Protein modification by ubiquitin and ubiquitin-like proteins is one of the most complex and intensely studied mechanisms of posttranslational protein regulation in eukaryotes. Conjugation of the 76-amino-acid protein ubiquitin is first and foremost a signal for targeting proteins to the proteasome for degradation, but evidence that ubiquitin also plays diverse roles in the regulation of numerous biological pathways is building. In addition, there are many structurally related ubiquitin-like modifiers (Ubls) that utilize mechanistic pathways similar to those utilized by ubiquitin for conjugation to protein substrates and deconjugation. Despite similarities in structure between ubiquitin and other Ubls, modification by Ubls regulates such diverse cellular processes as transcriptional regulation, cell cycle control, and autophagy (see Kerscher et al. [22] for a review of Ubls and known functions). Ubiquitin has been identified in the majority of parasitic protozoa, but most Ubls in these organisms have not been characterized. Even less attention has been paid to the enzymes that regulate protein modification by ubiquitin or Ubls.
The essential roles of ubiquitin and Ubls in both protein turnover and transcriptional regulation in other organisms suggest that ubiquitin and Ubl pathways should be explored to better understand basic parasite biology. For this reason, we have compiled a comprehensive list of homologs of known Ubls and Ubl-deconjugating enzymes in medically important protozoa. We also discuss potential differences and unique characteristics of Ubls and deconjugating enzymes in parasites compared to those in mammals and yeast such as Saccharomyces cerevisiae and Schizosaccharomyces pombe. Notably absent from this review are the enzymes that conjugate ubiquitin and Ubls to their substrates. Although conjugation machinery is also important to the pathway, the essential role of deconjugating enzymes in multiple biological pathways and recent publications describing the identification of inhibitors of these enzymes indicate that they may represent a potentially important class of protease drug targets in parasites. Therefore, we have chosen to focus this review on these enzymes and the modifiers they regulate.
REGULATING THE REGULATORS: THE UBIQUITIN MODIFICATION PATHWAY
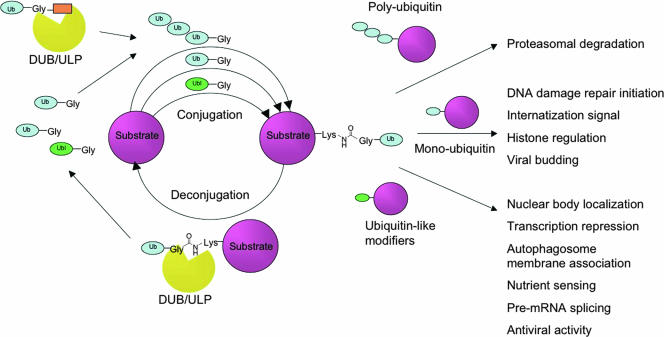
Like ubiquitin itself, the mechanistic steps that add ubiquitin to and remove it from proteins are conserved across the Eukaryota (see Kerscher et al. [22] and Hemelaar et al. [12] for reviews of enzymatic details). Before conjugation, ubiquitin must first be proteolytically processed from its precursor form by ubiquitin-specific proteases (USPs) to reveal a C-terminal diglycine. Processed ubiquitin is then conjugated by a series of ligases to the ɛ-amino group of a protein lysine side chain via an isopeptide bond. Both the number of ubiquitin molecules (monoubiquitin or polyubiquitin) and the location of the modification determine the fate of the modified substrate. In addition to targeting proteins for degradation, ubiquitylation regulates protein localization and DNA damage repair (17). Ubiquitin is removed by selective proteases called deubiquitinating proteases (DUBs) that hydrolyze the isopeptide linkage. Many of these hydrolases both process ubiquitin to expose the C-terminal diglycine and cleave ubiquitin from conjugated substrates; therefore, the term DUBs is generally applied to hydrolases involved in either function. The general process of the maturation of ubiquitin, the conjugation of ubiquitin to substrates, and deconjugation is summarized in Fig. 1. It is a dynamic balance of conjugation and deconjugation that determines the fate of the protein being modified.
FIG. 1.
Maturation, conjugation, and deconjugation of ubiquitin and Ubls. Before conjugation, ubiquitin (Ub) and Ubls are processed from a precursor form to expose their C termini. The C-terminal extension varies in both length and sequence. They are then conjugated to substrates by a series of conjugation enzymes. Modified substrates are then subjected to or stimulate a variety of biological processes, depending on the modification type. Eventually the modifier is removed and recycled by ubiquitin- or Ubl-specific proteases that cleave the isopeptide bond generated during conjugation.
Although the majority of ubiquitin and Ubl pathways in mammalian and yeast cells have been studied and characterized, relatively little is known about how these systems are used by parasites. The complex life cycles and multiple disease-causing states of parasitic protozoa offer a unique context in which to study ubiquitin and Ubl modification pathways. The life cycles of most protozoan parasites within single or multiple hosts rely on strict timing of protein regulation and gene expression for both survival and virulence. The application of genomics and proteomics to numerous parasite species has confirmed that many genes and proteins are regulated in a life cycle-dependent manner (4, 6, 32). In the most striking example, the transcriptional profile of the intraerythrocytic life cycle of Plasmodium falciparum shows periodic waves of regulated gene expression for 80% of all genes expressed during the 48-h life cycle whereas only 15% of mammalian and yeast genes show such regulated expression patterns (4). While the regulation of gene expression and protein turnover is clearly critical for both life cycle and disease progression in medically important protozoa, the mechanisms regulating these processes are not well understood. Given the known functions of ubiquitin and Ubls in other organisms, a better understanding of these posttranslational modifiers is likely to be critical to understanding how parasites control many basic biological processes.
UBIQUITIN AND Ubls
In addition to ubiquitin, a number of Ubls exist in most organisms (Table 1). While these Ubls all share general secondary and tertiary structures with ubiquitin, they each carry out diverse functional roles when used for the posttranslational modification of proteins. In order to begin to address the roles of ubiquitin and Ubl modification pathways in parasitic protozoa, it is first necessary to identify all ubiquitin and Ubl genes from sequenced genomes (Table 2). We searched the literature and conducted BLASTP homology searches, followed by reciprocal best-hit analysis, to assemble a list of parasite homologs of the Ubls. We identified homologs for six of the nine major Ubl families, including ubiquitin, Nedd8 (neural precursor cell-expressed developmentally down-regulated 8), small ubiquitin-related modifier (SUMO), Hub1, ubiquitin-related modifier 1 (Urm1), and autophagy-8 (Atg8), but failed to identify homologs for the interferon-stimulated gene protein 15 (ISG15), FAT10, or autophagy-12 (Atg12). Of the identified families of Ubls, only ubiquitin and Atg8 have been characterized in parasitic protozoa.
TABLE 1.
