Abstract
The second-generation Histoplasma antigen immunoassay is semiquantitative, expressing results as a comparison to a negative control, which requires repeat testing of the prior specimen with the current specimen to accurately determine a change in antigen. Reporting results in this manner often is confusing to the ordering physician and laboratory. Development of a quantitative assay could improve accuracy, reduce interassay variability, and eliminate the need to test the prior sample with the current sample in the same assay. Calibrators with known concentrations of Histoplasma antigen were used to quantitate antigen in specimens from patients with histoplasmosis and from controls. Samples from cases of disseminated histoplasmosis or other mycoses and controls were tested to evaluate the performance characteristics of the quantitative assay. Paired specimens were evaluated to determine if quantitation eliminated the need to test the current and prior specimens in the same assay to assess a change in antigen. The sensitivity in samples from patients with AIDS and disseminated histoplasmosis was 100% in urine and 92.3% in serum. Cross-reactions occurred in 70% of other endemic mycoses, but not in aspergillosis. Specificity was 99% in controls with community-acquired pneumonia, medical conditions in which histoplasmosis was excluded, or healthy subjects. A change in antigen level categorized as an increase, no change, or decrease based on antigen units determined in the same assay agreed closely with the category of change in nanograms/milliliter determined from testing current and prior specimens in different assays. Sensitivity, specificity, and interassay precision are excellent in the new third-generation quantitative Histoplasma antigen immunoassay.
Antigen detection is a useful method for the diagnosis of histoplasmosis (8). Until now, antigen results have been expressed semiquantitatively as antigen units (calculated as enzyme immunoassay [EIA] units [EU]) based upon comparison to a negative control. Antigen levels decline with effective therapy (9, 12) and increase with relapse (13), providing a useful method for monitoring treatment. Based upon analysis of the course of antigen clearance during treatment of histoplasmosis in patients with AIDS (4, 6, 7), a ≥4-EU increase in antigen was chosen as evidence suggesting a relapse of histoplasmosis. In that evaluation, the change in antigen units between current and prior specimens tested concurrently rarely exceeded 4 EU in patients who did not relapse clinically (unpublished observation).
Due to interassay variability, prior specimens have been tested simultaneously with current specimens to accurately assess changes in antigen levels. Test reports including current and prior results often are confusing, however, and do not always reach the ordering physician. In this report, a method for quantitation of Histoplasma capsulatum antigen is described, and its performance characteristics, including accuracy, specificity, sensitivity in patients with AIDS and disseminated histoplasmosis, and interassay precision, are determined along with its potential usefulness for monitoring therapy.
MATERIALS AND METHODS
Preparation of purified Histoplasma galactomannan.
Galactomannan was purified from Histoplasma yeast by following published methods (1). Briefly, galactomannan was extracted from H. capsulatum yeast cells with 0.25 M sodium hydroxide and precipitated with 3 volumes of absolute ethanol. The ethanol precipitate was further purified by concanavalin A chromatography, followed by Sepharose CL-6B size-exclusion and DEAE ion-exchange chromatographies. The resulting galactomannan was studied using gas chromatography/mass spectrometry for glycosyl composition analysis, and both one-dimensional and two-dimensional nuclear magnetic resonances were used to measure carbon shifts; terminal (1→6)-α-d-galactofuranose was identified in the major portion of the Histoplasma galactomannan, the characterization and structure of which have been published previously (L. J. Wheat, U.S. patent application 20,070,020,711).
Preparation of urine calibrators.
Antigen calibrators were prepared from a pool of urine specimens from patients with antigen results above 10 EU by the second-generation assay. Dilutions of the urine pool were prepared, and the concentration of H. capsulatum galactomannan antigen in those dilutions was determined by comparing them to a standard curve of the galactomannan purified from the yeast as described above. Final calibrators have been selected at the following concentrations: 39, 28, 19, 14, 10, 6, 3.4, 1.7, and 0.6 ng/ml.
Clinical samples.
