Abstract
Vegetative incompatibility is a programmed cell death reaction that occurs when fungal cells of unlike genotypes fuse. Genes defining vegetative incompatibility (het genes) are highly polymorphic, and most if not all incompatibility systems include a protein partner bearing the fungus-specific domain termed the HET domain. The nonallelic het-C/het-E incompatibility system is the best-characterized incompatibility system in Podospora anserina. Cell death is triggered by interaction of specific alleles of het-C, encoding a glycolipid transfer protein, and het-E, encoding a HET domain and a WD repeat domain involved in recognition. We show here that overexpression of the isolated HET domain from het-E results in cell death. This cell death is characterized by induction of autophagy, increased vacuolization, septation, and production of lipid droplets, which are hallmarks of cell death by incompatibility. In addition, the HET domain lethality is suppressed by the same mutations as vegetative incompatibility, but not by the inactivation of het-C. These results establish the HET domain as the mediator of cell death by incompatibility and lead to a modular conception of incompatibility systems whereby recognition is ensured by the variable regions of incompatibility proteins and cell death is triggered by the HET domain.
Most living organisms have developed genetic systems to discriminate self from nonself, such as the immune system in vertebrates (28) or self-incompatibility systems in flowering plants (30). Self/nonself discrimination is particularly critical for organisms that spontaneously form somatic chimeras, such as protists (dictyostelids and myxomycota), sponges, tunicates, ascidians, and filamentous fungi (10). In filamentous fungi, nonself recognition is ensured at the sexual stage by the mating-type system, while vegetative nonself recognition leads to the process known as vegetative incompatibility (VI) (36). Filamentous fungi readily form heterokaryotic cells after vegetative fusion of genetically different isolates by a process called anastomosis. However, most hyphal fusions between natural isolates trigger the VI-associated programmed cell death reaction (PCD) (22, 42). VI is ubiquitous among filamentous fungi and is genetically controlled by specific het loci. Usually, a dozen het loci can be identified in a given species, a difference at any of them being enough to trigger VI. Vegetative incompatibility restricts horizontal transmission of deleterious cytoplasmic elements, such as viruses (33), and prevents resource plundering by parasitic genotypes (12).
Vegetative incompatibility is triggered by the coexpression of incompatible alleles from the same locus (allelic incompatibility) or from different loci (nonallelic incompatibility) (22, 41, 42). So far, het genes have been cloned only in the two model species Neurospora crassa and Podospora anserina. Although unrelated, genes forming VI systems share two characteristics. First, extensive polymorphism can be observed between het alleles. For instance, in N. crassa alleles at the het-6 incompatibility locus are only 68% identical (44), and alleles at the het-C locus are characterized by the presence of a highly divergent region that defines allele specificity (43) and are subjected to balancing selection (46). Polymorphism is also observed between alleles of pin-C, a gene closely linked to het-C, both forming allelic and nonallelic vegetative incompatibility systems with each other (23). In P. anserina, alleles of het-C are more variable within the open reading frame than in noncoding flanking or intron sequences (39, 40). Finally, alleles at het-D and het-E loci involved in nonallelic incompatibility with het-C belong to the NWD gene family, whose members share the presence of a WD repeat domain made of a variable number of WD40 sequence units. The WD repeat domain defines allele specificity (16, 39), and four positions of the elementary WD40 sequence are subjected to positive selection (34), promoting accumulation of nonsynonymous mutations. The second feature shared by VI systems is a protein domain termed HET, found in most VI systems so far. The HET domain, a fungus-specific sequence, is characterized by three conserved blocks of 15 to 30 amino acids comprised within a sequence of about 200 amino acids (44). The HET domain is encoded either by the het gene itself or by another gene involved in the system. In N. crassa, a HET domain is encoded by pin-C, a gene closely linked and essential to het-C incompatibility (23), by tol, a mediator of mating type VI, and by het-6 (22, 44). In P. anserina, a HET domain is located at the N-terminal end of HET-D and HET-E proteins (34). Note that for the P. anserina HET-s prion system, no HET domain-containing partner has been described yet, while in N. crassa the VI gene un-24 lacks a HET domain but forms haplotypes with het-6, their combination being described as a supergene (29). As all these VI systems trigger a PCD, one might hypothesize that the HET domain is the trigger of VI-associated PCD (22). Circumstantial evidence supports this idea. A point mutation in the HET domain of pin-C abolishes the VI reaction (23), while mutations in vib-1 suppressing VI reactions in N. crassa result in low expression of het-6, tol, and pin-C, all HET domain-encoding genes (22, 47, 48). Put together, these features lead to a modular conception of VI whereby recognition is ensured by the polymorphic regions of HET proteins and PCD is triggered by the HET domain included in the system (Fig. 1). Interestingly, in addition to being found in various VI systems, analysis of several fungal genomes uncovered numerous HET domain-encoding genes whose functions remain unknown. For example, 55 HET domain-encoding genes have been annotated in the N. crassa genome (19), and up to 38 were found in aspergilli genomes (18, 20).
FIG. 1.
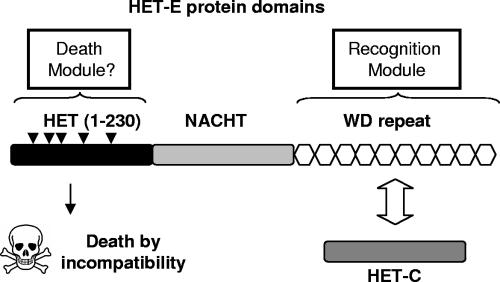
Schematic representation of the het-C/het-E vegetative incompatibility system. The recognition module is made of the HET-C protein and the WD repeat domain of HET-E. The death module is made of the HET domain of HET-E. Arrowheads represent mutated tryptophan residues.
