Abstract
The objectives of this study were to test the efficacy and safety of planned exposure to porcine reproductive and respiratory syndrome virus (PRRSV) in protecting naïve and previously exposed pigs against PRRSV challenge and to gain information on the dose of PRRSV necessary to induce a protective immune response. Fifty 2-week-old pigs were randomly assigned to one of five groups: a group exposed to a low dose of autogenous PRRSV vaccine (the L-VAC group), a group exposed to a high dose of autogenous vaccine (the H-VAC group), a group exposed to a low dose of a heterologous PRRSV strain (strain SDSU73) prior to planned exposure (the SDSU73-L-VAC group), a group exposed to a high dose of a heterologous PRRSV strain (strain SDSU73) prior to planned exposure (the SDSU73-H-VAC group), and a control group. All groups were challenged with PRRSV VR2385 5 weeks after the planned exposure. Necropsy was done 2 weeks after the PRRSV challenge. The H-VAC, SDSU73-L-VAC, and SDSU73-H-VAC groups had significantly (P < 0.05) less severe clinical disease (sneezing, respiratory scores, and weight gain), significantly (P < 0.05) less severe macroscopic and microscopic lung lesions, and significantly (P < 0.05) lower numbers of PRRSV genomic copy numbers in their sera compared to the results for the control group. Planned exposure to live PRRSV can be used as an inexpensive and effective way to decrease the severity of PRRSV-induced disease following subsequent challenge.
A syndrome known as porcine reproductive and respiratory syndrome (PRRS) emerged simultaneously in Europe and North America at the beginning of the 1990s (11, 22). The “Lelystad virus” was isolated from pigs with PRRS in The Netherlands in 1991 (22). A similar virus was identified in the United States in 1992 (2, 4) and was subsequently found to be closely related to the European isolate, and both viruses were designated porcine reproductive and respiratory syndrome virus (PRRSV). PRRSV is a small, enveloped, positive-sense, single-stranded RNA virus classified in the order Nidovirales, family Arteriviridae, genus Arterivirus (3). PRRSV is associated with reproductive failure in adults and is characterized by mid- to late-term abortions, increased numbers of mummified fetuses, early embryonic death, and infertility (14). In growing pigs, PRRSV infection is associated with respiratory disease and is characterized by labored breathing, sneezing, fever, and increased susceptibility to bacterial diseases, such as those caused by Streptococcus suis (21). PRRSV isolates vary antigenically, genetically, and in their virulence properties (9, 13). North American PRRSV isolates have been found to be more virulent than the Lelystad PRRSV strain when they are compared in the growing pig model (7, 8). It is also clear that North American PRRSV isolates vary in their virulence (9). The degree of immunity generated against heterologous isolates based on genetic or antigenic characteristics is still poorly understood.
PRRSV-induced abortion storms affecting 10 to 25% of the breeding-age females in a herd are not uncommon (6), and the losses due to the postweaning form of the disease can be similarly severe and financially devastating. It has been estimated that the economic impact of PRRSV in the United States is approximately $66.75 million for breeding herds and $493.57 million for growing pigs (16). Strategies that can be used to control PRRSV include the elimination of PRRSV through partial de- and repopulation, herd closure followed by the replacement and introduction of naïve pigs, segregated early weaning, multisite production, and parity segregation strategies. Vaccination approaches with modified live virus products have proved successful in experimental settings; however, failures have been reported in the field (10) and vaccine-like isolates of PRRSV have been recovered from cases of reproductive failure in breeding herds or respiratory disease in growing pigs, suggesting that reversion to virulence may occur under some circumstances (17).
Veterinarians are increasingly relying on natural or planned exposure to the farm-specific strain of PRRSV to induce long-term immunity in the herd. Planned exposure in the past has most commonly been done by putting infected pigs in contact with naïve breeding stock or feeding naïve breeding stock aborted fetuses or tissues from infected pigs. These approaches may be unreliable in getting the targeted population consistently infected in a timely manner. Because of this, some veterinarians have initiated the injection of serum from PRRSV-infected pigs into breeding-age animals to ensure exposure to the resident strain (1, 20). This procedure has not been well described or tested in a controlled setting for safety or efficacy. The main objective of this study was to test the efficacy and safety of a live autogenous PRRSV vaccine to protect naïve or previously exposed pigs against PRRSV challenge. The second objective was to gain more information on the PRRSV dose that is able to induce a protective immune response yet minimal disease.
MATERIALS AND METHODS
Animals.
