One of the major advances serving to define the beginning of the era of modern medicine was the development of penicillin in the early 1940s as the first widely used antibiotic effective against microorganisms (reviewed in reference 111). In what has become an all-too-common trend, antibiotic-resistant isolates emerged soon after the large-scale use of penicillin to treat bacterial infections (7). This cycle has been repeated many times over the years as new antibiotics are generated (reviewed in reference 66). This has led to obvious concern about the continuing efficacy of antibiotics to control infectious disease (1).
Resistance to antibiotics in pathogenic fungi is a problem of special importance in the control of infections caused by these organisms. The same extraordinary conservation of the basic eukaryotic cellular biology exhibited by fungal and animal cells that has allowed these smaller eukaryotes to serve as outstanding model organism limits the range of fungus-specific antibiotics that have been described. In addition, mutant fungi are readily isolated, both in the laboratory and in the clinic, that demonstrate resistance to a wide range of antibiotics beyond that initially used for treatment. This broad-spectrum drug tolerance is referred to as multidrug resistance and occurs in organisms ranging from bacteria to humans (67). The limited number of antifungal drugs makes this phenotype an acute problem in the chemotherapeutic eradication of fungal infections.
Much of our understanding of multidrug resistance in fungi comes from studies in the generally nonpathogenic yeast Saccharomyces cerevisiae, in which the multidrug-resistant phenotype is referred to as pleiotropic drug resistance or Pdr (see reference 5 for a historical review). Genes influencing this phenotype are typically designated PDR loci. With the development of powerful new genetic and molecular biological techniques, workers have provided important new insights into the physiology of multidrug resistance from experiments performed directly in pathogenic organisms. This review focuses on providing an introduction to the various pathways influencing multidrug resistance in S. cerevisiae and compares these pathways to similar ones from pathogenic fungi such as Candida albicans, Candida glabrata, and Aspergillus fumigatus.
PLEIOTROPIC DRUG RESISTANCE IN SACCHAROMYCES CEREVISIAE
The facile genetics of S. cerevisiae led to the identification of an allelic series of pleiotropic drug-resistant mutations mapping to a gene present on chromosome VII that define the PDR1 gene (94). Pdr1p is a zinc cluster-containing positive transcriptional regulator related to the well-known Gal4p transcription factor (4). Although Pdr1p was the first multidrug resistance determinant identified in S. cerevisiae, this factor is not a direct mediator of drug resistance. Pdr1p and other transcriptional regulators modulate expression of a variety of proteins that act to prevent the toxic action of drugs. We first consider here the proteins that are direct detoxifiers of antifungal agents, then examine the transcription factors regulating these factors, and finally discuss the signals that regulate expression of the PDR genes. We refer here to the set of genes defined by their regulation by Pdr1p and its homologue Pdr3p collectively as the Pdr pathway (Fig. 1).
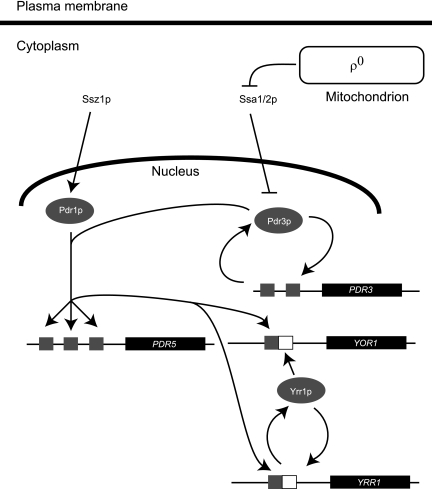
FIG. 1.
Multidrug resistance pathway in Saccharomyces cerevisiae. A general scheme describing key genetic interactions regulating pleiotropic drug resistance is shown. Standard arrows indicate positive interactions, while the blunt arrows denote negative regulation. The small gray boxes represent Pdr1p/Pdr3p response elements (PDREs), and the white boxes indicate Yrr1p response elements.
MEMBRANE TRANSPORTERS AND OTHER DIRECT EFFECTORS OF PLEIOTROPIC DRUG RESISTANCE
The first identified gene that fulfilled the criteria for a direct effector of drug resistance in the Pdr pathway was the PDR5 locus (6, 9, 50). This gene encodes an ATP-binding cassette transporter protein that is a member of the ABCG class of transporters (21). Early experiments indicated that the loss of Pdr5p led to a dramatic increase in drug sensitivity to a wide range of different compounds (65, 73). In addition, direct biochemical assays demonstrated that pdr5Δ cells were defective in the efflux of various dyes and radiolabeled probes (58). Overproduction of Pdr5p by use of high-copy-number plasmids carrying the gene or the presence of hyperactive PDR1 alleles correlated with a robust Pdr phenotype (29, 51, 64). Together, these data strongly support the model that elevated Pdr5p levels contribute to the pleiotropic drug-resistant phenotype by increasing the activity of this multispecific drug pump.
Comparison of cells carrying hyperactive PDR1 alleles and containing or lacking PDR5 argued that, although Pdr5p is an important determinant in the Pdr phenotype, the presence of this gene is not sufficient to explain the entire spectrum of drug resistance seen. For example, hyperactive PDR1-6 mutants are resistant to both cycloheximide and oligomycin. Removal of the PDR5 gene eliminates the increased cycloheximide tolerance in a PDR1-6 cell but does not reduce the high-level oligomycin resistance (51, 73). Screening a high-copy-number plasmid library for genes that influence oligomycin resistance allowed the recovery of the YOR1 gene (19, 52). YOR1 encodes an ABC transporter of the ABCC family that is required for normal oligomycin resistance. Loss of YOR1 from a PDR1-6 background reduces oligomycin resistance but has no effect on the elevated cycloheximide tolerance conferred by this Pdr1p derivative (52). Similarly, removal of the PDR5 homologue SNQ2 gene from cells carrying a hyperactive allele of PDR1 reduced 4-nitroquinoline-N-oxide resistance but did not influence cycloheximide or oligomycin resistance (23).
These data serve to illustrate a major theme in the Pdr phenotype in S. cerevisiae, as well as fungal multidrug resistance in general. Overproduction of multiple ABC transporter proteins is required for the expression of the full range of drug resistance seen in these multidrug-tolerant cells. This typically occurs due to a change in the activity of a transcription factor and shares striking similarity with the multidrug resistance seen in mammalian cells. Isolation of the human MDR1 gene was accomplished by use of cell lines that massively overproduced this multidrug resistance ABC transporter protein (97, 101). Most multidrug-resistant mammalian cells appear to emerge via amplification of the gene encoding a particular ABC transporter gene (see references 41 and 100) for reviews) rather than producing an altered transcription factor but, irrespective of the exact mechanism, both types of cells can become multidrug resistant through the elevation of ABC transporter expression.
Along with the ABC transporter proteins, proteins of the major facilitator superfamily (MFS) also contribute to pleiotropic drug resistance. Compared to the ABC transporter-encoding genes, our understanding of the function and regulation of the MFS proteins is at an earlier stage. At least 20 different MFS proteins exhibit structural characteristics consistent with or have already been demonstrated to play a role in drug resistance (reviewed in references 79 and 102). The large number of these membrane transporters suggests that their contributions to drug tolerance are likely underappreciated. The MFS proteins often share overlapping drug specificity with ABC transporters, which may explain in part why MFS transporter involvement in multidrug resistance has been obscured by their better-publicized relatives. For example, the MFS protein Flr1p contributes to resistance to the antifungal agent fluconazole, as well as cycloheximide (2). The ABC transporter Pdr5p also mediates tolerance to both of these compounds and is clearly a major determinant in the resistance phenotype to these and other drugs (59). Interestingly, while a pdr5Δ strain is exquisitely sensitive to cycloheximide (65), this sensitivity can be fully suppressed if the transcription factor Yap1p is overproduced (29). This suppression is likely due to the activation of FLR1, a Yap1p target gene (2). Much remains to be learned of the participation of MFS proteins in eukaryotic multidrug resistance.
