Abstract
Upon starvation, Dictyostelium discoideum cells halt cell proliferation, aggregate into multicellular organisms, form migrating slugs, and undergo morphogenesis into fruiting bodies while differentiating into dormant spores and dead stalk cells. At almost any developmental stage cells can be forced to dedifferentiate when they are dispersed and diluted into nutrient broth. However, migrating slugs can traverse lawns of bacteria for days without dedifferentiating, ignoring abundant nutrients and continuing development. We now show that developing Dictyostelium cells revert to the growth phase only when bacteria are supplied during the first 4 to 6 h of development but that after this time, cells continue to develop regardless of the presence of food. We postulate that the cells’ inability to revert to the growth phase after 6 h represents a commitment to development. We show that the onset of commitment correlates with the cells’ loss of phagocytic function. By examining mutant strains, we also show that commitment requires extracellular cyclic AMP (cAMP) signaling. Moreover, cAMP pulses are sufficient to induce both commitment and the loss of phagocytosis in starving cells, whereas starvation alone is insufficient. Finally, we show that the inhibition of development by food prior to commitment is independent of contact between the cells and the bacteria and that small soluble molecules, probably amino acids, inhibit development during the first few hours and subsequently the cells become unable to react to the molecules and commit to development. We propose that commitment serves as a checkpoint that ensures the completion of cooperative aggregation of developing Dictyostelium cells once it has begun, dampening the response to nutritional cues that might inappropriately block development.
Dictyostelium discoideum is a soil amoeba that feeds on bacteria by phagocytosis and can grow axenically in nutrient broth by macropinocytosis (3, 12, 21). Upon nutrient depletion, the solitary amoebae enter a multicellular developmental program. They stop replicating chromosomal DNA, start attracting each other by secretion of pulsatile cyclic AMP (cAMP) signals, aggregate into mounds of about 50,000 cells, and differentiate into prespore and prestalk cells. The aggregates become enveloped in an acellular sheath and form slug-like structures capable of phototactic and thermotactic migration. Eventually, the aggregates form fruiting bodies in which 20% of the cells become stalk cells and die, while 80% become viable spores (17, 20). Developing cells can be forced to dedifferentiate by mechanical disaggregation, dilution, and incubation in nutrients (16, 36).
The growth-to-differentiation transition (GDT) is a conserved process that allows rapid adaptation to changing growth conditions. In unicellular organisms, the GDT facilitates responses to nutrient starvation and to harsh conditions by mediating differentiation into dormant cells. Even mammalian cells can be induced to differentiate by amino acid starvation (29, 30). Sensing nutrient availability is a key step in controlling the GDT. In Dictyostelium, YakA and the Gdt kinases link nutrient sensing to the initiation of the prestarvation response (6, 41). The GDT is accompanied by induction of cAMP signaling components such as the cAMP receptor CarA, the aggregative adenylyl cyclase AcaA, the protein kinase PKA-C, and the trimeric G-protein alpha subunit GpaB. Upon starvation, cAMP signaling is a key component in the progression of development.
Dictyostelium differentiation is reversible, as seen in dedifferentiation and in transdifferentiation. When multicellular structures are disaggregated and exposed to nutrient media for several hours, cells at almost any developmental stage can dedifferentiate and resume vegetative growth (16, 36). Transcriptional profiling of dedifferentiation revealed that the process is genetically regulated, and several genes have been shown to participate in this regulation (16). Physical removal of the prestalk or the prespore zone from a slug results in transdifferentiation and a reproportioning of the remaining portion (1, 31, 32). Genetic ablation of prespore cells results in the transdifferentiation of prestalk cells into prespore cells and the reestablishment of the correct cell type proportions (34).
In light of its reversibility, Dictyostelium development might be vulnerable to inappropriate inhibition by transient or “false” nutrient signals. However, it is surprising that Dictyostelium slugs can migrate across bacterial lawns and continue their development without dedifferentiation. Therefore, we hypothesized that cells commit to development at a critical time and that this mechanism is an integral part of development. The cellulose sheath which surrounds developing Dictyostelium cells could serve as an insulator that prevents cells from interacting with surrounding bacteria, which would provide a solution to the problem of false nutritional cues. We sought factors that may be involved in the commitment to development at the cellular level. We found that commitment is a cAMP-dependent process that occurs after a few hours of starvation, before or during early aggregation, concomitantly with the loss of phagocytosis. We also found that the loss of phagocytosis is not a causative factor in commitment and that commitment is inhibited by small soluble factors, probably amino acids, which are secreted by bacteria.
MATERIALS AND METHODS
Growth, development, and commitment.
Wild-type and mutant Dictyostelium strains (Table 1) were grown axenically in shaking suspension at 22°C in HL5 medium with the appropriate supplements (34). For development, cells were washed with 20 mM potassium phosphate buffer (pH 6.4) (KK2) and deposited at a density of 5 × 105 cells/cm2 on 1.5% agar made with KK2 (KK2 agar). Agar plates were incubated at 22°C.
TABLE 1.
