Abstract
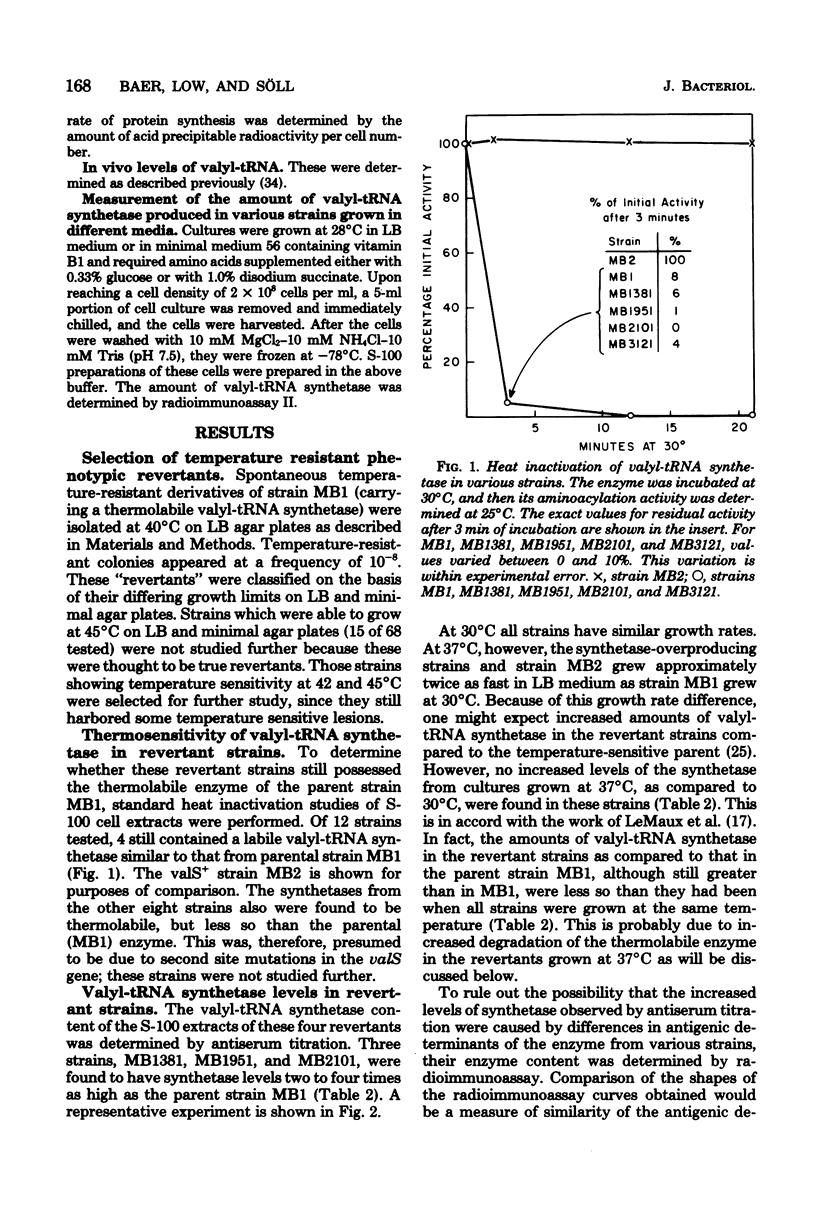
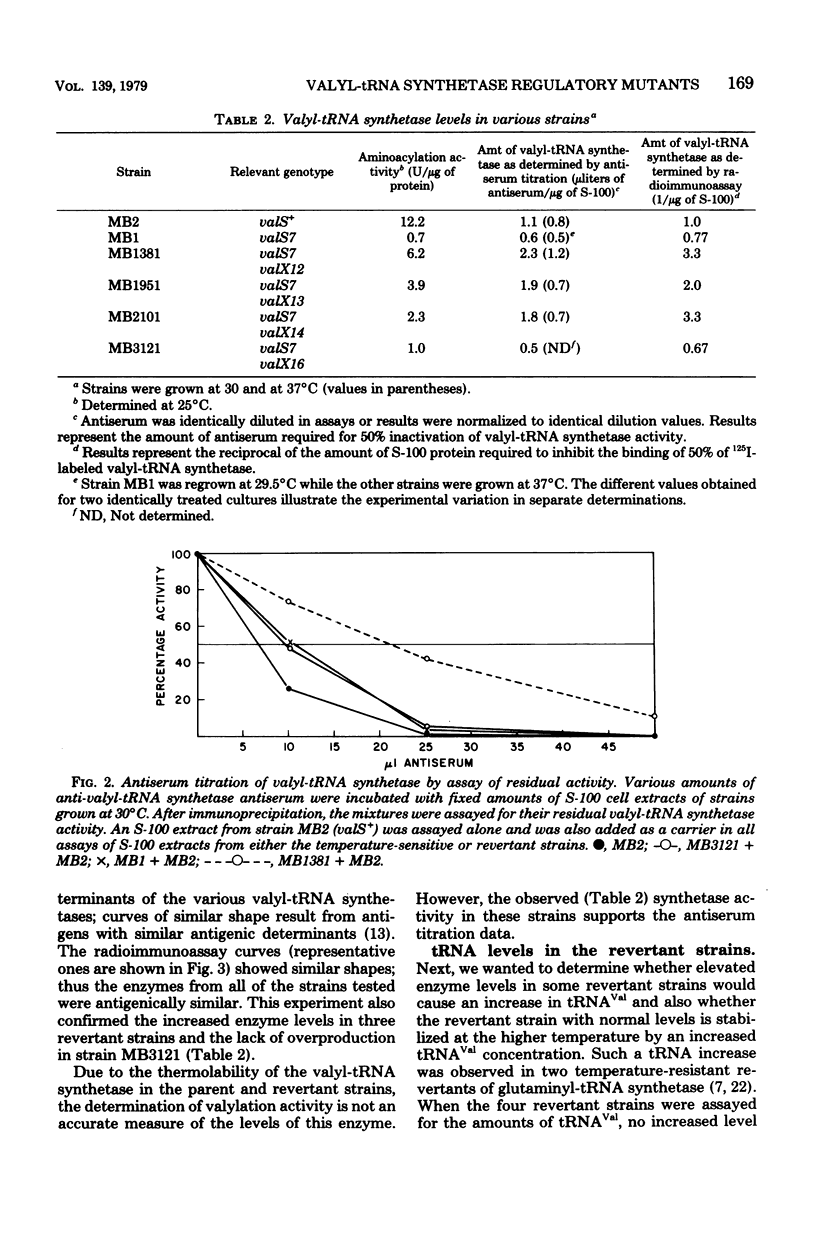
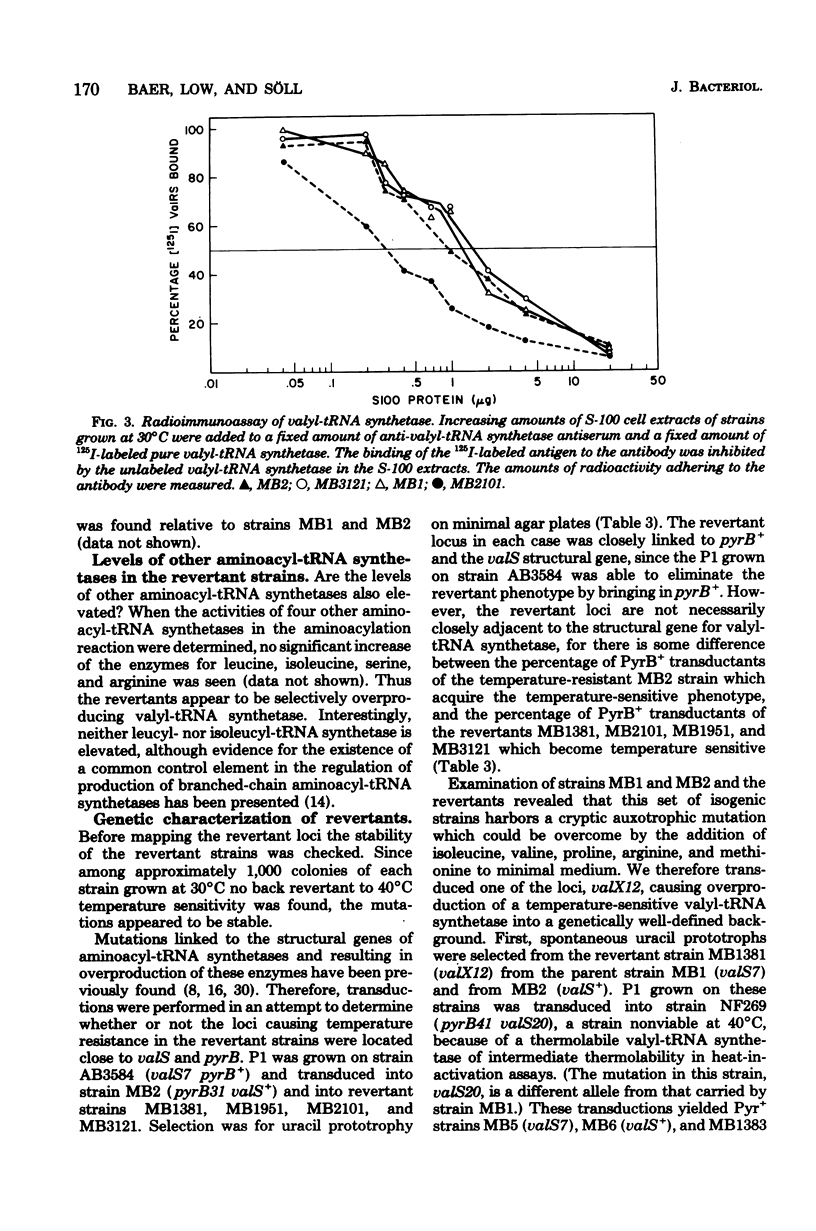
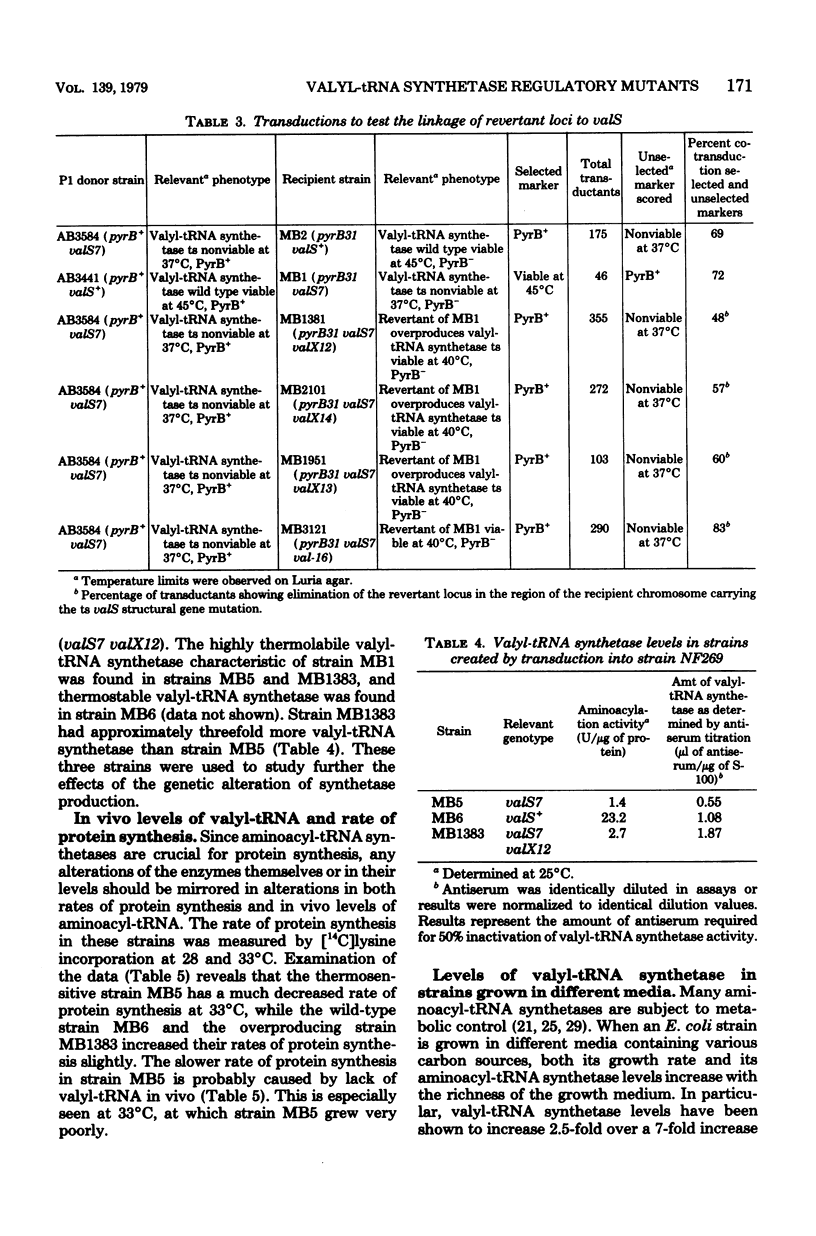
Spontaneous revertants of a temperature-sensitive Escherichia coli strain harboring a thermolabile valyl-transfer ribonucleic acid (tRNA) synthetase were selected for growth at 40 degrees C. Of these, a large number still contain the thermolabile valyl-tRNA synthetase. Three of these revertants contained an increased level of the thermolabile enzyme. The genetic locus, valX, responsible for the enzyme overproduction, is adjacent to the structural gene, valS, of valyl-tRNA synthetase. Determination (by radioimmunoassay) of the turnover rates of valyl-tRNA synthetase showed that the increased level of valyl-tRNA synthetase is due to new enzyme synthesis rather than decreased rates of protein degradation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenase P. W., Leonard E. J. Solid phase radioimmunoassay of human beta 1C globulin. Immunochemistry. 1970 Jan;7(1):29–41. doi: 10.1016/0019-2791(70)90028-5. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Cassio D., Mathien Y., Waller J. P. Enhanced level and metabolic regulation of methionyl-transfer ribonucleic acid synthetase in different strains of Escherichia coli K-12. J Bacteriol. 1975 Aug;123(2):580–588. doi: 10.1128/jb.123.2.580-588.