SPHINGOLIPID HOMEOSTASIS IS COREGULATED WITH PDR GENES
The lipid composition of the plasma membrane is the central determinant regulating passage of compounds from the external environment to the interior of the cell. The distribution of lipid components in the inner and outer leaflets of the plasma membrane is asymmetric and controlled in part by the Pdr pathway. Both Pdr5p and Yor1p have been found to enhance the outward movement (flop) of the phospholipid phosphatidylethanolamine (22, 90). Phosphatidylethanolamine is normally maintained at low levels in the outer leaflet by the rapid inward movement (flip) carried out by aminophospholipid translocases, five of which can be found in the S. cerevisiae genome (reviewed in reference 89). Intriguingly, two phosphatidylinositol transfer protein homologues (PDR16 and PDR17) were also found to be target genes of the Pdr pathway and to influence phospholipid levels and drug resistance (115). These observations predict that activation of the Pdr pathway may trigger changes in phospholipid composition of the plasma membrane, but the consequences of these changes remain uncertain.
Eukaryotic membrane lipids include sterols and sphingolipids in addition to phospholipids. In S. cerevisiae, ergosterol is the major sterol in the cell and is produced via the action of ERG pathway (see reference 112 for a review). A well-known phenotype of many erg-null mutants is extreme sensitivity to drug challenges, which is consistent with a requirement for ergosterol in normal membrane function. Analyses of erg mutant strains indicate that Pdr5p transport activity is unaffected in these mutants and suggest that loss of ergosterol may enhance passive diffusion of compounds across the altered membrane (33, 53).
The final class of membrane lipid, sphingolipids, is thought to associate with ergosterol to form microdomains called lipid rafts that are concentrated in the outer leaflet of the plasma membrane (reviewed in references 30 and 46). Analysis of the expression of the IPT1 gene, encoding the last step in sphingolipid biosynthesis (31), established a direct connection between the Pdr pathway and biosynthesis of this class of membrane lipid. IPT1 transcription is controlled by Pdr1p and/or Pdr3p and responds to signals known to induce the S. cerevisiae Pdr pathway (see below). Interestingly, loss of IPT1 altered drug resistance of the resulting mutants and suggests that normal sphingolipid content is required for wild-type levels of drug tolerance (43). Later experiments provided evidence that several biosynthetic steps upstream of the Ipt1p-catalyzed reaction were also responsive to increases in Pdr pathway-mediated regulation (57). The known interactions are summarized in Fig. 2.
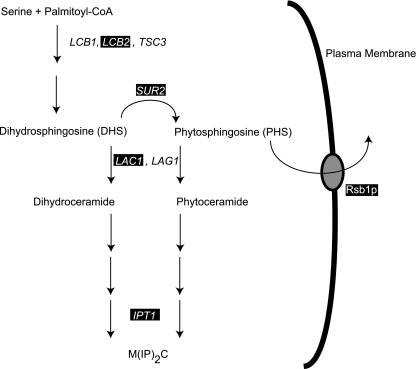
FIG. 2.
Interactions between the Pdr pathway and sphingolipid biosynthesis. An outline of the biosynthetic pathway producing sphingolipids is depicted. Arrows indicate enzymatic reactions. Genes encoding proteins involved in selected pathway reactions are shown. The black boxes denote genes that are believed to be regulated by Pdr1p and/or Pdr3p. Rsb1p can transport both DHS and PHS (56). M(IP)2C is mannosyl diinositolphospho-ceramide (31), the final product of the sphingolipid pathway in S. cerevisiae.
The genetic interactions between the Pdr and sphingolipid pathways were extended by the finding that the RSB1 gene is regulated by Pdr1p and Pdr3p (55, 83). Rsb1p is thought to act as an effluxer of ceramide precursors called long-chain bases (LCBs) (56). LCBs can be cytotoxic if allowed to accumulate, and Rsb1p can act to prevent inappropriate buildup of these sphingolipid intermediates. Surprisingly, loss of Pdr5p and Yor1p from cells strongly elevates LCB resistance (55) in a Rsb1p-dependent fashion, again emphasizing the interconnections between sphingolipid biosynthesis and the Pdr pathway. These multiple interfaces between pleiotropic drug resistance and the homeostasis of membrane lipids suggest the possibility that the physiological role of the Pdr pathway is to assist in regulation of the function of the plasma membrane at the level of both lipids and membrane transporters.
TRANSCRIPTIONAL CONTROL OF PLEIOTROPIC DRUG RESISTANCE
A central determinant in the drug resistance phenotype of S. cerevisiae is provided by the regulation of transactivation capability of a limited number of regulatory proteins. Pdr1p and its homologue Pdr3p are Zn2Cys6-containing transcriptional regulatory proteins that exert major influences on the multidrug resistance phenotypes of cells. There are a large number of Zn2Cys6-containing transcription factors in S. cerevisiae (>50), and many of these have been shown or are believed to be involved in pleiotropic drug resistance (recently reviewed in reference 68). We will focus on Pdr1p and Pdr3p as illustrative of the larger number of Zn2Cys6-containing factors that contribute to drug resistance. More detailed considerations of these and other transcriptional regulatory proteins involved in multidrug resistance in S. cerevisiae are available in several reviews (34, 68, 76).
As mentioned above, hyperactive mutant forms of Pdr1p drove the initial identification of the pleiotropic drug resistance phenotype in S. cerevisiae (4, 12). Similar mutant alleles of PDR3 have also been described (81). These single amino acid substitution forms of Pdr1p and Pdr3p produce transcriptional regulatory proteins that behave as strong, constitutive activators of downstream gene expression (12, 81). Pdr1p and Pdr3p both bind to elements referred to as Pdr1p/Pdr3p response elements (PDREs) located upstream of target genes (24, 51). In vivo footprinting experiments indicate that Pdr1p and Pdr3p are likely to be constitutively bound to relevant PDREs (35, 69), an observation consistent with the constitutive nuclear localization of these proteins (25). In addition, both Pdr1p and Pdr3p can activate the expression of another zinc cluster transcription factor-encoding gene called YRR1 (20, 123). Increased expression of Yrr1p can amplify the transcriptional effects of activation of either Pdr1p or Pdr3p since this factor recognizes a sequence different from the PDRE (63).
Although significant similarities are shared by Pdr1p and Pdr3p, important differences are known. First, these factors are expressed at dramatically different levels. Use of epitope-tagged forms of both proteins indicates that Pdr1p is present at nearly 10 times the level of Pdr3p (39). Second, the regulation of these factors is responsive to different signals. Overproduction of the DnaK protein Ssz1p (42) or the DnaJ Zuo1p (32) induces Pdr1p-dependent gene transcription but has no effect on Pdr3p. Conversely, cells lacking their mitochondrial genome (ρ°) activate Pdr3p function but have no effect on Pdr1p (45). PDR1 expression levels are constant, whereas PDR3 is both autoregulated and highly induced in ρ° cells (25, 44, 45). Finally, recent work from our laboratory demonstrates the Hsp70 protein Ssa1p can negatively regulate Pdr3p but not Pdr1p activity (107a). Although these two transcription factors share >30% identity across their roughly 1,000-amino-acid lengths (26), these differences indicate that Pdr1p and Pdr3p have nonidentical roles to play in the control of multidrug resistance.
The second class of transcription factor that has been associated with the regulation of multidrug resistance is the basic region-leucine zipper (bZip) family of regulatory proteins. Although a number of these bZip-containing factors are present in S. cerevisiae, we will restrict our discussion to Yap1p, the first of these factors shown to be involved in pleiotropic drug resistance. Yap1p is better known for its important role in oxidative stress tolerance (see references 75, 82, and 98 for reviews), and its regulation by oxidants has been the subject of intensive study. Briefly, Yap1p normally cycles between the nucleus and the cytoplasm in the absence of stress (62). Upon oxidant challenge, Yap1p rapidly accumulates in the nucleus, where it activates antioxidant gene expression (16, 61, 62, 122). Mutant forms of Yap1p have been described that are constitutively located in the nucleus and hyper-resistant to certain oxidants (16, 62, 119).
