Abstract
Antibodies to influenza virus and human immunodeficiency virus are detectable in B cells during the early stages of the immune response, prior to their occurrence in plasma. To investigate similar phenomena in a model of immunization against hepatitis B virus (HBV) infection, medical students in Ghana were screened for HBV markers, HBV surface (HBs) antigen (HBsAg), and HBV core antibodies (anti-HBc). Consenting volunteers, 24 of whom were seronegative (susceptible) and 2 of whom were positive for anti-HBc (prior infection), were vaccinated on day 0, day 40, and 6 months. Two sets of 10 blood samples, sequentially collected at intervals of 2 days following each immunization on days 0 and 40, were processed into B-cell lysates and plasma. Solid-phase HBsAg coated on microtiter plates for enzyme immunoassay or nitrocellulose membranes for dot blot assay was used to detect anti-HBs activity by an indirect antiglobulin assay. A commercially procured sandwich immunoassay was used, along with an enzyme-linked immunosorbent assay and a dot blot assay, for the detection of anti-HBs in B-cell lysates and plasma. Following the first injection of vaccine, a single sample of B-cell lysate collected between 5 and 21 days revealed anti-HBs in 18/21 subjects with no plasma antibodies detectable by sandwich immunoassay. After the booster dose was injected on day 40, a single sample of B-cell lysate collected between 44 and 49 days showed anti-HBs in 16/19 subjects, and this was accompanied by plasma antibodies in 8 subjects. In contrast, between 8 and 13 days, both subjects with prior HBV infection showed anti-HBs in B-cell lysates and plasma. Thus, primary immunization with the HBV vaccine appears to transiently elicit low-affinity anti-HBs in B-cell lysates into plasma.
The enzyme-linked immunospot assay is a well-established method for the study of the immune response and the secretion of antibodies from B lymphocytes in both natural infections and vaccine experiments (5, 6, 9, 11, 12), although the procedures are laborious and include time-consuming incubation steps. Further development based on this technology has revealed that specific antibodies can be detected after purified lymphocytes were placed in appropriately coated enzyme-linked immunosorbent assay (ELISA) wells and kept for 1 to 2 h at 37°C to allow the spontaneous secretion of antibodies, referred to here as the PlasmAcute technology (9). It has since been discovered that after the separation of the B cells from plasma and other blood components, disruption of the B cells will, surprisingly, directly release functional antibodies that can be measured in immunoassays (11). In clinical studies performed in South Africa, human immunodeficiency virus-specific antibodies were detected in B cells before PCR and before classical seroconversion (12). It has been shown that the immune response to antigenic stimulation by an influenza vaccine can be detected in B lymphocytes by the enzyme-linked immunospot assay or the PlasmAcute technology. This response can be detected at about day 2 or 3 to day 16 or 17 following vaccination. Plasma immunoglobulin G (IgG) can usually be detected from day 12 onwards in this system (5, 6).
The synthesis and assembly of Ig molecules as a consequence of their transport through the secretory pathway of the B cell are well documented (13, 16, 17, 19). Heavy (H) and Light (L) chains are synthesized separately on different polyribosomes and are assembled in the endoplasmic reticulum. H chains are normally assembled first and are intermittently bound to the chaperon molecules or binding protein, and these act like surrogate L chains. The binding protein is later replaced by proper L chains (15). Both antibodies designated for secretion and membrane-bound antibodies are produced through similar pathways (4, 18, 20, 30). If lymphocytes were disrupted at this point and antibodies were recovered, these B-cell-associated antibodies would therefore appear to be in different stages of assembly and maturity (8, 14, 27). The transport of antibody molecules through the secretory pathway ensures that only fully assembled and correctly folded antibody molecules can be secreted from the cell. Incomplete molecules are retained in the endoplasmic reticulum (17). Most modern sandwich ELISAs for antibody detection utilize antigen on the solid phase both for capture and in the liquid phase as a conjugate for detection (Murex package insert; Abbott, Dartford, United Kingdom). These test designs require completely assembled antibodies with two binding sites, i.e., two H chains and two L chains (H2L2), in order to produce a detectable signal. Incomplete combinations of H and L chains, like HL or H2L, can specifically bind to the capture antigens or conjugate but not both. An ELISA with a format that uses antihuman conjugate detects incompletely assembled antibodies as well as completely assembled antibodies.
