Abstract
Foot-and-mouth disease (FMD) is a highly contagious disease affecting cloven-hoofed animals. Vaccination against FMD is a routine practice in many countries where the disease is endemic. This study was designed first to investigate the extract of the seeds of Momordica cochinchinensis (Lour.) Spreng. (ECMS) for its adjuvant effect on vaccination of inactivated FMDV antigens in a guinea pig model and then to evaluate the supplement of ECMS in oil-emulsified FMD vaccines for its immunopotentiation in pigs. The results indicated that ECMS and oil emulsion act synergistically as adjuvants to promote the production of FMDV- and VP1-specific immunoglobulin G (IgG) and subclasses in guinea pigs. A supplement of ECMS in a commercial FMD vaccine significantly enhanced FMDV-specific indirect hemagglutination assay titers as well as VP1-specific IgG and subclasses in pigs. Therefore, ECMS could be an alternative approach to improving swine FMD vaccination when the vaccine is poor to induce an effective immune response.
Foot-and-mouth disease (FMD) is a highly contagious disease affecting cloven-hoofed animals, particularly domestic species, such as cattle, swine, and sheep. Financial losses due to FMD are huge, and it is believed to be the most economically important animal disease in the world (26). Vaccination against FMD is a routine practice in many countries where the disease is endemic. Most commercially available FMD vaccines are inactivated whole-virus preparations containing oil emulsions as an adjuvant to improve their efficacy. However, some FMD vaccines have been reported to induce poor immune responses. For example, Xie et al. (33) observed that only 20.9% of piglets produced immune responses with antibody titers high enough for protection following vaccination against FMD (type O) in the Ningxia province of China. Hao et al. (12) analyzed 91 serum samples of pigs having received vaccination against FMD (type O) and found that only 31.9% of the samples had antibody titers required for immune protection immunity. Therefore, there is a need to improve currently available FMD vaccines in order to effectively protect animals from FMD infection.
Our previous research has demonstrated that a supplement of saponin extracted from the bark of Quillaja saponaria Molina (Quil A) in vaccines significantly enhances immune responses to FMD vaccination (32). However, Quil A may be limited in veterinary use because of its hemolytic activity (23). Another study has shown that an extract from a traditional Chinese medicine, the seeds of Momordica cochinchinensis (Lour.) Spreng. (ECMS), has an adjuvant effect on the immune responses elicited by ovalbumin (OVA), with a much lower hemolytic activity (30). ECMS has also been proven effective in enhancing immune responses to vaccination against avian influenza (H5N1) in chickens (24). The medical use of the seeds has been described in ancient Chinese medical literature, Kai Bao Materia Medica, from the Song Dynasty (AD 793) (10) and is currently included in both Chinese pharmacopoeia (5) and Chinese veterinary pharmacopoeia (6). Traditionally, the seeds are used for the treatment of inflammatory swelling, scrofula, tinea, and diarrhea as well as suppurative skin infections, such as sores, carbuncles, furuncles, and boils, in humans and animals (5, 6, 10). This study was designed first to investigate the adjuvant effect of ECMS and its combination with oil emulsion on vaccination of inactivated FMDV antigens in a guinea pig model and then to evaluate the supplement of ECMS in oil-emulsified FMD vaccines for its immunopotentiation in pigs. As protection against FMDV infection has been believed to be correlated closely with serum indirect hemagglutination assay (IHA) titers (12) as well as FMDV peptide VP1-specific immunoglobulin G (IgG) (29), these parameters were used to measure the efficacy of FMD vaccination.
MATERIALS AND METHODS
Animals.
Thirty female guinea pigs were purchased from Zhejiang University Experimental Animal Center (Hangzhou, China) and housed in polypropylene cages with sawdust bedding. Forty-eight large white crossbred Landrace piglets at 40 days old, with a reciprocal of maternal antibodies to FMD less than 1:32 of IHA titers, were used and kept on a pig farm in Xiaoshan, Hangzhou, China, where sows were immunized against FMD twice annually. Feed and water were supplied ad libitum.