Less is known of the response mediated by Yap1p upon drug challenge, but the YAP1 gene was isolated as a high-copy-number mediator of pleiotropic drug resistance along with PDR5 (65). As mentioned above, Yap1p defines a pathway for drug resistance parallel to that of PDR5. Interestingly, Yap1p is known to activate the expression of at least two different MFS protein-encoding genes: ATR1 (17) and FLR1 (2). At least in S. cerevisiae, the control of multidrug resistance gene expression appears to be divided between zinc cluster-containing factors primarily driving transcription of ABC transporter-encoding genes, while bZip-containing proteins mainly act by regulating mRNA levels of MFS proteins. The rationale underlying this division of transcriptional regulatory circuits remains to be determined.
CANDIDA ALBICANS
While the powerful genetics associated with the use of S. cerevisiae as a model fungus have allowed the most rapid progress in our understanding of fungal multidrug resistance, this organism is not a significant cause of human disease. The major fungal source of bloodstream infections in humans is from the genus Candida which has grown in importance until candidemia now represents the fourth most common nosocomial infection (110). The primary Candida species associated with candidemia is Candida albicans (87), and this organism has been the most intensively studied in terms of multidrug resistance.
The first multidrug resistance gene from C. albicans was recovered by selection of a segment of C. albicans genomic DNA that altered drug resistance in S. cerevisiae when carried on an appropriate plasmid (8). The first gene isolated in this fashion was designated BENr and is now referred to as MDR1 (40). The protein encoded by this gene is a member of the major facilitator superfamily of membrane transporters (see reference 70 for a review). Shortly after this finding, two genes encoding homologues of the S. cerevisiae Pdr5p ABC transporter protein were identified (91, 104). These proteins were given the acronyms CDR1 and CDR2 for Candida drug resistance 1 and 2. Experiments in several labs established that mutant C. albicans strains lacking these ABC transporters were multidrug sensitive (104, 105) and that a number of different resistant isolates were found to overproduce CDR1 and CDR2 transcripts (106, 120). Measurements of drug transport in strains engineered to overproduce Cdr1p support the idea that this protein acts as an ATP-dependent drug efflux pump (77). Green fluorescent protein fusions to Cdr1p indicated that this protein was primarily found in the plasma membrane, similar to the location of Pdr5p in S. cerevisiae (108).
More recent work has focused on the control of transcription of CDR1 and CDR2. Experiments from two different laboratories provided characterization of the promoter region of CDR1 (27, 93). CDR1 transcriptional control was found to be complex and the product of multiple DNA elements of either positive or negative nature in the promoter region. Analysis of the CDR2 promoter suggests that this gene is regulated in parallel with CDR1 since azole-resistant C. albicans isolates often exhibit elevated mRNA levels corresponding to both of these ABC transporter-encoding genes (27).
Several different transcriptional regulatory proteins have been implicated in the modulation of CDR1 and CDR2 expression. The best characterized of these is the Zn2Cys6 cluster protein Tac1p (18). This factor binds to a single element in the CDR2 promoter that contains tandem repeats of a CGG sequence. These short trinucleotide repeats are commonly associated with the binding sites of Zn2Cys6 cluster proteins (68). Tac1p exhibits the highest degree of sequence similarity with a protein from S. cerevisiae designated Hal9p, a factor involved in the control of expression of the ENA1 sodium-potassium ATPase (71). Interestingly, the presence of a CDR2-lacZ fusion in S. cerevisiae does not lead to the production of significant β-galactosidase activity unless Tac1p is heterologously provided (18), suggesting that Hal9p cannot stimulate CDR2 expression. Although Pdr1p does share significant sequence similarity with Tac1p (E value = 10−13), there are clear differences between these two Zn2Cys6 cluster proteins at the level of DNA binding specificity and protein sequence. An interesting commonality between PDR1 and TAC1 gain-of-function mutants is the spectrum of highly responsive target genes that these two important regulators of multidrug resistance induce in their respective organisms. In S. cerevisiae, PDR1 hyperactive mutants strongly induce PDR5 and PDR15, as well as another membrane protein called Rsb1p that is required for resistance to the long-chain base phytosphingosine (28). In C. albicans, TAC1 hyperactive alleles induce CDR1 (Pdr5p homologue), CDR2 (Pdr15p homologue), and RTA3 (Rsb1p homologue).
A second positive regulator of CDR1 was found by screening a C. albicans expression library in a S. cerevisiae cell carrying a CDR1-lacZ reporter gene for clones that could elevate expression of this heterologous reporter gene (13). This gene was designated CaNDT80 since it was found to encode a homologue of the S. cerevisiae Ndt80p transcription factor. ScNdt80p serves to activate genes involved in sporulation in S. cerevisiae (15). CaNdt80p appears to have functionally diverged from the role of its S. cerevisiae cognate protein since the C. albicans factor is important in positive regulation of CDR1 during vegetative growth.
Evidence implicating an important role for a negative regulator of CDR1 expression has also accrued (38). To date, the identity of this factor is still undetermined, but its binding site has been mapped to an element located downstream of the Tac1p recognition element (37). A 55-kDa protein has been shown to be cross-linked to this negative regulatory element, and reductions in the level of this factor are believed to cause azole hyper-resistance in some clinical isolates. Together, these data suggest that control of expression of CDR1 in C. albicans involves more complexity at the level of the transcription factors compared to the control of ScPDR5 expression that is primarily dependent on only Pdr1p and Pdr3p (26, 51).
Transcriptional control of CaMDR1 has also been examined by several laboratories. Detailed deletion mapping experiments have been described in several publications (47, 49, 96, 99). These studies have identified several different regions in the CaMDR1 promoter that are involved in basal, oxidant- or drug-induced expression. There are differences in the precise roles of these multiple elements but clearly the CaMDR1 promoter is a complex transcriptional control region that integrates multiple inputs to determine the proper expression of CaMdr1p.
At least two different factors have been linked to the trans-regulation of CaMDR1 expression. Isolation of the C. albicans Yap1p homologue (Cap1p) indicated that CaMDR1 was a likely downstream target of this transcription factor (3). This has been supported by direct mutagenesis of putative Cap1p regulatory elements present in CaMDR1 (99). Surprisingly, other analyses demonstrated that loss of CAP1 either had no effect on azole resistance or negatively influenced tolerance to this drug (3). However, overproduction of a carboxy-terminal truncation mutant of Cap1p led to a dramatic increase in azole resistance, an observation that correlates with strongly elevated CaMDR1 transcription (3). This behavior is similar to that observed in S. cerevisiae when YAP1 is deleted as yap1Δ cells exhibit a modest (twofold) increase while overproduction of Yap1p produced a striking increase in the MIC for fluconazole (14).
Along with Cap1p, the C. albicans homologue of S. cerevisiae Mcm1p has also been implicated in control of CaMDR1 transcription (96, 99). Laboratory strains of C. albicans that were selected for high azole resistance were found to be dependent on the presence of a functional CaMcm1p binding site for high level production of CaMDR1. Cap1p was not required for this effect arguing that CaMcm1p is the primary if not the sole factor that is upregulated in these azole-resistant isolates. Azole resistance has also been shown to be impacted by changes in expression of the C. albicans IPT1 gene (92), suggesting a further conservation with S. cerevisiae. The picture that emerges from studies in C. albicans on the molecular basis of multidrug resistance is consistent with this organism sharing a very similar range of effector genes with S. cerevisiae, but with the regulation of gene expression exhibiting differences. This may be due to the very different milieus in which these organisms normally reside. Further study of the resistance pathways in these yeasts will clarify this notion.
CANDIDA GLABRATA
C. glabrata is a haploid species of Candida that has emerged as the second most common Candida organism associated with fungemia (87). A likely contributing factor to the rapid growth (2% in the 1970s to 20% now) is the robust ability of C. glabrata to acquire tolerance to commonly deployed antifungal agents such as azoles. Long-term monitoring of C. glabrata-associated disease indicates that azole-resistant isolates are increasing in frequency, even from geographic regions in which C. glabrata was originally sensitive to these drugs (85). This is not seen in the case of C. albicans since clinical data indicate that azole drugs retain their efficacy in controlling disease associated with this species (48). A second complicating factor for C. glabrata comes from the routine multidrug resistance of azole-tolerant isolates (107). Not only does C. glabrata relatively easily convert to an azole-resistant form, but it also frequently appears to become simultaneously multidrug resistant.
