Abstract
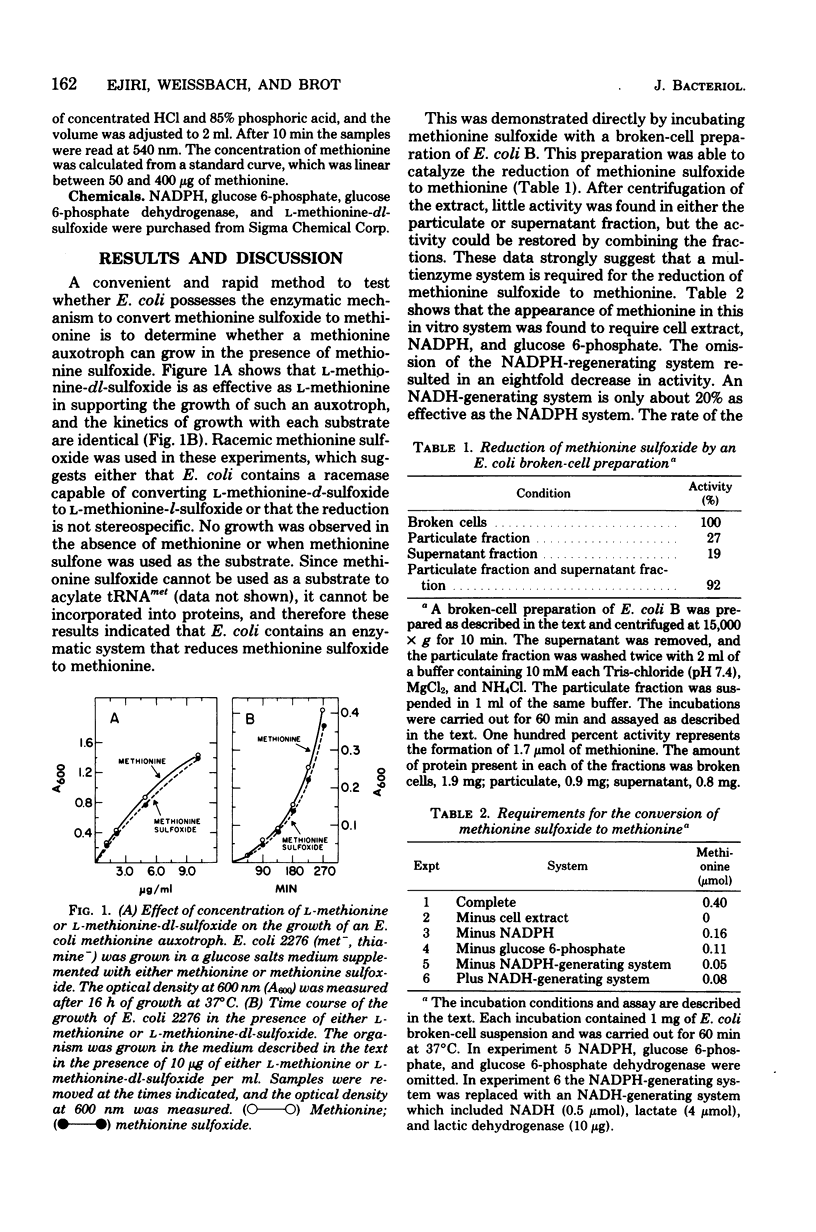
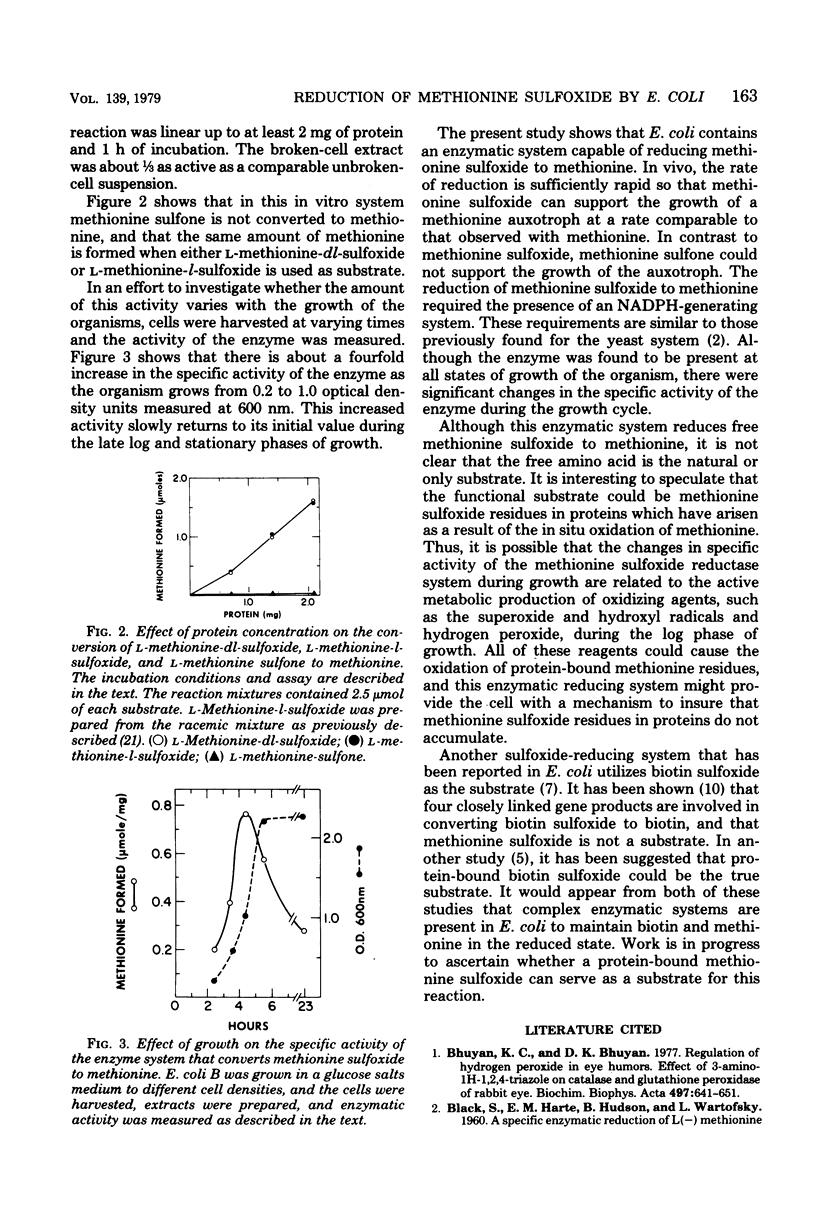
L-Methionine-dl-sulfoxide can support the growth of an Escherichia coli methionine auxotroph, suggesting the presence of an enzyme(s) capable of reducing the sulfoxide to methionine. This was verified by showing that a cell-free extract of E. coli catalyzes the conversion of methionine sulfoxide to methionine. This reaction required reduced nicotinamide adenine dinucleotide phosphate and a generating system for this compound. The specific activity of the enzyme increased during logarithmic growth and was maximal when the culture attained a density of about 10(9) cells per ml.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhuyan K. C., Bhuyan D. K. Regulation of hydrogen peroxide in eye humors. Effect of 3-amino-1H-1,2,4-triazole on catalase and glutathione peroxidase of rabbit eye. Biochim Biophys Acta. 1977 May 26;497(3):641–651. doi: 10.1016/0304-4165(77)90284-7. [DOI] [PubMed] [Google Scholar]
- Brot N., Goodwin J., Fales H. In vivo and in vitro formation of 2,3-dihydroxybenzoylserine by Escherichia coli K12. Biochem Biophys Res Commun. 1966 Nov 22;25(4):454–461. doi: 10.1016/0006-291x(66)90227-0. [DOI] [PubMed] [Google Scholar]
- Caldwell P., Luk D. C., Weissbach H., Brot N. Oxidation of the methionine residues of Escherichia coli ribosomal protein L12 decreases the protein's biological activity. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5349–5352. doi: 10.1073/pnas.75.11.