Common Ubls
| Ubl | Known function(s) | Protozoa with predicted homologs | Protozoa for which characterization of Ubl has been published |
|---|---|---|---|
| Ubiquitin | Protein degradation, internalization, histone regulation | Plasmodium, Toxoplasma, Leishmania, Trypanosoma, Entamoeba, Giardia, Cryptosporidium, and Theileria spp. | Plasmodium, Leishmania, Trypanosoma, Entamoeba, and Giardia spp. |
| Nedd8 | Ubiquitin conjugation | Plasmodium spp. | None |
| ISG15 | Interferon response | None | None |
| SUMO | Transcriptional regulation, protein localization | Plasmodium, Toxoplasma, Leishmania, Trypanosoma, Entamoeba, Cryptosporidium, and Theileria spp. | None |
| FAT10 | Ubiquitin-independent degradation | None | None |
| Hub1 | Pre-mRNA splicing | Plasmodium, Toxoplasma, Cryptosporidium, Theileria, and Entamoeba spp. | None |
| Urm1 | Starvation response | Plasmodium, Leishmania, Trypanosoma, Entamoeba, Cryptosporidium, and Giardia spp. | None |
| Atg8 | Autophagy | Plasmodium, Toxoplasma, Leishmania, Trypanosoma, and Theileria spp. | Trypanosoma and Leishmania spp. |
| Atg12 | Autophagy | None | None |
TABLE 2.
Ubls and their putative parasite homologs as determined by reciprocal best-hit analysis
| Ubl | Species | Ubl gene accession no. (parasite genome identifier) | BLASTP reciprocal best-hit
|
Reference(s) | |
|---|---|---|---|---|---|
| Accession no. (organism, protein name) | Expectation value | ||||
| Ubiquitin | Plasmodium falciparum | NP_701482 (PFL0585w) | AAH53371 (Homo sapiens, ribosomal fusion protein S27a) | 4e−36 | 16 |
| Toxoplasma gondii | 38.m01076a | AAH08955.2 (Homo sapiens, ubiquitin C) | 7e−150 | ||
| Theileria annulata | CAI73380 | AAH08955.2 (Homo sapiens, ubiquitin C) | 4e−72 | ||
| Theileria parva | EAN33959 | AAH08955.2 (Homo sapiens, ubiquitin C) | 3e−72 | ||
| Leishmania major | CAJ09316 | BAA23486 (Homo sapiens, polyubiquitin) | 0 | 23 | |
| Trypanosoma brucei | XP_829056 | BAA23486 (Homo sapiens, polyubiquitin) | 0 | 23 | |
| Trypanosoma cruzi | P08565 | BAA23486 (Homo sapiens, polyubiquitin) | 4e−36 | ||
| Cryptosporidium hominis | XP_667472 | AAH08955.2 (Homo sapiens, ubiquitin C) | 8e−112 | ||
| Cryptosporidium parvum | XP_626192 | AAH08955.2 (Homo sapiens, ubiquitin C) | 2e−112 | ||
| Entamoeba histolytica | CAA67177 | AAH53371 (Homo sapiens, ribosomal fusion protein S27a) | 3e−34 | 46 | |
| Giardia lamblia | X70050 | AAH53371 (Homo sapiens, ribosomal fusion protein S27a) | 2e−33 | 26 | |
| Nedd8 | Plasmodium falciparum | NP_705038 (MAL13P1.64) | AAI04202 (Homo sapiens, Nedd8) | 7e−17 | |
| Plasmodium chabaudi | CAH83092 | AAI04202 (Homo sapiens, Nedd8) | 1e−16 | ||
| Plasmodium berghei | CAH95491 | AAI04202 (Homo sapiens, Nedd8) | 1e−16 | ||
| ISG15 | None | CAI15574 (Homo sapiens, ISG15) | |||
| SUMO | Trypanosoma cruzi | EAN92418 | CAA67896 (Homo sapiens, SUMO3) | 3e−19 | |
| Trypanosoma cruzi | EAN95569 | CAA67896 (Homo sapiens, SUMO3) | 4e−19 | ||
| Trypanosoma brucei | AAX79561 | AAH66306 (Homo sapiens, SUMO1) | 9e−19 | ||
| Leishmania major | CAJ02226 | CAA67896 (Homo sapiens, SUMO3) | 4e−18 | ||
| Plasmodium falciparum | NP_703403 (PFE0285c) | CAA67896 (Homo sapiens, SUMO3) | 5e−18 | ||
| Theileria annulata | CAI73057 | CAA67896 (Homo sapiens, SUMO3) | 8e−13 | ||
| Entamoeba histolytica | XP_655984 | AAI07854 (Homo sapiens, SUMO2) | 5e−18 | ||
| Theileria parva | EAN34278 | CAA67896 (Homo sapiens, SUMO3) | 8e−13 | ||
| Cryptosporidium hominis | XP_665282 | AAH66306 (Homo sapiens, SUMO1) | 6e−13 | ||
| Cryptosporidium parvum | XP_627315 | AAH66306 (Homo sapiens, SUMO1) | 6e−13 | ||
| Toxoplasma gondii | 57.m01794 | AAH66306 (Homo sapiens, SUMO1) | 2e−13 | ||
| FAT10 | None | AAD52982 (Homo sapiens, FAT10) | |||
| Hub1 | Plasmodium falciparum | XP_001350772 (PFL1830w) | NP_014430 (Saccharomyces cerevisiae, Hub1p) | 4e−18 | |
| Plasmodium berghei | XP_680294 | NP_014430 (Saccharomyces cerevisiae, Hub1p) | 1e−18 | ||
| Plasmodium yoelii | XP_726593 | NP_014430 (Saccharomyces cerevisiae, Hub1p) | 1e−18 | ||
| Toxoplasma gondii | 55.m04782 | NP_014430 (Saccharomyces cerevisiae, Hub1p) | 3e−20 | ||
| Cryptosporidium parvum | XP_001388147 | NP_014430 (Saccharomyces cerevisiae, Hub1p) | 9e−18 | ||
| Theileria parva | XP_762746 | NP_014430 (Saccharomyces cerevisiae, Hub1p) | 3e−17 | ||
| Theileria annulata | XP_955328 | NP_014430 (Saccharomyces cerevisiae, Hub1p) | 9e−17 | ||
| Entamoeba histolytica | XP_648708 | NP_014430 (Saccharomyces cerevisiae, Hub1p) | 4e−16 | ||
| Urm1 | Plasmodium chabaudi | XP_740984 | NP_012258 (Saccharomyces cerevisiae, Urm1p) | 8e−8 | |
| Plasmodium falciparum | NP_701252 (PF11_0393) | NP_012258 (Saccharomyces cerevisiae, Urm1p) | 4e−8 | ||
| Plasmodium berghei | CAH95991 | NP_012258 (Saccharomyces cerevisiae, Urm1p) | 1e−7 | ||
| Giardia lamblia | XP_779378 | NP_012258 (Saccharomyces cerevisiae, Urm1p) | 5e−6 | ||
| Plasmodium yoelii | EAA18635 | NP_012258 (Saccharomyces cerevisiae, Urm1p) | 2e−7 | ||
| Cryptosporidium hominis | XP_668249 | NP_012258 (Saccharomyces cerevisiae, Urm1p) | 2e−13 | ||
| Cryptosporidium parvum | EAK90632 | NP_012258 (Saccharomyces cerevisiae, Urm1p) | 5e−13 | ||
| Trypanosoma cruzi | EAN88200 | NP_012258 (Saccharomyces cerevisiae, Urm1p) | 1e−12 | ||
| Trypanosoma brucei | AAX79740 | NP_012258 (Saccharomyces cerevisiae, Urm1p) | 2e−12 | ||
| Leishmania major | CAJ08004 | NP_012258 (Saccharomyces cerevisiae, Urm1p) | 4e−11 | ||
| Entamoeba histolytica | XP_657081 | NP_012258 (Saccharomyces cerevisiae, Urm1p) | 3e−10 | ||
| Atg8 | Plasmodium falciparum | NP_700667 (PF10_0193) | NP_009475 (Saccharomyces cerevisiae, Atg8p) | 1e−24 | |
| Plasmodium berghei | XP_678543 | NP_009475 (Saccharomyces cerevisiae, Atg8p) | 5e−28 | ||
| Plasmodium yoelii | EAA17180 | NP_009475 (Saccharomyces cerevisiae, Atg8p) | 5e−28 | ||