In P. anserina, het-C encoding a glycolipid transfer protein forms nonallelic VI systems with both het-D and het-E (C/D and C/E systems). As already mentioned, het-D and het-E are paralogues belonging to the NWD gene family (34). NWD proteins share a central NACHT domain (24) and a C-terminal WD repeat domain (45). Within this family, five members, including het-D and het-E, also encode an N-terminal HET domain and are named HNWD genes. A combination of high mutation supply through high WD40 repeat number and repeat-induced point mutation (a fungus-specific mutagenic process [21]), positive Darwinian selection favoring accumulation of nonsynonymous mutations (32), and concerted evolution leading to shuffling of WD40 sequences between members of the NWD gene family (31) promotes diversification of the WD40 repeat sequences (34). The WD40 repeat domain of HET-E defines allele specificities of genetic interactions with het-C (16, 39). HNWD proteins also harbor a central GTP binding site (16, 39) whose functionality is essential to VI (15). The GTP binding site of HET-E was a defining member for the NACHT type of GTP binding site (24). HET-D and HET-E belong to the STAND class of proteins. STAND proteins associate the P-loop NTP binding site with numerous domains that include PCD-controlling proteins (25). STAND proteins are believed to transduce signals through a conformational change of the protein after hydrolysis of the bound NTP. Thus, in the modular conception of VI systems, the recognition modules of the C/D and C/E systems are constituted of HET-C proteins and of the WD repeat domains of HET-D and HET-E proteins, and the HET domain would trigger PCD upon recognition (Fig. 1).
The existence of nonallelic VI systems allowed for easy identification of mutations suppressing vegetative incompatibility. Indeed, appropriate crosses led to the production of spores harboring incompatible alleles from different loci in the same nucleus. Such spores can germinate, but after about 12 h, growth stops and the VI is induced. Both C/D and C/E VI systems are suppressed by mutations in mod-A1 and mod-B1 (3, 5, 7). mod-A1 partially suppresses the VI reaction, while its association with mod-B1 results in complete suppression of the process. Note that no phenotype could be associated to the mod-B1 mutation alone in or out of the VI reaction. Cloning and analysis of mod-A1 revealed a proline-rich protein with little homology to known proteins (2, 27), and current work suggests that mod-B is paralogous to mod-A (M. Paoletti, unpublished data). Other mod genes were selected from complex screens (for a review, see reference 27), but mod-A1 and mod-B1 remain the only two mutations suppressing all nonallelic incompatibility systems in P. anserina.
In the present paper we report that overexpression of the HET domain from het-E, alone, triggers a MOD-A- and MOD-B-dependent PCD reaction. HET domain- and VI-induced PCD reactions display identical cytological and morphological characteristics, including induction of autophagy (36). Finally, the HET domain-associated PCD is independent of HET-C, the normal protein partner for HET-D and HET-E in VI. These results establish the HET domain as a central mediator of PCD in fungi.
MATERIALS AND METHODS
Fungal strains and transformation.
All media, growth conditions, and genetic crosses for P. anserina were described previously (17). The P. anserina reference wild-type (WT) strain is the s strain. mod-A1 mod-B1 is a double mutant harboring mutations mod-A1 and mod-B1, that totally suppress the VI reaction when combined (27), GFP-PaATG8 is an s strain harboring an ectopic copy of the green fluorescent protein (GFP)-PaATG8 fusion (37), and Δhet-C is an s strain deleted for het-C. P. anserina cotransformations were performed using 1.5 μg of selectable plasmid conferring resistance to hygromycin or phleomycin and 5 μg of the plasmid of interest, as described in reference 4 or in reference 14, when heat shock was omitted to avoid heat-induced expression. Under these conditions, most protoplasts are transformed by both plasmids, which integrate at the same locus (V. Razanamparany, unpublished data); consequently, efficiency of cotransformation is a measurement of the toxicity of the transformed plasmids (14, 44). The presence of the entire expression cassette was confirmed in 85% of transformants by PCR using the primers pMODE-F and HET-Kpn-R (Table 1).
TABLE 1.