Our approach in this study was to detect specific antibodies at very early stages of the immunological process following vaccine immunization, before antibodies are secreted from B cells into plasma. Immunocytochemistry of B cells has detected specific IgG within 2 to 3 days after antigenic stimulation (10).
The immune response to hepatitis B virus (HBV) infection and vaccination is well documented (2, 23, 25, 26), although early events were mostly investigated by determining the number of cultured antibody-secreting B cells. HBV vaccination was therefore used as a model for studies of the early events of intracellular antibody development and detection.
The main goal of this study was to assess the feasibility of detecting HBV surface (HBs) antigen (HBsAg)-specific antibodies in B-cell lysates as a tool to reveal the early mechanisms of vaccine-induced immunity.
MATERIALS AND METHODS
Study design.
The objective of the study was to monitor at close intervals the development of the humoral immune response after the first two injections of HBV vaccine both in B cells and in the plasma of volunteers. For this purpose volunteer medical students were first screened for HBV markers (HBsAg and HBV core antibodies [anti-HBc]), and those without markers were considered susceptible to HBV infection and were eligible for vaccination. After the collection of personal information and signature of an informed consent, 24 volunteers entered the study, which was approved by the Ethics and Publication Committee of the Kwame Nkrumah University of Science and Technology School of Medical Sciences, Kumasi, Ghana. In addition, as controls, two students with low levels of anti-HBc were vaccinated and monitored according to the protocol applied to the susceptible students. Randomization of the sampling was not feasible due to the local organization and logistics. The control group was limited to two anti-HBc (HBV core antigen)-positive subjects.
On day 0, all students were vaccinated with 0.5 ml of HBvacPRO recombinant vaccine (Sanofi Pasteur, Lyon, France) intramuscularly. The second and third doses were administered after 40 days and 6 months, respectively. EDTA-anticoagulated blood samples (6 ml) were collected from the two groups every other day starting on day 1 and day 2, respectively, for a total of 21 or 22 days after the first vaccine injection. Forty days later, the second vaccine dose was injected and samples were collected by use of the same protocol. Whole-blood samples were stored at 4°C for no longer than 3 days until the B-cell lysate assay was performed. Plasma was then separated and kept frozen at −20°C until it was used.
Testing for HBV markers.
The samples were screened for HBsAg by the Determine rapid test (Abbott, Delkenheim, Germany), which is routinely used for blood donor screening (21).
The samples were tested for anti-HBc and anti-HBs with the Murex (Abbott) microtiter plate assays, according to the manufacturer's instructions. A sandwich assay with conjugated HBsAg, which, in principle, should detect any antibody, i.e., IgM as well as intact IgG (Murex anti-HBs package insert; Abbott), was used to measure the samples for anti-HBs reactivity. B-cell lysates and plasma samples were initially tested for anti-HBs by the commercial assay, but when it became clear that this sandwich assay was inadequate for the purposes of the study, an in-house antiglobulin indirect assay was developed and utilized.
Recombinant HBsAg (100 μl; 1 μg/ml) was coated onto microtiter plates overnight at 4°C in 1 M bicarbonate buffer (pH 8.2). The plates were then washed in phosphate-buffered saline (PBS) and blocked with PBS buffer containing 4% bovine serum albumin (Sigma) overnight at 4°C. After the plates were washed with PBS containing 3% bovine serum albumin and 0.05% Tween 20 (pH 7.4), 100 μl of a lysate diluted 1:2 or a plasma sample diluted 1:100 in PBS containing 0.05% Tween 20 buffer was added and the plates were incubated for 1 h at 37°C. After five washes as described above, 100 μl of horseradish peroxidase (HRPO)-conjugated mouse anti-human IgG (Sigma) diluted 1:4,000 was added, and the plates were incubated for 1 h at 37°C. The plates were then washed five times, and 100 μl of substrate was added. The plates were allowed to develop for 30 min at 37°C, the reaction was stopped with 50 μl of 1 M H2SO4, and the plates were read at a wavelength of 450 nm in a spectrophotometer. The assay cutoff was calculated on the basis of the mean of four negative controls plus 3 standard deviations. The results were expressed as the optical density as well as the sample-to-cutoff ratio (S/CO).
