Abstract
To investigate whether the production of an antigen-specific antibody is associated with Neospora caninum-induced bovine abortion, 62 serum samples were tested with an enzyme-linked immunosorbent assay using the recombinant antigens NcSAG1, NcSRS2, and NcGRA7. Our study suggested that NcGRA7 would be a new marker for the serodiagnosis of N. caninum infection resulting in abortion.
Neospora caninum is an obligate intracellular apicomplexa protozoan parasite that is closely related to Toxoplasma gondii (6). Domestic dogs are the known definitive host for N. caninum (17), and, recently, coyotes were also demonstrated to be final hosts of N. caninum (11). The life cycle of N. caninum is typified by three infectious stages: tachyzoites, tissue cysts, and oocysts. Tachyzoites can be vertically transmitted from a persistently infected mother to her fetus (4), and horizontal transmission could also occur by the uptake of sporulated oocysts shed by the definitive hosts or tissue cysts (10). Vertical transmission and horizontal transmission increase the possibility of the wide prevalence of neosporosis.
N. caninum infection has been reported in various animals, including cattle, goats, sheep, horses, deer, foxes, dingoes, raccoons, and coyotes (5). Current evidence strongly suggests that infection with N. caninum represents a major cause of reproductive failure and abortion in cattle, resulting in alarming economic losses to the livestock industry worldwide (7). Infected cows at any age may abort from 3 months of gestation to term, and most abortions occur at 5 to 6 months of gestation (5). Quantitative studies in the United States, New Zealand, The Netherlands, and Germany have indicated that 12 to 42% of aborted fetuses from dairy cattle were infected with N. caninum and that up to 90% of cattle in some herds were infected (5).
Many diagnostic methods have been developed to determine N. caninum infection in animals and bovine abortion associated with N. caninum infection. Although a definitive diagnosis of bovine abortion caused by N. caninum is needed to demonstrate that there are parasites in the lesions and exclude other causes of abortion, serologic diagnosis, such as that with the enzyme-linked immunosorbent assay (ELISA), is important and widely used. A number of antigens have been evaluated as potential diagnosis antigens for the detection of an antibody to N. caninum, including tachyzoite lysates, protein fragments from tachyzoites, and recombinant proteins. The surface proteins NcSAG1 and NcSRS2 (14) and the dense-granule protein NcGRA7 (1) are three important immunodominant proteins of N. caninum. These proteins have been used for the serologic diagnosis of N. caninum infection (7).
Although N. caninum has been found to be a major cause of bovine abortion, a marker for the serodiagnosis of Neospora infection in an aborting cow has not been identified. In this study, we compared ELISAs based on the recombinant antigens NcSAG1, NcSRS2, and NcGRA7 and the N. caninum tachyzoite lysate antigen (NLA) for the detection of the N. caninum-specific antibody in the aborting cows.
The recombinant proteins NcSAG1, NcSRS2, and NcGRA7 were expressed in Escherichia coli as glutathione S-transferase (GST) fusion proteins and then purified using glutathione-Sepharose 4B (Amersham Pharmacia Biotech, Sweden) as described previously (3, 9, 13). The NLA was prepared as previously reported (15). Fifty microliters of purified NcSAG1, NcSRS2, NcGRA7, and their control GST as well as NLA at a final concentration of 5 μg/ml in a carbonate-bicarbonate buffer (pH 9.6) was used to coat the ELISA plates (Nunc, Denmark) overnight at 4°C. After blocking with phosphate-buffered saline (PBS) containing 3% skim milk (PBS-SM) for 1 h at 37°C, the plates were washed twice with PBS containing 0.05% Tween 20 (PBS-T), and 100 μl of serum samples diluted at 1:250 with PBS-SM was added to duplicate wells. Plates were incubated at 37°C for 1 h. After being washed five times with PBS-T, plates were incubated with horseradish peroxidase-conjugated goat anti-bovine immunoglobulin G (IgG) plus IgA and IgM (Bethyl Laboratories) diluted at 1:4,000 with PBS-SM at 37°C for 1 h. The plates were washed five times, and then substrate solution (0.1 M citric acid, 0.2 M sodium phosphate, 0.003% H2O2, and 0.3 mg/ml 2,2′-azide-bis [3-ethylbenzthiazoline-6-sulfonic acid]; Sigma, St. Louis, MO) was added to each well in 100-μl aliquots. The absorbance at 415 nm was read after 1 h of incubation at room temperature by using an ELISA reader (microplate reader MTP-120; Corona, Tokyo, Japan). The ELISA result was determined by the difference in mean optical densities at a value of 415 nm (OD415) between the recombinant antigen (NcSGA1, NcSRS2, or NcGRA7) and the GST protein. For ELISA using NLA, the result was determined by simply taking the OD415 value. The cutoff point of 0.04 was determined as the OD415 value for Neospora-negative sera plus 3 standard deviations.