Specimens used in this analysis were retrieved from those stored frozen at MiraVista Diagnostics. Serum and urine specimens obtained at baseline or treatment week 1 or 2 that were collected from AIDS patients who underwent treatment for disseminated histoplasmosis with fluconazole were used to determine the sensitivity of the new assay in this population (7). These samples, from histoplasmosis cases in patients with AIDS used in the receiving operator characteristic (ROC) curve analysis, were obtained through a clinical trial that was approved by the institution's institutional review board. Additionally, 228 serum- or urine-paired samples from 92 patients, including 20 patients from the above-described trial who were known to have relapsed and 72 with multiple positive urine specimens previously submitted for Histoplasma antigen testing at MiraVista Diagnostics, were tested twice on different days, and results were calculated as EU using the cutoff value or as nanograms/milliliter by extrapolation from the standard curve. These sample pairs were chosen to include a full spectrum of antigen levels and changes in levels between current and prior specimen pairs, including pairs showing an increase in antigen of at least 4 EU, a decrease of at least 4 EU, or no change. The 4-EU threshold was based upon an analysis of changes in antigen levels during successful suppressive therapy for histoplasmosis (4), in which increases of ≥4 EU were rare, and was used as a criterion for relapse in a study of treatment withdrawal in patients with AIDS who achieved immune reconstitution (3). Controls included patients with other endemic mycoses (blastomycosis, 10 patients; coccidioidomycosis, 10 patients; paracoccidioidomycosis, 5 patients; and penicilliosis marneffei, 5 patients), patients with aspergillosis with Aspergillus fumigatus galactomannan antigenemia (n = 9), patients with mycoplasma pneumonia (n = 25), H. capsulatum-antigen-negative patients with other diseases (n = 25), and healthy subjects (n = 25 urine, 25 sera). Retrieval of clinical data on the patients who had a negative H. capsulatum antigen test and who were later determined to have other diseases was conducted under an institutional-review-board-approved protocol, as was the collection of samples from patients with Mycoplasma pneumonia. For all clinical samples included in this analysis, results were unlinked to patient identifiers or current procedural terminology codes and were not made available to the ordering physicians for use in patient care.
H. capsulatum second-generation antigen assay.
The H. capsulatum second-generation antigen assay is modified (10) from the original EIA (2). In brief, the test is a sandwich EIA using microplates coated with polyclonal rabbit anti-Histoplasma antibodies (capture antibody). Patient specimens and controls are incubated in the precoated plates, permitting binding of antigen to the capture antibody. Bound antigen then is detected with a biotinylated detector antibody, followed by detection with streptavidin horseradish peroxidase and tetramethylbenzidine. The detector antibody has been modified (10) to reduce false-positive results caused by anti-rabbit antibodies (11). Results are calculated by establishing a cutoff value for positivity that is a function of the mean negative control values. Individual specimen results are divided by that cutoff to generate EU, and EU of ≥1.0 are considered positive.
For quantitation, the antigen content of the assay calibrators was determined by comparison to reference standards containing known concentrations of H. capsulatum galactomannan. The optimal antigen concentrations for the calibrators, which were calculated using a four-parameter standard curve fit, were determined to range from 0.6 to 39 ng/ml. Results were classified as positive or negative as described above, using the cutoff value for positivity. Results for positive specimens were determined by extrapolation from the calibration curve and were expressed in nanograms/milliliter. Specimens with optical density (OD) results that were greater than or equal to the cutoff for positivity but that were less than the 0.6-ng/ml standard were reported as being “positive, <0.6 ng/ml,” and those with results exceeding the 39-ng/ml standard were reported as being “positive, >39 ng/ml.” Samples with OD values less than the cutoff for positivity were reported as “none detected.”
Statistical analysis.
ROC curve analysis was performed to determine the cutoff for positivity that would give the optimal sensitivity and specificity and to calculate positive and negative predictive values and likelihood ratios. Comparisons of changes in antigen in paired samples, as calculated using the semiquantitative or quantitative assay methods, were evaluated by linear regression analysis. To test the hypothesis that quantitation eliminated the need to include the prior specimen in the same assay, changes in antigen levels determined using current and prior specimen pair results tested simultaneously were compared to those using current and prior results from different assays.
RESULTS
ROC analysis.
Serum and urine specimens (n = 130) from 65 cases of disseminated histoplasmosis and 100 controls without fungal infection were tested simultaneously. ROC analysis identified the optimal cutoff for positivity to be the optical density that was equal to three times the assay negative control (Fig. 1). At this cutoff, using a 10% disease prevalence, the sensitivity was 95.4%, the specificity was 99.0%, the positive predictive value was 91.4%, the negative predictive value was 99.6%, and the area under the curve was 0.997. The positive likelihood ratio was 95.4, and the negative likelihood ratio was 0.05.
FIG. 1.

ROC curve depicting assay sensitivity and specificity, based upon testing serum and urine specimens from 65 patients with AIDS and disseminated histoplasmosis and 100 controls with mycoplasma pneumonia, other conditions for which histoplasmosis was excluded, and healthy subjects. With a cutoff OD of 0.065 (three times the mean normal), the sensitivity is 95.4% and the specificity is 99.0%.