The samples were also tested for anti-HBs by an in-house dot blot enzyme immunoassay (EIA). Purified HBsAg (1 μg/ml; 100 μl) was diluted in 1 M bicarbonate buffer (pH 8.2) and applied to a nitrocellulose membrane by using a 96-well vacuum manifold (Biodot; Bio-Rad, Hercules, CA). The samples were diluted and processed by the protocol recommended by the manufacturer. Reactivity to HBsAg was visualized with a mouse anti-human IgG HRPO conjugate and HRPO substrate (Sigma). The results were interpreted with a computer-assisted light intensity analyzer for each dot.
B-cell lysate preparation and testing.
Lymphocytes were separated from whole blood by the standard PlasmAcute procedure. In brief, 500 μl of EDTA-anticoagulated blood was diluted in PBS containing Tween 20 and citrate in 1.5-ml Eppendorf tubes. Twelve microliters of anti-CD19-coated paramagnetic microparticles (Fluorobeads B; One Lambda, Canoga Park, CA) was added to each tube, and the contents were mixed for 5 min. The lymphocytes attached to the beads were separated from the liquid phase with a magnetic stand, washed three times, and lysed by adding 50 μl of disruption buffer containing protease inhibitors. The lysates were diluted in 200 μl of storage buffer containing protease inhibitors and milk proteins. The lysates were stored at 4°C for <24 h or at −20°C until they were tested.
RESULTS
Among the members of a class of 110 medical students, 24 (21.8%) did not carry detectable markers of HBV, and all 24 of these individuals entered the study. Only 20 of these students participated in the sample collection after the second vaccine injection, although all students completed the full course of vaccination. The two volunteers with low levels of anti-HBc who entered the study also participated in the second injection follow-up.
Serial samples from both B-cell lysates and plasma obtained from the volunteers after the first vaccine injection were initially tested by the commercial Murex anti-HBs assay (an antigen sandwich EIA), and all results were negative except for those for the two anti-HBc-positive volunteers, who developed a secondary immune response detectable 7 days after vaccination (Table 1; Fig. 1). That is, all the HBV-naïve volunteers failed to seroconvert after the primary inoculation.
TABLE 1.
Anti-HBs markers in B-cell lysates and plasma samples after the primary and first booster injections of HBV recombinant vaccine
| Volunteer no. | Primary injection
|
First booster injection
|
||||||
|---|---|---|---|---|---|---|---|---|
| Daya | S/CO
|
Dayb | S/CO
|
|||||
| Lysate
|
EIA of plasma | Lysate
|
EIA of plasma | |||||
| EIA | Dot blot assay | EIA | Dot blot assay | |||||
| 1 | 19 | 3.3 | <1 | <1 | 49 | 1.4 | <1 | <1 |
| 2 | 18 | 2.4 | 6.8 | <1 | 44 | 1.5 | 1.6 | 2.0 (47)c |
| 3 | 15 | 3.1 | <1 | <1 | 47 | 1.9 | <1 | <1 |
| 4 | 10 | 3.1 | <1 | <1 | 49 | 1.4 | 1.5 | 3.0 |
| 5 | 11 | 2.7 | NDd | <1 | 47 | 2.0 | 2.1 | >3 (51) |
| 6 | 16 | 2.2 | 1.3 | <1 | 47 | 1.7 | <1 | <1 |
| 7 | 15 | 2.8 | <1 | <1 | 47 | 1.7 | 1.3 | <1 |
| 8 | 6 | 2.5 | ND | <1 | 47 | 1.7 | ND | 1.4 |
| 9 | 17 | 2.3 | ND | <1 | 47 | 1.3 | 2.0 | <1 |
| 10 | 14 | 2.0 | <1 | <1 | 47 | 1.2 | <1 | 2.1 (49) |
| 11 | 9 | 2.1 | ND | <1 | 47 | <1 | <1 | >3 (49) |
| 12 | 12 | 2.2 | ND | <1 | ND | |||
| 13 | 21 | 6.7 | 2.6 | <1 | 47 | 1.5 | <1 | 2 (51) |
| 14 | 16 | 2.3 | <1 | <1 | ND | |||
| 15 | NRe | <1 | <1 | <1 | 47 | 1.5 | <1 | <1 |
| 16 | 16 | 2.4 | ND | <1 | 47 | 2.3 | 1.0 | <1 |
| 17 | 5 | 3.3 | ND | <1 | 47 | 1.6 | ND | <1 |
| 18 | 18 | 2.4 | ND | <1 | 47 | 1.6 | ND | <1 |
| 19 | NR | <1 | <1 | <1 | 47 | 2.3 | 1.1 | <1 |
| 20 | NR | <1 | ND | <1 | ND | |||
| 21 | 9 | 3.0 | <1 | 2.5 (11) | ND | |||
| 22 | 12 | 1.3 | ND | <1 | 47 | 1.5 | <1 | >3 (51) |
| 23 | NR | <1 | <1 | <1 | 44 | 1.4 | <1 | <1 |
| 24 | NR | <1 | ND | <1 | 49 | 2.3 | 3.2 | <1 |
| 25f | 9 | 2.1 | 2.2 | 5.5 | 47 | 2.4 | ND | >3 (51) |
| 26f | 13 | 6.0 | <1 | 2.8 | 47 | 2.3 | <1 | 2.8 (51) |
Day after the primary injection when B-cell lysate reactivity was observed.