Bovine serum samples (n = 62), which were a gift from the Souya Livestock Hygiene Service Center, Hokkaido, Japan, and obtained from three Holstein dairy herds with a history of Neospora-associated abortions, were used in this study. All the sera were seropositive for N. caninum, as confirmed by an indirect-fluorescent-antibody test (IFAT; sera with antibody titers of >200). In the case of abortion, fetal samples (cerebrum, cerebellum, liver, and skeletal muscle) were examined through an immunohistochemical test and the tachyzoites were detected in the lesions. In addition, their mothers' sera used in this study were seropositive for N. caninum by IFAT. The serum samples were classified into three groups, i.e., group 1, 16 samples from aborting cows (gestation ranging from 3 to 7 months); group 2, 36 samples from nonaborting cows; and group 3, 10 samples from heifers.
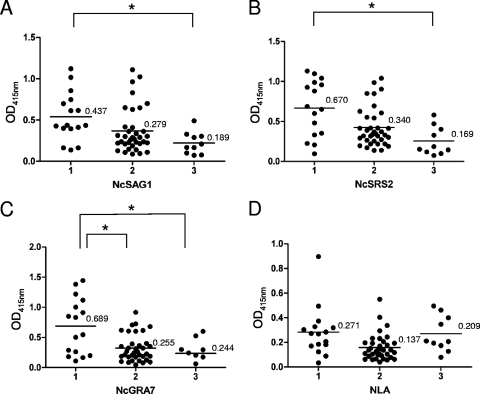
To detect the specific antibody associated with parasite-induced abortion, bovine sera from the above three groups were examined by ELISA with four antigens, NcSAG1, NcSRS2, NcGRA7, and NLA (Fig. 1). Among the three serum groups, the mean values of OD415 for group 1 were higher than those for groups 2 and 3 in the ELISA with recombinant antigens. The ELISA with recombinant antigens could discriminate between group 1 and group 3 (P < 0.01), while there was no statistically significant difference among the groups by the ELISA with NLA. These results indicated that the specific antibodies against NcSAG1, NcSRS2, and NcGRA7 were produced in the aborting cows. However, in the ELISA with NcSAG1 and NcSRS2, there was no statistically significant difference between aborting and nonaborting cows. More importantly, the ELISA with NcGRA7 could discriminate the aborting cows from the parasite-infected animals (P < 0.01).
FIG. 1.
Detection of antibody to N. caninum by ELISA with NcSAG1 (A), NcSRS2 (B), NcGRA7 (C), and the parasite lysates (NLA) (D). Group 1 includes serum samples from aborting cows. Group 2 includes samples from nonaborting cows. Group 3 includes samples from heifers. The mean OD415 values are shown. Data were analyzed by analysis of variance, and the differences among the mean OD415 values were then analyzed using Turkey-Kramer multiple-comparison tests. *, statistically significant difference among the samples (P < 0.05). The OD415 values were representative of at least three repeated experiments.