Fifty-four 2-week-old, segregated, early weaned, crossbred pigs were purchased from a herd free of PRRSV and Mycoplasma hyopneumoniae, as determined on the basis of regular serological testing. The sows in this herd were routinely vaccinated for swine influenza virus (SIV) and porcine parvovirus (PPV).
Experimental design, housing, and feeding.
The experimental design of the study is summarized in Table 1. The pigs were included in one of five groups: a group exposed to a low dose of autogenous PRRSV vacccine (the L-VAC group), a group exposed to a high dose of autogenous homologous vaccine (the H-VAC group), a group exposed to a low dose of a heterologous PRRSV strain (strain SDSU73) prior to planned exposure (the SDSU73-L-VAC group), a group exposed to a high dose of a heterologous PRRSV strain (strain SDSU73) prior to planned exposure (the SDSU73-H-VAC group), and a control group. On the day of delivery, the pigs were randomly assigned to five groups with 10 pigs and one group with four pigs (serum donor pigs). All groups were housed in separate rooms in pens (2 by 2.5 m) on the floor and equipped with one nipple drinker and one self-feeder. The experimental protocol was approved by the Iowa State University Institutional Animal Care and Use Committee.
TABLE 1.
Experimental design used to test the efficacy of planned injection of PRRSV to protect pigs against subsequent exposure to PRRSV
| Groupa | Designation | Inoculum at 3 wks of age | Content of serum injected at 8 wks of ageb |
|---|---|---|---|
| 1 | L-VAC | None | Low |
| 2 | H-VAC | None | High |
| 3 | SDSU73-L-VAC | SDSU73c | Low |
| 4 | SDSU73-H-VAC | SDSU73c | High |
| 5 | Controls | None | None |
Each group had 10 pigs. The pigs in all groups were inoculated with strain VR2385 at 13 weeks of age.
PRRSV genomic copy numbers were determined by quantitative PCR. Low, approximately 102 PRRSV strain VR2385 genomic copies per 2 ml; high, approximately 105 PRRSV strain VR2385 copies per 2 ml.
Groups 3 and 4 received approximately 105 TCID50s of SDSU73 in a 2-ml volume.
PRRSV isolates.
PRRSV isolate VR2385 was recovered from a 160-sow herd in southwestern Iowa that had experienced severe respiratory disease in 3- to 16-week-old pigs and high numbers of late-term abortions in 1991 (8). VR2385 has a restriction fragment length polymorphism pattern of 1-3-4. Highly virulent isolate SDSU73 was recovered from a sow herd that experienced a severe epidemic of atypical PRRS in 1996 (14). The restriction fragment length polymorphism pattern of SDSU73 is 1-4-4. The nucleic acid homology between SDSU73 and the VR2385 is 76%, based on sequencing of open reading frame 5 (ORF5).
Serology.
Blood samples were collected on the arrival of the pigs at the research facility (when they were 2 weeks of age) and weekly thereafter throughout the duration of the study.
(i) PRRSV ELISA.
The serum samples taken on the arrival of the pigs at the research facility, on the day of VR2385 serum injection, on the day of VR2385 challenge, and at necropsy were tested for the presence of antibodies to PRRSV by a commercial PRRSV enzyme-linked immunosorbent assay (ELISA; HerdChek PRRS virus antibody test kit 2XR; IDEXX Laboratories, Inc., Westbrook, MA). Samples were considered positive if the sample-to-positive ratio was 0.4 or greater.
(ii) VR2385-specific PRRSV FFN assay.
A fluorescent focus neutralization (FFN) assay for determination of the amount of VR2385-specific neutralizing antibodies was done with sera collected on the day of VR2385-positive serum injection (5 weeks after SDSU73 inoculation) and 5 weeks later, on the day of VR2385 challenge, according to the protocols routinely performed at the Veterinary Diagnostic Laboratory at Iowa State University.
(iii) Miscellaneous serology.
Serum samples collected on the arrival of the pigs at the research facility and at the termination of the study were tested by a PCV2 ELISA based on the recombinant ORF2 capsid protein of PCV2 (15). Samples were considered positive if the calculated sample-to-positive ratio was 0.2 or greater. The serum samples taken on the arrival of the pigs at the research facility and at necropsy were tested for the presence of antibodies to PPV, SIV H1N1, and SIV H3N2 by hemagglutination inhibition assay.
PRRSV quantification.