Protein and DNA sequence similarity analyses indicate that C. glabrata and S. cerevisiae are closely related organisms (121). This close relationship is also illustrated by the similarities in the multidrug resistance pathways in these two yeasts. As described above for S. cerevisiae, ρ° C. glabrata cells are highly multidrug resistant (103). This mitochondrial control of multidrug resistance proceeds through the activation of the CgPdr1p protein (114). A notable difference between S. cerevisiae and C. glabrata is that CgPdr1p represents the single best homologue of the Pdr1p/Pdr3p pair of proteins found in S. cerevisiae (117). CgPDR1 may correspond to a fusion gene between the S. cerevisiae PDR1 and PDR3 loci. CgPDR1 shares more sequence similarity with ScPdr1p but exhibits the ρ° induction seen for ScPDR3 (114). Single amino acid substitution mutant forms of CgPdr1p have been found that are linked with high-level transcription of both CgPDR1 and CgCDR1, as well as robust multidrug resistance (117). This behavior is directly analogous to that seen for the hyperactive alleles of ScPDR1 and ScPDR3.
Although it is clear that ρ° C. glabrata cells are highly multidrug resistant and occur frequently, the role of these cells in disease is less certain. C. glabrata clinical isolates that were converted to petite status by either ethidium bromide or fluconazole treatment were found to have reduced virulence in a mouse model of fungal pathogenesis (11). One concern with these experiments (noted by the authors of that study) is that evaluation of virulence was carried out without the administration of fluconazole. Since the absence of drug selection would remove the possible selective advantage provided by the high-level expression of the CgCDR genes and associated multidrug resistance, the petite cells may be lost due to competition from the healthy endogenous microbial flora. There have been reports of petite C. glabrata strains isolated from patients (10), but a larger number of other clinical isolates are not apparently linked with a petite character (107).
While the finding that petite strains of C. glabrata and S. cerevisiae exhibit a multidrug-resistant phenotype illustrates the similar mitochondrial regulatory basis in these organisms, an interesting difference has been uncovered by screening a collection of transposon-generated C. glabrata mutants (54). Insertion of a transposon into several genes was found to elevate azole resistance but also trigger petite formation. When the selective pressure was removed, the petite phenotype appeared to abate. Previous studies on both C. glabrata and S. cerevisiae petite mutants have not uncovered multidrug-resistant mutants that exhibit reversible behavior (103, 124).
One feature of the C. glabrata petite multidrug-resistant mutants that remains unclear is the status of their mitochondrial genome. The so-called high-frequency azole-resistant mutants were demonstrated to lack a mitochondrial genome (103) and closely resemble the ρ° multidrug-resistant mutants of S. cerevisiae. Three of the transposon-induced C. glabrata petite mutants were examined for mitochondrial genome status (54) and intriguingly found to still retain cytoplasmic nucleoids. This is an important potential difference since a number of different S. cerevisiae mutants that are petite but not ρ° fail to express the multidrug-resistant phenotype (124). The petite transposon insertion mutants in C. glabrata include an insertion in the CgSHE9 gene. Disruption mutants lacking SHE9 (aka MDM33) (72) do not appear to lose its mitochondrial genome in either S. cerevisiae or C. glabrata, but multidrug resistance has only been assessed for C. glabrata mutants. Further work is required to determine whether the reversible multidrug-resistant status of the three C. glabrata genes is also exhibited by their cognate S. cerevisiae mutants. As suggested previously (54), mutants that exhibit a gradual diminution of mitochondrial function without complete loss of the mitochondrial genome may give rise to this reversible multidrug resistance. Mutants of this type would have clear advantages in terms of survival of chemotherapy in a patient since they could combine the benefits of robust multidrug resistance of a petite cell without permanently acquiring the fragile growth of these mitochondrially deficient strains.
As described above for S. cerevisiae, multidrug resistance in C. glabrata is also influenced by transcriptional control of MFS protein expression. The CgFLR1 gene is induced by the C. glabrata Yap1p homologue (CgAP-1) and confers resistance to a range of agents, including fluconazole (14). C. glabrata Cgap-1 mutants had normal fluconazole tolerance, while Cgpdr1 mutants were hypersensitive to this drug. The Yap1p-FLR1 fluconazole resistance pathway seems to serve an ancillary role in tolerance to this antifungal agent in both S. cerevisiae and C. glabrata. Somewhat surprisingly, while Cgap-1 mutants are hypersensitive to oxidants, there is no detectable effect on virulence (14). Although CgAP-1 is an important determinant of oxidative stress resistance, this does not appear to influence the ability of C. glabrata to colonize a mouse model.
The similarities between multidrug-resistant isolates of S. cerevisiae and C. glabrata have been extended by analysis of a genes that are transcriptionally responsive to a hyperactive mutant form of CgPdr1p by microarray (116). While a number of genes that are induced in C. glabrata represent homologues that are also elevated in response to S. cerevisiae hyperactive Pdr1p, the largest group of induced transcripts correspond to genes that are uniquely induced in the pathogenic yeast (116). These C. glabrata-specific genes may represent loci that are required for the organism to successfully withstand the challenges of toxic compounds when exposed in an animal host.
ASPERGILLUS SPP.
The primary human pathogen among the filamentous fungi is Aspergillus fumigatus (95). Infections associated with this organism are have a high morbidity and an intrinsically high resistance to the standard cadre of antifungal agents (86). Not surprisingly, continued use of azole-based drugs has the undesirable consequence of elevating the resistance of subsequent isolates from these patients. Fortunately, acquisition of a multidrug resistance phenotype is relatively rare among Aspergillus species, but this statement must be qualified by the recognition that analysis of this phenotype is complicated by technical difficulties in establishing this phenotype (74). In addition, molecular characterization of genes involved in Aspergillus multidrug resistance has been hindered by lack of a complete genomic sequence. This has recently been accomplished (80) and will accelerate study of the loci involved in drug resistance in this important human pathogen.
Previous studies have provided some information concerning multidrug resistance genes in Aspergillus. Degenerate PCR cloning allowed the isolation of two different ABC transporter-encoding genes, and expression of one of these in S. cerevisiae elevated resistance to an antifungal drug of the echinocandin family (113). Using a probe for C. albicans CDR1, A. fumigatus atrF was isolated and shown to be inducible in azole-resistant isolates but not inducible in azole-susceptible strains (109). Azole-resistant isolates selected in vitro were found to have elevated expression of ABC transporter and MFS-encoding genes (78). These data are consistent with similar systems for drug detoxification existing in Aspergillus as have been described in yeasts.
The availability of the genomic sequence of Aspergillus has already provided interesting insights into the likely composition of multidrug resistance systems in this filamentous fungus. The A. fumigatus genome is ca. 50% larger than that of S. cerevisiae and yet predicts the presence of 96 potential MFS multidrug transporters compared to 24 from budding yeast (36). Similarly, S. cerevisiae contains 13 ABC transporter genes thought to participate in multidrug resistance, whereas A. fumigatus is believed to have 35. Analyses of these proteins in A. fumigatus is required to confirm that these proteins are involved in drug resistance in this organism, but the presence of such a proportionally larger number suggests a hardening of the drug defensive capability of this pathogenic organism. It is striking to note that the increased proportion of MFS proteins compared to ABC transporters found in the Aspergillus genome compared to S. cerevisiae resembles the situation in pathogenic bacteria. Currently, there are no examples of ABC transporters in clinically relevant bacteria pathogens that are important in drug resistance, whereas MFS protein participation in evasion of antibacterial therapy is very common (88).
SUMMARY
Multidrug resistance is a problem in chemotherapy in situations ranging from bacterial infections to cancer. Fungal drug resistance is an especially acute issue due to the limited number of antifungal compounds (60). Understanding the regulation and function of multidrug resistance pathways in fungi is still very much a work in progress, but its importance continues to grow with the increasing number of immunocompromised patients worldwide and their increasing reliance on chemotherapy to control fungal infections (see references 84 and 118 for recent reviews). Much of our current understanding of the molecular basis of fungal multidrug resistance springs from work in the typically nonpathogenic yeast S. cerevisiae. However, rapid advances in the experimental tractability of pathogenic fungi such as the Candida and Aspergillus species will bring the pathways in these clinically important organisms into focus. This is an important goal since the systems used for drug resistance in these pathogens share some similarity with those in S. cerevisiae but have important differences that can only be uncovered in the native organism.