Primers for constructions and site-directed mutagenesis
| Name | Sequencea |
|---|---|
| TRPC-F | CAGCGGCCGCGGTACCACTTAACGTTACTGAAATCATC |
| GPD-R | TAGCGGCCGCTTAATTAAGGTGAAGAGAGAGAGAGAGATG |
| pmodE-F | TGAATTCTGGAGCCTTCTCTG |
| pmodE-pac-R | GTTAATTAACTTGAAAGGGTATCTGAAG |
| HET-Pac-F | GTTAATTAATGCGTCTCCTGGAACGTGATG |
| HET-Kpn-R | TGGTACCGGAGAAAGAAGATGAAGGTCG |
| W33A-F | GATTCTTTCACACACAGCGGGGCCCGATGAGG |
| W33A-R | CCTCATCGGGCCCCGCTGTGTGTGAAAGAATC |
| W75A-F | GGGCGTAAATTCTTCGCGGTAGACACATGCTG |
| W75A-R | CAGCATGTGTCTACCGCGAAGAATTTACGCCC |
| W99A-F | CAACTCTATGTTCAGGGCGTATCGCGATGCGGC |
| W99A-R | GCCGCATCGCGATACGCCCTGAACATAGAGTTG |
| W135A-F | TTTCAGAAATGCAAAGCGTTTACTCGAGGATGGACCC |
| W135A-R | GGGTCCATCCTCGAGTAAACGCTTTGCATTTCTGAAA |
| W140A-F | GGTTTACTCGAGGAGCGACCCTCCAAGAAC |
| W140A-R | GTTCTTGGAGGGTCGCTCCTCGAGTAAACC |
| W199A-F | CATGACCGGATGGCAGCGATGAAGCAACGC |
| W199A-R | GCGTTGCTTCATCGCTGCCATCCGGTCATG |
| qRT-hist-F | TCCGTCGAGTCTTACCTCGT |
| qRT-hist-R | ATGTCCTTGCTCTGGATGGT |
| qRT-HET-F | GACGGGCGTAAATTCTTCTG |
| qRT-HET-R | AGAGTTGATCGCCTCCTGAA |
Restriction sites added for constructs are underlined.
Plasmid construction and site-directed mutagenesis.
First, a 1.5-kb EcoRI fragment was deleted from the pRP81.1 plasmid (38) bearing the P. anserina GPD promoter and trpC terminator to remove nonessential GPD promoter sequence (11). Unique PacI, NotI, and KpnI sites were added between the GPD promoter and trpC terminator by amplifying the whole plasmid with primers GPD-F and TRPC-R (Table 1) to produce plasmid pOP1.2. An 892-bp fragment corresponding to the strong promoter of the mod-E gene encoding a heat shock protein (27) was amplified from P. anserina genomic DNA with primers pmodE-F and pmodE-pac-R and cloned in EcoRI and PacI sites in place of the GPD promoter, resulting in the plasmid pMODE. The DNA sequence encoding the HET domain of the het-E gene from strain s (amino acids 1 to 230, encoded by the allele het-E4s) (Fig. 2) was amplified using primers HET-Pac-F and HET-Kpn-R from genomic DNA and cloned into both pOP1.2 and pMODE with PacI and KpnI, yielding the plasmids pOP-HET and pHET, respectively. All PCR amplifications were conducted with the Expand Long Template PCR system from Roche following the manufacturer's recommendations. Site-directed mutagenesis was performed with the QuikChange II mutagenesis kit by Stratagene. All primers are listed in Table 1.
FIG. 2.
Alignment of the HET domains from the HNWD family. Amino acids defining the HET domain (44) are underlined, tryptophan residues conserved with N. crassa HET domain sequences are in bold, residues mutated to alanine are highlighted, and mutations abolishing lethality are indicated with a §. Numbering is according to the HET-E sequence.
RNA extraction and expression analysis.
Fungal strains were grown on cellophane sheets on complete medium at 26°C for 2 days before transfer to 37°C for 3 h. Mycelia were scrapped off the cellophane, lyophilized, and ground to powder, and total RNA was extracted with the RNeasy plant mini-kit from Qiagen and RQ1 DNase treated (Promega). Quantitative reverse transcription-PCR experiments were conducted using the DyNAmo SYBR green two-step quantitative RT-PCR kit from Finnzymes, following the provider's recommendations. A 500-ng aliquot of total RNA was reverse transcribed using the random hexamer primer set, and quantitative PCR was carried out on an MW3000P thermocycler (Stratagene) with gene-specific primers (Table 1). After an initial 2 min at 50°C and 15 min at 95°C, 45 cycles of 10 s at 95°C, 30 s at 58°C, and 30 s at 72°C were performed before a 10-min final extension at 72°C and a melting/re-annealing curve analysis of the final products. Data were normalized to histone H3 gene expression (Table 1). The data were analyzed with the MxPro QPCR software (version 3.0).
Cytological analysis and microscopy.
Lipid droplets and septa were observed after Nile Red and Congo Red staining, and all microscopy observations were made as previously described (37). Light and fluorescence microscopy observations were conducted on a Leica DMRXA confocal microscope. Dead mycelium was revealed by Evans blue staining followed by fluorescence microscopy observation. Quantification of the amount of dead fungal material was conducted as described in reference 35. Basically, mortality was estimated as the ratio of the length of dead fungal material over the total length of fungal mycelium.
RESULTS
Isolated HET domain expression is lethal.
In a preliminary experiment, we placed the HET domain-encoding DNA sequence from the het-E gene of WT strain s (amino acids 1 to 230 of allele het-E4s) (Fig. 2) (39) under the control of the strong and constitutive GPD promoter on the pOP1.2 plasmid, resulting in plasmid pOP-HET. Transformation of P. anserina WT reference isolate s, a self-compatible genetic background for the het-E4s allele, yielded only 15 transformants compared to the 65 transformants obtained in the control transformation with the empty pOP1.2 plasmid. Transformations of the same plasmids in a mod-A1 mod-B1 double mutant background, suppressing VI, yielded about 45 transformants after transformation with either the pOP1.2 or pOP-HET plasmid. These data suggested a mod-A mod-B-dependent toxicity of HET domain overexpression and prompted us to analyze inducible expression of the HET domain.