The extraction of RNA from the sera collected on the day of PRRSV inoculation and at 7 and 14 days after PRRSV inoculation was performed by using a QIAamp viral RNA mini kit (Qiagen, Valencia, CA). Primer-probe combinations specific for ORF7 of the North American PRRSV strain (primer PRRSORF7F [TGTCAGATTCAGGGAGRATAAGTTAC], primer PRRSORF7R [ATCARGCGCACAGTRTGATGC], and probe PRRSORF7P [FAM-TGTGGAGTTYAGTYTGCC, where FAM is 6-carboxyfluorescein]) and the ORF7 of the European PRRSV (primer LELYRTF [GCTGAAGATGACRTYCGGCA], primer LELYRTR [GCAGTYCCTGCGCCTTGAT], and probe LELYRTP [VIC-TGCAATCGATYCAGAC]) were used (18). The PCR mixture consisted of 5 μl RNA template and 20 μl PCR master mixture. The master mixture contained 0.35 μl of the mixture from the QuantiTect probe reverse transcriptase PCR kit (Qiagen) with the magnesium chloride concentration adjusted to 6 mM; 025 μl (1.25 U) of HotStar Taq (Qiagen); 800, 800, and 275 nM forward primers, reverse primers, and detection probes for the North American strain, respectively; and 400, 400, and 100 nM forward primers, reverse primers, and detection probes for the European PRRSV strain, respectively. Each reaction mixture included five progressive 1:10 dilutions of a known genomic copy number of PRRSV that served to generate a standard curve. Each plate was run in a sequence detection system (GeneAmp 7900; Applied Biosystems, Foster City, CA) under company-specific conditions (30 min at 50°C and 15 min at 95°C, followed by 35 cycles of 15 s at 94°C and 60 s at 60°C).
Serum donor pigs.
Four serum donor pigs were inoculated intranasally at 3 weeks of age with 2 ml of VR2385 at 105 50% tissue culture infective doses (TCID50s). At 7 days postinoculation, blood was drawn and was confirmed to be positive for PRRSV nucleic acids by reverse transcriptase PCR. The pigs were killed, and blood was collected for serum injection. Within 30 min, the blood was transported to the laboratory on ice, centrifuged, and stored at −80°C until further use.
Inoculation of pigs with a heterologous isolate of PRRSV.
At 3 weeks of age, 5 weeks before VR2385-positive serum injection, the pigs in the SDSU73-L-VAC and SDSU73-H-VAC groups were inoculated intranasally with 2 ml of passage 2 of SDSU73 at 105 TCID50s (Table 1).
PRRSV-positive serum injection.
On the day of planned exposure by serum injection, the donor serum was thawed and the amount of PRRSV genomic copies was determined by quantitative real-time PCR. During the time period necessary for real-time PCR testing, the sera were stored at +4°C on ice. Serum from one pig that contained approximately 2.18 × 106 PRRSV copies and that was negative for PRRSV-specific antibodies was selected for use for serum injections.
One gram ceftiofur sodium (Naxcel; lot number 1253NX; Pharmacia & Upjohn Company, Kalamazoo, MI) was dissolved in 250 ml sterile saline. The ceftiofur sodium-saline solution was used to dilute the serum. Two solutions with different concentrations were prepared: 30 ml was prepared to contain approximately 105 virus particles in 2 ml, and another 30 ml was prepared to contain approximately 102 virus particles in 2 ml. The final serum-saline-ceftiofur sodium mixtures were kept on ice for approximately 30 min until the use of the product as an injectable autogenous live vaccine. All pigs except the controls received 2 ml of the autogenous vaccine intramuscularly in the right neck. The pigs in the L-VAC and SDSU73-L-VAC groups received approximately 102 PRRSV copies, and the pigs in the H-VAC and SDSU73-H-VAC groups received approximately 105 PRRSV copies (Table 1). After injection, the amount of viable PRRSV in the vaccine preparations was determined by titration and was found to be 104.17 TCID50s in the high-dose groups and 102.17 TCID50s in the low-dose groups.
Postvaccination PRRSV challenge.
Five weeks after the injection with the live autogenous vaccine, all pigs were challenged intranasally with 2 ml PRRSV strain VR2385 at a dose of 104.25 TCID50s. At this time the pigs were 13 weeks old.
Clinical evaluation.
The pigs were monitored daily and were scored for the severity of clinical respiratory disease, with the scores ranging from 0 (normal) to 6 (severe dyspnea and abdominal breathing) (8). In addition, the pigs were evaluated daily for clinical signs and behavioral changes, including sneezing and lethargy. Rectal temperatures were recorded daily. The pigs were weighed weekly until PRRSV challenge and at necropsy.
