Abstract
Cryptococcosis is a significant infection with a high mortality in solid-organ transplant recipients. Nonetheless, the pathogenesis of this disease is poorly understood. It has been hypothesized that cryptococcosis may result from either primary infection or reactivation of a latent infection. Sera were obtained from transplant recipients prior to transplantation and at the time they developed cryptococcosis. Control sera were obtained before and after transplant from patients who did not develop cryptococcosis. Sera were tested for antibodies against Cryptococcus neoformans by using an immunoblot assay. Antibody responses were also compared with those observed in sera from rats with experimental pulmonary cryptococcosis. In all, 52% of the transplant recipients who developed cryptococcosis exhibited serologic evidence of cryptococcal infection before transplantation. These patients developed cryptococcosis significantly earlier after transplant than patients without preexisting reactivity did (5.6 ± 3.4 months compared to 40.6 ± 63.8 months, respectively [P = 0.0011]). The results from our study suggest that a substantial proportion of transplant-associated cryptococcosis cases result from the reactivation of a latent infection. These findings also highlight the potential utility of serologic studies in identifying patients at risk for the development of cryptococcosis after transplantation.
Cryptococcosis is a significant opportunistic infection in solid-organ transplant recipients, with a reported incidence of 1 to 5% and mortality of 20 to 40% (8, 9). Cryptococcus neoformans infection is acquired via inhalation of aerosolized particles from the environment. Nonetheless, the pathogenesis of the disease is poorly understood. C. neoformans is hypothesized to cause in immunocompetent individuals a subclinical pulmonary infection which can evolve to a quiescent latent state with the potential for later reactivation in the context of acquired immunosuppression. Alternatively, it has been suggested that symptomatic disease results from a primary progressive process. Evidence for both mechanisms exists (3, 7, 10).
In previous studies, we developed an immunoblot assay to study subclinical cryptococcosis in immunocompetent individuals (1, 4). Using this approach, we documented that subclinical cryptococcosis was common among children living in the Bronx, NY (4), but not among children living in a northern suburb of New York (2). In the present study, we used serology to study the pathogenesis of cryptococcosis in solid-organ transplant recipients. Results from our studies provide evidence for reactivation of cryptococcosis in a significant proportion of affected transplant recipients. Our findings also highlight the potential for serology to identify transplant recipients at risk for reactivation-type cryptococcosis.
MATERIALS AND METHODS
Strains and growth conditions.
C. neoformans strain 24067 (serotype D) and Candida albicans (BSMY 212) were obtained from the American Type Culture Collection. Fungi were grown in Sabouraud dextrose broth for 2 days at 30°C prior to protein isolation.
Fungal protein extracts.
Whole-cell and cytosolic protein extracts of C. neoformans were used in these studies. Cells were centrifuged at 4,000 × g for 20 min at 4°C, and the pellet was washed twice with phosphate-buffered saline (PBS). The pellet was resuspended in PBS containing a protease inhibitor cocktail buffer (Roche, Mannheim, Germany) and 0.5-mm zirconia-silica beads (Sigma). Cells were disrupted using a mini bead beater. The resulting suspension was centrifuged at 4,000 × g for 15 min at 4°C to obtain whole-cell 32 extracts and at 100,000 × g for 1 h at 4°C to obtain cytosolic extracts. The membrane fractions were washed and centrifuged at 100,000 × g for 30 min at 4°C. The resulting supernatant was pooled with the previous supernatant as part of the cytosolic fraction. Protein extracts were stored at −80°C prior to use. The same approach was used to obtain cytosolic C. albicans protein antigens.
Rat studies.
Rats (three to five per group) were infected intratracheally with 1 × 107 C. neoformans (ATCC 24067) organisms as described previously (1). At different times, rats were sacrificed and sera were obtained. To establish a model of resolved cryptococcal infection, another group of rats were intratracheally infected with 1 × 104 of the unencapsulated strain Cap 67. Sera were collected at 3 months. No lung fungal burden was detected in rats with resolved infection (limit of detection, 50 organisms per lung).
Study population.