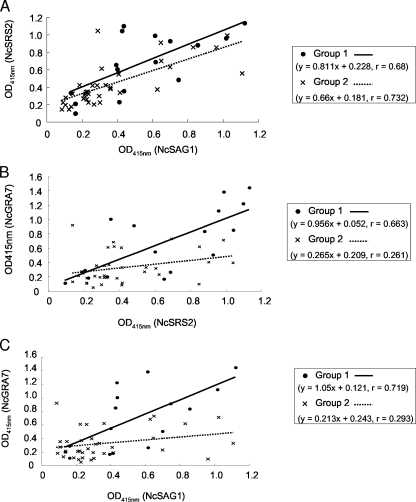
In order to examine the distribution of the OD415 values between aborting and nonaborting cows, a further comparison of the ELISA with recombinant antigens was performed (Fig. 2). In Fig. 2A, positive correlations between the OD415 values of the ELISA with NcSAG1 and NcSRS2 in both aborting cows (r = 0.68, P < 0.01) and nonaborting cows (r = 0.732, P < 0.01) were found. However, when the difference in the correlation coefficients of the regression lines obtained from aborting and nonaborting cows was examined, no statistically significant difference was found. This result indicates that the patterns of production of antibodies against NcSAG1 and NcSRS2 in aborting and nonaborting cows were not different. We then tried to determine whether the production of antibodies against NcGRA7 and the other two molecules had a correlation among animals (Fig. 2B and C). A simple regression analysis revealed a correlation between the antibody responses against NcGRA7 and other recombinant antigens in aborting cows (NcGRA7 and NcSRS2, r = 0.663, P < 0.01; NcGRA7 and NcSAG1, r = 0.719, P < 0.01). In contrast, there was no correlation in the antibody responses from nonaborting cows. These results indicate that the production of the anti-NcGRA7 antibody is upregulated in aborting cows.
FIG. 2.
Comparison of the correlation between the OD415 values from ELISA with two antigens. (A) Correlation between the OD415 values from ELISA with NcSAG1 and NcSRS2. (B) Correlation between the OD415 values from ELISA with NcSRS2 and NcGRA7. (C) Correlation between the OD415 nm values from ELISA with NcSAG1 and NcGRA7. Group 1 includes serum samples from aborting cows. Group 2 includes samples from nonaborting cows. Pearson's correlation coefficient analysis and simple regression were used to assess the relationship between the OD415 values from ELISA with two antigens. The difference in the correlation coefficients of the regression lines obtained from two groups was determined by testing the t value.
Evidence has shown that cattle aborting due to neosporosis have a higher N. caninum-specific antibody response than infected but nonaborting cattle (7). However, a previous study has shown that no serological test could be used to establish definitively that N. caninum caused an abortion in an individual cow (8). In order to estimate whether the production of an antigen-specific antibody is associated with abortion induced by N. caninum infection, 62 serum samples were classified into three groups, namely, those from aborting cows, nonaborting cows, and heifers, and subjected to ELISA using NcSAG1, NcSRS2, NcGRA7, and the parasite lysates as the antigens. Our results show that a higher level of antibodies against NcSAG1 and NcSRS2 was detectable in aborting cows than in heifers. The recombinant antigens NcSAG1 and NcSRS2 could not discriminate between aborting and nonaborting cows. Therefore, production of these antibodies might be induced in parasite-infected cows through their pregnancy. On the other hand, the levels of the anti-NcGRA7 antibody in aborting cows were significantly higher than those in nonaborting cows and heifers. Although an analysis covering the time points of NcGRA7-specific antibody production during the pregnancy will be required in a future study, these data suggest that NcGRA7 might be a new marker for the serodiagnosis of N. caninum infection resulting in abortion in cows.
In cattle herds with endemic abortion due to neosporosis, there is often a positive association between the serostatus of the mother and that of the daughters (19, 21). There is evidence that the recrudescence of latent infection during gestation is responsible for an increased abortion risk (12, 18, 20). Moreover, a small percentage (less than 5%) of cows may repeatedly abort due to neosporosis (2). On the other hand, epidemiologic data have shown evidence for protective immunity to N. caninum-associated abortion when chronically infected dams are reinfected horizontally (16). This observation was confirmed when naturally infected dams received an experimental challenge infection (22). These reports make it difficult to understand why N. caninum infection could induce abortion. Our study suggested that NcSAG1 and NcSRS2 might be upregulated through parasite infection and gestation and that NcGRA7 might play a role in parasite-induced abortion. Therefore, the identification of parasite molecules specifically induced in the aborting cows might trigger an investigation into the mechanism of parasite-associated abortion.