Necropsy.
Necropsies were performed 14 days after PRRSV challenge, when the pigs were 15 weeks of age. The total amount of lung affected by macroscopic lesions (ranging from 0 to 100% of the affected lung) was estimated, and the lungs were insufflated with fixative, as described previously (8). Sections of the lymph nodes (superficial inguinal, mediastinal, tracheobronchial, mesenteric), tonsil, thymus, ileum, kidney, colon, spleen, and liver were collected at necropsy; fixed in 10% neutral-buffered formalin; and routinely processed for histological examination.
Histopathology.
Microscopic lesions were evaluated in a blinded fashion. Lung tissue sections were scored for the presence and the severity of interstitial pneumonia, with the scores ranging from 0 (normal) to 6 (severe diffuse) (8). Sections of the heart, liver, kidney, ileum, colon, lymph nodes, spleen, and tonsil were evaluated for the presence of lymphohistiocytic inflammation and were scored from 0 (none) to 3 (severe) (8).
Statistical analysis.
Summary statistics were calculated to assess the overall quality of the data. Analysis of variance (ANOVA) was used for cross-sectional assessment of the average daily weight gain and nonrepeated continuous measures. The significance level was a P value of <0.05; pairwise testing by use of the Tukey-Kramer adjustment was used to identify the groups that were different. In order to summarize and simplify the clinical observations, response feature analysis and the chi-square test were used. The clinical scores for each pig were reduced to one weekly mean score, and the resulting values were subject to statistical analysis. Nonrepeated measures of the necropsy and the histopathology data were assessed by use of the nonparametric Kruskal-Wallis ANOVA. If a nonparametric ANOVA test was significant (P < 0.05), then Wilcoxon tests were used to assess the differences for pairs of groups. Differences in incidence were evaluated by using Fisher's exact test.
RESULTS
PRRSV-specific IgG antibodies.
On their arrival at the research facilities, all pigs were negative for PRRSV-specific immunoglobulin G (IgG) antibodies (Fig. 1). At the time of injection with the live autogenous vaccine, all pigs in the SDSU73-L-VAC and SDSU73-H-VAC groups, which had been exposed to SDSU73 5 weeks earlier, had seroconverted to PRRSV, whereas the PRRSV-naïve groups (the L-VAC, H-VAC, and control groups) had not. On the day of VR2385 challenge, all groups except the controls had seroconverted to PRRSV. At the termination of the study, all pigs in all groups were positive for PRRSV-specific antibodies, as determined by ELISA (Fig. 1).
FIG. 1.
Anti-PRRSV IgG response as measured by ELISA in the different treatment groups. Postinoculation day (DPI) −70, SDSU73 inoculation; postinoculation day −35, serum injection; postinoculation day 0, VR2385 inoculation; postinoculation day 14, necropsy.
Anti-VR2385-specific neutralizing antibodies.
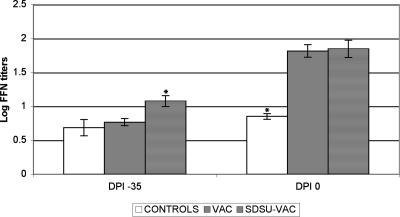
On the day of injection with the live autogenous vaccine, the strain SDSU73-treated groups had significantly (P < 0.003) larger amounts of neutralizing antibodies compared to those in all other groups (Fig. 2). On the day of VR2385 challenge, all groups that received an injection with the live autogenous PRRSV vaccine had significantly (P < 0.001) larger amounts of neutralizing antibodies than the controls (Fig. 2).
FIG. 2.
Anti-VR2385-specific neutralizing antibody response in the controls, pigs inoculated with SDSU73 on postinoculation day (DPI) −70 (SDSU73-VAC groups), and pigs injected with serum on postinoculation day −35 (VAC and SDSU73-VAC groups) prior to VR2385 inoculation on postinoculation day 0. Asterisks, significant differences (P < 0.05) between groups on a given day postinoculation.
Additional serology.
On their arrival at the research facility, all pigs had some level of passively acquired antibodies to PPV and PCV type 2 (PCV2), the titers of antibodies to both of which decayed over time. None of the control pigs seroconverted to PPV or PCV2 during the course of the experiment. At the termination of the experiment, all pigs were negative for antibodies specific to PPV and PCV2 and for antibodies specific to H3N2 and H1N1 SIV.
Clinical disease.