| Plasmodium chabaudi | XP_745350 | NP_009475 (Saccharomyces cerevisiae, Atg8p) | 5e−28 | ||
| Toxoplasma gondii | 52.m00003 | NP_009475 (Saccharomyces cerevisiae, Atg8p) | 4e−30 | ||
| Theileria parva | EAN32621 | NP_009475 (Saccharomyces cerevisiae, Atg8p) | 9e−18 | ||
| Theileria annulata | CAI74649 | NP_009475 (Saccharomyces cerevisiae, Atg8p) | 3e−17 | ||
| Trypanosoma brucei | AAX78826 (Tb07.10C21.40) | NP_009475 (Saccharomyces cerevisiae, Atg8p) | 7e−33 | 13 | |
| AAX78827 | 3e−30 | ||||
| AAX70074 | 3e−18 | ||||
| Trypanosoma cruzi | EAN97061 (Tc00.1047053510533.180) | NP_009475 (Saccharomyces cerevisiae, Atg8p) | 6e−20 | 13 | |
| EAN96431 | 2e−34 | ||||
| Leishmania major | CAJ07266 (LmjF19.1630) | NP_009475 (Saccharomyces cerevisiae, Atg8p) | 2e−31 | 13, 40 | |
| Atg12 | None | P38316 (Saccharomyces cerevisiae, ATG12) | 13, 40 | ||
Toxoplasma protein sequences are not yet available in NCBI, so all accession numbers are for ToxoDB only.
We began our search with perhaps the most ancient Ubl, Urm1. Ubiquitin and Ubls are evolutionarily related to prokaryotic sulfur carrier proteins that utilize similar enzymatic methods of conjugation. Knowledge of this evolutionary link came from the structural comparison of the Escherichia coli sulfur carrier protein MoaD to the yeast Ubl Urm1, a protein involved in oxidative stress response and nutrient sensing but which is apparently nonfunctional in higher eukaryotes (48). Like yeast, parasitic protozoa contain homologs of Urm1, although to date none have been characterized functionally. Urm1 may or may not be functional, but it provides evidence that parasite ubiquitin and Ubls have an origin similar to that of Ubls of other organisms. Additionally, the study of parasitic protozoa may provide information about the evolutionary origins of ubiquitin conjugation systems, since functional urmylation pathways are not known to exist in organisms other than yeast.
Unlike Urm1, ubiquitin is both highly conserved and functional in all Eukaryota, including parasitic protozoa. The best-known function of ubiquitin is the targeting of proteins modified by a chain of four or more ubiquitins to the proteasome for degradation (39). Polyubiquitin chains with two different linkages, Lys48 and Lys63, have been observed in vivo in yeast (1). Lys48 linkages are utilized in polyubiquitin that targets proteins for degradation. The function of Lys63-linked polyubiquitin is less well understood, but this chain is known to play a role in the localization of the mitosis-regulatory protein survivin to the centromere (10). Additionally, monoubiquitylation is known to regulate histones and signal internalization by membrane proteins (14).
Parasitic protozoan ubiquitin and ubiquitin modification have been most extensively studied in Trypanosoma spp. Ubiquitin genes were first identified in Trypanosoma cruzi by two independent research groups nearly two decades ago (23, 43). Unlike humans, which have two polyubiquitin and two ubiquitin fusion proteins (45), Trypanosoma cruzi has at least five genes encoding proteins comprising ubiquitin fused to unrelated proteins and at least five genes encoding polyubiquitin. Further study of the ubiquitin-proteasome pathway in Trypanosoma cruzi revealed ubiquitin-dependent degradation of cytoskeletal proteins associated with the parasite flagellum during trypomastigote-to-amastigote transformation (7) and evidence that the many ubiquitin-encoding genes of Trypanosoma cruzi are differentially regulated during the parasite life cycle and growth phases (31). The polyubiquitin gene of Plasmodium falciparum is also regulated in a life cycle-dependent manner (16), and recent analysis of Plasmodium targets by a yeast two-hybrid assay has linked ubiquitin-regulating proteins to mRNA stability and transcriptional regulation (27). These data suggest a role for ubiquitin in the regulation of the life cycles of Plasmodium and Trypanosoma spp. and possibly other parasites.
Ubls vary greatly in their degree of conservation across species. The Ubl most closely related to ubiquitin, Nedd8, is 49% identical to mammalian ubiquitin and is regulated by the DUB UCH-L3 in addition to its own specific deconjugating enzyme (11, 28). Interestingly, a Nedd8 homolog was identified only in Plasmodium spp. Nedd8 may not actually be missing from other protozoa, but the high level of sequence homology of Nedd8 to ubiquitin within a single species and the relatively low level of sequence homology of Ubls other than ubiquitin across species suggests that Nedd8 homologs in parasites may be identified as second copies of ubiquitin, thus masking them in the reciprocal best-hit analysis. The function of Nedd8 in ubiquitin conjugation and Cullin regulation suggests that it is an important Ubl and therefore requires further parasite-to-parasite comparisons and experimental study to determine if it is in fact functional in Plasmodium falciparum.
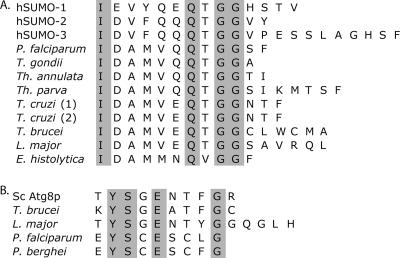
Like Nedd8 and all Ubls, SUMO is nearly identical to ubiquitin in overall structural fold but is divergent in both amino acid sequence and function (33). The primary function of SUMO is transcriptional regulation, usually in the form of repression, but other functions include the regulation of protein interaction and localization (20). SUMO homologs were identified in all of the organisms surveyed except Giardia spp. Unlike humans, which have four forms of SUMO, all of the parasite species surveyed (except Trypanosoma cruzi) have a single SUMO homolog, similar to yeast. The examination of alignments revealed that all parasite homologs have at least one amino acid after the final diglycine motif, confirming the necessity of a processing enzyme before SUMO can be conjugated (Fig. 2A).
FIG. 2.