Acknowledgments
We thank J. P. Dubey (United States Department of Agriculture, Agriculture Research Service, Livestock and Poultry Sciences Institute, and Parasite Biology and Epidemiology Laboratory) for supplying the N. caninum NC-1 isolate, the Souya Livestock Hygience Service Center (Hokkaido, Japan) for supplying bovine sera, and Eriko Nishikawa (Obihiro University of Agriculture and Veterinary Medicine) for helpful discussions of statistical analysis.
This work was supported by a grant from the Ministry of Education, Science, Sports, Science and Technology of Japan.
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Alvarez-Garcia, G., A. Pitarch, A. Zaballos, A. Fernandez-Garcia, C. Gil, M. Gomez-Bautista, A. Aguado-Martinez, and L. M. Ortega-Mora. 2007. The NcGRA7 gene encodes the immunodominant 17 kDa antigen of Neospora caninum. Parasitology 134:41-50. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, M. L., C. W. Palmer, M. C. Thurmond, J. P. Picanso, P. C. Blanchard, R. E. Breitmeyer, A. W. Layton, M. McAllister, B. Daft, H. Kinde, D. H. Read, J. P. Dubey, P. A. Conrad, and B. C. Barr. 1995. Evaluation of abortions in cattle attributable to neosporosis in selected dairy herds in California. J. Am. Vet. Med. Assoc. 207:1206-1210. [PubMed] [Google Scholar]
- 3.Chahan, B., I. Gaturaga, X. Huang, M. Liao, S. Fukumoto, H. Hirata, Y. Nishikawa, H. Suzuki, C. Sugimoto, H. Nagasawa, K. Fujisaki, I. Igarashi, T. Mikami, and X. Xuan. 2003. Serodiagnosis of Neospora caninum infection in cattle by enzyme-linked immunosorbent assay with recombinant truncated NcSAG1. Vet. Parasitol. 118:177-185. [DOI] [PubMed] [Google Scholar]
- 4.Davison, H. C., C. S. Guy, J. W. McGarry, F. Guy, D. J. Williams, D. F. Kelly, and A. J. Trees. 2001. Experimental studies on the transmission of Neospora caninum between cattle. Res. Vet. Sci. 70:163-168. [DOI] [PubMed] [Google Scholar]
- 5.Dubey, J. P. 2003. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 41:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubey, J. P., and D. S. Lindsay. 1996. A review of Neospora caninum and neosporosis. Vet. Parasitol. 67:1-59. [DOI] [PubMed] [Google Scholar]
- 7.Dubey, J. P., and G. Schares. 2006. Diagnosis of bovine neosporosis. Vet. Parasitol. 140:1-34. [DOI] [PubMed] [Google Scholar]
- 8.Dubey, J. P., M. C. Jenkins, D. S. Adams, M. M. McAllister, R. Anderson-Sprecher, T. V. Baszler, O. C. Kwok, N. C. Lally, C. Bjorkman, and A. Uggla. 1997. Antibody responses of cows during an outbreak of neosporosis evaluated by indirect fluorescent antibody test and different enzyme-linked immunosorbent assays. J. Parasitol. 83:1063-1069. [PubMed] [Google Scholar]
- 9.Gaturaga, I., B. Chahan, X. Xuan, X. Huang, M. Liao, S. Fukumoto, H. Hirata, Y. Nishikawa, Y. Takashima, H. Suzuki, K. Fujisaki, and C. Sugimoto. 2005. Detection of antibodies to Neospora caninum in cattle by enzyme-linked immunosorbent assay with truncated NcSRS2 expressed in Escherichia coli. J. Parasitol. 91:191-192. [DOI] [PubMed] [Google Scholar]
- 10.Gondim, L. F., L. Gao, and M. M. McAllister. 2002. Improved production of Neospora caninum oocysts, cyclical oral transmission between dogs and cattle, and in vitro isolation from oocysts. J. Parasitol. 88:1159-1163. [DOI] [PubMed] [Google Scholar]