After injection of the pigs with the live autogenous PRRSV vaccine, individual pigs in all groups developed fevers, with their temperatures ranging from 40 to 40.5°C, and had periods of labored breathing. For the 5 weeks postvaccination, there were no significant differences among the groups injected with the live autogenous PRRSV vaccine, as determined by measurement of their respiratory scores and rectal temperatures. However, the nonexposed controls had significantly (P < 0.0014) higher average daily weight gains compared to those for the pigs in all other groups that received the injection of live autogenous PRRSV vaccine. In the period after challenge with VR2385, there were pigs in all groups that experienced increased respiratory scores; however, there were no differences in respiratory scores among the groups. The rectal temperatures were significantly (P < 0.001) higher in the controls, and the controls had significantly (P < 0.05) reduced average weight gains compared to those for pigs in the groups that had previously been inoculated with heterologous isolate SDSU73 and subsequently injected with the live autogenous PRRSV vaccine containing VR2385 (Table 2).
TABLE 2.
Average daily weight gain from the time of PRRSV strain VR2385 challenge to the day of necropsy
| Group | Avg daily wt gain (g)a |
|---|---|
| L-VAC | 986.4 ± 48.6*,** |
| H-VAC | 954.9 ± 71.5*,** |
| SDSU73-L-VAC | 1,101.6 ± 42.1* |
| SDSU73-H-VAC | 1,016.1 ± 107.3* |
| Control | 711.6 ± 67.6** |
Average daily weight gain from 0 to 14 days after PRRSV strain VR2385 inoculation. Values are means ± SE. Different symbols (* and **) within the column indicate significant (P < 0.05) differences in group mean values. Values with both symbols are not significantly different from the other values.
PRRSV genomic copy numbers in sera.
The data on the PRRSV genomic copy numbers in sera are summarized in Table 3. There was a significantly (P < 0.001) higher incidence of PCR-positive pigs, and on postinoculation days 7 and 14 the PRRSV genomic copy numbers were significantly (P < 0.001) higher in the controls than in all other groups. Among the controls, 80% of the pigs were positive for PRRSV nucleic acids in serum by real-time PCR on postinoculation days 7 and 14.
TABLE 3.
Incidence of PRRSV genomic copies in serum on day of PRRSV challenge and days 7 and 14 postinoculation
| Group | Incidence (no. of PRRSV-positive pigs/total no. of pigs in group [group mean of log-transformed data ± SE]) on postinoculation daya:
|
||
|---|---|---|---|
| 0 | 7 | 14 | |
| L-VAC | 0/10 (0.0 ± 0.0) | 2/10 (0.6 ± 0.5)* | 0/10 (0.0 ± 0.0)* |
| H-VAC | 0/10 (0.0 ± 0.0) | 0/10 (0.0 ± 0.0)* | 0/10 (0.0 ± 0.0)* |
| SDSU73-L-VAC | 1/10 (0.2 ± 0.2) | 1/10 (0.3 ± 0.3)* | 0/10 (0.0 ± 0.0)* |
| SDSU73-H-VAC | 0/10 (0.0 ± 0.0) | 0/10 (0.00 ± 0.0)* | 0/10 (0.0 ± 0.0)* |
| Control | 0/10 (0.0 ± 0.0) | 8/10 (3.9 ± 0.7)** | 8/10 (2.0 ± 0.5)** |
Different symbols (* and **) within a column indicate significant (P < 0.001) differences in group mean values and incidences.
Macroscopic lesions.
The macroscopic lesion data are summarized in Table 4. Macroscopic lung lesions were characterized by failure of the lungs to collapse and by mottled tan, well-demarcated areas of pneumonia. The controls had the highest mean lung lesion scores, with 34.9% ± 5.1% of the lung affected. For all vaccinated groups except the L-VAC group, the mean gross lung lesion scores were significantly (P < 0.001) lower than those for the control group.
TABLE 4.
Comparison of severities of macroscopic and microscopic lung lesions in PRRSV-infected pigsa
| Group (no. of pigs) | Macroscopic lung lesion incidence (%) | Microscopic lung lesion score |
|---|---|---|
| L-VAC (10) | 20.3 ± 3.1*,** | 1.6 ± 0.3*,** |
| H-VAC (10) | 18.2 ± 3.4* | 0.7 ± 0.2* |
| SDSU73-L-VAC (10) | 10.0 ± 3.6* | 1.1 ± 0.3* |
| SDSU73-H-VAC (10) | 11.6 ± 3.1* | 1.4 ± 0.2* |
| Control (9) | 34.9 ± 5.1** | 2.7 ± 0.4** |
The range for the macroscopic lung lesion incidence is 0 to 100%; the range for the microscopic lung lesion score is 0 to 6. Different symbols (* and **) within a column indicate significant (P < 0.001) differences in group mean values and incidences. Values with both symbols are not significantly different from the other values.