C termini of aligned sequences of Ubls. (A) The alignment of parasite and human SUMO (hSUMO) homologs reveals that all species have one or more amino acids after the diglycine motif required for conjugation, thus indicating that processing is required before conjugation. P. falciparum, Plasmodium falciparum; T. gondii, Toxoplasma gondii; Th. annulata, Theileria annulata; Th. parva, Theileria parva; T. cruzi, Trypanosoma cruzi; T. brucei, Trypanosoma brucei; L. major, Leishmania major; E. histolytica, Entamoeba histolytica. (B) The alignment of parasite and yeast Atg8 homologs reveals that yeast and members of the kinetoplastid family, represented here by Trypanosoma brucei and Leishmania major, have residues beyond the conserved glycine but that members of the apicomplexan family, represented here by Plasmodium falciparum and Plasmodium berghei, have no additional residues, questioning the necessity of C-terminal processing before conjugation. Sc Atg8p, Saccharomyces cerevisiae Atg8p.
The functions of SUMO are not limited to transcriptional regulation; protein interactions and localization can also be affected by SUMOylation. A yeast two-hybrid assay of Plasmodium falciparum provided evidence for interaction between SUMO and serine repeat antigen 4, an essential papain fold protease localized to the parasitophorous vacuole and hypothesized to play a role in erythrocyte rupture (27) (34). These data must be confirmed in vivo, but they offer another example of the potential for the regulation of proteins unique to parasitic protozoa.
Although ubiquitin and SUMO have different functions, evidence indicates that these modifiers act as competing controls of several biological pathways. During S phase in the yeast cell cycle, both ubiquitin and SUMO can modify proliferating cell nuclear antigen (PCNA) at the same lysine residue (38). Ubiquitylation of PCNA at K164 is required for DNA damage repair, while SUMOylation of PCNA at K164 prevents recombination events in replicating regions of DNA. Although the relationship between ubiquitylation and SUMOylation is not fully understood, their competing roles in essential cell cycle process controls in yeast suggest that they also play interesting roles in the unique life cycles of parasitic protozoa.
Atg8 is a unique Ubl that is conjugated to lipids rather than proteins. Autophagy is the process by which cells engulf and degrade proteins and organelles during differentiation or as a defense under starvation conditions (29). The process of autophagy is characterized by the formation of autophagosomes, membranous structures that engulf cellular matter for degradation. The formation of the autophagosome is dependent on the conjugation of Atg8 to the amine group of phosphoethanolamine (PE). In addition, Atg12, another Ubl, must be conjugated to the ɛ-amino lysine (L128) side chain of Atg5 (44). Parasite homologs show 30 to 50% conservation compared to yeast Atg8. Homologs in kinetoplastids, the family of parasites that includes Trypanosoma and Leishmania spp., have one or more amino acid residues after the single C-terminal diglycine that need to be processed before conjugation to PE. However, apicomplexans, the family of parasites that includes Plasmodium and Toxoplasma spp., have no additional residues (Fig. 2B). Surprisingly, Atg12 is missing from all protozoa examined despite the observation of functional autophagosomes and autophagy in Leishmania major (3).
Several Ubls, such as the diubiquitins FAT10 and ISG15, are noticeably absent in parasitic protozoa. As Ubls that appear to function in response to cancer and immune stimuli, respectively, it is not surprising that these Ubls identified in multicellular organisms are not found in unicellular organisms. Although parasites have homologs of many, but not all, of the conserved Ubls, further study will be required to determine if protozoa have their own unique Ubls.
UBIQUITIN- AND Ubl-DECONJUGATING ENZYMES
The deconjugating enzymes of ubiquitin and Ubls are mainly cysteine proteases but include representatives of multiple cysteine protease clans, as well as metalloproteases. To identify parasite homologs, BLASTP homology searches were performed with representative proteases from each class of deconjugating enzymes. Representative proteases were chosen based on available crystallographic or experimental data identifying relevant catalytic residues for that enzyme. This method allowed for subsequent ClustalW alignments. Only those homologs that were identified by both BLASTP homology searches and active-site-residue alignment are included.
DUBs.
DUBs can carry out a number of processing events, including the maturation of the C termini of ubiquitin precursors, the removal of a single ubiquitin from a polyubiquitin chain, and the removal of ubiquitin from conjugated substrates. There are close to 90 DUBs in humans, and these DUBs fall into five subclasses: ubiquitin C-terminal hydrolases (UCHs), USPs, otubain proteases, Machado-Joseph disease (MJD) proteases, and JAB1/MPN/Mov34 metalloenzymes (see Nijman et al. [35)] for structural and functional comparisons). Parasitic protozoa have homologs of four out of the five major classes of DUBs as well as homologs of a predicted class, that of permuted papain fold peptidases of double-stranded RNA viruses and eukaryotes (PPPDEs) (Table 3).
TABLE 3.
Ubl-deconjugating enzymes and their putative parasite homologs based on BLASTP results and active-site-residue alignment
| Enzyme (organism, accession no.) | Parasite species with homolog | Parasite homolog gene NCBI accession no. (parasite genome identifier) | Expectation value for BLASTP vs genome | Reference(s) |
|---|---|---|---|---|
| Ubiquitin-deconjugating enzymes | ||||