Virus, light white oil, and vaccine.
FMDV antigen (type O) was supplied by the Lanzhou Veterinary Research Institute (LVRI), China, and the concentration of antigen protein was determined by the Bradford protocol (3). FMD vaccine (type O) mixed with an oil emulsion as an adjuvant is available commercially in China. Light white oil (veterinary pharmaceutical grade) was a product of Hangzhou Refinery China Petrochemical Corporation (Hangzhou, China).
ECMS.
The seeds of M. cochinchinensis were appraised by the Zhejiang Institute of Veterinary Drug Control and were the standard product as described in the Chinese veterinary pharmacopoeia (6). The procedure for preparation of ECMS is described in a Chinese patent (13). Briefly, 10 kg of dried cochinchina momordica seeds was crushed into powder and then immersed in 50% ethanol for 24 h. The powder-and-ethanol mixture was put into a round-bottom flask. The mixture was refluxed three times at 90°C, with each reflux taking 2 h. The remaining ethanol was removed using a R502B rotary evaporator (Shenko Tech Co. Ltd., Shanghai, China). The extract was then dissolved in water and washed with diethyl ether to remove the ether-soluble substance. After that, the saponin fraction was extracted with water-saturated n-butanol. The butanol-soluble fraction was purified by passage through a chromatography column with macro-porous resin D101A (Hai Guang Chemical Co. Ltd., Tianjin, China). Purified ECMS was obtained by evaporating the liquid eluted from the column.
Experiment A.
Guinea pigs were randomly divided into five groups with six animals in each group and intramuscularly injected with inactivated FMD antigen (350 μg) in saline with or without 100 μg of ECMS or in oil emulsion with or without ECMS (100 μg). The fifth group of animals was not immunized, as a control. The oil was emulsified in water with a ratio of 1:1 (vol/vol) by being pushed back and forth between two syringes connected with a plastic tube for 15 min, as described by Koh et al. (16). Each animal was injected twice, with a 3-week interval between injections. Blood samples were collected on the day before initiating and 2, 4, and 6 weeks after the boosting injection. Serum was separated and stored at −20°C until use.
Experiment B.
Twenty-four piglets were randomly assigned to four groups with six animals in each group. The animals were immunized by a single intramuscular injection of an FMD vaccine (2 ml) mixed with ECMS (0, 1, 2, or 4 mg per dose). Blood samples were collected before and 3 weeks after immunization. Serum was separated for measurement of IHA titers.
Experiment C.
Twenty-four piglets were randomly divided into two groups with 12 animals in each group. The animals were immunized by a single intramuscular injection of an FMD vaccine (2 ml) supplemented with or without ECMS. Since the optimal dose of ECMS was 4 mg, as analyzed in experiment B, 4 mg of ECMS was used in each dose of FMD vaccine. Blood samples were collected before and 3 and 6 weeks after immunization. Serum was separated for measurement of IHA titers and FMDV peptide VP1-specific antibodies.
FMDV-specific IgG and subclasses of guinea pig samples.