Subjects included in the study were identified from a larger cohort of organ transplant recipients with cryptococcosis in a prospective study (12). Cryptococcosis was defined as having cultures positive for C. neoformans in a clinical specimen, including blood cultures, or positive cryptococcal antigen in the blood or cerebrospinal fluid in a patient with compatible clinical presentation (12). Sera obtained before and after solid-organ transplantation from patients who developed cryptococcosis and those who did not develop cryptococcosis were studied. For patients with cryptococcosis, the posttransplant sera were collected at the time of diagnosis of cryptococcal disease. Pre- and posttransplant sera from patients who did not develop cryptococcosis at the same follow-up as the patients with cryptococcosis cases were also studied. All serum samples were stored at −80°C prior to use. Upon thawing, the samples were heat inactivated at 56°C for 30 min and then stored at 4°C.
Definitions.
Sera were divided into four groups as follows: (i) sera obtained before transplant from patients who did not develop cryptococcosis (over 10 years) (Pre/−) (n = 11), (ii) sera obtained before transplant from patients who developed cryptococcosis after transplant (Pre/+) (n = 21), (iii) sera obtained after transplant from patients who did not have cryptococcosis (Post/−) (n = 10), and (iv) sera obtained after transplant from patients with active cryptococcosis (Post/+) (n = 21).
Patients.
A total of 63 sera from 48 transplant recipients were studied. The patients were recipients of a lung, liver, kidney, or heart transplant. Sera were available from 21 transplant recipients with cryptococcosis; this group of recipients included 15 patients from whom paired (i.e., pre- and posttransplant) sera were available. The clinical characteristics of study patients are shown in Table 1.
TABLE 1.
Clinical characteristics of the patients with cryptococcosisa
| Patient parameter | Value |
|---|---|
| Age (yr) | |
| Median | 56 |
| Range | 39-77 |
| No. of patients with: | |
| Transplant type | |
| Liver | 8 |
| Lung | 3 |
| Kidney | 8 |
| Heart | 2 |
| Immunosuppressive regimen | |
| Tacrolimus | 19 |
| Cyclosporine A | 2 |
| Prednisoneb | 17 |
| Time to onset of cryptococcosis | |
| Median | 11.5 mo |
| Range | 25 days-18 yrs |
| No. (%) of patients with indicated infection | |
| Pulmonary only | 18 (85) |
| Central nervous system | 8 (38) |
| Disseminatedc | 12 (57) |
| Serum cryptococcal antigen titer range | 1:32-1:2,048 |
A total of 21 patients were included.
The median prednisone dose at the onset of disseminated infection was 10 mg (range, 5 to 40 mg).
Disseminated infection was defined as central nervous system disease, fungemia, or involvement of two or more noncontiguous organ sites (12).
Immunoblotting.
Electrophoresis was done in the Bio-Rad Mini-Protean II system with a 10% resolving gel at 100 V. Gels were transferred to nitrocellulose membranes, which were then blocked overnight in a buffer containing 5% milk in PBS with 0.1% Tween 20. Individual channels on a blotting frame were incubated with diluted rat (1:200) or human (1:1,600) sera for 1 h at room temperature or overnight at 4°C. Channels were washed three times with blocking buffer and then incubated with a horseradish peroxidase-conjugated goat antibody to human immunoglobulin G or rat immunoglobulin G. Blots were developed with luminol.
Interpretation of data.
Reactivity to cytoplasmic proteins was analyzed with respect to overall reactivity (i.e., to all proteins) or to a set of nine designated proteins most commonly recognized by the sera of infected patients. Reactivity to these nine designated bands was further characterized as extensive (≥6), moderate (3 to 5) and minimal (0 to 2) (4). Investigator scoring was blinded with respect to cohort.
Statistical analysis.
The median numbers of reactive bands among various groups were compared with the Mann-Whitney test or, if the comparisons were done between multiple groups, the Kruskal-Wallis test. The proportions of sera demonstrating reactivity were compared by a chi-square test. Comparisons of clinical characteristics based on serologic findings were done with either Mann-Whitney or chi-square tests. Statistics were calculated using GraphPad InStat software (San Diego, CA). A P value of <0.05 was considered significant.
RESULTS
Rat studies.