| UCHs | ||||
| UCH-L3 (Homo sapiens, P15374) | Plasmodium falciparum | AAN37189 (PF14_0576) | 6e−20 | 9, 47 |
| Plasmodium yoelii | EAA21121 | 6e−15 | ||
| Toxoplasma gondii | 55.m05062 | 8e−36 | ||
| Cryptosporidium hominis | XP_668440 | 6e−18 | ||
| Cryptosporidium parvum | XP_627961 | 4e−19 | ||
| Trypanosoma cruzi | EAN94987 | 8e−38 | ||
| Trypanosoma brucei | XP_828117 | 6e−42 | ||
| Leishmania major | CAJ04230 | 4e−27 | ||
| UCH-L5 (Homo sapiens, Q9Y5K5) | Plasmodium falciparum | NP_701037 (PF11_0177) | 1e−29 | 47 |
| Plasmodium berghei | CAH95599 | 1e−30 | ||
| Plasmodium yoelii | XP_724692 | 1e−33 | ||
| Plasmodium chabaudi | XP_740948 | 6e−30 | ||
| Toxoplasma gondii | 50.m00034 | 3e−49 | ||
| Cryptosporidium hominis | XP_668440 | 3e−52 | ||
| Cryptosporidium parvum | XP_627961 | 9e−52 | ||
| Trypanosoma cruzi | EAN86456 | 1e−52 | ||
| EAN81045 | 2e−33 | |||
| Trypanosoma brucei | XP_828589 | 3e−51 | ||
| Leishmania major | CAJ04230 | 4e−50 | ||
| Entamoeba histolytica | XP_654194 | 2e−42 | ||
| USPs | ||||
| USP7 (Mus musculus, AAI0067) | Plasmodium falciparum | NP_704193 (MAL7P1.147) | 5e−52 | 47 |
| Plasmodium yoelii | XP_729206 | 2e−52 | ||
| Toxoplasma gondii | 80.m00082 | 7e−61 | ||
| Theileria annulata | CAI75715 | 6e−58 | ||
| Cryptosporidium hominis | XP_666360 | 4e−61 | ||
| Cryptosporidium parvum | XP_627060 | 4e−61 | ||
| Trypanosoma cruzi | EAN91491 | 2e−60 | ||
| EAN98443 | 1e−51 | |||
| EAN95845 | 1e−32 | |||
| Trypanosoma brucei | EAN77302 | 4e−66 | ||
| EAN76617 | 5e−51 | |||
| Leishmania major | AAZ14396 | 1e−78 | ||
| CAJ03358 | 4e−35 | |||
| CAJ08130 | 3e−25 | |||
| Entamoeba histolytica | EAL48197 | 3e−25 | ||
| Other USPs | Plasmodium falciparum | PFA0220w | 47 | |
| PFD0165w | ||||
| PFD0608c | ||||
| PFE1355c | ||||
| PFE0835w | ||||
| PFI0225w | ||||
| PF13_0096 | ||||
| PF14_0145 | ||||
| MJD Ataxin-3(Mus musculus, NP_08391) | Plasmodium falciparum | NP_701621 (PFL1295w) | 4e−16 | 42 |
| Plasmodium berghei | XP_670958 | 6e−15 | ||
| Plasmodium yoelii | EAA19332 | 1e−14 | ||
| Toxoplasma gondii | 44.m02555 | 2e−35 | ||
| Cryptosporidium hominis | XP_667276 | 3e−20 | ||
| Cryptosporidium parvum | XP_627894 | 3e−20 | ||
| Otubain proteases | ||||
| A20 | None | |||
| VCIP135 | None | |||
| JAB1/MPN/Mov34 metalloenzyme POH1 (Homo sapiens, NP_005796) | Plasmodium falciparum | NP_705563 (MAL13P1.343) | 5e−105 | |
| Plasmodium berghei | XP_676818 | 6e−103 | ||
| Plasmodium yoelii | EAA22608 | 1e−103 | ||
| Toxoplasma gondii | 59.m00030 | 2e−112 | ||
| Theileria parva | EAN32483 | 5e−108 | ||
| Theileria annulata | CAI74788 | 5e−108 | ||
| Cryptosporidium parvum | CAD98369 | 3e−104 | ||
| Cryptosporidium hominis | XP_667262 | 3e−103 | ||
| Trypanosoma brucei | AAL72634 | 9e−78 | ||
| Trypanosoma cruzi | EAN85253 | 8e−76 | ||
| EAN93016 | 5e−70 | |||
| Leishmania major | CAJ07770 | 1e−77 | ||
| Entamoeba histolytica | XP_650487 | 1e−93 | ||
| Giardia intestinalis | CAB97491 | 2e−48 | ||
| Giardia lamblia | XP_778570 | 2e−48 | ||
| PPPDEa (Cryptosporidium parvum, XP_627971) | Plasmodium falciparum | NP_701537 (PFL0865w) | 8e−27 | 19 |
| Plasmodium berghei | XP_679861 | 8e−27 | ||
| Plasmodium yoelii | XP_725065 | 6e−19 | 19 | |
| Plasmodium chabaudi | XP_741893 | 2e−27 | ||
| Cryptosporidium hominis | XP_668431 | 0 | ||
| Toxoplasma gondii | 50.m03185 | 1e−43 | ||
| Trypanosoma cruzi | EAN94109 | 1e−7 | ||
| EAN87232 | 1e−7 | |||
| Trypanosoma brucei | EAN80399 | 5e−5 | ||
| Leishmania major | CAJ08653 | 8e−8 | ||
| Entamoeba histolytica | EAL51330 | 2e−4 | ||
| Giardia lamblia | XP_768551 | 1e−24 | 19 | |
| Nedd8-specific deconjugating enzyme NEDP1 (Homo sapiens, Q96LD8) | None | |||
| SUMO-deconjugating enzymes | ||||
| Ubiquitin-like proteases | ||||
| Ulp1 (Saccharomyces cerevisiae, Q02724) | Plasmodium falciparum | NP_701689 (PFL1635w) | 1e−23 | 47 |
| NP_704529 (MAL8P1.157) | 1e−5 | |||
| Plasmodium berghei | XP_671926 | 2e−18 | ||
| XP_677733 | 3e−5 | |||
| Plasmodium yoelii | EAA21830 | 5e−22 | ||
| EAA23028 | 9e−5 | |||
| Plasmodium chabaudi | XP_736612 | 1e−19 | ||
| XP_743639 | 4e−5 | |||
| XP_741227 | 4e−5 | |||
| Theileria parva | EAN31525 | 4e−18 | ||
| EAN32232 | 2e−6 | |||
| Theileria annulata | CAI76227 | 2e−12 | ||
| CAI76877 | 1e−7 | |||
| Toxoplasma gondii | 33.m01285 | 9e−23 | ||
| 57.m01727 | 5e−15 | |||
| Trypanosoma cruzi | EAN82253 | 6e−11 | ||
| EAN90516 | 8e−11 | |||
| Trypanosoma brucei | EAN76330 | 3e−10 | ||
| Entamoeba histolytica | XP_657158 | 9e−16 | ||
| Cryptosporidium parvum | XP_626217 | 3e−27 | ||
| Cryptosporidium hominis | XP_665558 | 1e−15 | ||
| Ulp2 (Schizosaccharomyces pombe, O13769) | Plasmodium falciparum | MAL8P1.157 | n/a | 47 |
| Wss1p metalloprotease (Saccharomyces cerevisiae, NP_012002) | Plasmodium falciparum | NP_700566 (PF10_0092) | 4e−12 | 19 |
| Plasmodium berghei | XP_676977 | 2e−8 | ||
| Trypanosoma brucei | EAN80397 | 5e−5 | 19 | |
| Trypanosoma cruzi | EAN87230 | 3e−12 | ||
| EAN80397 | 5e−12 | |||
| Leishmania major | CAJ08651 | 9e−12 | ||
| Autophagy-related deconjugating enzyme | Plasmodium falciparum | NP_702059 (PF14_0171) | 4e−4 | |
| Atg4 (Saccharomyces cerevisiae, P53867) | Plasmodium yoelii | EAA22584 | 1e−5 | |
| Cryptosporidium parvum | XP_626849 | 3e−15 | ||
| Theileria annulata | CAI74479 | 2e−8 | ||
| Trypanosoma cruzi | EAN87801 (Tc00.1047053509443.30) | 7e−25 | 13 | |
| EAN84153 | ||||
| EAN91243 | 7e−9 | |||
| 2e−8 | ||||
| Trypanosoma brucei | EAN80574 (Tb11.01.7979) | 5e−12 | 13 | |
| AAX79730 (Tb06.28P18.550) | 5e−7 | |||
| Leishmania major | CAJ08920 (Lmj32.3890) | 3e−17 | 13, 40 | |
| CAJ05824 (Lmj30.0270) | 2e−11 | |||
| Entamoeba histolytica | XP_656724 | 7e−10 | ||
| XP_653798 | 6e−9 | |||
| XP_652043 | 2e−5 |
Predicted protease family.