Microscopic lesions.
Microscopic lung lesions were typical of those associated with PRRSV infection and were characterized by interstitial pneumonia with alveolar septal thickening due to type 2 pneumocyte hypertrophy and hyperplasia and the infiltration of septae with macrophages, necrotizing alveolitis, and the accumulation of seroproteinaceous fluid in the alveolar spaces. The group mean scores for interstitial pneumonia are summarized in Table 4. With the exception of the L-VAC group, the microscopic lung lesion scores were significantly (P < 0.001) lower in the vaccinated groups than in the control group.
Effect of planned exposure dose.
The dose had no effect on macroscopic or microscopic lung lesions, the average daily weight gain after challenge, or rectal temperatures after challenge.
Effect of previous exposure to a heterologous strain.
There was no evidence that neutralizing antibodies to SDSU73 interfered with the development of protective immunity to VR2385. There were significantly (P < 0.01) less severe macroscopic lung lesions in the strain SDSU73-inoculated pigs. There was no effect of exposure to SDSU73 on microscopic lung lesions, average daily weight gain after VR2385 challenge, or rectal temperature after challenge.
DISCUSSION
The main objective of this study was to test the efficacy and safety of the live autogenous PRRSV vaccine for the protection of naïve or previously exposed pigs against homologous PRRSV challenge in a controlled setting. Under the conditions of this study, the live autogenous PRRSV vaccine utilized was effective in partially protecting naïve pigs and pigs previously exposed to a heterologous PRRSV stain against subsequent PRRSV challenge, as measured by the decreased length and levels of viremia and the decreased severity of PRRSV-induced gross and microscopic lesions. This is the first controlled experiment to have further documented the efficacy of a procedure that has been used extensively in the field.
On the basis of the macroscopic lung lesion scores, repeated exposure was more protective than a single exposure. However, the microscopic lung lesion scores neither completely reflected the macroscopic lesions scores nor completely supported cross-protection. The slight discordance of the macroscopic lesion scores and the microscopic lesion scores can be explained by the fact that the macroscopic lesion score accounts for the severity and the extent of lesions, whereas the microscopic lung lesion score accounts for severity only. For example, a lesion might be mild but diffuse. This would result in a low microscopic lesion score but a moderate macroscopic lesion score.
Fano et al. (5) reported a significant (P < 0.05) reduction in the prevalence of PRRSV antibody-positive pigs 1 year after they implemented live autogenous PRRSV vaccination of incoming gilts in a Mexican swine production system. Similarly, Hill et al. (9a) reported a marked improvement of sow herd stability after serum autogenous vaccine interventions, as determined by measurement of the reproductive performance of the breeding herd and the incidence of viremia in weaned pigs in the United States. In contrast to those studies, we tested our protocol under experimental conditions with the growing pig model.
The use of a live autogenous PRRSV vaccine may be justified in herds in which currently available commercial modified live virus PRRSV vaccines have failed. However, those who use planned exposure must understand the risks. Experiences from the field document that short-term losses immediately following the use of the live autogenous product in pregnant animals are variable but can be substantial (R. Baker, personal communication). Similarly, there is evidence that under certain conditions, the commercially approved, live attenuated PRRSV vaccines can also revert to virulence (17).
Reports of abortion induction in sows previously exposed to apparently homologous isolates also frequently occur (R. Baker, personal communication). This may be a consequence of determining the degree of homology entirely on the basis of the sequences of ORF5, which may not fully represent antigenic diversity or similarity.
Killed autogenous vaccines have also been used in breeding herds, particularly to booster immunity from previous natural exposure (M. McCaw, personal communication).
It has been shown that PRRSV-specific antibodies are effective in reducing PRRSV-associated disease, and some level of heterologous protection has been demonstrated; however, the protection appears to be strain specific (12). Osorio et al. (19) found that a cocktail of PRRSV IgG can confer protective immunity against PRRSV-induced reproductive failure in gilts. In contrast, there is also research that supports the idea that small amounts of antibodies accelerate PRRSV binding and entrance into macrophages and, subsequently, increase the incidence of disease (23). Practitioners have expressed concern that the immunity present in breeding-age animals previously exposed to an isolate of PRRSV may interfere with the development of protective immunity against new isolates (anergy) that enter the herd and against isolates that are utilized in updated autogenous live vaccine regimens.