Day after the primary injection when peak anti-HBs activity was observed after the booster injection was given 40 days after the first injection.
The numbers in parentheses indicate the number of days after the primary injection, if it is different from the second peak of anti-HBs activity.
ND, not done.
NR, no reactivity.
Volunteers 25 and 26 were anti-HBc positive.
FIG. 1.
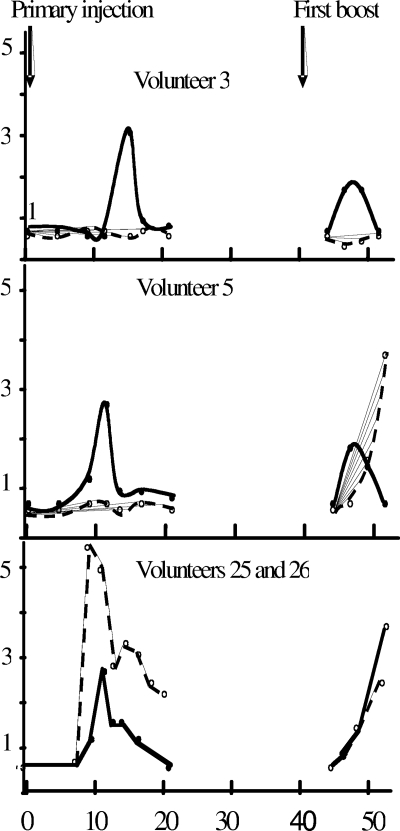
Anti-HBs expressed as optical density/cutoff values in B-cell lysate and plasma samples from two susceptible volunteers (volunteers 3 and 5) and two volunteers with anti-HBc only (volunteers 25 and 26). (Top and middle panels) The anti-HBs in B-cell lysates is shown as solid lines and circles, and that in plasma is shown as dashed lines and open circles; (bottom panel) the anti-HBs in plasma is shown as a solid line and circles for volunteer 25 and as dashed lines and open circles for volunteer 26. In all cases the first HBV vaccine injection was at time zero and the first booster injection was at 40 days. The ordinate indicates the EIA S/CO.
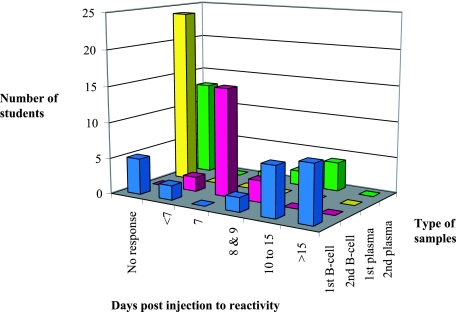
An indirect assay was developed in-house. That assay captured anti-HBs with coated recombinant HBsAg and detected anti-HBs with an HRPO-conjugated anti-human IgG. The assay was applied to the same B-cell lysates and plasma samples from each volunteer as described above. As shown in Table 1 and Fig. 2, evidence of anti-HBs was obtained only in B-cell lysates from 18 volunteers (S/COs ≥ 2) and not in plasma. In two more individuals the reactivity was equivocal (S/COs = 1.3 and 1.4, respectively). In all cases, only 1 of the 10 serial samples from each subject was reactive. This unique positive sample was found at different intervals after primary vaccine injection: 5 to 9 days in four cases, 10 to 15 days in seven cases, and 16 to 21 days in eight cases. In only one volunteer (volunteer 21) a low level of anti-HBs reactivity was also found in plasma (S/CO = 2.5). The B-cell lysate at the unique peak of anti-HBs reactivity of eight samples was available for confirmatory testing by a dot blot assay (see below).