Acknowledgments
Work on multidrug resistance in our laboratory was supported by NIH grants GM49825 and GM75120.
We thank Puja Shahi for a critical reading of the manuscript.
Footnotes
Published ahead of print on 14 September 2007.
REFERENCES
- 1.Alanis, A. J. 2005. Resistance to antibiotics: are we in the post-antibiotic era? Arch. Med. Res. 36:697-705. [DOI] [PubMed] [Google Scholar]
- 2.Alarco, A. M., I. Balan, D. Talibi, N. Mainville, and M. Raymond. 1997. AP-1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J. Biol. Chem. 272:19304-19313. [DOI] [PubMed] [Google Scholar]
- 3.Alarco, A. M., and M. Raymond. 1999. The bZip transcription factor Cap1p is involved in multidrug resistance and oxidative stress response in Candida albicans. J. Bacteriol. 181:700-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzi, E., W. Chen, S. Ulaszewski, E. Capieaux, and A. Goffeau. 1987. The multidrug resistance gene PDR1 from Saccharomyces cerevisiae. J. Biol. Chem. 262:16871-16879. [PubMed] [Google Scholar]
- 5.Balzi, E., and A. Goffeau. 1995. Yeast Multidrug Resistance: the PDR network. J. Bioenerget. Biomembr. 27:71-76. [DOI] [PubMed] [Google Scholar]
- 6.Balzi, E., M. Wang, S. Leterme, L. Van Dyck, and A. Goffeau. 1994. PDR5: a novel yeast multidrug resistance transporter controlled by the transcription regulator PDR1. J. Biol. Chem. 269:2206-2214. [PubMed] [Google Scholar]
- 7.Barber, M. 1948. Infection by penicillin resistant staphylococci. Lancet ii:641-644. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Yaacov, R., S. Knoller, G. A. Caldwell, J. M. Becker, and Y. Koltin. 1994. Candida albicans gene encoding resistance to benomyl and methotrexate is a multidrug resistance gene. Antimicrob. Agents Chemother. 38:648-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bissinger, P. H., and K. Kuchler. 1994. Molecular cloning and expression of the S. cerevisiae STS1 gene product. J. Biol. Chem. 269:4180-4186. [PubMed] [Google Scholar]
- 10.Bouchara, J. P., R. Zouhair, S. Le Boudouil, G. Renier, R. Filmon, D. Chabasse, J. N. Hallet, and A. Defontaine. 2000. In-vivo selection of an azole-resistant petite mutant of Candida glabrata. J. Med. Microbiol. 49:977-984. [DOI] [PubMed] [Google Scholar]
- 11.Brun, S., F. Dalle, P. Saulnier, G. Renier, A. Bonnin, D. Chabasse, and J. P. Bouchara. 2005. Biological consequences of petite mutations in Candida glabrata. J. Antimicrob. Chemother. 56:307-314. [DOI] [PubMed] [Google Scholar]
- 12.Carvajal, E., H. B. van den Hazel, A. Cybularz-Kolaczkowska, E. Balzi, and A. Goffeau. 1997. Molecular and phenotypic characterization of yeast PDR1 mutants that show hyperactive transcription of various ABC multidrug transporter genes. Mol. Gen. Genet. 256:406-415. [DOI] [PubMed] [Google Scholar]
- 13.Chen, C. G., Y. L. Yang, H. I. Shih, C. L. Su, and H. J. Lo. 2004. CaNdt80 is involved in drug resistance in Candida albicans by regulating CDR1. Antimicrob. Agents Chemother. 48:4505-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, K. H., T. Miyazaki, H. F. Tsai, and J. E. Bennett. 2007. The bZip transcription factor Cgap1p is involved in multidrug resistance and required for activation of multidrug transporter gene CgFLR1 in Candida glabrata. Gene 386:63-72. [DOI] [PubMed] [Google Scholar]
- 15.Chu, S., and I. Herskowitz. 1998. Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol. Cell 1:685-696. [DOI] [PubMed] [Google Scholar]
- 16.Coleman, S. T., E. A. Epping, S. M. Steggerda, and W. S. Moye-Rowley. 1999. Yap1p activates gene transcription in an oxidant-specific fashion. Mol. Cell. Biol. 19:8302-8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman, S. T., E. Tseng, and W. S. Moye-Rowley. 1997. Saccharomyces cerevisiae basic region-leucine zipper protein regulatory networks converge at the ATR1 structural gene. J. Biol. Chem. 272:23224-23230. [DOI] [PubMed] [Google Scholar]
- 18.Coste, A. T., M. Karababa, F. Ischer, J. Bille, and D. Sanglard. 2004. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 3:1639-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui, Z., D. Hirata, E. Tsuchiya, H. Osada, and T. Miyakawa. 1996. The mutidrug resistance-associated protein (MRP) subfamily (Yrs1/Yor1) of Saccharomyces cerevisiae is important for the tolerance to a broad range of organic anions. J. Biol. Chem. 271:14712-14716. [DOI] [PubMed] [Google Scholar]
- 20.Cui, Z., T. Shiraki, D. Hirata, and T. Miyakawa. 1998. Yeast gene YRR1, which is required for resistance to 4-nitroquinoline-N-oxide, mediates transcriptional activation of the multidrug resistance transporter gene SNQ2. Mol. Microbiol. 29:1307-1315. [DOI] [PubMed] [Google Scholar]
- 21.Dean, M., Y. Hamon, and G. Chimini. 2001. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 42:1007-1017. [PubMed] [Google Scholar]
- 22.Decottignies, A., A. M. Grant, J. W. Nichols, H. de Wet, D. B. McIntosh, and A. Goffeau. 1998. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem. 273:12612-12622. [DOI] [PubMed] [Google Scholar]
- 23.Decottignies, A., L. Lambert, P. Catty, H. Degand, E. A. Epping, W. S. Moye-Rowley, E. Balzi, and A. Goffeau. 1995. Identification and characterization of SNQ2, a new multidrug ABC transporter of the yeast plasma membrane. J. Biol. Chem. 270:18150-18157. [DOI] [PubMed] [Google Scholar]
- 24.Delahodde, A., T. Delaveau, and C. Jacq. 1995. Positive autoregulation of the yeast transcription factor Pdr3p, involved in the control of the drug resistance phenomenon. Mol. Cell. Biol. 15:4043-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delahodde, A., R. Pandjaitan, M. Corral-Debrinski, and C. Jacq. 2001. Pse1/Kap121-dependent nuclear localization of the yeast multidrug resistance (MDR) transcription factor Pdr1. Mol. Microbiol. 39:304-312. [DOI] [PubMed] [Google Scholar]
- 26.Delaveau, T., A. Delahodde, E. Carvajal, J. Subik, and C. Jacq. 1994. PDR3, a new yeast regulatory gene, is homologous to PDR1 and controls the multidrug resistance phenomenon. Mol. Gen. Genet. 244:501-511. [DOI] [PubMed] [Google Scholar]
- 27.de Micheli, M., J. Bille, C. Schueller, and D. Sanglard. 2002. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol. Microbiol. 43:1197-1214. [DOI] [PubMed] [Google Scholar]
- 28.DeRisi, J., B. van den Hazel, P. Marc, E. Balzi, P. Brown, C. Jacq, and A. Goffeau. 2000. Genome microarray analysis of transcriptional activation in multidrug resistance yeast mutants. FEBS Lett. 470:156-160. [DOI] [PubMed] [Google Scholar]
- 29.Dexter, D., W. S. Moye-Rowley, A.-L. Wu, and J. Golin. 1994. Mutations in the yeast PDR3, PDR4, PDR7 and PDR9 pleiotropic drug resistance loci affect the transcript level of an ATP binding cassette transporter encoding gene, PDR5. Genetics 136:505-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickson, R. C., and R. L. Lester. 2002. Sphingolipid functions in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1583:13-25. [DOI] [PubMed] [Google Scholar]
- 31.Dickson, R. C., E. E. Nagiec, G. B. Wells, M. M. Nagiec, and R. L. Lester. 1997. Synthesis of mannose-(inositol-P)2-ceramide, the major sphingolipid in Saccharomyces cerevisiae, requires the IPT1 (YDR072c) gene J. Biol. Chem. 272:29620-29625. [DOI] [PubMed] [Google Scholar]