The first proteolytically active DUBs to be found in protozoan parasites were recently identified in Plasmodium falciparum and Toxoplasma gondii. Using an activity-based probe, which contains full-length human ubiquitin and had the C-terminal glycine residue replaced with a reactive functional group that irreversibly binds the active-site cysteine of deconjuating enzymes, Artavanis-Tsakonas et al. (2) and Frickel et al. (9) selectively labeled and identified UCH-54 (corresponding to accession no. PF11_0177) in Plasmodium falciparum and UCH-L3 (corresponding to accession no. 55.m050682) in Toxoplasma gondii. These DUBs also showed cross-reactivity with a similar probe for human Nedd8, suggesting that the same deconjugating enzyme may regulate both ubiquitin and Nedd8 homologs. This possibility may explain why Plasmodium falciparum has a Nedd8 homolog but no Nedd8-specific protease homolog (Tables 2 and 3). Since the human Nedd8 used to make the probe is more closely related to human and Plasmodium falciparum ubiquitin (58% conserved) than to Plasmodium falciparum Nedd8 (52.6% conserved), parasite-derived Nedd8 probes will be required to confirm cross-reactivity.
Recent genetic analysis of variants of the rodent malaria parasite Plasmodium chabaudi resistant to the antimalarial drugs artesunate and chloroquine identified mutations in a ubiquitin-deconjugating enzyme with strong genetic linkage to drug resistance (18). This DUB was found to be most similar to the Plasmodium falciparum MAL7P1.147 DUB described as a USP7 homolog in Table 3. Although subsequent analysis of drug-resistant Plasmodium falciparum did not identify mutations in the MAL7P1.147 enzyme, the authors speculate that this result was due to the transient nature of the Plasmodium falciparum resistance compared to the stable resistance found in Plasmodium chabaudi. Further work to characterize this DUB in stably artemisinin-resistant Plasmodium falciparum is necessary to determine what, if any, role this enzyme may play in parasite drug resistance.
Interestingly, Plasmodium, Toxoplasma, and Cryptosporidium spp. all have homologs of the MJD subclass protease Ataxin-3, a ubiquitin-deconjugating enzyme that has been linked to neurodegenerative disease in mammals (5). The parasite homologs are 19.9 to 29.4% conserved compared to human Ataxin-3, but the catalytic triad consists of conserved cysteine, histidine, and aspartate rather than asparagine. This aspartate-for-asparagine substitution has been observed in previous Ataxin-3 homolog sequence alignments (42). Both aspartate and asparagines are found in the catalytic triads of cysteine proteases, but the functional significance of these residue substitutions in parasite homologs remains to be explored. Surprisingly, no homolog of Ataxin-3 in yeast has been identified. The parasite homologs identified do not appear to have an expanded glutamine repeat region, the hallmark of the disease-causing form of Ataxin-3. Parasitic protozoa offer a potential model system in which to study the normal function of Ataxin-3, which is still not well understood.
The parasite homologs of Ataxin-3 do not have a glutamine-rich region, but the recently identified DUB Plasmodium falciparum UCH-54 has an asparagine repeat region in the predicted protein sequence. The predominant protein identified by mass spectrometry was nearly double the predicted size (100 kDa compared to the predicted 54 kDa), possibly as a result of protein aggregation that was stable under sodium dodecyl sulfate-polyacrylamide gel electrophoresis conditions (2). Our own alignments revealed asparagine repeats in the Plasmodium falciparum USP7 and Ulp1 homologs and unusual glutamine-glutamate (QEEQ) and glutamine-glutamate-lysine (QEKK) repeats in the Ulp1 homolog in regions not homologous to any other protozoan sequences aligned (E.L. Ponder, unpublished data). In agreement with this assessment, asparagine- and glutamine-asparagine-rich regions of yeast prion-forming proteins are sufficient to form self-seeding protein aggregates similar to those that cause Alzheimer's and Huntington's diseases (37). Further study of both parasites and other eukaryotes is required to determine the significance of these repeat regions and their potential role in protein aggregation.
An additional subclass of predicted DUBs included in this survey was the PPPDEs. Using a bioinformatics approach, Iyer et al. (19) identified the PPPDE class of DUBs, whose prototype is a hypothetical protein from the apicomplexan Cryptosporidium parvum. Although this study did not provide confirmation of DUB activity for any members of this class, bioinformatics approaches did identify the majority of accepted classes of DUBs (35). As exemplified by the identification of PPPDEs, the study of ubiquitin and Ubls in protozoa has the potential to identify new players in these pathways as well as novel functions.
Ubl proteases.
Like ubiquitin-deconjugating enzymes, SUMO-deconjugating enzymes cleave precursor SUMO to the active form containing the required C-terminal diglycine motif and cleave SUMO from substrates (20). Although SUMO and SUMOylation pathways have not been characterized in any parasite, the conservation of SUMO across yeast and mammals suggests that SUMO is a candidate for the regulation of transcription in parasite development. Of the parasites surveyed in this study, the majority have only one homolog of the essential yeast de-SUMOylation enzyme Ulp1 (19.9 to 30.4% conserved) (Table 3). Plasmodium spp. and Theileria spp. parasites, however, have two homologs. Although Plasmodium was previously predicted to have homologs of both yeast Ulp1 and Ulp2 (a nonessential second homolog of Ulp1) (47), we found that both of these homologs aligned better with Ulp1 in our own searches for the alignment of active-site residues (Ponder, unpublished). Further genomic and functional characterization is needed to understand the evolutionary origins of the corresponding genes and their functions in parasites.
Autophagy-related proteases.
Autophagy is the only proven example of a classic Ubl pathway with a novel function and importance in protozoa. Autophagy-related protein 4 (Atg4) is a papain fold cysteine protease that processes Atg8 to expose a C-terminal glycine for conjugation and cleaves Atg8 from its conjugated PE on the outer layer of the autophagosome (24, 25). Homologs of Atg4 in both apicomplexans and kinetoplastids (16.5 to 21.8% conserved) have been identified (Table 3). The apicomplexans have one homolog, while the kinetoplastids and Entamoeba histolytica have multiple copies. The disruption of Atg4 leads to defects in autophagosome trafficking in Leishmania major (3). Additionally, parasites expressing a mutant ATPase that results in the accumulation of autophagosomes and increased susceptibility to starvation (i.e., an autophagy defect) are unable to transition from the promastigote to the infective metacyclic stage (3). Atg8 and Atg4 are highly conserved across protozoa, while all parasitic protozoa lack Atg12, a finding that is in agreement with the results of previous bioinformatics searches for Atg12 in kinetoplastids (13). Autophagy is functional in Leishmania major even without an Atg12 homolog (3), confounding the hypothesis that the conjugation of both Atg8 and Atg12 is necessary for autophagy in response to starvation. More extensive experimental evaluation of the autophagy pathway in protozoa is necessary to understand this discrepancy.