Dictyostelium strains used in this study
| Strain name | Genotype | Parental strain | Description | Reference |
|---|---|---|---|---|
| AX4 | axeABC− | AX3 | Lab wild type (axenic) | 19 |
| DH1 | pyr5-6− | AX3 | Uracil auxotroph | 7 |
| carA− | AX3 | cAMP receptor 1-null mutant | 4 | |
| acaA− | JH10 | Adenylyl cyclase-null mutant | 38 | |
| MP2 | gpaB− | HPS400 | gpaB-null mutant | 24 |
| AK800 | yakA− | HL328 (AX4 pyr5-6−) | yakA null with insertion in the middle of the kinase domain | 37 |
| AK127 | lagC− | HL328 (AX4 pyr5-6−) | Loose aggregate C mutant lacks membrane glycoprotein gp150 | 8 |
| AK228 | tagB− | HL328 (AX4 pyr5-6−) | tagB null (blocked at the tight aggregate stage) | 33 |
| sadA− | AX3 | Substrate adhesion molecule (sadA)-null mutant | 9 | |
| HG1664 | talA− | AX2 | Talin-null mutant | 26 |
| HG1772 | cnx−/crt− | HG1768 (AX2 derivative) | Calreticulin/calnexin double null; neomycin resistant | 25 |
| HG1768 | crt− | AX2 | Calreticulin-null mutant | 25 |
| HG1770 | cnx− | AX2 | Calnexin-null mutant | 25 |
The bacterial food source was Klebsiella pneumoniae. Lawns of bacteria were grown on SM agar (34) at 22°C for 2 to 3 days, harvested, and resuspended in water or in KK2. The bacterial cell density was adjusted to an optical density of 100 at 600 nm per 1 × 107 Dictyostelium cells. To test commitment, bacteria were deposited over the developing Dictyostelium cells at various time points. The morphological progression of the developing Dictyostelium cells was observed by stereomicroscopy. Spores were collected after 30 h and counted using phase-contrast microscopy.
In some cases, nitrocellulose filters (0.45 μm) and dialysis tubing (12 to 14 kDa) were used to examine the effect of bacterial suspension, glucose, folic acid, or amino acids on development. Dictyostelium cells were first deposited on nitrocellulose filters and the filters placed on dialysis tubing filled with KK2 (time zero). The filters were incubated at 22°C and, at various developmental time points, the filters were placed on top of dialysis tubing filled with KK2 containing bacterial suspension, 1% glucose, 10 μM folic acid, or 1× amino acid solution, trace minerals, and salts, following the FM medium recipe (10). Morphological progression was observed by stereomicroscopy.
Phagocytosis and macropinocytosis.
Cells were harvested from KK2 agar plates and resuspended in KK2 to a final density of 1 × 108 cells/ml. To test phagocytosis, fluorescent beads (Dragon Green-labeled polystyrene beads of 0.52-μm diameter, 480-nm excitation, and 520-nm emission; Bangs Laboratories, Inc.) were added at 5 μl of beads per 20 μl of cell suspension, and the mixture was spotted on KK2 agar. After a 30-minute incubation, the cells were harvested, washed three times with KK2 containing 20 mM EDTA, fixed with 2% formaldehyde, and stored at 4°C. After one more wash with KK2, the number of cells that engulfed beads was counted using fluorescence microscopy, and the total number of cells was counted by phase-contrast microscopy. Phagocytosis efficiency was determined as follows: % phagocytosis = number of cells containing ≥3 beads/total number of cells × 100. At least 250 cells were counted for each time point. To test macropinocytosis, cells were incubated in suspension for 10 min with (2-mg/ml) Texas Red dextran (molecular weight, 70,000; Invitrogen) instead of fluorescent beads.
Development in suspension.
Vegetative cells were washed, resuspended at a density of 1 × 106 cells/ml in KK2, and shaken at 200 rpm at 22°C. For cAMP pulses, 30 nM cAMP was added every 6 min. As a control, KK2 was added in pulses of equal volume. Cell samples were harvested at various time points by centrifugation and processed for commitment and phagocytosis assays as described above.
RESULTS
Commitment occurs during early development.
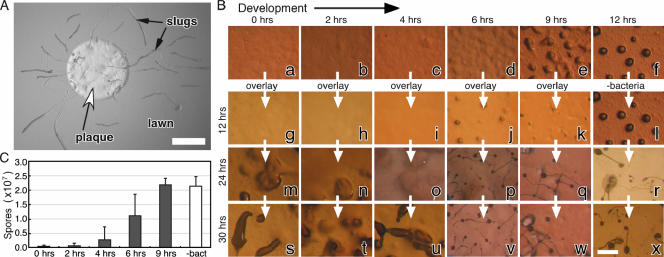
Dictyostelium cells feed on bacteria and develop upon starvation. Developing cells are capable of dedifferentiation if they are disaggregated and exposed to nutrients at a low cell density (16, 36). Surprisingly, Dictyostelium slugs continue to develop even if they are exposed to food. Figure 1A illustrates the well-known phenomenon of slugs migrating across a lawn of food bacteria. This observation raises the hypothesis that Dictyostelium development includes a commitment point, at which time cells become unable to revert to the vegetative phase absent dedifferentiation conditions.
FIG. 1.
Commitment after 4 h of development. (A) Dictyostelium slugs migrate across a bacterial lawn, illustrating that developing amoebae are insensitive to nutrients. The lighter circle in the center of the bacterial lawn is a plaque (white arrowhead) caused by Dictyostelium cells that have consumed the bacteria. The elongated rods emanating from the plaques are slugs (black arrows). Scale bar, 2 mm. (B) Dictyostelium cells were starved on nonnutrient agar plates (time zero) and morphology was recorded for the times indicated above the pictures (panels a to f). Bacteria were added (overlay) to duplicate plates at the times indicated above the pictures, and morphology was recorded after 12 h (panels g to k), 24 h (panels m to q), and 30 h (panels s to w). Controls without added bacteria were photographed at the same times (panels l, r, and x, respectively). All of the time points are relative to the initiation of starvation (time zero). White arrows indicate that photographs of the same plate were taken at different times. Some of the pictures represent the same field over time. Scale bar, 1 mm. (C) Cells (1 × 107) were developed as described above, and spores were collected and counted after 30 h from the time of initial starvation. The sporulation levels of cultures overlaid with bacteria at the indicated times (0, 2, 4, 6, and 9 h) are represented by dark bars, and the sporulation of the untreated culture (−bact) is represented by a white bar. Data are averages ± standard deviations (SD) of four independent replications.