FIG. 2.
Days of occurrence and level of anti-HBs reactivity in B-cell lysate and plasma samples after the primary and the first booster injections of recombinant HBV vaccine to 24 susceptible volunteers. First row, numbers of volunteer with B-cell lysate reactivity at different time intervals after either injection; second row, reactivity and timing of B-cell lysate reactivity after the first boost injection; third row, reactivity of antigen sandwich anti-HBs assay with plasma after the primary injection of the vaccine; fourth row, numbers of volunteers with plasma anti-HBs reactivity after the first booster injection of the vaccine.
In contrast, as shown in Fig. 1, the pattern of B-cell-associated anti-HBs reactivity tested by the indirect assay was considerably modified after the second injection of the HBV vaccine. Only four B-cell lysate samples had S/COs above 2, although 15 more had some reactivity below the two times S/CO confidence limit (S/COs = 1.2 to 1.7). Of these 19 reactive lysate samples, 16 were detected 4 to 7 days after the booster injection. The last three lysate samples (one positive, two equivocal) were detected at 9 days. In all cases an anti-HBs signal was no longer present at day 11 postinjection. Among the five individuals who had no B-cell response after the primary vaccine injection (volunteers 15, 19, 20, 23, and 24), four consented to provide samples after the second injection, and all of them had detectable B-cell-associated anti-HBs 4 to 9 days after vaccine booster administration. However, none of these four volunteers demonstrated detectable anti-HBs in plasma at this time.
In contrast, the two control volunteers presenting the serological pattern of anti-HBc as the only marker of HBV infection had a low level of B-cell antibody 9 and 13 days, respectively, after the primary vaccine injection but presented with a relatively high level of circulating anti-HBs 9 to 11 days postinjection. However, this response was not sustained, and this antibody was no longer detectable after 21 and 44 days after the primary immunization (Fig. 1). The lysates of 14 volunteers with anti-HBs reactivity by EIA were submitted to confirmatory testing by the dot blot assay. The value of the dot blot assay as confirmation of the result of the indirect ELISA was evaluated with only 47 lysate samples that were available and that had been kept frozen at −20°C. Concordance was observed for 33 samples (70%; 13 positive and 20 negative). For 14 samples the results were discordant (12 EIA positive only and 2 dot blot positive only). The reactivity observed in the B-cell lysates was confirmed after the primary vaccine injection in 3/8 samples and after the second injection in 8/12 samples.
The abilities of the indirect and antigen sandwich EIAs to detect of anti-HBs in B-cell lysates were compared by using samples collected after the second vaccine injection (first booster). Nineteen of 20 samples were positive by the indirect assay, and 10 also reacted by the antigen sandwich assay. No samples which were positive by the antigen sandwich assay were negative by the indirect assay.
After the primary vaccine injection, no reactivity was observed in the plasma of the susceptible students (Fig. 2). In contrast, after the second vaccine injection, plasma samples from eight volunteers contained anti-HBs and the plasma of one volunteer was equivocal when it was tested by the antigen sandwich assay (Murex; Abbott). The peak of the antibody reactivity was reached between 7 and 11 days postinjection. Contrary to what was observed with the lysates, in five cases, anti-HBs became detectable at 9 days and the reactivity continued to rise, while in two cases, the antibody level initially detectable at 7 days stabilized at 9 and 11 days postinjection, respectively. The last positive case had clearly detectable anti-HBs on days 7 and 9, but it was no longer detectable on day 11.
The ability of the antigen sandwich assay to detect anti-HBs in the lysates and the ability of the antigen sandwich assay to detect anti-HBs in plasma samples after the second immunizations were compared. In five cases, both types of samples were positive, but the lysate reactivity preceded the plasma reactivity by 2 to 3 days. In five cases, antibody was detectable in lysate but not in plasma at 7 days postinjection. In three cases, antibody was detectable in plasma but not in lysate, and in seven cases, antibody was not detectable in either type of sample.