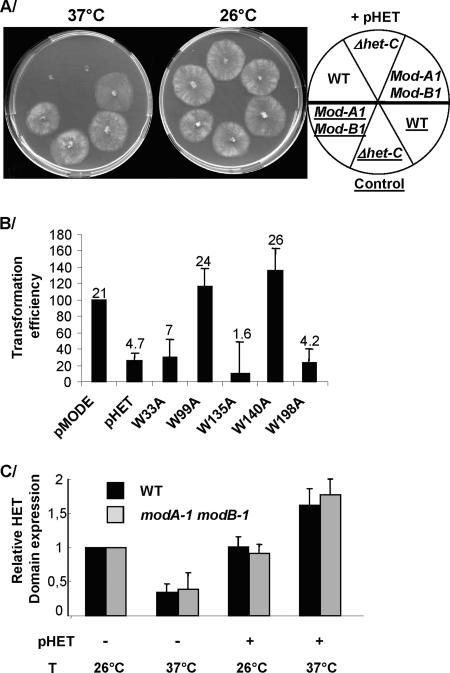
We placed the same HET domain-encoding DNA under the control of the heat-inducible mod-E promoter (27) on the pMODE plasmid, yielding the pHET plasmid. mod-E encodes a heat shock protein of the HSP90 family, and expression of the mod-E gene is induced upon transfer from 26°C to 37°C (26). The same promoter was successfully used to drive expression of idi-4, another P. anserina gene whose overexpression is lethal (14). WT s strain was transformed with pHET or with empty pMODE as a control. Transformants were recovered and growth compared at 26°C (repressing conditions) and 37°C (inducing conditions). After 48 h of incubation, 20 out of 28 transformants did not grow at 37°C but grew at 26°C, while 8 displayed severe alterations when grown at 37°C compared to 26°C. Control transformants grew equally well at 26°C and 37°C (Fig. 3A). These results indicate that induction of the HET domain leads to growth arrest in P. anserina.
FIG. 3.
HET domain toxicity in different genetic backgrounds. (A) Strains were grown under conditions promoting (37°C) or restricting (26°C) expression of the HET domain. (B) Transformation efficiencies of the WT strain with pCSN43 and plasmids expressing WT or mutated HET domains compared to pMODE and pHET controls. The data are cumulative over three separate transformation experiments, and average numbers of transformants are indicated along with standard error bars. (C) The relative HET domain expression was analyzed by quantitative RT-PCR in WT and mod-A1 mod-B1 strains. The presence or absence of the pHET plasmid is indicated by + and − signs; inducing (37°C) or repressing (26°C) temperatures are also indicated. The expression level of the histone gene was used as an internal control to normalize expression levels in the different samples. Expression of the HET domain in the untransformed strains grown at the restrictive temperature was used as a reference and was arbitrarily set to 1. Standard errors were determined over three replicates of the RT-PCR analysis.
Mutations of conserved residues of the HET domain suppress lethality.
To ensure that growth arrest was related to HET domain activity rather than an unspecific effect of overexpression, we sought to inactivate the HET domain. Kaneko et al. (23) found a loss-of-function point mutation in the HET domain of N. crassa pin-C; unfortunately, this position is not conserved in the P. anserina HET domain. Instead, we identified six tryptophan residues present in each HET domain of the HNWD gene family, all located in highly conserved regions (Fig. 2.), four of which are conserved in HET domains from N. crassa het-6, het-C, and tol (Fig. 2) (44). As tryptophan residues are rare, their conservation is highly significant and could indicate that these positions are important to HET domain function. We successfully mutated five of the corresponding codons to alanine codons. Note that none of these mutations is predicted to affect the secondary structure of the HET domain (see Fig. S1 in the supplemental material). Mutated HET domain alleles were transformed into the WT strain. Efficiency of transformation was compared to control experiments (Fig. 3B). Mutations at position 198, and at positions 33 and 135 conserved in N. crassa, led to a low transformation efficiency, similar to a nonmutated HET domain. Substitutions at these positions thus did not alter lethality. However, the W99A and W140A mutations lead to high transformation efficiencies and, thus, suppress HET domain expression lethality. We conclude that W residues at positions 99 and 140 are essential to the HET domain activity.
Lethality is suppressed in a mod-A1 mod-B1 mutant but not in a Δhet-C mutant background.
Cell death by incompatibility is partially suppressed by the mod-A1 mutation alone, while complete suppression of VI is achieved when the mod-A1 and mod-B1 mutations are combined (3, 5, 6, 27). We first transformed a mod-A1 mod-B1 double mutant strain with the pHET plasmid. Transformation efficiency was equally high with control or HET domain-expressing plasmids. Out of 20 randomly selected transformants, 18 did not display any growth alteration after transfer to 37°C compared to 26°C and grew as well as control strains (Fig. 3A); the remaining two displayed the same growth alterations at 26°C and 37°C, most likely due to the site of transgene integration in the genome. The presence of the entire expression cassette was confirmed in 17 of the transformants by PCR using the primers pMODE-F and HET-Kpn-R.
Next, we chose to introduce the pHET expression plasmid into a mod-A1 genetic background by genetic crossing to avoid any risks of mod-A1 protoplasts failing to regenerate due to partial suppression. A total of 64 homokaryotic progenies of the cross WT::pHET × mod-A1 were tested for growth at 26°C and 37°C and compared to 32 homokaryotic progenies of a control cross, WT::pHET × WT. All progenies grew normally at 26°C, but for both crosses half the progenies failed to grow at 37°C, indicating that mod-A1 alone does not suppress the HET domain expression lethality.