To test the commitment hypothesis, we examined when developing Dictyostelium becomes unable to resume feeding upon the addition of bacteria. Under standard starvation conditions, the cells formed ripples after 6 h, aggregated at 9 h, and progressed to the tight aggregate stage by 12 h (Fig. 1B, panels a to f). Later, they developed into fruiting bodies (Fig. 1B, panels r to x). In parallel, we deposited bacteria on top of the starving amoebae at several times and documented morphological changes after 12, 24, and 30 h from the initial starvation. We observed that when bacteria were added at 0, 2, or 4 h of development, the amoebae did not develop with normal timing (Fig. 1B, compare panels g through i to panel l). Instead, they consumed the bacteria first and then started aggregation. At 24 h, we observed loose and tight aggregates (Fig. 1B, panels m to o) compared to the canonical developmental stage of fruiting bodies (Fig. 1B, panel r), and at 30 h, we observed fingers and culminants but almost no mature fruiting bodies (Fig. 1B, compare panels s through u to panel x).
Adding bacteria to the starving amoebae later had a markedly different effect. When bacteria were supplied after 6 or 9 h, the amoebae continued to develop without any obvious delay. At 12 h, we observed tight aggregates (Fig. 1B, panels j and k), and at 24 and 30 h, we observed fruiting bodies on top of the bacterial lawn (Fig. 1B, panels p to q and v and w, respectively). Adding bacteria later than 9 h did not delay development either (data not shown).
Spores form after 24 h, so we used the number of spores produced as a quantitative measure of commitment. When the cells were exposed to bacteria at 0, 2, or 4 h of development, few or no spores were produced at the 30-hour time point (Fig. 1C). When developing cells were exposed to bacteria after 6 or 9 h of starvation, they produced large numbers of spores, and the spore totals in the 9-hour overlay samples were essentially indistinguishable from those for the control amoebae developed without any bacterial overlay. These observations are highly suggestive of a specific commitment phase in development, after which cells appear to ignore the bacteria as a food source. Developmental progression is delayed by the presence of bacteria only very early in development, and cells appear to commit to development between 4 and 6 h, prior to aggregation and prior to sheath formation. This observation suggests that commitment is a physiological property of the cells rather than a physical property of the aggregate, such as the acellular sheath that surrounds the cells after 12 hours (11), which could sequester the amoebae from the bacteria.
Commitment is accompanied by a loss of phagocytic ability.
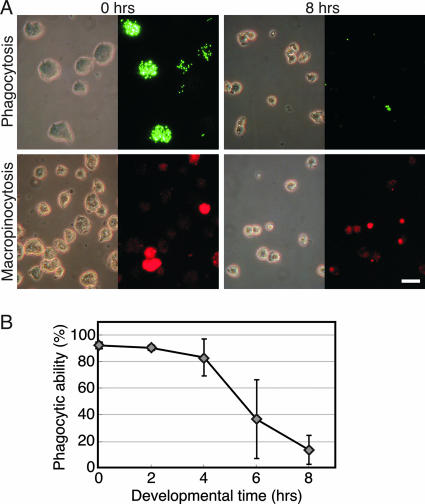
Dictyostelium cells feed on bacteria by phagocytosis and on axenic liquid media by macropinocytosis (3, 12, 21). To test whether developing cells become unable to take up nutrients, we tested them for phagocytosis and for macropinocytosis. Vegetative (0-h) and 8-hour developing cells were incubated with fluorescently labeled beads (Fig. 2A, top) and the number of bead-containing cells was determined by fluorescence microscopy. The vegetative cells (0 h) ingested many labeled particles, whereas the committed cells (8 h) ingested almost none (Fig. 2A, top). Essentially identical results were obtained with bacteria instead of beads (data not shown). Macropinocytosis was somewhat reduced but to a lesser extent than phagocytosis (Fig. 2A, bottom). We also quantified phagocytosis during the first 8 h of development. We defined cells that engulfed three or more beads as phagocytic and the phagocytic index as the proportion (%) of phagocytic cells in the entire population. We found that nearly all the vegetative cells (0 h) were phagocytic and most of the cells after 2 and 4 h of development retained their phagocytic ability. Between 4 and 6 h after starvation, the phagocytic index decreased about twofold, and it was reduced almost completely at 8 h (Fig. 2B). Thus, the loss of phagocytosis correlates temporally with commitment.
FIG. 2.
Phagocytosis and macropinocytosis are decreased during development. (A) Cells were harvested from growth media at 0 h or after 8 h of development on KK2 agar as indicated, washed, and incubated with fluorescent Dragon Green-conjugated polystyrene beads for phagocytosis or Texas Red dextran for macropinocytosis assays, as indicated on the left. Photographs were taken of representative fields by use of phase-contrast microscopy (left) and fluorescence microscopy (right). Scale bar, 20 μm. (B) Phagocytosis was quantified, and data are presented as averages ± SD of four independent replications.
cAMP signaling is necessary for commitment and for loss of phagocytosis.