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A., Morgan S., Low K. B., Söll D. Regulation of the biosynthesis of aminoacyl-transfer ribonucleic acid synthetases and of transfer ribonucleic acid in Escherichia coli. VI. Mutants with increased levels of glutaminyl-transfer ribonucleic acid synthetase and of glutamine transfer ribonucleic acid. J Bacteriol. 1979 Jul;139(1):176–184. doi: 10.1128/jb.139.1.176-184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. J., Low B., Konigsberg W. H. Close linkage of the genes serC (for phosphohydroxy pyruvate transaminase) and serS (for seryl-transfer ribonucleic acid synthetase) in Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1091–1095. doi: 10.1128/jb.113.3.1091-1095.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V., Wittel F. P. Effect of the RelA gene on the synthesis of individual proteins in vivo. Cell. 1976 May;8(1):115–122. doi: 10.1016/0092-8674(76)90192-6. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Hirshfield I. N., Zamecnik P. C. Thiosine-resistant mutants of Escherichia coli K-12 with growth-medium-dependent lysl-tRNA synthetase activity. I. Isolation and physiological characterization. Biochim Biophys Acta. 1972 Feb 15;259(3):330–343. [PubMed] [Google Scholar]
- Jackson J., Williams L. S., Umbarger H. E. Regulation of synthesis of the branched-chain amino acids and cognate aminoacyl-transfer ribonucleic acid synthetases of Escherichia coli: a common regulatory element. J Bacteriol. 1974 Dec;120(3):1380–1386. doi: 10.1128/jb.120.3.1380-1386.