Autophagy may also be linked to the effects of chloroquine on mammalian cells and intraerythrocytic Plasmodium falciparum. In the early 1980s, it was reported that chloroquine induces the formation of autophagic vacuoles in treated lymphocytes (21) and the accumulation of endocytic vesicles in treated Plasmodium falciparum parasites (49). Although significant efforts have been made to understand chloroquine-mediated killing, its mechanism of action still remains unclear (see Olliaro and Goldberg [36] for a review of chloroquine-mediated killing). The vacuolarization of Plasmodium falciparum upon treatment with antimalarial agents has also been postulated to be an early sign of apoptotic blebbing. However, the potential role of apoptosis in a unicellular organism remains the subject of debate (see Deponte and Becker [8] for a review of apoptosis in protozoa). Further characterization of these vacuoles using a marker for the autophagosome, such as Atg8, may help clarify the mode of killing by antimalarial drugs. Analysis of autophagy in other protozoa will likely provide additional information to explain how parasites use autophagy in their normal development as well as to combat drug-induced starvation.
CONCLUSIONS AND FUTURE DIRECTIONS
Medically important parasitic protozoa have homologs of many key Ubls, ranging from Urm1, a minimally understood relative of a bacterial sulfur carrier protein, to ubiquitin, one of the most well-conserved proteins in all Eukaryota. Although these Ubls may have similar functions in both parasites and other eukaryotes, evidence of life cycle-dependent ubiquitin gene regulation in Trypanosoma cruzi and Plasmodium falciparum, the potential interaction of SUMO and serine repeat antigen 4 in Plasmodium falciparum, and the identification of a genetic linkage between a DUB and artesunate resistance in Plasmodium chabaudi demonstrate the need to identify unique parasite targets of these and other Ubls. Evidence of the essential roles of the ubiquitin-proteasome and autophagy pathways in the development of Trypanosoma cruzi and Leishmania major, respectively, also suggest that further study of Ubl pathways will lead to a better understanding of parasite life cycle regulation.
Proteases have generally been identified as potential drug targets in parasites including Plasmodium falciparum and Trypansoma cruzi (41). Therefore, further characterization of DUBs may help validate proteases as a new class of drug targets while also providing insight into the regulation of basic parasite biology. This characterization may be further facilitated as more inhibitors of these classes of enzymes are identified (12, 15, 30). As additional deconjugating enzymes are further characterized functionally, we hope our compilation of homologs will allow easier extrapolation of findings to other medically relevant parasites.
Acknowledgments
This work was funded by a Burroughs Wellcome Trust Pathogenesis of Infectious Disease award (to M.B.). E.L.P. was funded by the National Science Foundation Graduate Research Fellowship Program.
Footnotes
Published ahead of print on 28 September 2007.
REFERENCES
- 1.Arnason, T., and M. J. Ellison. 1994. Stress resistance in Saccharomyces cerevisiae is strongly correlated with assembly of a novel type of multiubiquitin chain. Mol. Cell. Biol. 14:7876-7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artavanis-Tsakonas, K., S. Misaghi, C. A. Comeaux, A. Catic, E. Spooner, M. T. Duraisingh, and H. L. Ploegh. 2006. Identification by functional proteomics of a deubiquitinating/deNeddylating enzyme in Plasmodium falciparum. Mol. Microbiol. 61:1187-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besteiro, S., R. A. Williams, L. S. Morrison, G. H. Coombs, and J. C. Mottram. 2006. Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. J. Biol. Chem. 281:11384-11396. [DOI] [PubMed] [Google Scholar]
- 4.Bozdech, Z., M. Llinas, B. L. Pulliam, E. D. Wong, J. Zhu, and J. L. DeRisi. 2003. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 1:E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett, B. G., and R. N. Pittman. 2005. The polyglutamine neurodegenerative protein ataxin 3 regulates aggresome formation. Proc. Natl. Acad. Sci. USA 102:4330-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleary, M. D., U. Singh, I. J. Blader, J. L. Brewer, and J. C. Boothroyd. 2002. Toxoplasma gondii asexual development: identification of developmentally regulated genes and distinct patterns of gene expression. Eukaryot. Cell 1:329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Diego, J. L., J. M. Katz, P. Marshall, B. Gutierrez, J. E. Manning, V. Nussenzweig, and J. Gonzalez. 2001. The ubiquitin-proteasome pathway plays an essential role in proteolysis during Trypanosoma cruzi remodeling. Biochemistry 40:1053-1062. [DOI] [PubMed] [Google Scholar]
- 8.Deponte, M., and K. Becker. 2004. Plasmodium falciparum: do killers commit suicide? Trends Parasitol. 20:165-169. [DOI] [PubMed] [Google Scholar]
- 9.Frickel, E. M., V. Quesada, L. Muething, M. J. Gubbels, E. Spooner, H. Ploegh, and K. Artavanis-Tsakonas. 2007. Apicomplexan UCHL3 retains dual specificity for ubiquitin and Nedd8 throughout evolution. Cell. Microbiol. 9:1601-1610. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez, G. J., and Z. Ronai. 2006. Ubiquitin and SUMO systems in the regulation of mitotic checkpoints. Trends Biochem. Sci. 31:324-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemelaar, J., A. Borodovsky, B. M. Kessler, D. Reverter, J. Cook, N. Kolli, T. Gan-Erdene, K. D. Wilkinson, G. Gill, C. D. Lima, H. L. Ploegh, and H. Ovaa. 2004. Specific and covalent targeting of conjugating and deconjugating enzymes of ubiquitin-like proteins. Mol. Cell. Biol. 24:84-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemelaar, J., P. J. Galardy, A. Borodovsky, B. M. Kessler, H. L. Ploegh, and H. Ovaa. 2004. Chemistry-based functional proteomics: mechanism-based activity-profiling tools for ubiquitin and ubiquitin-like specific proteases. J. Proteome Res. 3:268-276. [DOI] [PubMed] [Google Scholar]
- 13.Herman, M., S. Gillies, P. A. Michels, and D. J. Rigden. 2006. Autophagy and related processes in trypanosomatids: insights from genomic and bioinformatic analyses. Autophagy 2:107-118. [DOI] [PubMed] [Google Scholar]
- 14.Hicke, L. 2001. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2:195-201. [DOI] [PubMed] [Google Scholar]
- 15.Hirayama, K., S. Aoki, K. Nishikawa, T. Matsumoto, and K. Wada. 2007. Identification of novel chemical inhibitors for ubiquitin C-terminal hydrolase-L3 by virtual screening. Bioorg. Med. Chem. 15:6810-6818. [DOI] [PubMed] [Google Scholar]
- 16.Horrocks, P., and C. I. Newbold. 2000. Intraerythrocytic polyubiquitin expression in Plasmodium falciparum is subjected to developmental and heat-shock control. Mol. Biochem. Parasitol. 105:115-125. [DOI] [PubMed] [Google Scholar]