If commitment and phagocytosis are causally related, they might be regulated by a specific developmental pathway. We postulated that the cAMP signaling apparatus might provide such regulation because it is both necessary and sufficient for the initiation of Dictyostelium development. We characterized commitment in the cAMP signaling-defective carA−, acaA−, gpaB−, and yakA− mutants by use of the bacterial overlay assay (strain definitions are shown in Table 1). In all cases, we found that the mutant cells ingested bacteria even after 9 h of incubation on KK2 agar. Data from the carA− cells (Fig. 3A) were similar to data from the other mutant strains (data not shown). These findings suggest that cAMP signaling is necessary for commitment.
FIG. 3.
The cAMP receptor CarA is required for commitment. carA− mutant cells were tested for commitment and phagocytosis as described for Fig. 1 and 2, respectively. (A) Cells were starved (time zero) on KK2 agar for the length of time indicated above the pictures (0, 2, 4, 6, or 9 h) and then overlaid with bacteria. After 24 and 30 h relative to time zero, the Dictyostelium cells (lighter shades, indicated by D in the pictures) ingested the bacteria (darker shades, indicated by B in the pictures) almost completely in the 0-, 2-, and 4-h samples and partly in the 6- and 9-h samples. Scale bar, 1 mm. (B) Wild-type (WT) AX4 cells and carA− mutant cells were washed, developed on KK2 agar, collected at the indicated time points, and tested for phagocytic ability. Phagocytosis was quantified, and data are presented as averages ± SD of five replications.
We also tested commitment in mutant strains that arrest at later developmental stages. lagC− mutants arrest at the loose aggregate stage without producing slime sheath (8, 18), and tagB− mutants exhibit attenuated development and arrested at the tight aggregate stage, after sheath production (33). We found that both mutant strains committed prior to aggregation despite the fact that they cannot complete the developmental process (data not shown). These findings support the notion that commitment is a regulated physiological process that takes place before the onset of obvious morphological changes. The establishment of commitment is probably not dependent on any known physical barriers such as tight cell-cell interactions, extracellular matrix, or the slime sheath.
Next we tested the phagocytosis ability of the carA− cells. Our results (Fig. 3B) show that carA− cells retain their phagocytic ability throughout the first 8 h of starvation, whereas the wild-type cells lose that ability between 4 and 6 h. These results correlate with the commitment ability of these strains (carA− and wild-type strains, Fig. 3A and 1B, respectively), supporting the idea that commitment is tightly associated with the loss of phagocytosis.
cAMP pulses induce commitment and a loss of phagocytosis.
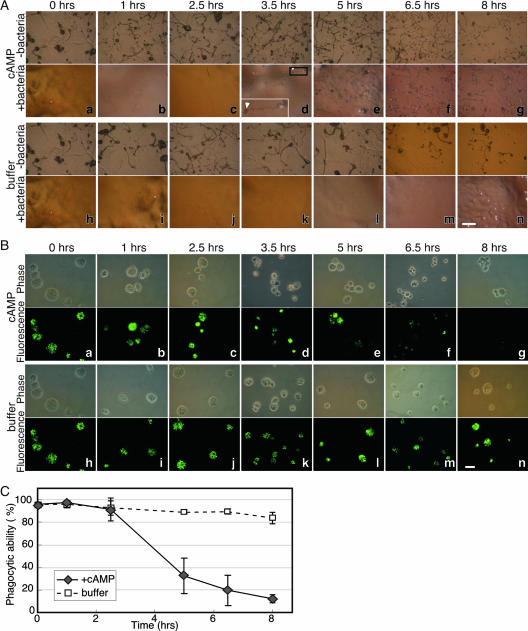
The above data show that cAMP signaling is necessary for the induction of commitment and for the loss of phagocytosis. We tested whether it was also sufficient to induce commitment. Recurring cAMP pulses induce Dictyostelium cells to differentiate in suspension without cell-cell contact (22). We resuspended vegetative wild-type cells in KK2, pulsed them with cAMP, plated them on KK2 agar with or without bacteria at certain time points, and observed their morphology and phagocytosis after 24 h from the initial starvation. We found that without bacteria, the cells formed fruiting bodies after 24 h regardless of the treatment (Fig. 4A, top, −bacteria). These results show that the treated cells were viable and capable of timely development. Conversely, we observed that cells pulsed with cAMP for 0, 1, and 2.5 h started eating the bacteria and eventually cleared the bacterial lawn (Fig. 4A, panels a to c). By definition, these cells were not committed. Cells pulsed with cAMP for 3.5 h or more continued to develop and formed fruiting bodies on the bacterial lawn after 24 h (Fig. 4A, panels d to g). These data suggest that starvation with cAMP pulses for 3.5 h is sufficient to induce commitment. The commitment of cAMP-pulsed cells (Fig. 4A) occurred between 2.5 and 3.5 h, faster than the commitment of untreated cells undergoing development on a solid surface (between 4 and 6 h) (Fig. 1B). It appears that pulsing the cells with cAMP from the beginning of starvation induces precocious commitment.
FIG. 4.