- 32.Eisenman, H. C., and E. A. Craig. 2004. Activation of pleiotropic drug resistance by the J-protein and Hsp70-related proteins, Zuo1 and Ssz1. Mol. Microbiol. 53:335-344. [DOI] [PubMed] [Google Scholar]
- 33.Emter, R., A. Heese-Peck, and A. Kralli. 2002. ERG6 and PDR5 regulate small lipophilic drug accumulation in yeast cells via distinct mechanisms. FEBS Lett. 521:57-61. [DOI] [PubMed] [Google Scholar]
- 34.Ernst, R., R. Klemm, L. Schmitt, and K. Kuchler. 2005. Yeast ATP-binding cassette transporters: cellular cleaning pumps. Methods Enzymol. 400:460-484. [DOI] [PubMed] [Google Scholar]
- 35.Fardeau, V., G. Lelandais, A. Oldfield, H. Salin, S. Lemoine, M. Garcia, V. Tanty, S. Le Crom, C. Jacq, and F. Devaux. 2007. The central role of PDR1 in the foundation of yeast drug resistance. J. Biol. Chem. 282:5063-5074. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira, M. E., A. L. Colombo, I. Paulsen, Q. Ren, J. Wortman, J. Huang, M. H. Goldman, and G. H. Goldman. 2005. The ergosterol biosynthesis pathway, transporter genes, and azole resistance in Aspergillus fumigatus. Med. Mycol. 43(Suppl. 1):S313-S319. [DOI] [PubMed] [Google Scholar]
- 37.Gaur, N. A., R. Manoharlal, P. Saini, T. Prasad, G. Mukhopadhyay, M. Hoefer, J. Morschhauser, and R. Prasad. 2005. Expression of the CDR1 efflux pump in clinical Candida albicans isolates is controlled by a negative regulatory element. Biochem. Biophys. Res. Commun. 332:206-214. [DOI] [PubMed] [Google Scholar]
- 38.Gaur, N. A., N. Puri, N. Karnani, G. Mukhopadhyay, S. K. Goswami, and R. Prasad. 2004. Identification of a negative regulatory element which regulates basal transcription of a multidrug resistance gene CDR1 of Candida albicans. FEMS Yeast Res. 4:389-399. [DOI] [PubMed] [Google Scholar]
- 39.Ghaemmaghami, S., W. K. Huh, K. Bower, R. W. Howson, A. Belle, N. Dephoure, E. K. O'Shea, and J. S. Weissman. 2003. Global analysis of protein expression in yeast Nature 425:737-741. [DOI] [PubMed] [Google Scholar]
- 40.Goldway, M., D. Teff, R. Schmidt, A. B. Oppenheim, and Y. Koltin. 1995. Multidrug resistance in Candida albicans: disruption of the BENr gene. Antimicrob. Agents Chemother. 39:422-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottesman, M. M., C. A. Hrycyna, P. V. Schoenlein, U. A. Germann, and I. Pastan. 1995. Genetic analysis of the multidrug transporter. Annu. Rev. Genet. 29:607-649. [DOI] [PubMed] [Google Scholar]
- 42.Hallstrom, T. C., D. J. Katzmann, R. J. Torres, W. J. Sharp, and W. S. Moye-Rowley. 1998. Regulation of transcription factor Pdr1p function by a Hsp70 protein in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:1147-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hallstrom, T. C., L. Lambert, S. Schorling, E. Balzi, A. Goffeau, and W. S. Moye-Rowley. 2001. Coordinate control of sphingolipid biosynthesis and multidrug resistance in Saccharomyces cerevisiae. J. Biol. Chem. 276:23674-23680. [DOI] [PubMed] [Google Scholar]
- 44.Hallstrom, T. C., and W. S. Moye-Rowley. 2000. Hyperactive forms of the Pdr1p transcription factor fail to respond to positive regulation by the Hsp70 protein Pdr13p. Mol. Microbiol. 36:402-413. [DOI] [PubMed] [Google Scholar]
- 45.Hallstrom, T. C., and W. S. Moye-Rowley. 2000. Multiple signals from dysfunctional mitochondria activate the pleiotropic drug resistance pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275:37347-37356. [DOI] [PubMed] [Google Scholar]
- 46.Hannun, Y. A., and L. M. Obeid. 2002. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 277:25847-25850. [DOI] [PubMed] [Google Scholar]
- 47.Harry, J. B., B. G. Oliver, J. L. Song, P. M. Silver, J. T. Little, J. Choiniere, and T. C. White. 2005. Drug-induced regulation of the MDR1 promoter in Candida albicans. Antimicrob. Agents Chemother. 49:2785-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hazen, K. C., E. J. Baron, A. L. Colombo, C. Girmenia, A. Sanchez-Sousa, A. del Palacio, C. de Bedout, and D. L. Gibbs. 2003. Comparison of the susceptibilities of Candida spp. to fluconazole and voriconazole in a 4-year global evaluation using disk diffusion. J. Clin. Microbiol. 41:5623-5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiller, D., S. Stahl, and J. Morschhauser. 2006. Multiple cis-acting sequences mediate upregulation of the MDR1 efflux pump in a fluconazole-resistant clinical Candida albicans isolate. Antimicrob. Agents Chemother. 50:2300-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirata, D., K. Yano, K. Miyahara, and T. Miyakawa. 1994. Saccharomyces cerevisiae YDR1, which encodes a member of the ATP-binding cassette (ABC) superfamily, is required for multidrug resistance. Curr. Genet. 26:285-294. [DOI] [PubMed] [Google Scholar]
- 51.Katzmann, D. J., P. E. Burnett, J. Golin, Y. Mahe, and W. S. Moye-Rowley. 1994. Transcriptional control of the yeast PDR5 gene by the PDR3 gene product. Mol. Cell. Biol. 14:4653-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katzmann, D. J., T. C. Hallstrom, M. Voet, W. Wysock, J. Golin, G. Volckaert, and W. S. Moye-Rowley. 1995. Expression of an ATP-binding cassette transporter encoding gene (YOR1) is required for oligomycin resistance in Saccharomyces cerevisiae. Mol. Cell. Biol. 15:6875-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaur, R., and A. K. Bachhawat. 1999. The yeast multidrug resistance pump, Pdr5p, confers reduced drug resistance in erg mutants of Saccharomyces cerevisiae. Microbiology 145(Pt. 4):809-818. [DOI] [PubMed] [Google Scholar]
- 54.Kaur, R., I. Castano, and B. P. Cormack. 2004. Functional genomic analysis of fluconazole susceptibility in the pathogenic yeast Candida glabrata: roles of calcium signaling and mitochondria. Antimicrob. Agents Chemother. 48:1600-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kihara, A., and Y. Igarashi. 2004. Cross talk between sphingolipids and glycerophospholipids in the establishment of plasma membrane asymmetry. Mol. Biol. Cell 15:4949-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kihara, A., and Y. Igarashi. 2002. Identification and characterization of a Saccharomyces cerevisiae gene, RSB1, involved in sphingoid long-chain base release. J. Biol. Chem. 277:30048-30054. [DOI] [PubMed] [Google Scholar]
- 57.Kolaczkowski, M., A. Kolaczkowska, B. Gaigg, R. Schneiter, and W. S. Moye-Rowley. 2004. Differential regulation of ceramide synthase components LAC1 and LAG1 in Saccharomyces cerevisiae. Eukaryot. Cell 3:880-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kolaczkowski, M., M. van der Rest, A. Cybularz-Kolaczkowski, J.-P. Soumillion, W. N. Konings, and A. Goffeau. 1996. Anticancer drugs, ionophoric peptides, and steroids as substrates of the yeast multidrug transporter Pdr5p. J. Biol. Chem. 271:31543-31548. [DOI] [PubMed] [Google Scholar]
- 59.Kontoyiannis, D. P. 1999. Genetic analysis of azole resistance by transposon mutagenesis in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 43:2731-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kontoyiannis, D. P., and R. E. Lewis. 2002. Antifungal drug resistance of pathogenic fungi. Lancet 359:1135-1144. [DOI] [PubMed] [Google Scholar]