Serum samples were analyzed for measurement of total serum IgG and subclasses by indirect double antibody sandwich enzyme-linked immunosorbent assay (ELISA). All of the wells of polyvinyl 96-well microtiter plates were coated with 50 μl of cattle anti-FMDV (type O) antibody (LVRI, China) diluted in 0.05 M carbonate-bicarbonate buffer, pH 9.6 (1:1,000) and incubated overnight at 4°C. After six washes with phosphate-buffered saline containing 0.05% Tween 20 (PBST), the wells were blocked with 5% skim milk and incubated at 37°C for 2 h. PBST was also used as a diluent and washing solution in all subsequent steps. Thereafter, 50 μl of FMDV (type O) antigen (LVRI) (1:3 dilution) was added and incubated at 4°C for 2 h. After washing; 50-μl serum samples (1:50) were added to duplicate wells and incubated at 25°C for 1 h. Following six washings, 50 μl of goat anti-guinea pig IgG, IgG1, or IgG2 (Bethyl Laboratory, Inc.) (1:1,000) was added to the wells and incubated at 25°C for 1 h. After six washes, 100 μl of horseradish peroxidase (HRP)-conjugated rabbit anti-goat IgG (Bethyl Laboratory, Inc.) diluted in PBST (1:10,000) was added to each well and incubated at 25°C for 1 h. Plates were washed again with PBST. After that, 100 μl of 3,3′,5,5-tetramethylbenzidine solution (100 μg per ml of 0.1 M citrate-phosphate, pH 5.0) was added to each well and incubated for 15 min at room temperature. The reaction was stopped by adding 50 μl of 2 M H2SO4 to each well. The optical density of the plate was read by an automatic ELISA plate reader at 450 nm.
FMDV peptide VP1-specific IgG and subclasses.
To an FMDV peptide VP1-coated microtiter plate (United Biomedical, Inc.), 100 μl of serum samples (1:20 dilution) was added. The plate was incubated at 37°C for 60 min and then washed six times with PBST. To measure IgG of guinea pigs, 100 μl of HRP-conjugated rabbit anti-guinea pig IgG (LVRI, Lanzhou, China) (1:800) was added. To measure IgG subclasses of guinea pigs, 100 μl of goat anti-guinea pig IgG1 or IgG2 (Bethyl Laboratory, Inc.) (1:1,000) was added and incubated at 37°C for 30 min. The wells were then washed six times, and 100 μl of HRP-conjugated rabbit anti-goat IgG (Bethyl Laboratory, Inc.) (1:10,000) was added. To measure IgG of pigs, 100 μl of HRP-conjugated protein A/G (United Biochemical, Inc.) (1:500) was added and incubated at 37°C for 30 min. To measure IgG subclasses of pigs, 100 μl of mouse anti-porcine IgG1 or IgG2 (Serotec, Oxford, United Kingdom) (1:100) was added and incubated at 37°C for 30 min. The wells were then washed six times, and 100 μl of HRP-conjugated goat anti-mouse IgG (Chemicon International, Inc., Temecula, CA) (1:500) was added. The plate was incubated at 37°C for 30 min and then washed six times. After that, 100 μl of 3,3′,5,5-tetramethylbenzidine substrate solution was added to each well and incubated at 37°C for 15 min. The reaction was stopped by adding 50 μl of 2 M H2SO4, and the optical density value of the plate was read by an automatic ELISA plate reader at 450 nm.
IHA against FMD (type O) in pigs.
Antibody titer was measured by IHA as described by Xiao et al. (32). Briefly, serum samples were heated at 56°C for 30 min to remove inhibitors that may interfere with the results. Dilution solution (0.1 M phosphate buffer solution, pH 7.6, containing 2% of rabbit serum) (50 μl) was added into each well of a 110° V-bottom 96-well plate from the first to the 12th well, and then 50 μl of serum or positive or negative serum was added in the first well. Serial dilution (twofold) was performed from the first to the 12th well. Twenty-five microliters of FMDV-sensitized erythrocyte from sheep (LVRI) was added to each well, and the mixture was incubated for 2 h at 37°C. Titers were expressed as the highest serum dilution where 50% hemagglutination was inhibited.
Statistical analysis.
Statistical analysis was performed using SPSS 10.0 for Windows. Data are expressed as means ± standard deviations (SD). For analysis of IHA titers, data were log transformed to calculate geometric means. Bonferroni's multiple-range test was used to compare the parameters between groups. A probability of less than 0.05 was considered significantly different.
RESULTS
FMDV-specific IgG, IgG1, and IgG2 in guinea pigs.