DISCUSSION
This study tends to indicate that following the first injection of the HBV vaccine to volunteers, B-cell-associated anti-HBs can be detected during a brief period of time but it remains undetectable in plasma. However, by the method used here the detection of anti-HBs in the lysates of purified B cells is considerably easier than their detection in cultured B cells or peripheral blood mononuclear cell (PBMC) supernatants, as reported by others (3, 18, 24). Intra-B-cell antibodies are not yet fully processed and so lack the correct glycosylation and are incompletely assembled (1, 28). This might have caused the differences in antibody detection observed between the two assay formats used in this study, i.e., the antigen sandwich assay and the indirect assay (Table 1). Monovalent antibodies or antibodies with very low affinities are more likely to be detected by the indirect assay than by the antigen sandwich assay.
This pattern was very different from that for the two individuals who, prior to vaccination, had a serological profile of anti-HBc only. These volunteers demonstrated a clear secondary anti-HBs response, as detected by the antigen sandwich assay, 7 days after primary injection of the vaccine (Fig. 2).
After the second vaccine injection, a considerable difference in reactivity between the lysate and the plasma samples in each assay format was observed. In all but 1 volunteer the lysate samples were reactive 7 to 9 days postinjection by the indirect assay, but they were reactive in only 10/20 volunteers by the antigen sandwich assay. In contrast, among the seven individuals whose plasma samples were reactive to anti-HBs after the second vaccine injection, no difference in antibody detection was found between the two assays. The fact that no such issue was observed in secreted antibody detected after the culture of PBMCs for a few days or in plasma (7, 22, 25) suggests that special attention should be paid to the method used to detect B-cell-associated antibodies.
Whether B-cell counts would in any way correlate with the quantification of B-cell-associated anti-HBs is questionable. Shokrgozar and Shokri (25) showed that the total number of B cells was similar or slightly higher in nonresponders than in responders. A low frequency of specific B cells is therefore unlikely to be the consequence of a low B-cell count. The number of B cells in plasma remains relatively constant whether the individual is infected or not. However, there is a close correlation between specific B-cell frequency and anti-HBs titer.
The discrepancies noted between the indirect and the confirmatory dot blot anti-HBs assays should also be considered (Table 1). Although the antibody binding processes of the two assays could be considered similar, the cutoff determination was radically different, and this might have affected the sensitivities of the assays. The dot blot assay may not have been as sensitive as the indirect EIA.
Keeping in mind the caveats presented above regarding the significance of the assays used, evidence of the presence of B-cell-associated anti-HBs was observed prior to the detection of any antibody in plasma (except in volunteer 21). Specific antibody was detected in only one plasma sample 15 to 21 days after primary injection of the vaccine (Table 1; Fig. 1). The presence of anti-HBs in the lysate was confirmed by the dot blot assay in three of the eight available samples collected after 10 days or more, while the results for the two samples reactive before 10 days postvaccination were not confirmed by dot blot analysis. This timing was compatible with data obtained with volunteers who received killed cholera vaccine and who were tested for secreted antibody in the supernatants of their cultured PBMCs (25), where the peak of antitoxin IgG was observed 21 days after primary vaccination. However, in our system antibody was detectable between 14 and 24 days postvaccination.
Following the second injection of HBV vaccine, anti-HBs was detectable in both B-cell lysate and plasma samples in 5/20 individuals, but in all cases reactivity in the lysate samples was observed after 4 to 9 days, preceding antibody detection in plasma by 2 to 4 days (Fig. 1). In five other volunteers, antibody was detected only in lysate samples, and contrary to what was observed after the primary injection, reactivity was observed 7 days postinjection in all cases. In three cases, anti-HBs was found only in plasma, and in the last seven cases, no antibody was detected in either medium. These data suggest that the antigen boost elicits the production of antibodies of better quality and in higher quantities than those elicited after primary injection and that this boost is possibly produced by plasma cells rather than lymphocytes.
These data suggest that specific antibodies can be detected in B-cell lysates before they become detectable in plasma, but the format of the assays used for the detection of intracellular antibodies needs to be carefully chosen. In addition, the HBV vaccine model relies on a single injection of antigen that remains in circulation in decreasing quantities for a matter of days. The pattern of detection of these antibodies might be different when one is dealing with a viral infection, in which a portfolio of antigens is produced during the preseroconversion window period. This situation might prolong the period of time during which B-cell-associated antibodies are detectable.