It is interesting that suppression by mod-A1 mod-B1 mutations is specific to the HET domain-induced PCD. idi-4 encodes a bZIP transcription factor specifically induced during the VI reaction and whose overexpression triggers a cell death reaction. However, mod-A1 and mod-B1 mutations fail to suppress lethality caused by idi-4 overexpression (14). This observation combined with the fact that W residues at positions 99 and 140 are essential to the HET domain toxicity suggest that HET domain toxicity results from its activity rather than an unspecific effect of its overexpression.
In wild-type isolates, the VI-associated PCD is triggered by a genetic interaction between het-E (or het-D) and het-C encoding a glycolipid transfer protein. One might hypothesize that HET-C is involved in mediating PCD, especially as ACD11, a protein homologous to HET-C in Arabidopsis thaliana, controls PCD (9). Alternatively, in the modular system as presented in Fig. 1, HET domain lethality would be independent of the glycolipid transfer protein activity. Consequently, we analyzed the lethality of HET domain overexpression in a Δhet-C strain. We introduced the pHET plasmid in a Δhet-C strain by genetic crossing with an s isolate in which HET domain induction is lethal. Following the segregation of selection and PCR markers, we selected four Δhet-C progeny harboring the pHET plasmid. These progeny grew normally at 26°C but could not grow at all when placed at 37°C (Fig. 3A). HET-C is then not necessary for HET domain-induced PCD.
From the above results, we conclude that HET domain expression leads to toxicity in P. anserina because of its intrinsic activity. This activity is independent of HET-C and is signaled through the same pathway as VI-associated PCD that involves the MOD-A and MOD-B proteins.
Expression of the HET domain.
Using quantitative RT-PCR, we analyzed HET domain expression, corresponding to the het-E gene expression alone in WT and mod-A1 mod-B1 untransformed strains, or to the combined expression of the het-E gene and mod-E promoter-driven expression of the HET domain in the pHET transformant strains (Fig. 3C). Unexpectedly, we observed that het-E relative expression was reduced when the WT and mod-A1 mod-B1 untransformed strains were transferred to 37°C. In contrast, transfer from 26°C to 37°C results in a 1.6-fold increase of HET domain expression in the WT transformant strain and a 2-fold increase of HET domain expression in the mod-A1 mod-B1 transformant strain. Although the observed induction is less than previously reported for het-E gene induction upon transfer to 37°C (26), we conclude that transfer of transformants to 37°C does result in the induction of the HET domain expression driven by the mod-E promoter in both WT and mod-A1 mob-B1 genetic backgrounds. Importantly, this is the first report of het-E expression, and we note that the het-E gene expression pattern does not appear to be modified by the mod-A1 and mod-B1 mutations under the conditions investigated.
Cytological consequences of isolated HET domain expression.
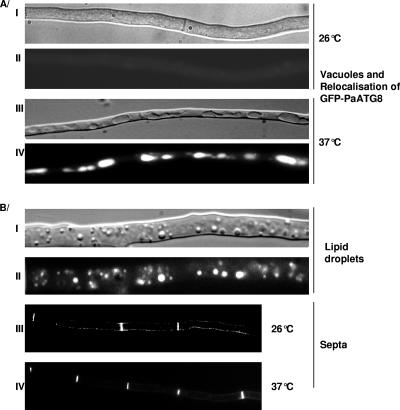
One of the major features of VI is the induction of autophagy (for review, see reference 36). Autophagy is evidenced by relocalization of the GFP-PaATG8 fusion protein from the cytoplasm into the vacuole (37). We introduced the pHET plasmid into a WT strain expressing a GFP-PaATG8 fusion. Upon transfer to 37°C (induction of HET domain expression), GFP-PaATG8 relocalizes from the cytosol to the vacuole (Fig. 4A). GFP-PaATG8 relocalization is not observed in the control GFP-PaATG8 strain when it is transferred to 37°C. Induction of HET domain expression also leads to vacuole enlargement, production of lipid droplets, and increased frequency of septation (Fig. 4B). Note that morphological alterations associated with HET domain expression were observed in the whole mycelium. These cytological alterations are all observed during VI-associated PCD (36). In conclusion, all known cytological/morphological markers of the VI reaction are induced by the expression of the HET domain.
FIG. 4.
Cytological effects of HET domain expression. All experiments were conducted as described elsewhere (37). (A) Induction of autophagy by HET domain expression. A WT strain expressing a GFP-PaATG8 fusion protein and the HET domain was grown on complete medium at 26°C for 24 h and transferred to 37°C for 4 h to induce expression of the HET domain. (I and III) light microscopy using a Nomarsky filter; (II and IV) fluorescence microscopy. (B) Production of lipid droplets and septa by HET domain expression. A HET domain-expressing transformant was grown for 24 h on complete medium at 26°C and transferred for 2 h, 4 h, or 6 h to 37°C before observation. After 2 h of incubation at 37°C, lipid droplets were visible. Pictures were taken after 6 h of incubation at 37°C. (I and III) Light microscopy with a Nomarsky filter; (II) Nile Red staining of lipid droplets; (IV) Congo Red staining of septa observed under fluorescence microscopy.
Quantification of HET domain-induced cell death.