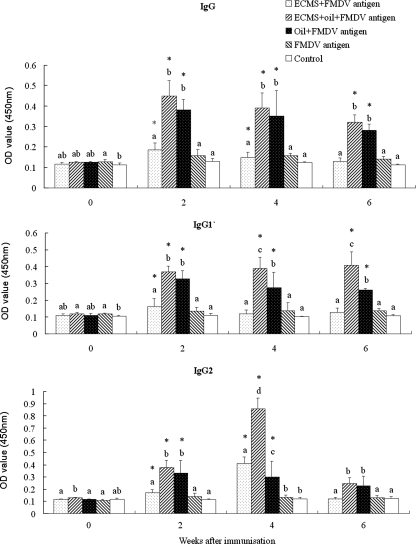
The changes in serum IgG and subclasses in guinea pigs are depicted in Fig. 1. IgG, IgG1, and IgG2 levels were increased significantly after the guinea pigs were immunized with FMDV antigens with an adjuvant of ECMS, oil emulsion, or oil emulsion containing ECMS, while no significantly increased IgG or subclasses were found with the guinea pigs receiving immunization of FMDV antigens alone. The highest levels of IgG, IgG1, and IgG2 were recorded for the animals immunized with FMDV antigens in oil emulsion plus ECMS.
FIG. 1.
FMDV-specific IgG, IgG1, and IgG2 in sera of guinea pigs (n = 6/group) immunized on days 1 and 21 by intramuscular injection of FMDV antigens (type O) in saline with or without ECMS (100 μg) or in oil emulsion with or without ECMS (100 μg). The animals without any injections were used as controls. Blood samples were collected before the first immunization and 2, 4, and 6 weeks after the boosting injection. FMDV-specific IgG and subclasses in the serum were measured by indirect double antibody sandwich ELISA as described in the text. The values are expressed as means ± SD. Bars with different letters at the same time point are statistically significantly different (P < 0.05). Bars with asterisks are statistically significantly different (P < 0.05) from those before immunization in the same group. OD, optical density.
FMDV peptide VP1-specific IgG, IgG1, and IgG2 in guinea pigs.
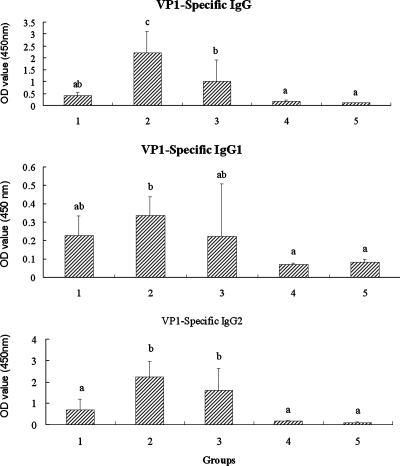
Serum FMDV peptide VP1-specific IgG and subclasses at 4 weeks after the boosting immunization are depicted in Fig. 2. FMDV peptide VP1-specific IgG, IgG1, and IgG2 levels were numerically higher in guinea pigs receiving immunization of FMDV antigens with adjuvant than in animals injected with antigens alone. Among the five groups, the highest IgG and subclass responses were recorded for the animals injected with the antigens in oil emulsion plus ECMS.
FIG. 2.
FMD VP1-specific IgG, IgG1, and IgG2 in sera of guinea pigs (n = 6/group) immunized on days 1 and 21 by intramuscular injection of FMDV antigens (type O) in saline with (group 1) or without (group 4) ECMS (100 μg) or in oil emulsion with (group 2) or without (group 3) ECMS (100 μg). Group 5 was a control without any injection. FMD VP1-specific IgG and subclasses in the serum collected 4 weeks after the boosting injection were measured by indirect double antibody sandwich ELISA as described in the text. The values are expressed as means ± SD (n = 6). Bars with different letters are statistically significantly different (P < 0.05). OD, optical density.
IHA titer against FMDV (type O) in pigs.
In experiment B, a commercial FMD vaccine was supplemented with different doses of ECMS (0, 1, 2, and 4 mg), and significantly increased IHA titers were found in the piglets 3 weeks after immunization with the vaccine plus 2 or 4 mg of ECMS, with the highest IHA titer in the pigs injected with the vaccine supplemented with 4 mg of ECMS (Fig. 3).