cAMP pulses induce commitment and loss of phagocytosis. (A) Growing cells were resuspended in KK2 (time zero) and subjected to cAMP or control buffer pulses as indicated on the left. At the times indicated above the pictures (h after time zero), the cells were plated on KK2 agar without (−bacteria, upper panels) or with (+bacteria, lower panels) bacteria. Morphology was documented after 24 h (relative to time zero). The cells formed fruiting bodies after 24 h in all the samples without bacteria (upper panel for each treatment). In the samples with bacteria, cells treated with cAMP did not form fruiting bodies (uncommitted) if they were first pulsed with cAMP for less than 3.5 h (panels a to c) and did form fruiting bodies (committed) if they were pulsed for 3.5 h or more. (The arrow in the magnified inset indicates the sorus of a fruiting body; the black rectangle indicates the location of the fruiting body in panel d.). Cells treated with buffer and overlaid with bacteria did not form fruiting bodies (uncommitted) even after 8 h of starvation (panels h to n). Scale bar, 1 mm. (B) Phagocytosis was tested after pulses with cAMP or with buffer (as indicated on the left) for the times indicated above the pictures. Photographs of representative fields from phase-contrast microscopy (upper panels) and fluorescence microscopy (lower panels) are shown. The cells retained phagocytic ability after 0, 1, and 2.5 h of cAMP pulsing (panels a, b, and c, respectively) and exhibited progressively reduced phagocytosis between 3.5 and 8 h after cAMP pulsing (panels d to g). Cells pulsed with buffer retained phagocytic ability (panels h to n). Scale bar, 10 μm. (C) Phagocytosis was quantified in cells treated with (+cAMP) and without (buffer) cAMP pulses for the indicated time course. Data are presented as averages ± SD of three independent replications.
To test whether starvation alone could induce commitment, we treated the cells in the same way but pulsed with KK2 buffer instead of cAMP (Fig. 4A, bottom). We found that starvation alone (up to 8 h) was not sufficient to induce commitment, as the cells made plaques in the bacterial lawn and did not develop after 24 h (Fig. 4A, panels h to n). These results, together with the results described in Fig. 3, suggest that cAMP pulses are both necessary and sufficient to induce developmental commitment.
If commitment and the loss of phagocytosis are causally related, then phagocytosis should also be lost upon pulsing with cAMP. Analysis of phagocytosis supported this idea (Fig. 4B and C). We found that the phagocytic ability of the cells was relatively unchanged in the first 2.5 h of cAMP pulsing (Fig. 4B, panels a to c), but after 3.5 h of cAMP pulses we observed a significant reduction in phagocytosis (Fig. 4B, panel d) and after 5 to 8 h we observed a near-total loss of phagocytosis (Fig. 4B, panels e to g). For the buffer-pulsed control, we found that most cells retained their phagocytic ability (Fig. 4B, panels h to n). Quantitative analysis of phagocytosis in three independent experiments confirmed that cAMP-pulsed cells lost their phagocytic ability between 2.5 and 5 h, whereas cells pulsed with buffer maintained high phagocytic ability even after 8 h of starvation (Fig. 4C). These data suggest that starvation accompanied by cAMP pulses, but not starvation alone, is sufficient for the loss of phagocytosis.
Commitment is inhibited by small soluble molecules.
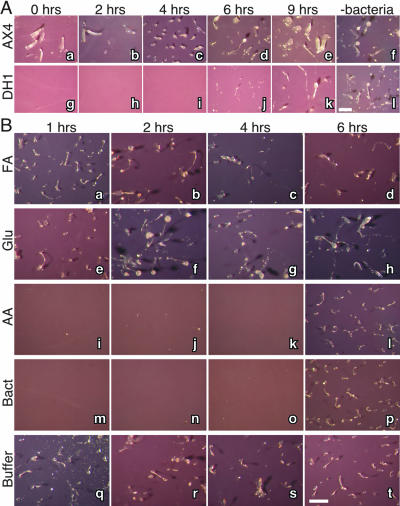
To examine whether phagocytosis is directly correlated with commitment, we prevented bacterial phagocytosis by separating the amoebae from the bacteria and monitored commitment. Two strains, AX4 and DH1, were starved on nitrocellulose filters to induce development. Bacteria were washed, resuspended in buffer, and placed inside dialysis tubing. The filters, carrying the developing amoebae, were then placed on top of the bacterium-filled tubes after 0, 2, 4, 6, or 9 h of development. The cells’ morphological progression was monitored after 26 h from the initial starvation (Fig. 5A). Intriguingly, when AX4 cells were placed on top of the bacterial suspension after less than 4 h of development, they exhibited delayed development and progressed to the finger stage only after 26 h of starvation (Fig. 5A, panels a to c). However, 6- or 9-hour cells developed into fruiting bodies after being placed on top of the bacteria (Fig. 5A, panels d and e) as if they were never exposed to bacteria at all (Fig. 5A, panel f). This phenomenon was even more dramatic in the other strain. When DH1 cells that had developed for less than 4 hours were placed on top of the bacterial suspension, they completely failed to develop (Fig. 5A, panels g to i), but after 6 or 9 h they exhibited continued development (Fig. 5A, panels j and k) as if they had not been exposed to bacteria (Fig. 5A, panel l). Therefore, commitment is observed even without direct contact with the bacteria.
FIG. 5.
Inhibition of commitment by small soluble molecules. Cells from two strains, AX4 (panels a to f) and DH1 (panels g to l), were washed (time zero) and deposited on nitrocellulose filters. The filters were placed on top of dialysis tubing containing KK2. (A) At the times indicated above the pictures, the filters were placed on top of fresh dialysis tubing filled with a bacterial suspension (0, 2, 4, 6, and 9 h) or left on the original dialysis tubing containing KK2 (−bacteria). Morphology was documented after 26 h relative to time zero. Cells that had developed for less than 6 h exhibited attenuated development (AX4, panels a to c) or arrested development (DH1, panels g to i), whereas more-advanced cells developed into fruiting bodies. (B) DH1 cells were developed as described above. After 1, 2, 4, and 6 h as indicated above the photographs, filters were placed on top of dialysis tubing containing 10 μM folic acid (FA, panels a to d), 10 mM glucose (Glu, panels e to h), and a mixture of amino acid solution (AA, panels i to l). Developmental morphology was documented after 24 h relative to time zero. Positive control filters were placed on dialysis tubing containing bacterial suspensions (Bact, panels m to p) and negative control filters on tubing filled with KK2 (Buffer, panels q to t). The amino acid mixture inhibited the development of cells starved for less than 6 h (panels i to k), as did the positive control (panels m to o). No effect was observed with the other conditions. Scale bar, 1 mm.