Results for histoplasmosis cases and controls using the ROC-defined cutoff.
The sensitivity was 100% in urine samples and 92.3% in serum samples from the disseminated histoplasmosis cases (Fig. 2). Cross-reactions were noted in 21 of 30 (70%) controls with other endemic mycoses (blastomycosis, 7/10 patients; coccidioidomycosis, 6/10 patients; paracoccidioidomycosis, 4/5 patients; and penicilliosis marneffei, 4/5 patients) but not with aspergillosis. The specificity in nonfungal controls was 99%. Five clinical specimens with results of >39 ng/ml of antigen were diluted 1:500 and retested. Multiplication of the nanograms/milliliter at that dilution by 500 showed that the antigen concentration ranged from 729 to >11,480 ng/ml. Interassay agreement for all specimens tested three separate times was evaluated by comparing the coefficients of variation. The mean coefficient of variation was 24.01% for results determined as EU, and that for results calculated as nanograms/milliliter was 11.19% (P < 0.0001).
FIG. 2.
Sensitivity in disseminated histoplasmosis in samples from patients with AIDS and specificity in samples from controls. The antigen concentration, in nanograms/milliliter, is shown on the vertical axis. The sensitivity in urine was 100%, and in serum it was 92.3%; the specificity was 99.0% in controls without fungal infections.
Interassay agreement of changes in nanograms/milliliter in histoplasmosis cases with paired current and prior specimens.
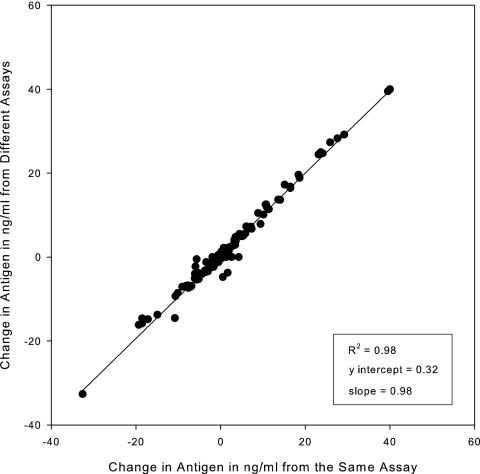
Paired current and prior specimens from patients with histoplasmosis were tested three times in separate assays. Changes in antigen levels between paired samples showed excellent agreement when calculated as nanograms/milliliter on the same day (from the same assay) as opposed to those calculated across different days (from different assays) (Fig. 3).
FIG. 3.
Interassay agreement of changes in nanograms/milliliter in histoplasmosis cases with paired current and prior specimens, comparing results determined in the same assay (same day) with those determined in different assays (different days). Data below the 0 value represent pairs in which the concentration decreased in the current specimen compared to that of the prior specimen, and data above 0 represent pairs in which the concentration increases in the current specimen compared to that of the prior specimen.
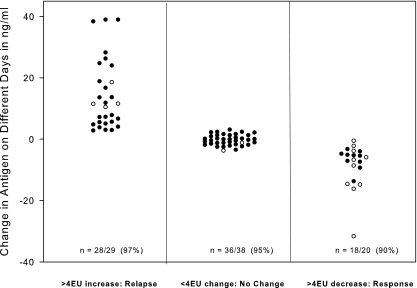
Changes in antigen concentration in nanograms/milliliter between a current and prior specimen calculated from separate assays according to category of change in EU in the same assay.
The changes in antigen levels from sequential samples from individual patients were determined by testing all samples simultaneously and repeating that for a total of three tests. The same-day test results of those samples were calculated as EU, and antigen level changes that were proven reproducible were used to categorize the change in EU as ≥4-EU increases, ≥4-EU decreases, or no change (<4-EU increase or decrease). Next, the change in antigen concentration, calculated quantitatively as nanograms/milliliter, for the current specimen compared to the antigen concentrations of prior specimens that were determined on different days (separate assays) was plotted according to the category determined from the change in EU that was determined on the same day (in the same assay) (Fig. 4). A >3-ng/ml increase for specimens with results below 20 ng/ml (n = 25) and >15% for those that were ≥20 ng/ml (n = 4) occurred in 97% of cases with a ≥4-EU increase, while a similar decrease in nanograms/milliliter occurred in 90% of cases with a ≥4-EU decrease (n = 10 with results of <20 ng/ml, and n = 10 with results of ≥20 ng/ml). No significant change in nanograms/milliliter occurred in 95% of cases without a ≥4-EU change.