FIG. 3.
IHA titers of sera from pigs (n = 6/group) immunized with a commercial FMDV (type O) vaccine (with oil as an adjuvant) alone (control) or mixed with ECMS (1, 2, or 4 mg). Blood samples were collected before and 3 weeks after immunization for measurement of IHA titers. Bars with different letters in the same time point are statistically significantly different (P < 0.01). Bars with asterisks are statistically significantly different (P < 0.05) from those before immunization in the same group.
In experiment C, serum IHA titers elicited by the commercial FMD vaccine supplemented with 4 mg of ECMS were significantly higher than those elicited by a commercial FMD vaccine without a supplement of ECMS (Fig. 4). Of 12 piglets receiving immunization of FMD vaccine containing ECMS, 10 were detected to have serum IHA titers of more than the reciprocal of 1:32, while only 3 of 12 did after injection of FMD vaccine alone.
FIG. 4.
IHA titers of sera from pigs (n = 12/group) immunized with a commercial FMDV (type O) vaccine (with oil as an adjuvant) alone (control) or in combination with ECMS (4 mg). Blood samples were collected before and 3 weeks after immunization for measurement of IHA titers. Bars with different letters in the same time point are statistically significantly different (P < 0.01). Bars with asterisks are statistically significantly different (P < 0.05) from those before immunization in the same group.
FMDV peptide VP1-specific IgG, IgG1, and IgG2 in pigs.
Compared with the control, serum peptide VP1-specific IgG, IgG1, and IgG2 levels were numerically at 3 weeks but significantly at 6 weeks increased in the pigs immunized with the vaccine supplemented with ECMS (Fig. 5).
FIG. 5.
FMDV-specific IgG, IgG1, and IgG2 in sera of pigs (n = 6/group) immunized by intramuscular injection of FMDV antigens (type O) in saline with or without ECMS (100 μg) or in oil emulsion with or without ECMS (100 μg). Blood samples were collected before the first immunization and 3 and 6 weeks after the boosting injection. FMDV-specific IgG antibody in the serum was measured by indirect double antibody sandwich ELISA as described in the text. The values are expressed as means ± SD (n = 6). Bars with different letters at the same time point are statistically significantly different (P < 0.05). Bars with asterisks are statistically significantly different (P < 0.05) from those before immunization in the same group. OD, optical density.
DISCUSSION
Our previous investigation has shown that an extract from a traditional Chinese medicine, ECMS, has an adjuvant effect on the immune response elicited by OVA in mice (31). In addition, ECMS has been found effective in stimulation of the immune response to vaccination against H5N1 avian influenza in chickens (24). In this study, we demonstrated that ECMS and oil emulsion synergistically promoted the production of IgG1 and IgG2 elicited by FMDV antigens in a guinea pig model, as shown in Fig. 1 and 2. A similar synergistic effect was observed previously when Quil A, extracted from the bark of Quillaja saponaria Molina, and oil emulsion were mixed as adjuvants to promote the production of IgG1, IgG2a, IgG2b, and IgG3 induced by OVA in a mouse model (32). There are different subclasses of IgG, such as IgG1 and IgG2, that provide the bulk of immunity to most infectious agents. During a T-cell-dependent immune response, there is a progressive change in the predominant Ig class of the specific antibody produced. This change is regulated by T cells and their cytokines. Interleukin-4 preferentially drives activated B cells to the IgG1 isotype (Th2-like immune response); gamma interferon enhances IgG2 responses (Th1 like) (27). As a Th1-like response is mainly against intracellular infection, this response seems more important in defense against FMDV infection. Figures 1 and 2 indicate that ECMS in combination with oil emulsion promotes the production of both IgG1 and IgG2.