Further investigation is required to reveal the full potential of detection of B-cell-associated antibodies to study the early events of the immune response to infection and vaccination and as a tool in diagnosis. Studies of the immune response during controlled infection in BALB/c mice as well as analysis of the structure and maturity of immunoglobulins both in B cells from human peripheral blood and in cell lines have been initiated to resolve some of the fundamental questions arising from the detection of B-cell-associated antibodies.
Acknowledgments
We are indebted to the medical students from the University of Science and Technology School of Medical Sciences, Kumasi, Ghana, for their invaluable collaboration in this study. The staff of the laboratory of molecular virology in Cambridge is thanked for their technical help with the conduct of some of the experiments.
Footnotes
Published ahead of print on 10 October 2007.
REFERENCES
- 1.Ambrosino, D. M., M. V. Kanchana, N. R. Delaney, R. W. Finberg, and B. Hum. 1991. Cells secrete predominantly L chains in the absence of H chain expression. J. Immunol. 146:599-602. [PubMed] [Google Scholar]
- 2.Bocher, W. O., S. Herzog-Hauff, J. Schlaak, K. H. Meyer zum Buschenfelde, and H. F. Lohr. 1999. Kinetics of hepatitis B surface antigen-specific responses in acute and chronic hepatitis B or after HBs vaccination: stimulation of the in vitro antibody response by interferon gamma. Hepatology 29:238-244. [DOI] [PubMed] [Google Scholar]
- 3.Chang, S., and D. A. Sack. 2001. Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin. Diagn. Lab. Immunol. 8:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherlet, M., S. J. Kromenaker, and F. Srienct. 1995. Surface IgG content of murine hybridomas: direct evidence for variation of antibody secretion rates during the cell cycle. Biotech. Bioeng. 47:535-540. [DOI] [PubMed] [Google Scholar]
- 5.Cox, R. J., K. A. Brokstad, and L. R. Haaheim. 1996. Kinetics of the early immune response induced after parenteral influenza vaccination, p. 561-571. In L. E. Brown, A. W. Hampson, and R. G. Webster (ed.), Options for the control of influenza III. Excerpta Medica, Elsevier, Amsterdam, The Netherlands.
- 6.Cox, R. J., K. A. Brokstad, M. A. Zuckerman, J. Wood, L. R. Haaheim, and J. S. Oxford. 1994. An early immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine 12:993-999. [DOI] [PubMed] [Google Scholar]
- 7.Crotty, S., R. D. Auberta, J. Glidewella, and R. Ahmeda. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286:111-122. [DOI] [PubMed] [Google Scholar]
- 8.Dul, J. L., and Y. Argon. 1990. A single amino acid substitution in the variable region of the light chain specifically blocks immunoglobulin secretion. Proc. Natl. Acad. Sci. USA 87:8135-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Madhun, A. S., R. J. Cox, A. Seime, O. Søvik, and L. R. Haaheim. 1998. Systemic and local immune responses after parenteral influenza vaccination in juvenile diabetic patients and healthy controls: results from a pilot study. Vaccine 16:156-160. [DOI] [PubMed] [Google Scholar]
- 10.El-Madhun, A. S., R. J. Cox, A. Søreide, J. Olofsson, and L. R. Haaheim. 1998. Systemic and mucosal immune responses in young children and adults after parenteral influenza vaccination. J. Infect. Dis. 178:933-939. [DOI] [PubMed] [Google Scholar]
- 11.Haaheim, L. R., M. W. Ibsen, M. Skogstrand, and R. J. Cox. 2001. Antibodies from lymphocytes used as diagnostic markers: a novel approach, p. 283-289. In A. D. M. E. Osterhaus et al. (ed.), Options for the control of influenza IV. International Congress Series 219. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 12.Haaheim, L. R., V. Maseko, O. Odinsen, D. Parker, F. Radebe, and H. Koornhof. 2003. The use of B-cell-derived antibodies to detect HIV infection earlier than NAT or p24 antigen, in a high-risk cohort. Abstr. 8th World STI-AIDS Congr.