- 61.Kuge, S., and N. Jones. 1994. YAP1-dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 13:655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuge, S., N. Jones, and A. Nomoto. 1997. Regulation of yAP-1 nuclear localization in response to oxidative stress. EMBO J. 16:1710-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Le Crom, S., F. Devaux, P. Marc, X. Zhang, W. S. Moye-Rowley, and C. Jacq. 2002. New insights into the pleiotropic drug resistance network from genome-wide characterization of the YRR1 transcription factor regulation system. Mol. Cell. Biol. 22:2642-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leonard, P. J., P. K. Rathod, and J. Golin. 1994. Loss of function mutation in the yeast multiple drug resistance gene PDR5 causes a reduction in chloramphenicol efflux. Antimicrob. Agents Chemother. 38:2492-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leppert, G., R. McDevitt, S. C. Falco, T. K. Van Dyk, M. B. Ficke, and J. Golin. 1990. Cloning by gene amplification of two loci conferring multiple drug resistance in Saccharomyces. Genetics 125:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levy, S. B., and B. Marshall. 2004. Antibacterial resistance worldwide: causes, challenges, and responses. Nat. Med. 10:S122-S129. [DOI] [PubMed] [Google Scholar]
- 67.Ling, V. 1997. Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother. Pharmacol. 40:S3-S8. [DOI] [PubMed] [Google Scholar]
- 68.MacPherson, S., M. Larochelle, and B. Turcotte. 2006. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol. Mol. Biol. Rev. 70:583-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mamnun, Y. M., R. Pandjaitan, Y. Mahe, A. Delahodde, and K. Kuchler. 2002. The yeast zinc finger regulators Pdr1p and Pdr3p control pleiotropic drug resistance (PDR) as homo- and heterodimers in vivo. Mol. Microbiol. 46:1429-1440. [DOI] [PubMed] [Google Scholar]
- 70.Marger, M. D., and M. H. Saier, Jr. 1993. A major superfamily of transmembrane facilitators that catalyse uniport, symport, and antiport. Trends Biochem. Sci. 18:13-20. [DOI] [PubMed] [Google Scholar]
- 71.Mendizabal, I., G. Rios, J. M. Mulet, R. Serrano, and I. F. de Larrinoa. 1998. Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 425:323-328. [DOI] [PubMed] [Google Scholar]
- 72.Messerschmitt, M., S. Jakobs, F. Vogel, S. Fritz, K. S. Dimmer, W. Neupert, and B. Westermann. 2003. The inner membrane protein Mdm33 controls mitochondrial morphology in yeast. J. Cell Biol. 160:553-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meyers, S., W. Schauer, E. Balzi, M. Wagner, A. Goffeau, and J. Golin. 1992. Interaction of the yeast pleiotropic drug resistance genes PDR1 and PDR5. Curr. Genet. 21:431-436. [DOI] [PubMed] [Google Scholar]
- 74.Moore, C. B., N. Sayers, J. Mosquera, J. Slaven, and D. W. Denning. 2000. Antifungal drug resistance in Aspergillus. J. Infect. 41:203-220. [DOI] [PubMed] [Google Scholar]
- 75.Moye-Rowley, W. S. 2003. Regulation of the transcriptional response to oxidative stress in fungi: similarities and differences. Eukaryot. Cell 2:381-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moye-Rowley, W. S. 2003. Transcriptional control of multidrug resistance in the yeast Saccharomyces. Prog. Nucleic Acids Res. Mol. Biol. 73:251-279. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura, K., M. Niimi, K. Niimi, A. R. Holmes, J. E. Yates, A. Decottignies, B. C. Monk, A. Goffeau, and R. D. Cannon. 2001. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob. Agents Chemother. 45:3366-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nascimento, A. M., G. H. Goldman, S. Park, S. A. Marras, G. Delmas, U. Oza, K. Lolans, M. N. Dudley, P. A. Mann, and D. S. Perlin. 2003. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob. Agents Chemother. 47:1719-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nelissen, B., R. De Wachter, and A. Goffeau. 1997. Classification of all putative permeases and other membrane plurispanners of the major facilitator superfamily encoded by the complete genome of Saccharomyces cerevisiae. FEMS Microbiol. Rev. 21:113-134. [DOI] [PubMed] [Google Scholar]
- 80.Nierman, W. C., A. Pain, M. J. Anderson, J. R. Wortman, H. S. Kim, J. Arroyo, M. Berriman, K. Abe, D. B. Archer, C. Bermejo, J. Bennett, P. Bowyer, D. Chen, M. Collins, R. Coulsen, R. Davies, P. S. Dyer, M. Farman, N. Fedorova, N. Fedorova, T. V. Feldblyum, R. Fischer, N. Fosker, A. Fraser, J. L. Garcia, M. J. Garcia, A. Goble, G. H. Goldman, K. Gomi, S. Griffith-Jones, R. Gwilliam, B. Haas, H. Haas, D. Harris, H. Horiuchi, J. Huang, S. Humphray, J. Jimenez, N. Keller, H. Khouri, K. Kitamoto, T. Kobayashi, S. Konzack, R. Kulkarni, T. Kumagai, A. Lafon, J. P. Latge, W. Li, A. Lord, C. Lu, W. H. Majoros, G. S. May, B. L. Miller, Y. Mohamoud, M. Molina, M. Monod, I. Mouyna, S. Mulligan, L. Murphy, S. O'Neil, I. Paulsen, M. A. Penalva, M. Pertea, C. Price, B. L. Pritchard, M. A. Quail, E. Rabbinowitsch, N. Rawlins, M. A. Rajandream, U. Reichard, H. Renauld, G. D. Robson, S. Rodriguez de Cordoba, J. M. Rodriguez-Pena, C. M. Ronning, S. Rutter, S. L. Salzberg, M. Sanchez, J. C. Sanchez-Ferrero, D. Saunders, K. Seeger, R. Squares, S. Squares, M. Takeuchi, F. Tekaia, G. Turner, C. R. Vazquez de Aldana, J. Weidman, O. White, J. Woodward, J. H. Yu, C. Fraser, J. E. Galagan, K. Asai, M. Machida, N. Hall, B. Barrell, and D. W. Denning. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151-1156. [DOI] [PubMed] [Google Scholar]
- 81.Nourani, A., D. Papajova, A. Delahodde, C. Jacq, and J. Subik. 1997. Clustered amino acid substitutions in the yeast transcription regulator Pdr3p increase pleiotropic drug resistance and identify a new central regulatory domain. Mol. Gen. Genet. 256:397-405. [DOI] [PubMed] [Google Scholar]
- 82.Paget, M. S., and M. J. Buttner. 2003. Thiol-based regulatory switches. Annu. Rev. Genet. 37:91-121. [DOI] [PubMed] [Google Scholar]
- 83.Panwar, S. L., and W. S. Moye-Rowley. 2006. Long chain base tolerance in Saccharomyces cerevisiae is induced by retrograde signals from the mitochondria. J. Biol. Chem. 281:6376-6384. [DOI] [PubMed] [Google Scholar]
- 84.Perlroth, J., B. Choi, and B. Spellberg. 2007. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med. Mycol. 45:321-346. [DOI] [PubMed] [Google Scholar]
- 85.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pfaller, M. A., and D. J. Diekema. 2004. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 42:4419-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, and S. A. Messer. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piddock, L. J. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pomorski, T., J. C. Holthuis, A. Herrmann, and G. van Meer. 2004. Tracking down lipid flippases and their biological functions. J. Cell Sci. 117:805-813. [DOI] [PubMed] [Google Scholar]
- 90.Pomorski, T., R. Lombardi, H. Riezman, P. F. Devaux, G. van Meer, and J. C. Holthuis. 2003. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell 14:1240-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prasad, R., P. Dewergifosse, A. Goffeau, and E. Balzi. 1995. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27:320-329. [DOI] [PubMed] [Google Scholar]