The humoral immune response against FMD infection has been well documented (4, 21), and the contribution of antibodies to the major immune defense against FMDV is clear (18, 20). Studies with animal models (19, 20) have shown that specific antibodies play an important role in the immune defense against FMD. However, a poor humoral immune response to vaccination of some commercial FMD vaccines has been reported previously for both experimental animals and pigs (28, 32). IHA is a routine approach to evaluate the humoral immune response to FMD vaccination in swine in China, as it is rapid and simple. Protection against FMDV infection has been reported to be correlated closely with serum IHA titers (33). Immune protection against FMD infection needs sufficient inhibitory activity to hemagglutination. According to the prevention and control norm for FMD (22), a serum IHA titer of 1:32 is believed to be the minimum titer for a pig to resist the challenge of FMDV infection. Experiment C demonstrated that a supplement of ECMS in a commercial FMD vaccine significantly increased IHA titers as well as the number of piglets with sufficient IHA titers, from two piglets in the control group to eight in the experiment group. Serum IHA titers in pigs tended to rise with increased dose of ECMS supplemented in vaccine, as shown in Fig. 3. Such a dose-dependent relationship was also found previously when OVA was used as the antigen in a mouse model (31).
The FMDV virion consists of an icosahedral capsid formed by 60 copies of each of the four structural proteins VP1 to VP4 and a RNA molecule (1, 8). VP1 is of particular interest because it is the only polypeptide among inducing neutralizing antibodies against the whole virus (2, 7, 14, 30). Unlike other picornaviruses, the FMDV virion is sensitive to digestion by protease to produce a specific cleavage of the VP1 molecule, suggesting that VP1 is the most exposed polypeptide of the capsid and responsible for the induction of an effective immune response to FMDV infection (25). Previous reports have shown that the purified VP1 polypeptide isolated from virus or biosynthesized by Escherichia coli is capable of eliciting neutralizing antibody in livestock and protecting animals against challenge with FMDV (15). Evaluation of antibodies against the FMD VP1 peptide (Fig. 2 and 5) has shown that addition of ECMS to FMD vaccine increases VP1-specific IgG, IgG1, and IgG2 levels in pigs compared to those with FMD vaccine used alone, indicating that ECMS has an adjuvant effect to promote production of protective antibodies in FMD vaccination.
Adjuvant combinations may combine various adjuvant components to achieve the desired mix of immunological responses. The best-known adjuvant combination is Freund's complete adjuvant (9), which combines the immunomodulatory properties of Mycobacterium tuberculosis with the depot effect of water-oil emulsion. This adjuvant generates very strong Th1 and Th2 responses. Although the combination of saponin-like adjuvant with oil is not common, the synergism between them for immunopotentiation has been reported previously. Gerber (11) found higher immune responses in guinea pigs vaccinated against canine parvovirus, in pigs vaccinated against pseudorabies virus, and in cats vaccinated against feline infectious virus when Quil A together with oil was used as an adjuvant than when Quil A or oil was used alone. Martínez-Fernández et al. (17) reported an increased immune response induced by Fh12 FABP of Fasciola hepatica in oil emulsion containing Quil A in sheep. Oil emulsion provides the depot effect with progressive release of the antigens at the site of injection. ECMS has immunomodulatory capacity (31). The synergism between ECMS and oil may be due to the depot effect of oil emulsion in combination with the immunomodulatory effect of ECMS.
In conclusion, our results show that ECMS and oil emulsion act synergistically as adjuvants to promote the production of FMDV- and VP1-specific IgG and subclasses in guinea pigs. A supplement of ECMS in a commercial FMD vaccine significantly enhances FMDV-specific IHA titers as well as VP1-specific IgG and subclasses in pigs and could be an alternative approach to improving swine FMD vaccination when the vaccine is poor to induce an effective immune response.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NSFC) (project no. 30471273).