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A., Campillo-Campbell A. D., Barker D. Repression of biotin biosynthesis in Escherichia coli during growth on biotin vitamers. J Bacteriol. 1978 Jul;135(1):90–98. doi: 10.1128/jb.135.1.90-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson F. H., Louw A. I. The oxidation of methionine and its effect of the properties of cardiotoxin VII1 from Naja melanoleuca venom. Biochim Biophys Acta. 1978 Jun 21;534(2):322–330. doi: 10.1016/0005-2795(78)90015-6. [DOI] [PubMed] [Google Scholar]
- Cleary P. P., Dykhuizen D. Enzymatic reduction of D-biotin-d-sulfoxide with cell-free extracts of Escherichia coli. Biochem Biophys Res Commun. 1974 Feb 4;56(3):629–634. doi: 10.1016/0006-291x(74)90651-2. [DOI] [PubMed] [Google Scholar]
- DEDMAN M. L., FARMER T. H., MORRIS C. J. Studies on pituitary adrenocorticotrophin. 3. Identification of the oxidation-reduction centre. Biochem J. 1961 Feb;78:348–352. doi: 10.1042/bj0780348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen D. Genetic analysis of the system that reduces biotin-d-sulfoxide in Escherichia coli. J Bacteriol. 1973 Aug;115(2):662–667. doi: 10.1128/jb.115.2.662-667.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Porqué P., Baldesten A., Reichard P. The involvement of the thioredoxin system in the reduction of methionine sulfoxide and sulfate. J Biol Chem. 1970 May 10;245(9):2371–2374. [PubMed] [Google Scholar]
- Jori G., Galiazzo G., Marzotto A., Scoffone E. Dye-sensitized selective photooxidation of methionine. Biochim Biophys Acta. 1968 Jan 22;154(1):1–9. doi: 10.1016/0005-2795(68)90252-3. [DOI] [PubMed] [Google Scholar]