- 92.Prasad, T., P. Saini, N. A. Gaur, R. A. Vishwakarma, L. A. Khan, Q. M. Haq, and R. Prasad. 2005. Functional analysis of CaIPT1, a sphingolipid biosynthetic gene involved in multidrug resistance and morphogenesis of Candida albicans. Antimicrob. Agents Chemother. 49:3442-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Puri, N., S. Krishnamurthy, S. Habib, S. E. Hasnain, S. K. Goswami, and R. Prasad. 1999. CDR1, a multidrug resistance gene from Candida albicans, contains multiple regulatory domains in its promoter and the distal AP-1 element mediates its induction by miconazole. FEMS Microbiol. Lett. 180:213-219. [DOI] [PubMed] [Google Scholar]
- 94.Rank, G. H., A. J. Robertson, and K. L. Phillips. 1975. Modification and inheritance of pleiotropic cross resistance and collateral sensitivity in Saccharomyces cerevisiae. Genetics 80:783-793. [PMC free article] [PubMed] [Google Scholar]
- 95.Richardson, M. D. 2005. Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 56(Suppl. 1):i5-i11. [DOI] [PubMed] [Google Scholar]
- 96.Riggle, P. J., and C. A. Kumamoto. 2006. Transcriptional regulation of MDR1, encoding a drug efflux determinant, in fluconazole-resistant Candida albicans strains through an Mcm1p binding site. Eukaryot. Cell 5:1957-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Riordan, J. R., K. Deuchars, N. Kartner, N. Alon, J. Trent, and V. Ling. 1985. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature 316:817-819. [DOI] [PubMed] [Google Scholar]
- 98.Rodrigues-Pousada, C. A., T. Nevitt, R. Menezes, D. Azevedo, J. Pereira, and C. Amaral. 2004. Yeast activator proteins and stress response: an overview. FEBS Lett. 567:80-85. [DOI] [PubMed] [Google Scholar]
- 99.Rognon, B., Z. Kozovska, A. T. Coste, G. Pardini, and D. Sanglard. 2006. Identification of promoter elements responsible for the regulation of MDR1 from Candida albicans, a major facilitator transporter involved in azole resistance. Microbiology 152:3701-3722. [DOI] [PubMed] [Google Scholar]
- 100.Roninson, I. B. 1992. From amplification to function: the case of the MDR1 gene. Mutat. Res. 276:151-161. [DOI] [PubMed] [Google Scholar]
- 101.Roninson, I. B., J. E. Chin, K. Choi, P. Gros, D. E. Housman, A. Fojo, D. W. Shen, M. M. Gottesman, and I. Pastan. 1986. Isolation of human MDR DNA sequences amplified in multidrug resistant KB carcinoma cells. Proc. Natl. Acad. Sci. USA 83:4538-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sa-Correia, I., and S. Tenreiro. 2002. The multidrug resistance transporters of the major facilitator superfamily, 6 years after disclosure of Saccharomyces cerevisiae genome sequence. J. Biotechnol. 98:215-226. [DOI] [PubMed] [Google Scholar]
- 103.Sanglard, D., F. Ischer, and J. Bille. 2001. Role of ATP-binding cassette transporter gene in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 45:1174-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 105.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1996. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob. Agents Chemother. 40:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 40:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanguinetti, M., B. Posteraro, B. Fiori, S. Ranno, R. Torelli, and G. Fadda. 2005. Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance. Antimicrob. Agents Chemother. 49:668-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107a.Shahi, P., K. Gulshan, and W. S. Moye-Rowley. 2007. Negative transcriptional regulation of multidrug resistance gene expression by an Hsp70 protein. J. Biol. Chem. 282:26822-26831. [DOI] [PubMed] [Google Scholar]
- 108.Shukla, S., S. V. Ambudkar, and R. Prasad. 2004. Substitution of threonine-1351 in the multidrug transporter Cdr1p of Candida albicans results in hypersusceptibility to antifungal agents and threonine-1351 is essential for synergic effects of calcineurin inhibitor FK520. J. Antimicrob. Chemother. 54:38-45. [DOI] [PubMed] [Google Scholar]
- 109.Slaven, J. W., M. J. Anderson, D. Sanglard, G. K. Dixon, J. Bille, I. S. Roberts, and D. W. Denning. 2002. Increased expression of a novel Aspergillus fumigatus ABC transporter gene, atrF, in the presence of itraconazole in an itraconazole resistant clinical isolate. Fungal Genet. Biol. 36:199-206. [DOI] [PubMed] [Google Scholar]
- 110.Slavin, M., J. Fastenau, I. Sukarom, P. Mavros, S. Crowley, and W. C. Gerth. 2004. Burden of hospitalization of patients with Candida and Aspergillus infections in Australia. Int. J. Infect. Dis. 8:111-120. [DOI] [PubMed] [Google Scholar]
- 111.Sternbach, G., and J. Varon. 1992. Alexander Fleming: the spectrum of penicillin. J. Emerg. Med. 10:89-91. [DOI] [PubMed] [Google Scholar]
- 112.Sturley, S. L. 2000. Conservation of eukaryotic sterol homeostasis: new insights from studies in budding yeast. Biochim. Biophys. Acta 1529:155-163. [DOI] [PubMed] [Google Scholar]
- 113.Tobin, M. B., R. B. Peery, and P. L. Skatrud. 1997. Genes encoding multiple drug resistance-like proteins in Aspergillus fumigatus and Aspergillus flavus. Gene 200:11-23. [DOI] [PubMed] [Google Scholar]
- 114.Tsai, H. F., A. A. Krol, K. E. Sarti, and J. E. Bennett. 2006. Candida glabrata PDR1, a transcriptional regulator of a pleiotropic drug resistance network, mediates azole resistance in clinical isolates and petite mutants. Antimicrob. Agents Chemother. 50:1384-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van den Hazel, H. B., H. Pichler, M. A. do Valle Matta, E. Leitner, A. Goffeau, and G. Daum. 1999. PDR16 and PDR17, two homologous genes of Saccharomyces cerevisiae, affect lipid biosynthesis and resistance to multiple drugs. J. Biol. Chem. 274:1934-1941. [DOI] [PubMed] [Google Scholar]
- 116.Vermitsky, J. P., K. D. Earhart, W. L. Smith, R. Homayouni, T. D. Edlind, and P. D. Rogers. 2006. Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Mol. Microbiol. 61:704-722. [DOI] [PubMed] [Google Scholar]
- 117.Vermitsky, J. P., and T. D. Edlind. 2004. Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob. Agents Chemother. 48:3773-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Warnock, D. W. 2006. Fungal diseases: an evolving public health challenge. Med. Mycol. 44:697-705. [DOI] [PubMed] [Google Scholar]
- 119.Wemmie, J. A., S. M. Steggerda, and W. S. Moye-Rowley. 1997. The Saccharomyces cerevisiae AP-1 protein discriminates between oxidative stress elicited by the oxidants H2O2 and diamide. J. Biol. Chem. 272:7908-7914. [DOI] [PubMed] [Google Scholar]
- 120.White, T. C. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wong, S., G. Butler, and K. H. Wolfe. 2002. Gene order evolution and paleopolyploidy in hemiascomycete yeasts. Proc. Natl. Acad. Sci. USA 99:9272-9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu, A., and W. S. Moye-Rowley. 1994. GSH1, which encodes γ-glutamylcysteine synthetase, is a target gene for yAP-1 transcriptional regulation. Mol. Cell. Biol. 14:5832-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang, X., Z. Cui, T. Miyakawa, and W. S. Moye-Rowley. 2001. Cross-talk between transcriptional regulators of multidrug resistance in Saccharomyces cerevisiae. J. Biol. Chem. 276:8812-8819. [DOI] [PubMed] [Google Scholar]
- 124.Zhang, X., and W. S. Moye-Rowley. 2001. Saccharomyces cerevisiae multidrug resistance gene expression inversely correlates with the status of the Fo component of the mitochondrial ATPase. J. Biol. Chem. 276:47844-47852. [DOI] [PubMed] [Google Scholar]