These findings indicate that Dictyostelium cells become insensitive to food after 6 to 9 h of development. They also indicate that bacteria inhibit early development even without direct physical contact with the amoebae, suggesting that phagocytic activity should not directly impinge on developmental commitment. To test this notion, we examined commitment in the phagocytosis-compromised sadA−, talA−, and cnx−/crt− mutants and found their commitment to be indistinguishable from that of the wild type (see Fig. S1A and S1B in the supplemental material; Table 1 shows strain information).
The results shown in Fig. 5 also suggest that the amoebae sense the presence of bacteria via small soluble molecules that can pass through the dialysis tubing (<14 kDa). Dictyostelium cells sense and chemotax towards folic acid, which is secreted from their bacterial prey (28). In addition, amino acid starvation is essential for the initiation of development, but glucose starvation is not (23). Therefore, we examined the effect of folic acid, glucose, and amino acids on commitment under the conditions described for Fig. 5A. The presence of folic acid or glucose in the buffer had no apparent effect on development (Fig. 5B, panels a to d and e to h, respectively). However, amino acids pause the development of cells even after 1, 2, or 4 h of starvation (Fig. 5B, panels i to k) but not after 6 h (Fig. 5B, panel l), as does the presence of bacteria (Fig. 5B, panels m to p). These results suggest that amino acids might be the soluble bacterial product that suppresses Dictyostelium development prior to commitment and that the cells become unable to react to amino acids and commit to development.
DISCUSSION
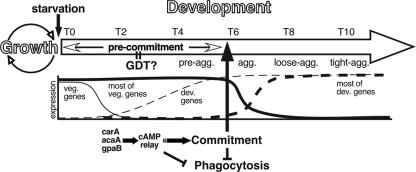
Dictyostelium development is accompanied by major physiological transitions and ends with the certain death of 20% of the cells. Upon starvation, the cells stop replicating chromosomal DNA (5, 35), reduce the expression of hundreds of vegetative genes, induce the expression of hundreds of developmental genes (39), and start to aggregate. The transcriptional transition and the ensuing cell movement must be metabolically costly, so it is not surprising that Dictyostelium has acquired a developmental commitment system as a regulatory mechanism. We propose that upon sensing starvation, the amoeba enter a precommitment stage (Fig. 6), in which they utilize internal energy stores, perhaps glycogen (2, 40). During precommitment, the developing cells maintain a vegetative-like physiology because they retain the mRNA of many vegetative genes (39). We therefore propose that commitment occurs upon completion of the GDT (Fig. 6). The precommitment stage may be selectively advantageous because it allows cells to feed if prey suddenly becomes available. This idea is supported by the persistent phagocytic ability during the first few hours of development and by the observation that the addition of bacteria to the precommitted amoebae resulted in an immediate resumption of growth.
FIG. 6.
The time course of commitment. The wide outlined arrow represents the developmental time from 0 to 10 h (T0 to T10) after starvation, the dashed lines represent examples of developmental gene expression patterns, and the solid lines represent examples of vegetative gene expression. Abbreviations: pre-agg., preaggregation; agg., aggregation; loose-agg., loose aggregate; tight-agg., tight aggregate; veg., vegetative; dev., developmental. See text for details.
cAMP plays a pivotal role in Dictyostelium development. We have found that cAMP pulses are necessary and sufficient to induce commitment. Iranfar et al. have shown that cAMP pulses of cells in suspension induce the expression of over 100 developmentally regulated genes, including genes required for cAMP signaling, whereas starvation alone induced only a few genes, including carA, gpaB, and the cAMP phosphodiesterase gene pdsA (13). In this paper, we show that cAMP signaling genes are essential for commitment and that starvation alone is not sufficient to induce commitment. We conclude that early cAMP signaling precedes commitment and is necessary for its establishment.
We also found that cAMP pulses induce the loss of phagocytosis. One of the simplest explanations would be that commitment results from the loss of phagocytosis, but our data argue against that. The most compelling argument is that bacteria inhibit the development of precommitted cells even when the amoebae are physically separated from the bacteria and cannot ingest them. In addition, the commitment of phagocytosis-compromised mutants is indistinguishable from that of the wild type. These findings are consistent with the idea that cAMP signaling induces commitment first and the loss of phagocytic ability follows (Fig. 6).
Commitment could have evolved as a mechanism that prevents dedifferentiation when food is insufficient, but the selective advantage of commitment when food is abundant is less clear. The reason could be the finding that dedifferentiation is a long and presumably costly process. When dedifferentiation is induced at the slug stage, the cells require 12 to 20 h until they divide (16, 36). Since development takes only 24 h, ignoring food completely might be advantageous. In addition to commitment, the loss of phagocytosis, the establishment of cell-cell adhesion, and the deposition of the slime sheath might further preclude dedifferentiation. However, low-density cells dedifferentiate despite the presumed metabolic cost (16, 36), probably because they fail to develop or redevelop without growth. We showed that cells retain their macropinocytotic capability even after losing phagocytosis. Cells at low density could obtain nutrients by pinocytosis, accounting for their ability to dedifferentiate after commitment. These ideas are supported by the fact that dedifferentiation requires both mechanical dissociation and cell dispersion in nutrition medium.