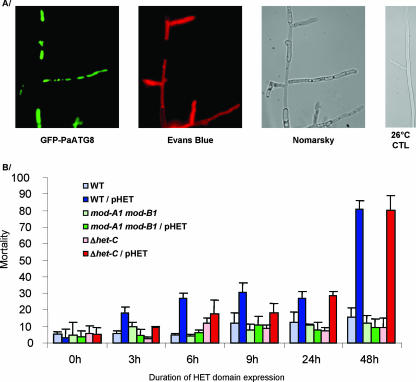
VI ultimately leads to up to 90% cell death in an auto-incompatible mycelium (36). We analyzed the outcome of HET domain expression in different genetic backgrounds. Using Evans blue staining and the GFP-PaATG8 fusion protein to differentiate dead from living cells (Fig. 5A), we estimated the amount of dead mycelia in HET domain-expressing strains (Fig. 5B). After 48 h of incubation at 37°C, 80% of fungal material was dead when the HET domain was expressed in the WT and in the Δhet-C strain. In contrast, a basal level of cell death (ca. 10%) was observed in the absence of HET domain expression or when it was expressed in a mod-A1 mod-B1 genetic background. We conclude that HET domain expression triggers cell death. This cell death is independent of the presence of HET-C but depends on the MOD-A and MOD-B proteins.
FIG. 5.
Cell death induced by HET domain expression. Isolates harboring control or pHET plasmids were grown for 24 h on complete medium at 26°C before transfer to 37°C. (A) Aspect of mycelia after 24-h induction under fluorescence or light microscopy. Dead and living cells were detected by combining Evans Blue staining and GFP-PaATG8 fluorescence. (B) Mortality expressed as the percent dead mycelium length over total mycelium length.
DISCUSSION
We have shown that the expression of the HET domain from het-E alone results in induction of all the phenotypes observed during VI-associated PCD, including autophagy, morphological and cytological changes, and ultimately massive cell death. The induction of all these phenotypes is independent of the presence of HET-C, the interacting partner of HET-D and HET-E in VI. VI- and HET domain-associated PCD is suppressed by the same combination of mod mutations. We conclude that the HET domain and VI cell death are closely related, if not identical. These data are consistent with the modular concept of VI systems. Our lab previously identified the recognition domain of C/D and C/E systems (16, 34), and we have now confirmed the role of the HET domain as the mediator of VI-associated PCD.
What mechanism could lead to activation of the HET domain during VI? HET-D and HET-E belong to the group of STAND proteins, a class of nucleotide binding proteins that control PCD (25), among which the mammalian APAF-1 controls cytochrome c-induced apoptosis (1). APAF-1 displays a structural organization similar to HET-D and HET-E, with a central NB-ARC domain (a class of P-loop NTP binding site) (25), a C-terminal WD repeat domain, and an N-terminal CARD domain effector of apoptosis. Under normal growth conditions, the WD domain binds to and inhibits the CARD domain activity. In contrast, binding of cytochrome c to the WD domain releases the CARD domain and triggers apoptosis (1). Release of the CARD domain is thought to result from a conformational change mediated by hydrolysis of the NTP bound to the NTP binding domain, a feature common to STAND proteins. By analogy, HET domain activity could be inhibited by binding of the NACHT and/or WD domain during vegetative growth. Interaction between the partners of the recognition module, the WD repeat domain and an incompatible HET-C protein (Fig. 6), would free the HET domain and allow it to trigger VI-associated PCD. Consistent with that view is the fact that a mutant affected in the NACHT domain of het-E and unable to bind GTP is inactive in incompatibility (15). All but two VI systems are known to include a HET domain partner, and so one might hypothesize that all VI systems work in a similar fashion. Under vegetative growth conditions, activity of the HET domain would be inhibited by binding to an inhibitor (intra- or extramolecular). Interactions between incompatible proteins would release the HET domain from its inhibitor and induce the cell death reaction (Fig. 6). In the C/D and C/E systems, the PCD would be mediated by MOD proteins.
FIG. 6.
Proposed model for control of HET domain activity. (A) Under vegetative growth conditions, the HET domain activity would be inhibited by binding with its own NACHT and/or WD domain. (B) Under incompatible conditions, binding of HET-C to the WD domain of HET-E releases the HET domain to mediate PCD via MOD proteins.
What could be the mechanism of HET domain induction? Although many aspects of the cell death by incompatibility have been described (36), too little is known at present about the molecular aspects of the reaction to propose a plausible mechanism for HET domain-induced cell death. However, since the HET domain toxicity requires at least the mod-B gene product, our results cannot be explained by a simple direct toxicity of the isolated HET domain. Importantly, our work establishes that MOD proteins are acting downstream of the HET domain, unlike the vib-1 suppressor in N. crassa, which acts upstream of the HET domain-containing genes by controlling their expression (13). It was suggested that MOD-A was involved in a signal transduction pathway (2), and a particular mutation of mod-B triggers a cell lysis reaction similar to cell death by incompatibility (8), and so one can speculate that the HET domain activates this putative MOD-controlled cell death pathway.
Several fungal genome sequences have been made publicly available in recent years, and it appears that HET domain-encoding genes are far more numerous than the dozen genetically characterized VI systems in each species. For instance, 50 genes have been annotated as heterokaryon incompatibility genes in N. crassa (19), up to 38 in Aspergillus species (18), and current annotation of P. anserina reveals even more HET domain-encoding genes (unpublished), 3 of which are considered paralogues to het-D and het-E (34). These numbers highlight the importance of this domain to fungal biology and raise two obvious questions. Do all these HET domains correspond to VI genes, and are they all controlling PCD? Preliminary work suggests that expression of some other HET domains from P. anserina is toxic to P. anserina (unpublished). In addition, as the HET domain is fungus specific, PCD-controlling HET domain proteins could represent favorable targets for the development of specific antifungal drugs.