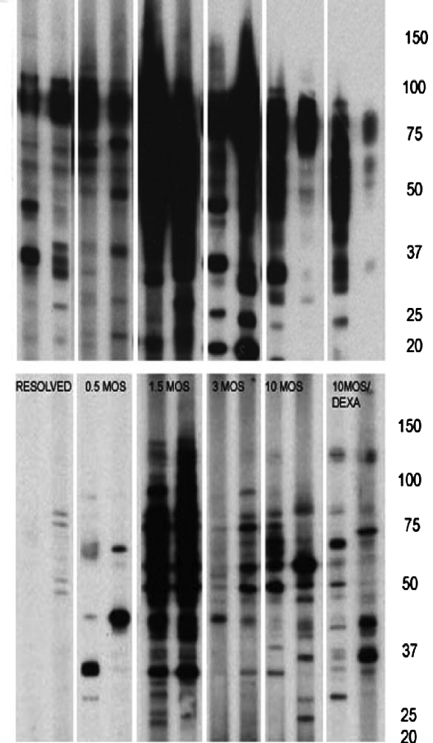
We have previously demonstrated that rats infected with C. neoformans develop a chronic asymptomatic pulmonary infection that persists for years (5). We also found that sera from infected rats exhibit extensive reactivity against whole-cell cryptococcal proteins (1). In the present studies, we observed that sera from infected rats also reacted with cytoplasmic extracts. Blots utilizing cytoplasmic extracts produced less smearing and were more easily interpreted than those with whole-cell extracts (Fig. 1). An increase in reactivity against cytoplasmic proteins over the course of pulmonary infection was observed, with maximal reactivity observed at 1.5 months. This increase in reactivity correlated with a decline in lung fungal burden (not shown). Several cytoplasmic proteins were consistently recognized by rat sera regardless of the stage of infection. This set included five proteins (approximate molecular masses of 82, 75, 71, 56, and 40 kDa), which were present in 65% of the infected rat sera. Dexamethasone treatment decreased the intensity of reactivity against cytoplasmic proteins. Sera from noninfected rats demonstrated no reactivity to cytoplasmic proteins. Rats infected with Cap 67 were able to resolve the infection, and their sera demonstrated between minimal reactivity and no reactivity (0 to 4 bands) against cytoplasmic proteins.
FIG. 1.
Immunoblots for rats infected intratracheally with Cryptococcus neoformans. Representative sera from rats at different times following infection are shown. Rats with resolved infection were inoculated intratracheally with an acapsular strain of C. neoformans and demonstrated to have no lung fungal burden. Some rats (10MOS/DEXA) were infected with C. neoformans for 9 months and then treated with dexamethasone for a month. The top and bottom panels show blots obtained with whole-cell protein and cytoplasmic extracts of C. neoformans, respectively. Molecular mass markers in kDa are shown on the right.
Transplant studies.(i) Reactivity to cryptococcal cytoplasmic proteins.
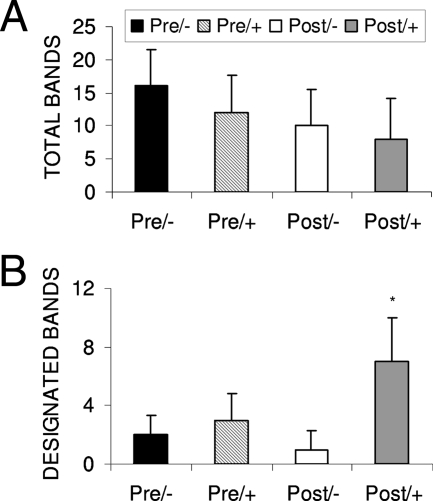
Reactivity against total cytoplasmic proteins was found in all groups. The median numbers of total proteins recognized by Pre/−, Pre/+, Post/−, and Post/+ sera were 16, 12, 10, and 8, respectively (P = 0.1). There was no difference between reactivity in pretransplant and posttransplant sera (P = 0.14) (Fig. 2A). Nine proteins (approximate molecular masses of 133, 122, 106, 97, 82, 75, 71, 56, and 40 kDa) were commonly recognized by Post/+ sera. Approximately 57% of Post/+ sera reacted with most (≥6) of the 9 designated proteins, while 24% reacted with all 9 proteins. Five of these proteins (82, 75, 71, 56, and 40 kDa) were also recognized by the majority (65%) of sera from infected rats. In contrast, sera from other groups demonstrated less reactivity against the nine designated proteins. The median numbers of designated proteins recognized by Pre/−, Pre/+ Post/−, and Post/+ sera were 2, 3, 1, and 7, respectively (P < 0.0001) (Fig. 2B).