Autophagy bears the function of bulk proteolysis of cytoplasmic proteins and organelles to scavenge amino acids when nutrient levels are low, so it represents a serious commitment to development. In Dictyostelium, autophagy is essential for development, and the expression of its key components, atg1 and atg8, is induced during the first 4 h of development (27). Autophagy in mammalian cells is also inhibited by amino acids and insulin (14, 15). The nutrient-mediated inhibition of development before commitment suggests that amino acid or energy levels might regulate developmental commitment. The onset of autophagy is coincident with commitment, suggesting that the two are functionally related.
We define commitment as a step that suppresses the response of Dictyostelium cells to food after the transition from growth to development. Commitment requires several hours of starvation and pulsatile extracellular cAMP signaling. Neither high cell density nor solid surfaces are essential, since pulsing low-density cells with cAMP in suspension induces commitment too. We propose that attaining the committed state provides the cells within a starving population with a physiological cue for participation in multicellular development, ensuring the cooperation needed to coordinate further development.
Supplementary Material
Acknowledgments
We thank R. H. Kessin and W. F. Loomis for insightful discussions and Peter Devreotes, Pierre Cosson, Jakob Franke, and the Dicty Stock Center for strains.
This work was supported by NIH grant GM52359.
Footnotes
Published ahead of print on 28 September 2007.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Abe, T., A. Early, F. Siegert, C. Weijer, and J. Williams. 1994. Patterns of cell movement within the Dictyostelium slug revealed by cell type-specific, surface labeling of living cells. Cell 77:687-699. [DOI] [PubMed] [Google Scholar]
- 2.Ashworth, J. M., and D. J. Watts. 1970. Metabolism of the cellular slime mould Dictyostelium discoideum grown in axenic culture. Biochem. J. 119:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardelli, J. 2001. Phagocytosis and macropinocytosis in Dictyostelium: phosphoinositide-based processes, biochemically distinct. Traffic 2:311-320. [DOI] [PubMed] [Google Scholar]
- 4.Caterina, M. J., J. L. Milne, and P. N. Devreotes. 1994. Mutation of the third intracellular loop of the cAMP receptor, cAR1, of Dictyostelium yields mutants impaired in multiple signaling pathways. J. Biol. Chem. 269:1523-1532. [PubMed] [Google Scholar]
- 5.Chen, G., G. Shaulsky, and A. Kuspa. 2004. Tissue-specific G1-phase cell-cycle arrest prior to terminal differentiation in Dictyostelium. Development 131:2619-2630. [DOI] [PubMed] [Google Scholar]
- 6.Chibalina, M. V., C. Anjard, and R. H. Insall. 2004. Gdt2 regulates the transition of Dictyostelium cells from growth to differentiation. BMC Dev. Biol. 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornillon, S., E. Pech, M. Benghezal, K. Ravanel, E. Gaynor, F. Letourneur, F. Bruckert, and P. Cosson. 2000. Phg1p is a nine-transmembrane protein superfamily member involved in Dictyostelium adhesion and phagocytosis. J. Biol. Chem. 275:34287-34292. [DOI] [PubMed] [Google Scholar]
- 8.Dynes, J. L., A. M. Clark, G. Shaulsky, A. Kuspa, W. F. Loomis, and R. A. Firtel. 1994. LagC is required for cell-cell interactions that are essential for cell-type differentiation in Dictyostelium. Genes Dev. 8:948-958. [DOI] [PubMed] [Google Scholar]
- 9.Fey, P., S. Stephens, M. A. Titus, and R. L. Chisholm. 2002. SadA, a novel adhesion receptor in Dictyostelium. J. Cell Biol. 159:1109-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franke, J., and R. Kessin. 1977. A defined minimal medium for axenic strains of Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 74:2157-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant, W. N., and K. L. Williams. 1983. Monoclonal antibody characterisation of slime sheath: the extracellular matrix of Dictyostelium discoideum. EMBO J. 2:935-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacker, U., R. Albrecht, and M. Maniak. 1997. Fluid-phase uptake by macropinocytosis in Dictyostelium. J. Cell Sci. 110:105-112. [DOI] [PubMed] [Google Scholar]
- 13.Iranfar, N., D. Fuller, and W. F. Loomis. 2003. Genome-wide expression analyses of gene regulation during early development of Dictyostelium discoideum. Eukaryot. Cell 2:664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadowaki, M., and T. Kanazawa. 2003. Amino acids as regulators of proteolysis. J. Nutr. 133:2052S-2056S. [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa, T., I. Taneike, R. Akaishi, F. Yoshizawa, N. Furuya, S. Fujimura, and M. Kadowaki. 2004. Amino acids and insulin control autophagic proteolysis through different signaling pathways in relation to mTOR in isolated rat hepatocytes. J. Biol. Chem. 279:8452-8459. [DOI] [PubMed] [Google Scholar]
- 16.Katoh, M., C. Shaw, Q. Xu, N. Van Driessche, T. Morio, H. Kuwayama, S. Obara, H. Urushihara, Y. Tanaka, and G. Shaulsky. 2004. An orderly retreat: dedifferentiation is a regulated process. Proc. Natl. Acad. Sci. USA 101:7005-7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessin, R. H. 2001. Dictyostelium—evolution, cell biology, and the development of multicellularity. Cambridge University Press, Cambridge, United Kingdom.
- 18.Kibler, K., J. Svetz, T. L. Nguyen, C. Shaw, and G. Shaulsky. 2003. A cell-adhesion pathway regulates intercellular communication during Dictyostelium development. Dev. Biol. 264:506-521. [DOI] [PubMed] [Google Scholar]
- 19.Knecht, D. A., S. M. Cohen, W. F. Loomis, and H. F. Lodish. 1986. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol. Cell. Biol. 6:3973-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loomis, W. F. 1975. Dictyostelium discoideum. A developmental system. Academic Press, New York, NY.