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRossa R., Vögell G., Low K. B., Söll D. Regulation of biosynthesis of aminoacyl-tRNA synthetases and of tRNA in Escherichia coli. II. Isolation of regulatory mutants affecting leucyl-tRNA synthetase levels. J Mol Biol. 1977 Dec 25;117(4):1033–1048. doi: 10.1016/s0022-2836(77)80011-9. [DOI] [PubMed] [Google Scholar]
- Lemaux P. G., Herendeen S. L., Bloch P. L., Neidhardt F. C. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. doi: 10.1016/0092-8674(78)90317-3. [DOI] [PubMed] [Google Scholar]
- Low B., Gates F., Goldstein T., Söll D. Isolation and partial characterization of temperature-sensitive Escherichia coli mutants with altered leucyl- and seryl-transfer ribonucleic acid synthetases. J Bacteriol. 1971 Nov;108(2):742–750. doi: 10.1128/jb.108.2.742-750.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeever W. G., Neidhardt F. C. Growth rate modulation of four aminoacyl-transfer ribonucleic acid synthetases in enteric bacteria. J Bacteriol. 1976 May;126(2):634–645. doi: 10.1128/jb.126.2.634-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan S. D., Söll D. Regulation of the biosynthesis of aminoacid: tRNA ligases and of tRNA. Prog Nucleic Acid Res Mol Biol. 1978;21:181–207. doi: 10.1016/s0079-6603(08)60270-6. [DOI] [PubMed] [Google Scholar]