Other risk factors from the use of serum injections for planned exposure to PRRSV must be considered. One issue is the risk of the spread of other known and unknown disease agents which may be present in raw serum. To minimize this risk, practitioners often request PCR for other agents, such as porcine circovirus type 2, in aliquots of the serum that they intend to use and use only those batches in which certain other agents were not found. It should be pointed out that diagnostic laboratories typically look for established economically important agents. Numerous other agents are potentially present in these autogenous serum vaccines. Porcine endogenous retroviruses, lymphotrophic herpesviruses, teschoviruses, pestiviruses, Chlamydia spp., and a host of other likely candidates should be considered potential risks. Although the distribution of these potential pathogens is likely ubiquitous within a herd, the long-term consequences of the synthetic transmission and propagation of these other viruses may largely be underappreciated.
The results of this study indicate that planned exposure to live PRRSV in serum can be used as an inexpensive and effective way to decrease the severity of PRRSV-induced disease following subsequent challenge, regardless of whether the pigs had been previously exposed to a heterologous strain of PRRSV. However, the success of this procedure also likely depends on the scenario for the individual herd. Precautions should be taken to confirm that the serum does not contain unintended viruses, and the use of mass serum injections should be monitored closely.
Acknowledgments
This study was funded by a grant from the Iowa Livestock Health Advisory Council.
PRRSV strain SDSU73 was kindly provided by Boehringer Ingelheim Vetmedica Inc. We thank Shan Yu and Eileen Thacker for providing PRRSV strain VR2385 and Josh Bowden for assistance with the animal work.
Footnotes
Published ahead of print on 10 October 2007.
REFERENCES
- 1.Batista, L., C. Pijoan, and M. Torremorell. 2001. Experimental injection of gilts with porcine reproductive and respiratory syndrome virus (PRRSV) during acclimatization. J. Swine Health Prod. 10:147-150. [Google Scholar]
- 2.Benfield, D. A., E. Nelson, J. E. Collins, L. Harris, S. M. Goyal, D. Robison, W. T. Christianson, R. B. Morrison, D. Gorcyca, and D. Chladek. 1992. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J. Vet. Diagn. Investig. 4:127-133. [DOI] [PubMed] [Google Scholar]
- 3.Cavanagh, D. 1997. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 142:629-633. [PubMed] [Google Scholar]
- 4.Collins, J. E., D. A. Benfield, W. T. Christianson, L. Harris, J. C. Hennings, D. P. Shaw, S. M. Goyal, S. McCullough, R. B. Morrison, H. S. Joo, D. Gorcyca, and D. Chladek. 1992. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Investig. 4:117-126. [DOI] [PubMed] [Google Scholar]
- 5.Fano, E., L. Olea, and C. Pijoan. 2005. Eradication of porcine reproductive and respiratory syndrome virus by serum inoculation of naïve gilts. Can. J. Vet. Res. 69:71-74. [PMC free article] [PubMed] [Google Scholar]
- 6.Halbur, P. G., and E. Bush. 1997. Update on abortion storms and sow mortality. J. Swine Health Prod. 5:73. [Google Scholar]
- 7.Halbur, P. G., P. S. Paul, M. L. Frey, J. Landgraf, K. Eernisse, X. J. Meng, J. J. Andrews, M. A. Lum, and J. A. Rathje. 1996. Comparison of the antigen distribution of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 33:159-170. [DOI] [PubMed] [Google Scholar]
- 8.Halbur, P. G., P. S. Paul, M. L. Frey, J. Landgraf, K. Eernisse, X. J. Meng, M. A. Lum, J. J. Andrews, and J. A. Rathje. 1995. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 32:648-660. [DOI] [PubMed] [Google Scholar]
- 9.Halbur, P. G., P. S. Paul, X. J. Meng, M. A. Lum, J. J. Andrews, and J. A. Rathje. 1996. Comparative pathogenicity of nine US porcine reproductive and respiratory syndrome virus (PRRSV) isolates in a five-week-old cesarean-derived, colostrum-deprived pig model. J. Vet. Diagn. Investig. 8:11-20. [DOI] [PubMed] [Google Scholar]
- 9a.Hill, H. T., J. A. Mulford, J. J. Kaisand, A. Holtcamp, C. Reyes. 2004. PRRS control: to hell and back. Proceedings of the American Association of Swine Veterinarians, 6 to 9 March 2004, p. 369-375. American Association of Swine Veterinarians, Perry, IA.