FIG. 2.
(A) Median numbers of total cytoplasmic proteins recognized by sera from Pre/− (n = 11), Pre/+ (n = 21), Post/+ (n = 21), and Post/− (n = 10) patients (P value was not significant). Panel B shows median numbers of designated cytoplasmic proteins recognized by sera from the same patients. (the asterisk indicates a P value of <0.01 for Post/+ sera compared to Post/− sera and for Post/+ sera versus Pre/− sera). Bars represent one standard deviation each.
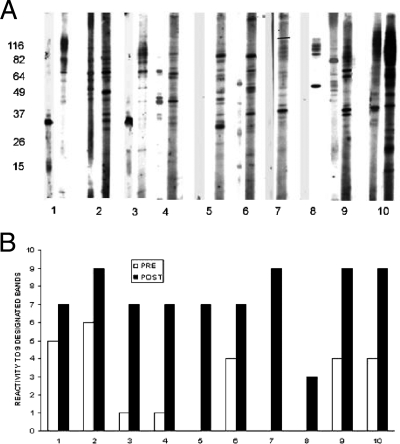
(ii) Paired sera.
The majority (13/15) of paired sera exhibited an increase in reactivity against the nine designated proteins in association with (Fig. 3) cryptococcosis.
FIG. 3.
(A) Representative immunoblots of paired sera from 10 transplant recipients that developed cryptococcosis. For each pair, blots on the left were made with sera obtained prior to transplant, while blots on right were made with sera obtained after transplant at the time that the diagnosis of cryptococcosis was made. (B) The corresponding median numbers of designated proteins recognized by these paired sera are shown.
(iii) Pretransplant sera.
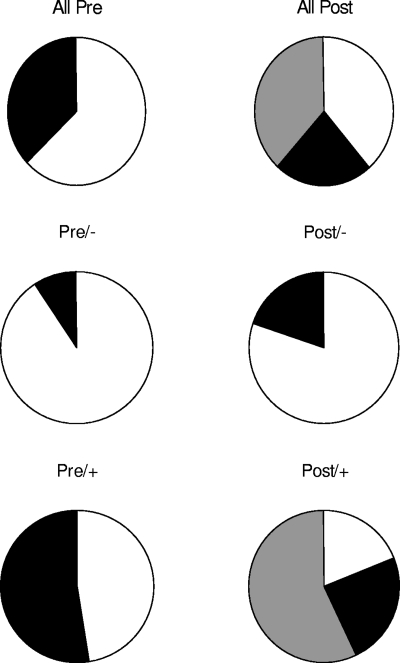
There was no difference in the median number of designated proteins recognized by Pre/− and Pre/+ sera. However, a greater proportion of Pre/+ sera demonstrated moderate reactivity (3 to 6 bands) to the 9 designated proteins compared to sera in the Pre/− group (P = 0.02) (Fig. 4). No sera from any of the pretransplant groups (i.e., Pre/− or Pre/+) recognized more than six proteins. Among Pre/+ patients, 11 had moderate reactivity to the nine cytoplasmic proteins, while 10 patients had little to no reactivity. The sensitivity and specificity of moderate reactivity to predict cryptococcosis in Pre patients were 52% (95% CI 30 to 74) and 91% (95% CI 59 to 100). The positive predictive value was 92% (95%CI 62 to 100). The likelihood ratio for a positive result was 5.8. For Pre/+ patients with moderate reactivity, the average time to diagnosis of cryptococcosis after transplant was 5.6 ± 3.4 months, compared to 40.6 ± 63.8 months for Pre/+ patients with little to no reactivity (P = 0.0011). No differences were detected between these groups with respect to the following features: patient age, proportion of central nervous system disease, organ rejection, and serum and cerebrospinal fluid antigen levels.
FIG. 4.
Proportions of sera demonstrating reactivity to designated cytoplasmic proteins among the various cohorts. White fills represent minimal reactivity (0 to 2 bands), black fills represent moderate reactivity (3 to 5 bands), and gray fills represent extensive reactivity (≥6 bands).