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Bachrach, H. L., D. O. Morgan, P. D. McKercher, D. M. Moore, and B. H. Robertson. 1982. Foot-and-mouth disease virus: immunogenicity and structure of fragments derived from capsid protein VP3 and of virus containing cleaved VP3. Vet. Microbiol. 7:85-96. [DOI] [PubMed] [Google Scholar]
- 2.Baxt, B., D. O. Morgan, B. H. Robertson, and C. A. Timpone. 1984. Epitopes on foot-and-mouth disease virus outer capsid protein VP1 involved in neutralization and cell attachment. J. Virol. 51:298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Brown, F. 1995. Antibody recognition and neutralisation of foot-and-mouth disease. Semin. Virol. 6:243-248. [Google Scholar]
- 5.Chinese Pharmaceutical Codex Evaluation Committee (ed.). 2005. Mu Bie Zi (Semen momordicae), p. 44. Chinese pharmaceutical codex edition 2005, part I. Chemical Industry Press, Beijing, China.
- 6.Chinese Veterinary Pharmaceutical Codex Evaluation Committee (ed.). 2000. Mu Bie Zi (Semen momordicae), p. 49-50. Chinese veterinary pharmaceutical codex edition 2000, part II. Chemical Industry Press, Beijing, China.
- 7.Collen, T., R. Dimarchi, and T. R. Doel. 1991. A T-cell epitope in VP1 of foot-and-mouth-disease virus is immunodominant for vaccinated cattle. J. Immunol. 146:749-755. [PubMed] [Google Scholar]
- 8.Domingo, E., G. Mateu, M. A. Martinez, J. Dopazo, A. Moya, and F. Sobrino. 1990. Genetic variability and antigenic diversity of foot and mouth disease virus. Appl. Virol. Res. 2:233-266. [Google Scholar]
- 9.Freud, J., K. J. Thomson, H. B. Hough, H. E. Sommer, and T. M. Pisani. 1948. Antibody formation and sensitization with the aid of adjuvants. J. Immunol. 60:383-398. [PubMed] [Google Scholar]
- 10.Gao, X. M (ed.). 2005. Mu Bie Zi (Semen momordicae), p. 601-602. Chinese materia medicia. China Traditional Chinese Materia Medica Press, Beijing, China.
- 11.Gerber, J. D. February 1989. Vaccine formulation. U.S. patent 4,806,350.
- 12.Hao, D. L., R. Luo, Y. F. Xu, and K. Li. 2005. Analysis of antibody titers to foot-and-mouth disease in pigs. J. Anim. Sci. Vet. Med. 24:42. (In Chinese.) [Google Scholar]
- 13.Hu, S. H., and C. W. Xiao. August 2005. Preparation of extract of cochinchina momordica seeds containing tri-terpenoid saponins. Chinese patent no. 2,005,100,604,341.
- 14.Kaaden, O. R., K. H. Adam, and K. Strohmaler. 1977. Induction of neutralizing antibodies and immunity in vaccinated guinea pigs by cyanogen bromide peptides of VP3 of foot-and-mouth disease virus. J. Gen. Virol. 34:397-400. [DOI] [PubMed] [Google Scholar]
- 15.Kleid, D. G., D. Yansura, B. Small, D. Dowbenko, D. M. Moore, M. J. Grubman, P. D. McKercher, D. O. Morgan, B. H. Robertson, and H. L. Bachrach. 1981. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science 214:1125-1129. [DOI] [PubMed] [Google Scholar]
- 16.Koh, Y. T., S. A. Higgins, J. S. Weber, and W. M. Kast. 2006. Immunological consequences of using three different clinical/laboratory techniques of emulsifying peptide-based vaccines in incomplete Freund's adjuvant. J. Trans. Med. 4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Fernández, A. R., J. J. Nogal-Ruiz, J. López-Abán, V. Ramajo, A. Oleaga, Y. Manga-González, G. V. Hillyer, and A. Muro. 2004. Vaccination of mice and sheep with Fh12 FABP from Fasciola hepatica using the new adjuvant/immunomodulator system ADAD. Vet. Parasitol. 126:287-298. [DOI] [PubMed] [Google Scholar]