FIG. 4.
Changes in antigen concentration between a current and prior specimen first categorized according to the change in antigen EU in the same assay and then plotted with the change in antigen concentration calculated quantitatively as nanograms/milliliter from separate days' assays. The number/total (percent) shown on the column designates agreement between the category of change in nanograms/milliliter as determined from different days' assays with change in antigen EU determined from the same assay. For the determination of agreement, a significant change in nanograms/milliliter was defined as >3 ng/ml when the concentration in the prior specimen was <20 ng/ml (designated by closed circles on the graph) and >15% when the concentration in the prior specimen was ≥20 ng/ml (open circles).
DISCUSSION
The sensitivity of the new assay was 100% for antigenuria and 92.3% for antigenemia in AIDS patients with disseminated histoplasmosis, higher than that previously reported (14). The specificity was 99%. Assuming the prevalence of disseminated histoplasmosis is about 10%, based upon the frequency of positive antigen results in new specimens tested at MiraVista Diagnostics, the positive predictive value is 91.4% and the negative predictive value is 99.5%. Studies are needed to assess the sensitivity in other clinical types of histoplasmosis, as well as in disseminated disease in patients with immunosuppressive disorders other than AIDS or with no underlying immunosuppression. Cross-reactions were noted for patients with other endemic mycoses, including coccidioidomycosis.
Quantitation eliminated the need to retest the prior specimen with the current specimen in the same assay to accurately assess changes in the antigen level. Change in ng/ml determined in different assays correlated well with change in antigen EU measured in the same assay. Furthermore, the category of change (increase, no change, decrease) in ng/ml determined in different assays showed excellent agreement with the category of change in EU determined in the same assay. A >3-ng/ml change for specimens with results below 20 ng/ml and a >15% change for those of ≥20 ng/ml correlated with a ≥4-EU change in more than 90% of cases: 97% for an increase in concentration and 90% for a decrease. Agreement for current/prior pairs showing no change, <4 EU, was 95%.
In the specimens obtained at baseline or week 1 or 2 of treatment with fluconazole from AIDS patients with disseminated histoplasmosis, the antigen concentration was >39 ng/ml in urine in 47.7% of cases and was >39 ng/ml in serum in 29.2% of cases. Changes in antigen concentration cannot be evaluated in specimens for which results exceed the highest point of calibration in the quantitative assay. Upon dilution, for specimens with results of >39 ng/ml, the antigen concentration was estimated to range from almost 1,000 to more than 10,000 ng/ml. When antigenuria exceeds the highest standard and measuring changes in antigen levels to assess response to therapy may not be possible, monitoring antigenemia is recommended. Once antigenemia has cleared, antigenuria should be monitored.
Persistent low-level antigenuria (<4 EU), however, may not represent active infection or predict relapse after stopping therapy. In the treatment withdrawal study, low-level antigenuria was present in 19% of cases when treatment was stopped, and none relapsed (3). Antigenemia was present in none. Studies are needed to determine if persistent antigenuria in the quantitative assay correlates with a risk for relapse after appropriate courses of treatment are discontinued.
In conclusion, these studies demonstrate that the method improvements that allow for the calculation of antigen results quantitatively have resulted in a test system superior to that of antigen results expressed as EU. The quantitative third-generation assay demonstrates excellent accuracy and precision, supporting the aim of no longer needing to test prior specimens alongside the current sample when using the test to monitor therapy.
Acknowledgments
We thank Chadi Hage and Deanna Fuller, Indiana University School of Medicine, for providing information about the clinical controls in which histoplasmosis was excluded. We also thank James Summersgill, University of Louisville School of Medicine, for providing the Mycoplasma pneumonia controls.
We disclose that all of the authors are employees of MiraVista Diagnostics and MiraBella Technologies, the company that developed and performs Histoplasma antigen testing.
The samples from histoplasmosis cases in patients with AIDS used in the ROC curve analysis were obtained through a clinical trial that was sponsored by The AIDS Clinical Trials Group and Mycoses Study Group of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract numbers AI25859 and NO-1-AI-65296.
Footnotes
Published ahead of print on 3 October 2007.