- 10.Hurd, H. S., E. J. Bush, W. Losinger, B. Corso, J. J. Zimmerman, R. Wills, S. Swenson, D. Pyburn, P. Yeske, and T. Burkgren. 2001. Outbreaks of porcine reproductive failure: report on a collaborative field investigation. J. Swine Health Prod. 9:103-108. [Google Scholar]
- 11.Keffaber, K. 1989. Reproductive failure of unknown aetiology. Am. Assoc. Swine Pract. Newsl. 1:1-10. [Google Scholar]
- 12.Lager, K. M., W. L. Mengeling, and S. L. Brockmeier. 1999. Evaluation of protective immunity in gilts inoculated with the NADC-8 isolate of porcine reproductive and respiratory syndrome virus (PRRSV) and challenge-exposed with an antigenically distinct PRRSV isolate. Am. J. Vet. Res. 60:1022-1027. [PubMed] [Google Scholar]
- 13.Meng, X. J. 2000. Heterogeneity of porcine reproductive and respiratory syndrome virus: implications for current vaccine efficacy and future vaccine development. Vet. Microbiol. 74:309-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mengeling, W. L., K. M. Lager, and A. C. Vorwald. 1998. Clinical consequences of exposing pregnant gilts to strains of porcine reproductive and respiratory syndrome (PRRS) virus isolated from field cases of “atypical” PRRS. Am. J. Vet. Res. 59:1540-1544. [PubMed] [Google Scholar]
- 15.Nawagitgul, P., P. A. Harms, I. Morozov, B. J. Thacker, S. D. Sorden, C. Lekcharoensuk, and P. S. Paul. 2002. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin. Diagn. Lab. Immunol. 9:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann, E. J., J. B. Kliebenstein, C. D. Johnson, J. W. Mabry, E. J. Bush, A. H. Seitzinger, A. L. Green, and J. J. Zimmerman. 2005. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 227:385-392. [DOI] [PubMed] [Google Scholar]
- 17.Opriessnig, T., P. G. Halbur, K. J. Yoon, R. M. Pogranichniy, K. M. Harmon, R. Evans, K. F. Key, F. J. Pallares, P. Thomas, and X. J. Meng. 2002. Comparison of molecular and biological characteristics of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (Ingelvac PRRS MLV), the parent strain of the vaccine (ATCC VR2332), ATCC VR2385, and two recent field isolates of PRRSV. J. Virol. 76:11837-11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opriessnig, T., N. E. McKeown, K. L. Harmon, X. J. Meng, and P. G. Halbur. 2006. Porcine circovirus type 2 infection decreases the efficacy of a modified live porcine reproductive and respiratory syndrome virus vaccine. Clin. Vaccine Immunol. 13:923-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osorio, F. A., J. A. Galeota, E. Nelson, B. Brodersen, A. Doster, R. Wills, F. Zuckermann, and W. W. Laegreid. 2002. Passive transfer of virus-specific antibodies confers protection against reproductive failure induced by a virulent strain of porcine reproductive and respiratory syndrome virus and establishes sterilizing immunity. Virology 302:9-20. [DOI] [PubMed] [Google Scholar]
- 20.Pugh, M. L., R. Main, N. DeBuse, and L. Karriker. 2005. Development of a quality-controlled protocol and resulting commercial sow farm production for on-farm live PRRSV virus inoculation. Proc. Am. Assoc. Swine Pract. 36:33-36. [Google Scholar]
- 21.Thanawongnuwech, R., G. B. Brown, P. G. Halbur, J. A. Roth, R. L. Royer, and B. J. Thacker. 2000. Pathogenesis of porcine reproductive and respiratory syndrome virus-induced increase in susceptibility to Streptococcus suis infection. Vet. Pathol. 37:143-152. [DOI] [PubMed] [Google Scholar]
- 22.Wensvoort, G., C. Terpstra, J. M. Pol, E. A. ter Laak, M. Bloemraad, E. P. de Kluyver, C. Kragten, L. van Buiten, A. den Besten, and F. Wagenaar. 1991. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet. Q. 13:121-130. [DOI] [PubMed] [Google Scholar]
- 23.Yoon, K. J., J. J. Zimmerman, S. L. Swenson, M. J. McGinley, K. A. Eernisse, A. Brevik, L. L. Rhinehart, M. L. Frey, H. T. Hill, and K. B. Platt. 1995. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J. Vet. Diagn. Investig. 7:305-312. [DOI] [PubMed] [Google Scholar]