- 18.McCullough, K. C., F. De Simone, E. Brocchi, L. Capucci, J. R. Crowther, and U. Kihm. 1992. Protective immune response against foot-and-mouth disease. J. Virol. 66:1835-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullough, K. C., D. Parkinson, and J. R. Crowther. 1988. Opsonization enhanced phagocytosis of foot-and-mouth disease virus. Immunology 65:187-191. [PMC free article] [PubMed] [Google Scholar]
- 20.McCullough, K. C., J. R. Crowther, R. N. Butcher, W. C. Carpenter, E. Brocchi, L. Capucci, and F. De Simone. 1986. Immune protection against foot-and-mouth disease virus studied using virus-neutralizing and non-neutralizing concentrations of monoclonal antibodies. Immunology 58:421-428. [PMC free article] [PubMed] [Google Scholar]
- 21.Meloen, R. H., D. J. Rowlands, and F. Brown. 1979. Comparison of the antibodies elicited by the individual structural polypeptides of foot and mouth disease and polio viruses. J. Gen. Virol. 45:761-763. [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Agriculture of China. 2000. Prevention and control norm for foot and mouth disease (NY/SY150-2000). http://www.scxmsp.gov.cn.
- 23.Oda, K., H. Matsuda, T. Murakami, S. Katayama, T. Ohgitani, and M. Yoshikawa. 2000. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 381:67-74. [DOI] [PubMed] [Google Scholar]
- 24.Rajput, Z. I., C. W. Xiao, S. H. Hu, A. G. Arijo, and N. M. Soomro. 2007. Improvement of the efficacy of influenza vaccination (H5N1) in chicken by using extract of cochinchina momordica seed (ECMS). J. Zhejiang Univ. Sci. B 8:331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson, B. H., D. M. Moore, M. J. Grubman, and D. G. Kleid. 1983. Identification of an exposed region of the immunogenic capsid polypeptide VP1 on foot and mouth disease virus. J. Virol. 46:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson, S. E., and R. M. Christley. 2007. Exploring the role of auction markets in cattle movements within Great Britain. Prev. Vet. Med. doi: 10.1016/j.prevetmed.2007.04.011. [DOI] [PubMed]
- 27.Roitt, I., J. Brostoff, and D. Male. 2001. Immunology, 6th ed. Mosby Publications, London, United Kingdom.
- 28.Shi, X. J., B. Wang, and M. Wang. 2007. Immune enhancing effects of recombinant bovine IL-18 on foot-and-mouth disease vaccination in mice model. Vaccine 25:1257-1264. [DOI] [PubMed] [Google Scholar]
- 29.Shi, X. J., B. Wang, C. Zhang, and M. Wang. 2006. Expressions of bovine IFN-γ and foot-and-mouth disease VP1 antigen in P. pastoris and their effects on mouse immune response to FMD antigens. Vaccine 24:82-89. [DOI] [PubMed] [Google Scholar]
- 30.Strohmaier, K., R. Franze, and K. H. Adam. 1982. Location and characterization of the antigenic portion of the FMDV immunizing protein. J. Gen. Virol. 59:295-306. [DOI] [PubMed] [Google Scholar]
- 31.Xiao, C. W., S. H. Hu, and Z. I. Rajput. 2007. Adjuvant effect of an extract from cochinchina momordica seeds on the immune responses to ovalbumin in mice. Front. Agric. China 1:90-95. [Google Scholar]
- 32.Xiao, C. W., Z. I. Rajput, and S. H. Hu. 2007. Improvement of a commercial foot-and-mouth disease vaccine by supplement of Quil A. Vaccine 25:4795-4800. [DOI] [PubMed] [Google Scholar]
- 33.Xie, Q., Y. G. Li, and M. L. Zhang. 2005. Detection of immune response to swine fever and FMD on a pig farm in Ningxia Province. Gansu J. Anim. Husb. Vet. 35:8-10. [Google Scholar]