Supplementary Material
Acknowledgments
We thank S. J. Saupe for critical reading of the manuscript and Olivier Picoche for constructing the expression vector pOP1.2.
M.P. is funded by an EU grant (Transdeath contract 511983).
Footnotes
Published ahead of print on 14 September 2007.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Bao, Q., and Y. Shi. 2007. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 14:56-65. [DOI] [PubMed] [Google Scholar]
- 2.Barreau, C., M. Iskandar, G. Loubradou, V. Levallois, and J. Begueret. 1998. The mod-A suppressor of non-allelic heterokaryon incompatibility in Podospora anserina encodes a proline-rich polypeptide involved in female organ formation. Genetics 149:915-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belcour, L., and J. Bernet. 1969. Sur la mise en évidence d'un gène dont la mutation supprime spécifiquement certaines manifestations d'incompatibilité chez les Podospora anserina. C. R. Acad. Sci. Paris 269:712-714. [Google Scholar]
- 4.Berges, T., and C. Barreau. 1989. Heat shock at an elevated temperature improves transformation efficiency of protoplasts from Podospora anserina. J. Gen. Microbiol. 135:601-604. [DOI] [PubMed] [Google Scholar]
- 5.Bernet, J. 1971. Sur un cas de suppression de l'incompatibilité cellulaire chez le champignon Podospora anserina. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 273:1120-1122. [Google Scholar]
- 6.Bernet, J. 1967. Systems of incompatibility in Podospora anserina. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 265:1330-1333. (In French.) [PubMed] [Google Scholar]
- 7.Bernet, J., and L. Belcour. 1967. On the possibility of selection of mutants of incompatibility genes in the Podospora anserina and on the properties of the first alleles obtained. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 265:1536-1539. (In French.) [PubMed] [Google Scholar]
- 8.Boucherie, H., and J. Bernet. 1977. Intracellular and extracellular phenoloxidases in the fungus Podospora anserina: effect of a constitutive mutation in a gene involved in their posttranscriptional control. Mol. Gen. Genet. 157:53-59. [Google Scholar]
- 9.Brodersen, P., M. Petersen, H. M. Pike, B. Olszak, S. Skov, N. Odum, L. B. Jorgensen, R. E. Brown, and J. Mundy. 2002. Knockout of Arabidopsis accelerated-cell-death 11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense. Genes Dev. 16:490-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buss, L. W. 1982. Somatic cell parasitism and the evolution of somatic tissue compatibility. Proc. Natl. Acad. Sci. USA 79:5337-5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppin, E., and R. Debuchy. 2000. Co-expression of the mating-type genes involved in internuclear recognition is lethal in Podospora anserina. Genetics 155:657-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debets, A. J. M., and A. J. F. Griffiths. 1998. Polymorphism of het genes prevents resource plundering in Neurospora crassa. Mycol. Res. 102:1343-1349. [Google Scholar]
- 13.Dementhon, K., G. Iyer, and N. L. Glass. 2006. VIB-1 is required for expression of genes necessary for programmed cell death in Neurospora crassa. Eukaryot. Cell 5:2161-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dementhon, K., S. J. Saupe, and C. Clave. 2004. Characterization of IDI-4, a bZIP transcription factor inducing autophagy and cell death in the fungus Podospora anserina. Mol. Microbiol. 53:1625-1640. [DOI] [PubMed] [Google Scholar]
- 15.Espagne, E., P. Balhadere, J. Begueret, and B. Turcq. 1997. Reactivity in vegetative incompatibility of the HET-E protein of the fungus Podospora anserina is dependent on GTP-binding activity and a WD40 repeated domain. Mol. Gen. Genet. 256:620-627. [DOI] [PubMed] [Google Scholar]
- 16.Espagne, E., P. Balhadere, M. L. Penin, C. Barreau, and B. Turcq. 2002. HET-E and HET-D belong to a new subfamily of WD40 proteins involved in vegetative incompatibility specificity in the fungus Podospora anserina. Genetics 161:71-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esser, K. 1976. Podospora anserina, p. 531-551. In R. C. King (ed.), Handbook of genetics. Plenum Press, New York, NY.