- Morgan S., Körner A., Low K. B., Söll D. Regulation of biosynthesis of aminoacyl-tRNA synthetases and of tRNA in Escherichia coli. I. Isolation and characterization of a mutant with elevated levels of tRNAGln 1. J Mol Biol. 1977 Dec 25;117(4):1013–1031. doi: 10.1016/s0022-2836(77)80010-7. [DOI] [PubMed] [Google Scholar]
- Nass G., Thomale J. Alteration of structure of level of threonyl-tRNA-synthetase in Borrelidin resistant mutants of E. coli. FEBS Lett. 1974 Feb 15;39(2):182–186. doi: 10.1016/0014-5793(74)80046-3. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neihardt F. C., Parker J., McKeever W. G. Function and regulation of aminoacyl-tRNA synthetases in prokaryotic and eukaryotic cells. Annu Rev Microbiol. 1975;29:215–250. doi: 10.1146/annurev.mi.29.100175.001243. [DOI] [PubMed] [Google Scholar]
- Paetz W., Nass G. Biochemical and immunological characterization of threonyl-tRNA synthetase of two borrelidin-resistant mutants of Escherichia coli K12. Eur J Biochem. 1973 Jun;35(2):331–337. doi: 10.1111/j.1432-1033.1973.tb02843.x. [DOI] [PubMed] [Google Scholar]
- Parker J., Flashner M., Mckeever W. G., Neidhardt F. C. Metabolic regulation of the arginyl and valyl transfer ribonucleic acid synthetases in bacteria. J Biol Chem. 1974 Feb 25;249(4):1044–1053. [PubMed] [Google Scholar]
- Parker J., Neidhardt F. C. Metabolic regulation of aminoacyl-tRNA synthetase formation in bacteria. Biochem Biophys Res Commun. 1972 Oct 17;49(2):495–501. doi: 10.1016/0006-291x(72)90438-x. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., McKitrick J., Tosa T. Characterization of a mutant of E. coli with elevated levels of seryl-tRNA synthetase. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1351–1357. doi: 10.1016/0006-291x(72)90615-8. [DOI] [PubMed] [Google Scholar]
- Platt T., Miller J. H., Weber K. In vivo degradation of mutant lac repressor. Nature. 1970 Dec 19;228(5277):1154–1156. doi: 10.1038/2281154a0. [DOI] [PubMed] [Google Scholar]
- Quay S. C., Oxender D. L. Regulation of amino acid transport in Escherichia coli by transcription termination factor rho. J Bacteriol. 1977 Jun;130(3):1024–1029. doi: 10.1128/jb.130.3.1024-1029.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theall G., Low K. B., Söll D. Regulation of the biosynthesis of aminoacyl-tRNA synthetases and of tRNA in Escherichia coli. IV. Mutants with increased levels of leucyl- or seryl-tRNA synthetase. Mol Gen Genet. 1979 Jan 31;169(2):205–211. doi: 10.1007/BF00271672. [DOI] [PubMed] [Google Scholar]
- Theall G., Low K. B., Söll D. Suppression of a defective alanyl-tRNA synthetase in Escherichia coli: a compensatory mutation to high alanine affinity. Mol Gen Genet. 1977 Nov 14;156(2):221–227. doi: 10.1007/BF00283495. [DOI] [PubMed] [Google Scholar]
- Zipser D., Bhavsar P. Missense mutations in the lacZ gene that result in degradation of beta-galactosidase structural protein. J Bacteriol. 1976 Sep;127(3):1538–1542. doi: 10.1128/jb.127.3.1538-1542.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



