedege, I. S. Aaberge, R. Rappuoli, and E. Rosenqvist. 2005. The concept of “tailor-made,” protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine 23:2202-2205. [DOI] [PubMed] [Google Scholar]
- 17.Ison, C. A., N. Anwar, M. J. Cole, R. Galassini, R. S. Heyderman, N. J. Klein, J. West, A. J. Pollard, S. Morley, R. Levin, et al. 1999. Assessment of immune response to meningococcal disease: comparison of a whole-blood assay and the serum bactericidal assay. Microb. Pathog. 27:207-214. [DOI] [PubMed] [Google Scholar]
- 18.Ison, C. A., N. Anwar, M. J. Cole, A. J. Pollard, S. L. Morley, K. Fidler, C. Sandiford, J. Banks, S. J. Kroll, and M. Levin. 2003. Age dependence of in vitro survival of meningococci in whole blood during childhood. Pediatr. Infect. Dis. J. 22:868-873. [DOI] [PubMed] [Google Scholar]
- 19.Ison, C. A., R. S. Heyderman, N. J. Klein, M. Peakman, and M. Levin. 1995. Whole blood model of meningococcal bacteraemia—a method for exploring host-bacterial interactions. Microb. Pathog. 18:97-107. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann, A. K., A. R. Gorringe, K. M. Reddin, K. West, I. Smith, and A. Halstensen. 1999. Human opsonins induced during meningococcal disease recognize transferrin binding protein complexes. Infect. Immun. 67:6526-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLennan, J., G. Kafatos, K. Neal, N. Andrews, J. C. Cameron, R. Roberts, M. R. Evans, K. Cann, D. N. Baxter, M. C. Maiden, and J. M. Stuart. 2006. Social behavior and meningococcal carriage in British teenagers. Emerg. Infect. Dis. 12:950-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madico, G., J. A. Welsch, L. A. Lewis, A. McNaughton, D. H. Perlman, C. E. Costello, J. Ngampasutadol, U. Vogel, D. M. Granoff, and S. Ram. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiden, M. C., and J. M. Stuart. 2002. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 359:1829-1831. [DOI] [PubMed] [Google Scholar]
- 24.Michaelsen, T. E., O. Ihle, K. J. Beckstrom, T. K. Herstad, R. H. Sandin, J. Kolberg, and A. Aase. 2003. Binding properties and anti-bacterial activities of V-region identical, human IgG and IgM antibodies, against group B Neisseria meningitidis. Biochem. Soc. Trans. 31:1032-1035. [DOI] [PubMed] [Google Scholar]
- 25.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. Melles, V. S. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 62:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley, S. L., M. J. Cole, C. A. Ison, M. A. Camaraza, F. Sotolongo, N. Anwar, I. Cuevas, M. Carbonero, H. C. Campa, G. Sierra, and M. Levin. 2001. Immunogenicity of a serogroup B meningococcal vaccine against multiple Neisseria meningitidis strains in infants. Pediatr. Infect. Dis. J. 20:1054-1061. [DOI] [PubMed] [Google Scholar]
- 27.Newcombe, R. G. 1998. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 17:857-872. [DOI] [PubMed] [Google Scholar]
- 28.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Hoiby, E. Rosenqvist, J. Holst, H. Nokleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 29.Platonov, A. E., I. V. Vershinina, H. Kayhty, C. A. Fijen, R. Wurzner, and E. J. Kuijper. 2003. Antibody-dependent killing of meningococci by human neutrophils in serum of late complement component-deficient patients. Int. Arch. Allergy Immunol. 130:314-321. [DOI] [PubMed] [Google Scholar]
- 30.Platonov, A. E., I. V. Vershinina, E. J. Kuijper, R. Borrow, and H. Kayhty. 2003. Long term effects of vaccination of patients deficient in a late complement component with a tetravalent meningococcal polysaccharide vaccine. Vaccine 21:4437-4447. [DOI] [PubMed] [Google Scholar]
- 31.Ram, S. 2006. A novel interaction between factor H SCR 6 and the meningococcal vaccine candidate GNA1870: implications for meningococcal pathogenesis and vaccine development, abstr. P6.2.05, p. 90. In IPNC 2006. Australia: 15th Int. Pathogenic Neisseria Conf., Cairns, Australia. Cambridge Publishing, West Leederville, Australia.
- 32.Sahu, A., and M. K. Pangburn. 1993. Identification of multiple sites of interaction between heparin and the complement system. Mol. Immunol. 30:679-684. [DOI] [PubMed] [Google Scholar]
- 33.Schneider, M. C., R. M. Exley, H. Chan, I. Feavers, Y. H. Kang, R. B. Sim, and C. M. Tang. 2006. Functional significance of factor H binding to Neisseria meningitidis. J. Immunol. 176:7566-7575. [DOI] [PubMed] [Google Scholar]
- 34.Sprong, T., P. Brandtzaeg, M. Fung, A. M. Pharo, E. A. Hoiby, T. E. Michaelsen, A. Aase, J. W. van der Meer, M. van Deuren, and T. E. Mollnes. 2003. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 102:3702-3710. [DOI] [PubMed] [Google Scholar]
- 35.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Hoiby, J. Holst, H. Nokleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 36.Toropainen, M., H. Kayhty, L. Saarinen, E. Rosenqvist, E. A. Hoiby, E. Wedege, T. Michaelsen, and P. H. Makela. 1999. The infant rat model adapted to evaluate human sera for protective immunity to group B meningococci. Vaccine 17:2677-2689. [DOI] [PubMed] [Google Scholar]
- 37.Toropainen, M., L. Saarinen, E. Wedege, K. Bolstad, T. E. Michaelsen, A. Aase, and H. Kayhty. 2005. Protection by natural human immunoglobulin M antibody to meningococcal serogroup B capsular polysaccharide in the infant rat protection assay is independent of complement-mediated bacterial lysis. Infect. Immun. 73:4694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trotter, C., J. Findlow, P. Balmer, A. Holland, R. Barchha, N. Hamer, N. Andrews, E. Miller, and R. Borrow. 2007. Seroprevalence of bactericidal and anti-outer membrane vesicle antibodies to Neisseria meningitidis group B in England. Clin. Vaccine Immunol. 14:863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trotter, C. L., N. J. Gay, and W. J. Edmunds. 2006. The natural history of meningococcal carriage and disease. Epidemiol. Infect. 134:556-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vu, D. M., A. W. de Boer, L. Danzig, G. Santos, B. Canty, B. M. Flores, and D. M. Granoff. 2006. Priming for immunologic memory in adults by meningococcal group C conjugate vaccination. Clin. Vaccine Immunol. 13:605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wedege, E., K. Bolstad, A. Aase, T. K. Herstad, L. McCallum, E. Rosenqvist, P. Oster, and D. Martin. 2007. Functional and specific antibody responses in adult volunteers in New Zealand who were given one of two different meningococcal serogroup B outer membrane vesicle vaccines. Clin. Vaccine Immunol. 14:830-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welsch, J. A., G. R. Moe, R. Rossi, J. Adu-Bobie, R. Rappuoli, and D. M. Granoff. 2003. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J. Infect. Dis. 188:1730-1740. [DOI] [PubMed] [Google Scholar]
- 43.Welsch, J. A., R. Rossi, M. Comanducci, and D. M. Granoff. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J. Immunol. 172:5606-5615. [DOI] [PubMed] [Google Scholar]