- 18.Fedorova, N. D., J. H. Badger, G. D. Robson, J. R. Wortman, and W. C. Nierman. 2005. Comparative analysis of programmed cell death pathways in filamentous fungi. BMC Genomics 6:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galagan, J. E., et al. 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422:859-868. [DOI] [PubMed] [Google Scholar]
- 20.Galagan, J. E., et al. 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438:1105-1115. [DOI] [PubMed] [Google Scholar]
- 21.Galagan, J. E., and E. U. Selker. 2004. RIP: the evolutionary cost of genome defense. Trends Genet. 20:417-423. [DOI] [PubMed] [Google Scholar]
- 22.Glass, N. L., and K. Dementhon. 2006. Non-self recognition and programmed cell death in filamentous fungi. Curr. Opin. Microbiol. 9:953-958. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko, I., K. Dementhon, Q. Xiang, and N. L. Glass. 2006. Non-allelic interactions between het-c and a polymorphic locus, pin-c, are essential for non-self recognition and programmed cell death in Neurospora crassa. Genetics 172:1545-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koonin, E. V., and L. Aravind. 2000. The NACHT family—a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem. Sci. 25:223-224. [DOI] [PubMed] [Google Scholar]
- 25.Leipe, D. D., E. V. Koonin, and L. Aravind. 2004. STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer. J. Mol. Biol. 343:1-28. [DOI] [PubMed] [Google Scholar]
- 26.Loubradou, G., J. Begueret, and B. Turcq. 1997. A mutation in an HSP90 gene affects the sexual cycle and suppresses vegetative incompatibility in the fungus Podospora anserina. Genetics 147:581-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loubradou, G., and B. Turcq. 2000. Vegetative incompatibility in filamentous fungi: a roundabout way of understanding the phenomenon. Res. Microbiol. 151:239-245. [DOI] [PubMed] [Google Scholar]
- 28.Medzhitov, R., and C. A. Janeway, Jr. 2002. Decoding the patterns of self and non-self by the innate immune system. Science 296:298-300. [DOI] [PubMed] [Google Scholar]
- 29.Micali, C. O., and M. L. Smith. 2006. A non-self recognition gene complex in Neurospora crassa. Genetics 173:1991-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasrallah, J. B. 2005. Recognition and rejection of self in plant self-incompatibility: comparisons to animal histocompatibility. Trends Immunol. 26:412-418. [DOI] [PubMed] [Google Scholar]
- 31.Nei, M., and A. P. Rooney. 2005. Concerted and birth-and-death evolution of multigene families. Annu. Rev. Genet. 39:121-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen, R. 2005. Molecular signatures of natural selection. Annu. Rev. Genet. 39:197-218. [DOI] [PubMed] [Google Scholar]
- 33.Nuss, D. L. 2005. Hypovirulence: mycoviruses at the fungal-plant interface. Nat. Rev. Microbiol. 3:632-642. [DOI] [PubMed] [Google Scholar]
- 34.Paoletti, M., S. J. Saupe, and C. Clavé. 2007. Genesis of a fungal non-self recognition repertoire. PLoS ONE 2:e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinan-Lucarre, B., A. Balguerie, and C. Clave. 2005. Accelerated cell death in Podospora autophagy mutants. Eukaryot. Cell 4:1765-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinan-Lucarre, B., M. Paoletti, and C. Clave. 2007. Cell death by incompatibility in the fungus Podospora. Semin. Cancer Biol. 17:101-111. [DOI] [PubMed] [Google Scholar]
- 37.Pinan-Lucarre, B., M. Paoletti, K. Dementhon, B. Coulary-Salin, and C. Clave. 2003. Autophagy is induced during cell death by incompatibility and is essential for differentiation in the filamentous fungus Podospora anserina. Mol. Microbiol. 47:321-333. [DOI] [PubMed] [Google Scholar]
- 38.Ridder, R., and H. D. Osiewacz. 1992. Sequence analysis of the gene coding for glyceraldehyde-3-phosphate dehydrogenase (gpd) of Podospora anserina: use of homologous regulatory sequences to improve transformation efficiency. Curr. Genet. 21:207-213. [DOI] [PubMed] [Google Scholar]
- 39.Saupe, S., B. Turcq, and J. Begueret. 1995. A gene responsible for vegetative incompatibility in the fungus Podospora anserina encodes a protein with a GTP-binding motif and G beta homologous domain. Gene 162:135-139. [DOI] [PubMed] [Google Scholar]
- 40.Saupe, S., B. Turcq, and J. Begueret. 1995. Sequence diversity and unusual variability at the het-c locus involved in vegetative incompatibility in the fungus Podospora anserina. Curr. Genet. 27:466-471. [DOI] [PubMed] [Google Scholar]
- 41.Saupe, S. J. 2000. Molecular genetics of heterokaryon incompatibility in filamentous ascomycetes. Microbiol. Mol. Biol. Rev. 64:489-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saupe, S. J., C. Clave, and J. Begueret. 2000. Vegetative incompatibility in filamentous fungi: Podospora and Neurospora provide some clues. Curr. Opin. Microbiol. 3:608-612. [DOI] [PubMed] [Google Scholar]
- 43.Saupe, S. J., and N. L. Glass. 1997. Allelic specificity at the het-c heterokaryon incompatibility locus of Neurospora crassa is determined by a highly variable domain. Genetics 146:1299-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, M. L., O. C. Micali, S. P. Hubbard, N. Mir-Rashed, D. J. Jacobson, and N. L. Glass. 2000. Vegetative incompatibility in the het-6 region of Neurospora crassa is mediated by two linked genes. Genetics 155:1095-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, T. F., C. Gaitatzes, K. Saxena, and E. J. Neer. 1999. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24:181-185. [DOI] [PubMed] [Google Scholar]
- 46.Wu, J., S. J. Saupe, and N. L. Glass. 1998. Evidence for balancing selection operating at the het-c heterokaryon incompatibility locus in a group of filamentous fungi. Proc. Natl. Acad. Sci. USA 95:12398-12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiang, Q., and N. L. Glass. 2004. The control of mating-type heterokaryon incompatibility by vib-1, a locus involved in het-c heterokaryon incompatibility in Neurospora crassa. Fungal Genet. Biol 41:1063-1076. [DOI] [PubMed] [Google Scholar]
- 48.Xiang, Q., and N. L. Glass. 2002. Identification of vib-1, a locus involved in vegetative incompatibility mediated by het-c in Neurospora crassa. Genetics 162:89-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.