(iv) Reactivity to candidal antigens.
No correlation between reactivity to cytoplasmic proteins of C. neoformans and reactivity to those of C. albicans was observed. Some sera with extensive reactivity to C. neoformans exhibited minimal reactivity to C. albicans (not shown). In addition, reactivity to cytoplasmic proteins of C. albicans did not increase in association with cryptococcal infection (not shown).
DISCUSSION
In previous studies, we demonstrated the utility of an immunoblot assay based on whole-cell extracts to define the incidence of subclinical cryptococcosis in children from the Bronx. Rat studies described here indicate the utility of the immunoblot assay in the study of cryptococcal infection and the potential advantages of using cytoplasmic proteins as opposed to whole-cell protein extracts. These studies also support the specificity of our findings from patients and provide a framework for their interpretation. For rats, the extent of reactivity to cytoplasmic proteins was related to the timing of infection. Reactivity was greatest at 1.5 months of infection, though persistent reactivity was present with ongoing infection at 10 months. Dexamethasone-induced immunosuppression resulted in a decrease in reactivity and resolved infection, induced by inoculation of an acapsular strain of C. neoformans, which produced minimal reactivity against cytoplasmic proteins.
The reactivity profile of transplant recipients with cryptococcosis was considerably more complex than that of experimentally infected rats. We hypothesize that this complexity reflects individual differences in the timing and extent of exposure to cryptococcal antigens as well as differences in the genetic makeup and immunosuppression of our patients. It is also possible that exposure to other fungi contributes to some of the observed reactivity to C. neoformans proteins. This may explain the reactivity to total cytoplasmic proteins observed in the Pre/− group. A decrease in reactivity to the total cytoplasmic proteins among the posttransplant groups likely reflects the immunosuppression of these groups.
Nonetheless, there was a commonality in reactivity to certain bands in sera of affected patients. Sera from Post/+ patients most commonly recognized nine proteins (approximate masses of 133, 122, 106, 97, 82, 75, 71, 56, and 40 kDa). The specificity of this profile to indicate cryptococcal infection is suggested by several findings. Reactivity against five of these proteins (82, 75, 71, 56, and 40 kDa) was also observed for infected rats. Reactivity against proteins of similar sizes has been reported by other investigators. Vecchiarelli et al. reported in their study that sera from the majority of the human immunodeficiency virus-positive individuals with cryptococcosis reacted with 106- and 82-kDa protein antigens (11). Hamilton et al. found reactivity to cryptococcal cytoplasmic proteins among sera from human immunodeficiency virus-infected patients with cryptococcosis (6). Several of these proteins were of the same approximate molecular masses as those observed in our study.
Among patients from whom sera were collected pretransplant, the proportion of those who developed cryptococcosis (Pre/+) exhibited seroreactivity to the designated cytoplasmic proteins was higher than that of the patients who did not develop cryptococcosis. Not all Pre/+ sera exhibited significant seroreactivity. We hypothesize that Pre/+ patients with moderate seroreactivity represent patients with reactivation-type cryptococcosis. Patients with preexisting reactivity against C. neoformans developed cryptococcosis earlier after transplant than patients without preexisting reactivity. These findings are consistent with a preexisting infection that reactivates in the setting of immunosuppression. In contrast, the relatively late presentation of cryptococcal disease among patients without preexisting reactivity could reflect the time needed to be exposed and acquire infection. The potential for C. neoformans to persist and cause reactivation-type disease is supported by several lines of evidence, including molecular typing (3), human pathology (7), and animal studies (5). An alternative explanation for this preexisting reactivity is the possibility of reinfection with C. neoformans. We note that rats that were infected with an avirulent strain of C. neoformans and allowed to resolve their infection did not demonstrate extensive reactivity to C. neoformans proteins. Nonetheless, the possibility of reinfection is difficult to exclude, though based on the timing of disease, we feel that reactivation is the most likely scenario.
Based on our findings, we suggest that an immunoblot assay may be useful in identifying transplant recipients at risk for cryptococcosis. This approach would be limited to identifying cases caused by reactivation. Identified patients could be potentially targeted to receive antifungal prophylaxis to prevent symptomatic disease. The utility of serology in this approach may be affected by a large number of factors, including the underlying prevalence of subclinical infection in the population.