- 11.Gondim, L. F., M. M. McAllister, W. C. Pitt, and D. E. Zemlicka. 2004. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int. J. Parasitol. 34:159-161. [DOI] [PubMed] [Google Scholar]
- 12.Guy, C. S., D. J. L. Williams, D. F. Kelly, J. W. McGarry, F. Guy, C. Bjorkman, R. F. Smith, and A. J. Trees. 2001. Neospora caninum in persistently infected, pregnant cows: spontaneous transplacental infection is associated with an acute increase in maternal antibody. Vet. Rec. 149:443-449. [DOI] [PubMed] [Google Scholar]
- 13.Hara, A. O., M. Liao, W. Baticados, H. Bannai, G. Zhang, S. Zhang, E. G. Lee, Y. Nishikawa, F. Claveria, M. Igarashi, H. Nagasawa, and X. Xuan. 2006. Expression of recombinant dense granule protein 7 of Neospora caninum and evaluation of its diagnostic potential for canine neosporosis. J. Protozool. Res. 16:34-41. [Google Scholar]
- 14.Howe, D. K., A. C. Crawford, D. Lindsay, and L. D. Sibley. 1998. The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii. Infect. Immun. 66:5322-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao, M., X. Xuan, X. Huang, H. Shirafuji, S. Fukumoto, H. Hirata, H. Suzuki, and K. Fujisaki. 2005. Identification and characterization of cross-reactive antigens from Neospora caninum and Toxoplasma gondii. Parasitology 130:481-488. [DOI] [PubMed] [Google Scholar]
- 16.McAllister, M. M., C. Bjorkman, R. Anderson-Sprecher, and D. G. Rogers. 2000. Evidence of point-source exposure to Neospora caninum and protective immunity in a herd of beef cows. J. Am. Vet. Med. Assoc. 217:881-887. [DOI] [PubMed] [Google Scholar]
- 17.McAllister, M. M., J. P. Dubey, D. S. Lindsay, W. R. Jolley, R. A. Wills, and A. M. McGuire. 1998. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 28:1473-1478. [PubMed] [Google Scholar]
- 18.Paré, J., M. C. Thurmond, and S. K. Hietala. 1997. Neospora caninum antibodies in cows during pregnancy as a predictor of congenital infection and abortion. J. Parasitol. 83:82-87. [PubMed] [Google Scholar]
- 19.Schares, G., A. Barwald, C. Staubach, P. Sondgen, M. Rauser, R. Schroder, M. Peters, R. Wurm, T. Selhorst, and F. J. Conraths. 2002. p38-avidity-ELISA: examination of herds experiencing epidemic or endemic Neospora caninum-associated bovine abortion. Vet. Parasitol. 106:293-305. [DOI] [PubMed] [Google Scholar]
- 20.Stenlund, S., H. Kindahl, U. Magnusson, A. Uggla, and C. Bjorkman. 1999. Serum antibody profile and reproductive performance during two consecutive pregnancies of cows naturally infected with Neospora caninum. Vet. Parasitol. 85:227-234. [DOI] [PubMed] [Google Scholar]
- 21.Thurmond, M. C., S. K. Hietala, and P. C. Blanchard. 1997. Herd-based diagnosis of Neospora caninum-induced endemic and epidemic abortion in cows and evidence for congenital and postnatal transmission. J. Vet. Diagn. Investig. 9:44-49. [DOI] [PubMed] [Google Scholar]
- 22.Williams, D. J., C. S. Guy, R. F. Smith, F. Guy, J. W. McGarry, J. S. McKay, and A. J. Trees. 2003. First demonstration of protective immunity against foetopathy in cattle with latent Neospora caninum infection. Int. J. Parasitol. 33:1059-1065. [DOI] [PubMed] [Google Scholar]