- 13.Hendershot, L., D. Bole, G. Köhler, and J. E. Kearney. 1987. Assembly and secretion of heavy chains that do not associate post-translationally with Immunoglobulin heavy chain-binding protein. J. Cell Biol. 104:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kline, G. H., L. Hartwell, G. B. Beck-Engeser, U. Keyna, S. Zaharevitz, N. R. Klinman, and H. M. Jäck. 1998. Pre-B cell receptor-mediated selection of pre-B cells synthesizing functional μ heavy chains. J. Immunol. 161:1608-1618. [PubMed] [Google Scholar]
- 15.Lee, Y. K., J. W. Brewer, R. Hellman, and L. M. Hendershot. 1999. BiP and immunoglobulin light chain cooperate to control the folding of heavy chain and ensure the fidelity of immunoglobulin assembly. Mol. Biol. Cell. 10:2209-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meilhoc, E. K., D. Wittrup, and J. E. Bailey. 1989. Application of flow cytometric measurement of surface IgG in kinetic analysis of monoclonal antibody synthesis and secretion by murine hybridoma cells. J. Immunol. Methods 121:167-174. [DOI] [PubMed] [Google Scholar]
- 17.Mielenz, D., C. Vetterman, M. Hampel, C. Lang, A. Avramidou, M. Karas, and H. M. Jäck. 2005. Lipid rafts associate with intracellular B cell receptors and exhibit a B cell stage-specific protein composition. J. Immunol. 174:3508-3517. [DOI] [PubMed] [Google Scholar]
- 18.Morris, L., J. M. Binley, B. A. Clas, S. Bonhoeffer, T. P. Astill, R. Kost, A. Hurley, Y. Cao, M. Markowitz, D. D. Ho, and J. P. Moore. 1998. HIV-1 antigen-specific and -nonspecific B cell responsesare sensitive to combination antiretroviral therapy. J. Exp. Med. 188:233-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oi, V. T., V. M. Bryan, L. A. Herzenberg, and L. A. Herzenberg. 1980. Lymphocyte membrane IgG and secreted IgG are structurally and allotypically distinct. J. Exp. Med. 151:1260-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen, M. J., and A. M. Kissonerghis. 1982. Immunoglobulin G biosynthesis in a human lymphoblastoid cell line differences between membrane-bound and secretory forms of γ chains. Eur. J. Biochem. 124:79-87. [DOI] [PubMed] [Google Scholar]
- 21.Owusu-Ofori, S., J. Temple, F. Sarkodie, M. Anokwa, D. Candotti, and J. P. Allain. 2005. Pre-donation screening of blood donors with rapid tests: implementation and efficacy of a novel approach to blood safety in resource-poor settings. Transfusion 45:133-140. [DOI] [PubMed] [Google Scholar]
- 22.Reference deleted.
- 23.Rahman, F., A. Dahmen, S. Herzog-Hauff, W. O. Bocher, P. R. Galle, and H. F. Lohr. 2000. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology 31:521-527. [DOI] [PubMed] [Google Scholar]
- 24.Raquib, R., J. Rahman, A. K. M. Kamaluddin, S. M. M. Kemal, F. A. Banu, S. Ahmed, Z. Rahim, P. K. Bardhan, J. Andersson, and D. A. Sack. 2003. Rapid diagnosis of active tuberculosis by detecting antibodies from lymphocyte secretions. J. Infect. Dis. 188:364-370. [DOI] [PubMed] [Google Scholar]
- 25.Shokrgozar, M. A., and F. Shokri. 2001. Enumeration of hepatitis B surface antigen-specific B lymphocytes in responder and non-responder normal individuals vaccinated with recombinant hepatitis B surface antigen. Immunology 104:75-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shokrgozar, M. A., and F. Shokri. 2002. Subtype specificity of anti-HBs antibodies produced by human B-cell lines isolated from normal individuals vaccinated with recombinant hepatitis B vaccine. Vaccine 20:2215-2220. [DOI] [PubMed] [Google Scholar]
- 27.Stott, D. I. 1972. Assembly of immunoglobulin G: the role of the light-chain pool. Biochem. J. 130:1151-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueki, Y., I. S. Goldfarb, N. Harindranath, M. Gore, H. Koprowski, A. L. Notkins, and P. Casali. 1990. Clonal analysis of a human antibody response: quantitation of precursors of antibody-producing cells and generation and characterization of monoclonal IgM, IgG, and IgA to rabies virus. J. Exp. Med. 171:19-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Vitetta, E. S., and J. W. Uhr. 1974. Cell surface immunoglobulin, a new method for the study of synthesis, intracellular transport, and exteriorization in murine splenocytes. J. Exp. Med. 139:1599. [DOI] [PMC free article] [PubMed] [Google Scholar]