- 21.Maniak, M. 2001. Fluid-phase uptake and transit in axenic Dictyostelium cells. Biochim. Biophys. Acta 1525:197-204. [DOI] [PubMed] [Google Scholar]
- 22.Mann, S. K., and R. A. Firtel. 1987. Cyclic AMP regulation of early gene expression in Dictyostelium discoideum: mediation via the cell surface cyclic AMP receptor. Mol. Cell. Biol. 7:458-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marin, F. T. 1976. Regulation of development in Dictyostelium discoideum. I. Initiation of the growth to development transition by amino acid starvation. Dev. Biol. 48:110-117. [DOI] [PubMed] [Google Scholar]
- 24.Milne, J. L., and P. N. Devreotes. 1993. The surface cyclic AMP receptors, cAR1, cAR2, and cAR3, promote Ca2+ influx in Dictyostelium discoideum by a G alpha 2-independent mechanism. Mol. Biol. Cell 4:283-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller-Taubenberger, A., A. N. Lupas, H. Li, M. Ecke, E. Simmeth, and G. Gerisch. 2001. Calreticulin and calnexin in the endoplasmic reticulum are important for phagocytosis. EMBO J. 20:6772-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niewohner, J., I. Weber, M. Maniak, A. Muller-Taubenberger, and G. Gerisch. 1997. Talin-null cells of Dictyostelium are strongly defective in adhesion to particle and substrate surfaces and slightly impaired in cytokinesis. J. Cell Biol. 138:349-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otto, G. P., M. Y. Wu, N. Kazgan, O. R. Anderson, and R. H. Kessin. 2004. Dictyostelium macroautophagy mutants vary in the severity of their developmental defects. J. Biol. Chem. 279:15621-15629. [DOI] [PubMed] [Google Scholar]
- 28.Pan, P., E. M. Hall, and J. T. Bonner. 1972. Folic acid as second chemotactic substance in the cellular slime moulds. Nat. New Biol. 237:181-182. [DOI] [PubMed] [Google Scholar]
- 29.Pilz, R. B., I. Huvar, J. S. Scheele, G. Van den Berghe, and G. R. Boss. 1997. A decrease in the intracellular guanosine 5′-triphosphate concentration is necessary for granulocytic differentiation of HL-60 cells, but growth cessation and differentiation are not associated with a change in the activation state of Ras, the transforming principle of HL-60 cells. Cell Growth Differ. 8:53-59. [PubMed] [Google Scholar]
- 30.Pilz, R. B., G. Van den Berghe, and G. R. Boss. 1987. Induction of HL-60 differentiation by starvation for a single essential amino acid but not by protein synthesis inhibitors. J. Clin. Investig. 79:1006-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rafols, I., A. Amagai, Y. Maeda, H. K. MacWilliams, and Y. Sawada. 2001. Cell type proportioning in Dictyostelium slugs: lack of regulation within a 2.5-fold tolerance range. Differentiation 67:107-116. [DOI] [PubMed] [Google Scholar]
- 32.Raper, K. B. 1940. Pseudoplasmodium formation and organization in Dictyostelium discoideum. J. Elisha Mitchell Sci. Soc. 56:241-282. [Google Scholar]
- 33.Shaulsky, G., A. Kuspa, and W. F. Loomis. 1995. A multidrug resistance transporter/serine protease gene is required for prestalk specialization in Dictyostelium. Genes Dev. 9:1111-1122. [DOI] [PubMed] [Google Scholar]
- 34.Shaulsky, G., and W. F. Loomis. 1993. Cell type regulation in response to expression of ricinA in Dictyostelium. Dev. Biol. 160:85-98. [DOI] [PubMed] [Google Scholar]
- 35.Shaulsky, G., and W. F. Loomis. 1995. Mitochondrial DNA replication but no nuclear DNA replication during development of Dictyostelium. Proc. Natl. Acad. Sci. USA 92:5660-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soll, D. R., and D. R. Waddell. 1975. Morphogenesis in the slime mold Dictyostelium discoideum. 1. The accumulation and erasure of “morphogenetic information.” Dev. Biol. 47:292-302. [DOI] [PubMed] [Google Scholar]
- 37.Souza, G. M., S. Lu, and A. Kuspa. 1998. YakA, a protein kinase required for the transition from growth to development in Dictyostelium. Development 125:2291-2302. [DOI] [PubMed] [Google Scholar]
- 38.Stepanovic, V., D. Wessels, K. Daniels, W. F. Loomis, and D. R. Soll. 2005. Intracellular role of adenylyl cyclase in regulation of lateral pseudopod formation during Dictyostelium chemotaxis. Eukaryot. Cell 4:775-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Driessche, N., C. Shaw, M. Katoh, T. Morio, R. Sucgang, M. Ibarra, H. Kuwayama, T. Saito, H. Urushihara, M. Maeda, I. Takeuchi, H. Ochiai, W. Eaton, J. Tollett, J. Halter, A. Kuspa, Y. Tanaka, and G. Shaulsky. 2002. A transcriptional profile of multicellular development in Dictyostelium discoideum. Development 129:1543-1552. [DOI] [PubMed] [Google Scholar]
- 40.Watts, D. J., and J. M. Ashworth. 1970. Growth of myxamoebae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem. J. 119:171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng, C., C. Anjard, K. Riemann, A. Konzok, and W. Nellen. 2000. Gdt1, a new signal transduction component for negative regulation of the growth-differentiation transition in Dictyostelium discoideum. Mol. Biol. Cell 11:1631-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.