- Jori G., Galiazzo G., Marzotto A., Scoffone E. Selective and reversibe photo-oxidation of the methionyl residues in lysozyme. J Biol Chem. 1968 Aug 25;243(16):4272–4278. [PubMed] [Google Scholar]
- Kido K., Kassell B. Oxidation of methionine residues of porcine and bovine pepsins. Biochemistry. 1975 Feb 11;14(3):631–635. doi: 10.1021/bi00674a026. [DOI] [PubMed] [Google Scholar]
- Koteliansky V. E., Domogatsky S. P., Gudkov A. T. Dimer state of protein L7/L12 and EF-G-dependent reactions of ribosomes. Eur J Biochem. 1978 Oct;90(2):319–323. doi: 10.1111/j.1432-1033.1978.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Morrissey J. J., Cupp L. E., Weissbach H., Brot N. Synthesis of ribosomal proteins L7L12 in relaxed and stringent strains of Escherichia coli. J Biol Chem. 1976 Sep 25;251(18):5516–5521. [PubMed] [Google Scholar]
- NEUMANN N. P., MOORE S., STEIN W. H. Modification of the methionine residues in ribonuclease. Biochemistry. 1962 Jan;1:68–75. doi: 10.1021/bi00907a011. [DOI] [PubMed] [Google Scholar]
- SCHACHTER H., DIXON G. H. Identification of the methionine involved in the active center of chymotrypsin. Biochem Biophys Res Commun. 1962 Sep 25;9:132–137. doi: 10.1016/0006-291x(62)90101-8. [DOI] [PubMed] [Google Scholar]
- Truscott R. J., Augusteyn R. C. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Biophys Acta. 1977 May 27;492(1):43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]