In summary, our findings suggest that approximately 52% of adult transplant recipients with cryptococcosis have had prior exposure to cryptococcal infection. These findings are consistent with the hypothesis that reactivation of latent infection may contribute significantly to the development of cryptococcosis in transplant recipients. Furthermore, these findings highlight the potential utility of serology in identifying transplant recipients at risk for cryptococcosis. We note that utility of this assay in predicting transplant recipients at risk for the development of cryptococcosis may be affected by several variables, including the prevalence of subclinical cryptococcal infection in the population and the type of immunosuppression used. We therefore recommend future studies to validate the utility of this approach.
Acknowledgments
This study was supported by NIH/NIAID grant RO1 AI054719-01 to N.S. D.C.S. was supported by the AIDS International Training and Research Program (NIH D43-TW001403) of the Albert Einstein College of Medicine and the Council of Scientific and Industrial Research-University Grants Commission of India (UGC-45/02-03/R.S.).
S. Husain has received grant support from Astellas and Enzon and has received honoraria from Pfizer and Schering Plough for speaking. K. Pursell serves on the speaker's bureau for Merck. N. Singh has received grant support from Schering Plough, Astellas, and Enzon and is on the speaker's bureau for Pfizer. There are no conflicts of interest for the other authors.
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Chen, L.-C., D. L. Goldman, T. L. Doering, L.-A. Pirofski, and A. Casadevall. 1999. The antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect. Immun. 67:2218-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis, J., W. Y. Zeng, A. Glatman-Freedman, J.-A. Navoa Ng, M. R. Pagcatipunan, H. Lessin, A. Casadevall, and D. L. Goldman. 2007. Serologic evidence for regional differences in pediatric cryptococcal infection. Pediatr. Infect. Dis. J. 26:549-551. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Hermoso, D., G. Janbon, and F. Dromer. 1999. Epidemiological evidence for dormant Cryptococcus neoformans infection. J. Clin. Microbiol. 37:3204-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman, D. L., H. Khine, J. Abadi, D. J. Lindenberg, L.-A. Pirofski, R. Niang, et al. 2001. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:e66. [DOI] [PubMed] [Google Scholar]
- 5.Goldman, D. L., S. C. Lee, A. J. Mednick, L. Montella, and A. Casadevall. 2000. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect. Immun. 68:832-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton, A. J., J. I. Figueroa, L. Jeavons, and R. A. Seaton. 1997. Recognition of cytoplasmic yeast antigens of Cryptococcus neoformans and Cryptococcus neoformans var. gattii by immune human sera. FEMS Immunol. Med. Microbiol. 17:111-119. [DOI] [PubMed] [Google Scholar]
- 7.Haugen, R. K., and R. D. Baker. 1954. The pulmonary lesions in cryptococcosis with special reference to subpleural nodules. Am. J. Clin. Pathol. 24:1381-1390. [DOI] [PubMed] [Google Scholar]
- 8.Husain, S., M. M. Wagener, and N. Singh. 2001. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg. Infect. Dis. 7:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabbour, N., J. Reyes, S. Kusne, M. Martin, and J. Fung. 1996. Cryptococcal meningitis after liver transplantation. Transplantation 61:156-167. [DOI] [PubMed] [Google Scholar]
- 10.Nosanchuk, J. D., S. Shoham, B. C. Fries, D. S. Shapiro, S. M. Levitz, and A. Casadevall. 2000. Evidence of zoonotic transmisssion of Cryptococcus neoformans from a pet cockatoo to an immunocompromised patient. Ann. Intern. Med. 132:205-208. [DOI] [PubMed] [Google Scholar]
- 11.Pitzurra, L., S. Perito, F. Baldelli, F. Bistoni, and A. Vecchiarelli. 2003. Humoral response against Cryptococcus neoformans mannoprotein antigens in HIV-infected patients. Clin. Exp. Immunol. 133:91-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh, N., B. D. Alexander, O. Lortholary, F. Dromer, K. L. Gupta, G. T. John, et al. 2007. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J. Infect. Dis. 195:756-764. [DOI] [PMC free article] [PubMed] [Google Scholar]