- 17.Huang, T. T., S. M. Nijman, K. D. Mirchandani, P. J. Galardy, M. A. Cohn, W. Haas, S. P. Gygi, H. L. Ploegh, R. Bernards, and A. D. D'Andrea. 2006. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat. Cell Biol. 8:339-347. [DOI] [PubMed] [Google Scholar]
- 18.Hunt, P., A. Afonso, A. Creasey, R. Culleton, A. B. Sidhu, J. Logan, S. G. Valderramos, I. McNae, S. Cheesman, V. D. Rosario, R. Carter, D. A. Fidock, and P. Cravo. 2007. Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Mol. Microbiol. 65:27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer, L. M., E. V. Koonin, and L. Aravind. 2004. Novel predicted peptidases with a potential role in the ubiquitin signaling pathway. Cell Cycle 3:1440-1450. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, E. S. 2004. Protein modification by SUMO. Annu. Rev. Biochem. 73:355-382. [DOI] [PubMed] [Google Scholar]
- 21.Jones, C. J., and M. I. Jayson. 1984. Chloroquine: its effect on leucocyte auto- and heterophagocytosis. Ann. Rheum. Dis. 43:205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerscher, O., R. Felberbaum, and M. Hochstrasser. 2006. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. [DOI] [PubMed]
- 23.Kirchhoff, L. V., K. S. Kim, D. M. Engman, and J. E. Donelson. 1988. Ubiquitin genes in trypanosomatidae. J. Biol. Chem. 263:12698-12704. [PubMed] [Google Scholar]
- 24.Kirisako, T., Y. Ichimura, H. Okada, Y. Kabeya, N. Mizushima, T. Yoshimori, M. Ohsumi, T. Takao, T. Noda, and Y. Ohsumi. 2000. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J. Cell Biol. 151:263-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klionsky, D. J. 2004. Cell biology: regulated self-cannibalism. Nature 431:31-32. [DOI] [PubMed] [Google Scholar]
- 26.Krebber, H., C. Wostmann, and T. Bakker-Grunwald. 1994. Evidence for the existence of a single ubiquitin gene in Giardia lamblia. FEBS Lett. 343:234-236. [DOI] [PubMed] [Google Scholar]
- 27.LaCount, D. J., M. Vignali, R. Chettier, A. Phansalkar, R. Bell, J. R. Hesselberth, L. W. Schoenfeld, I. Ota, S. Sahasrabudhe, C. Kurschner, S. Fields, and R. E. Hughes. 2005. A protein interaction network of the malaria parasite Plasmodium falciparum. Nature 438:103-107. [DOI] [PubMed] [Google Scholar]
- 28.Larsen, C. N., and H. Wang. 2002. The ubiquitin superfamily: members, features, and phylogenies. J. Proteome Res. 1:411-419. [DOI] [PubMed] [Google Scholar]
- 29.Levine, B., and D. J. Klionsky. 2004. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell 6:463-477. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Y., H. A. Lashuel, S. Choi, X. Xing, A. Case, J. Ni, L. A. Yeh, G. D. Cuny, R. L. Stein, and P. T. Lansbury, Jr. 2003. Discovery of inhibitors that elucidate the role of UCH-L1 activity in the H1299 lung cancer cell line. Chem. Biol. 10:837-846. [DOI] [PubMed] [Google Scholar]
- 31.Manning-Cela, R., S. Jaishankar, and J. Swindle. 2006. Life-cycle and growth-phase-dependent regulation of the ubiquitin genes of Trypanosoma cruzi. Arch. Med. Res. 37:593-601. [DOI] [PubMed] [Google Scholar]
- 32.McNicoll, F., J. Drummelsmith, M. Muller, E. Madore, N. Boilard, M. Ouellette, and B. Papadopoulou. 2006. A combined proteomic and transcriptomic approach to the study of stage differentiation in Leishmania infantum. Proteomics 6:3567-3581. [DOI] [PubMed] [Google Scholar]
- 33.Melchior, F. 2000. SUMO: nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 16:591-626. [DOI] [PubMed] [Google Scholar]
- 34.Miller, S. K., R. T. Good, D. R. Drew, M. Delorenzi, P. R. Sanders, A. N. Hodder, T. P. Speed, A. F. Cowman, T. F. de Koning-Ward, and B. S. Crabb. 2002. A subset of Plasmodium falciparum SERA genes are expressed and appear to play an important role in the erythrocytic cycle. J. Biol. Chem. 277:47524-47532. [DOI] [PubMed] [Google Scholar]
- 35.Nijman, S. M., M. P. Luna-Vargas, A. Velds, T. R. Brummelkamp, A. M. Dirac, T. K. Sixma, and R. Bernards. 2005. A genomic and functional inventory of deubiquitinating enzymes. Cell 123:773-786. [DOI] [PubMed] [Google Scholar]
- 36.Olliaro, P. L., and D. E. Goldberg. 1995. The plasmodium digestive vacuole: metabolic headquarters and choice drug target. Parasitol. Today 11:294-297. [DOI] [PubMed] [Google Scholar]
- 37.Osherovich, L. Z., B. S. Cox, M. F. Tuite, and J. S. Weissman. 2004. Dissection and design of yeast prions. PLoS Biol. 2:E86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfander, B., G. L. Moldovan, M. Sacher, C. Hoege, and S. Jentsch. 2005. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature 436:428-433. [DOI] [PubMed] [Google Scholar]
- 39.Pickart, C. M., and D. Fushman. 2004. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 8:610-616. [DOI] [PubMed] [Google Scholar]
- 40.Rigden, D. J., M. Herman, S. Gillies, and P. A. Michels. 2005. Implications of a genomic search for autophagy-related genes in trypanosomatids. Biochem. Soc. Trans. 33:972-974. [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal, P. J. 1999. Proteases of protozoan parasites. Adv. Parasitol. 43:105-159. [DOI] [PubMed] [Google Scholar]
- 42.Scheel, H., S. Tomiuk, and K. Hofmann. 2003. Elucidation of ataxin-3 and ataxin-7 function by integrative bioinformatics. Hum. Mol. Genet. 12:2845-2852. [DOI] [PubMed] [Google Scholar]
- 43.Swindle, J., J. Ajioka, H. Eisen, B. Sanwal, C. Jacquemot, Z. Browder, and G. Buck. 1988. The genomic organization and transcription of the ubiquitin genes of Trypanosoma cruzi. EMBO J. 7:1121-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson, A. R., J. H. Doelling, A. Suttangkakul, and R. D. Vierstra. 2005. Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 138:2097-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webb, G. C., R. T. Baker, M. Coggan, and P. G. Board. 1994. Localization of the human UBA52 ubiquitin fusion gene to chromosome band 19p13.1-p12. Genomics 19:567-569. [DOI] [PubMed] [Google Scholar]
- 46.Wostmann, C., D. Liakopoulos, A. Ciechanover, and T. Bakker-Grunwald. 1996. Characterization of ubiquitin genes and -transcripts and demonstration of a ubiquitin-conjugating system in Entamoeba histolytica. Mol. Biochem. Parasitol. 82:81-90. [DOI] [PubMed] [Google Scholar]
- 47.Wu, Y., X. Wang, X. Liu, and Y. Wang. 2003. Data-mining approaches reveal hidden families of proteases in the genome of malaria parasite. Genome Res. 13:601-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu, J., J. Zhang, L. Wang, J. Zhou, H. Huang, J. Wu, Y. Zhong, and Y. Shi. 2006. Solution structure of Urm1 and its implications for the origin of protein modifiers. Proc. Natl. Acad. Sci. USA 103:11625-11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yayon, A., and H. Ginsburg. 1983. Chloroquine inhibits the degradation of endocytic vesicles in human malaria parasites. Cell Biol. Int. Rep. 7:895. [DOI] [PubMed] [Google Scholar]