REFERENCES
- 1.Azuma, I., F. Kanetsuna, Y. Tanaka, Y. Yamamura, and L. M. Carbonell. 1974. Chemical and immunological properties of galactomannans obtained from Histoplasma duboisii, Histoplasma capsulatum, Paracoccidioides brasiliensis and Blastomyces dermatitidis. Mycopathol. Mycol. Appl. 54:111-125. [DOI] [PubMed] [Google Scholar]
- 2.Durkin, M. M., P. A. Connolly, and L. J. Wheat. 1997. Comparison of radioimmunoassay and enzyme-linked immunoassay methods for detection of Histoplasma capsulatum var. capsulatum antigen. J. Clin. Microbiol. 35:2252-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman, M., R. Zackin, C. J. Fichtenbaum, D. J. Skiest, S. L. Koletar, R. Hafner, L. J. Wheat, P. M. Nyangweso, C. T. Yiannoutsos, C. T. Schnizlein-Bick, S. Owens, and J. A. Aberg. 2004. Safety of discontinuation of maintenance therapy for disseminated histoplasmosis after immunologic response to antiretroviral therapy. Clin. Infect. Dis. 38:1485-1489. [DOI] [PubMed] [Google Scholar]
- 4.Hecht, F. M., J. Wheat, A. H. Korzun, R. Hafner, K. J. Skahan, R. Larsen, M. T. Limjoco, M. Simpson, D. Schneider, M. C. Keefer, R. Clark, K. K. Lai, J. M. Jacobson, K. Squires, J. A. Bartlett, and W. Powderly. 1997. Itraconazole maintenance treatment for histoplasmosis in AIDS: a prospective, multicenter trial. J. Acquir. Immun. Defic. Syndr. Hum. Retrovir. 16:100-107. [DOI] [PubMed] [Google Scholar]
- 5.Reference deleted.
- 6.Wheat, J., R. Hafner, A. H. Korzun, M. T. Limjoco, P. Spencer, R. A. Larsen, F. M. Hecht, W. Powderly, et al. 1995. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am. J. Med. 98:336-342. [DOI] [PubMed] [Google Scholar]
- 7.Wheat, J., S. MaWhinney, R. Hafner, D. McKinsey, D. Chen, A. Korzun, K. J. Shakan, P. Johnson, R. Hamill, D. Bamberger, P. Pappas, J. Stansell, S. Koletar, K. Squires, R. A. Larsen, T. Cheung, N. Hyslop, K. K. Lai, D. Schneider, C. Kauffman, M. Saag, W. Dismukes, W. Powderly, et al. 1997. Treatment of histoplasmosis with fluconazole in patients with acquired immunodeficiency syndrome. Am. J. Med. 103:223-232. [DOI] [PubMed] [Google Scholar]
- 8.Wheat, L. J. 2003. Current diagnosis of histoplasmosis. Trends Microbiol. 11:488-494. [DOI] [PubMed] [Google Scholar]
- 9.Wheat, L. J., G. Cloud, P. C. Johnson, P. Connolly, M. Goldman, A. Le Monte, D. E. Fuller, T. E. Davis, and R. Hafner. 2001. Clearance of fungal burden during treatment of disseminated histoplasmosis with liposomal amphotericin B versus itraconazole. Antimicrob. Agents Chemother. 45:2354-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheat, L. J., P. Connolly, M. Durkin, B. K. Book, and M. D. Pescovitz. 2006. Elimination of false-positive Histoplasma antigenemia caused by human anti-rabbit antibodies in the second-generation Histoplasma antigen assay. Transplant. Infect. Dis. 8:219-221. [DOI] [PubMed] [Google Scholar]
- 11.Wheat, L. J., P. Connolly, M. Durkin, B. K. Book, A. J. Tector, J. Fridell, and M. D. Pescovitz. 2004. False-positive Histoplasma antigenemia caused by antithymocyte globulin antibodies. Transplant. Infect. Dis. 6:23-27. [DOI] [PubMed] [Google Scholar]
- 12.Wheat, L. J., P. Connolly, N. Haddad, A. Le Monte, E. Brizendine, and R. Hafner. 2002. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob. Agents Chemother. 46:248-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheat, L. J., P. Connolly-Stringfield, R. Blair, K. Connolly, T. Garringer, and B. P. Katz. 1991. Histoplasmosis relapse in patients with AIDS: detection using Histoplasma capsulatum variety capsulatum antigen levels. Ann. Intern. Med. 115:936-941. [DOI] [PubMed] [Google Scholar]
- 14.Williams, B., M. Fojtasek, P. Connolly-Stringfield, and J. Wheat. 1994. Diagnosis of histoplasmosis by antigen detection during an outbreak in Indianapolis, Ind. Arch. Pathol. Lab. Med. 118:1205-1208. [PubMed] [Google Scholar]