Abstract
Cobinamide (Cbi) salvaging is impaired, but not abolished, in a Salmonella enterica strain lacking a functional cobU gene. CobU is a bifunctional enzyme (NTP:adenosylcobinamide [NTP:AdoCbi] kinase, GTP:adenosylcobinamide-phosphate [GTP:AdoCbi-P] guanylyltransferase) whose AdoCbi kinase activity is necessary for Cbi salvaging in this bacterium. Inactivation of the ycfN gene in a ΔcobU strain abrogated Cbi salvaging. Introduction of a plasmid carrying the ycfN+ allele into a ΔcobU ΔycfN strain substantially restored Cbi salvaging. Mass spectrometry data indicate that when YcfN-enriched cell extracts were incubated with AdoCbi and ATP, the product of the reaction was AdoCbi-P. Results from bioassays confirmed that YcfN converted AdoCbi to AdoCbi-P in an ATP-dependent manner. YcfN is a good example of enzymes that are used by the cell in multiple pathways to ensure the salvaging of valuable precursors.
Many bacteria, archaea, and eukaryotes (including humans) have enzymes that require adenosylcobalamin (AdoCbl) (also known as coenzyme B12) as a cofactor, yet synthesis of AdoCbl is limited to archaea and bacteria (29). The gram-negative enterobacterium Salmonella enterica serovar Typhimurium LT2 (hereafter referred to as serovar Typhimurium) synthesizes AdoCbl de novo only under anoxic conditions but can salvage exogenous, preformed corrinoids (e.g., cobinamide [Cbi], cobyric acid [Cby]) from the environment under oxic and anoxic conditions (15). When Cbi or Cby enters the cell, it is adenosylated by the housekeeping corrinoid adenosyltransferase CobA enzyme, yielding adenosylcobinamide (AdoCbi) or adenosylcobyric acid (AdoCby), respectively (13, 23). The adenosylated precursor is converted to AdoCbl by the enzymes of the nucleotide loop assembly pathway (12, 17) (Fig. 1).
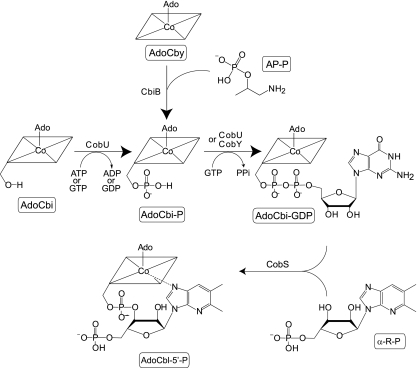
FIG. 1.
Nucleotide loop assembly pathway in Salmonella enterica serovar Typhimurium LT2. Intermediates are boxed and shown below structures; enzyme names are shown above or left of arrows. Abbreviations: AP-P, R-1-amino-2-propanol O-2-phosphate; PPi, pyrophosphate; α-R-P, α-ribazole-5′-phosphate; CbiB, putative AdoCbi-P synthase; CobU, ATP/GTP:AdoCbi kinase, GTP:AdoCbi-P guanylyltransferase; CobS, AdoCbl (5′-phosphate) synthase.
The CobU enzyme of the nucleotide loop assembly pathway is bifunctional (ATP/GTP:AdoCbi kinase, GTP:AdoCbi-phosphate [GTP:AdoCbi-P] guanylyltransferase) (19) and is important for de novo synthesis and for Cbi salvaging. The AdoCbi kinase activity is necessary only for Cbi salvaging, while the GTP:AdoCbi-P guanylyltransferase activity is required both for de novo synthesis of AdoCbl and for Cbi/Cby salvaging (26).
Archaea lack CobU (26). In lieu of CobU, archaea use CobY (ATP/GTP:AdoCbi-P nucleotidyltransferase) for de novo synthesis of AdoCbl and CbiZ (AdoCbi amidohydrolase) to salvage Cbi (32, 33). CbiZ converts Cbi to Cby (32), which, when condensed with aminopropanol-phosphate, yields AdoCbi-P (8), the substrate for CobY (26, 33) (Fig. 1).
De novo synthesis of AdoCbl can be restored in a ΔcobU mutant strain of serovar Typhimurium when a plasmid carrying the archaeal cobY gene is introduced into the strain (26).
Here, we report that a ΔcobU/pcobY+ strain of serovar Typhimurium can salvage Cbi, albeit at a reduced rate, and that the ability of the ΔcobU strain to salvage Cbi is due to the existence of an alternate AdoCbi kinase enzyme in this bacterium. Results from in vivo experiments identified the ycfN gene as the one encoding the alternate AdoCbi kinase enzyme, and results from in vitro experiments support the idea that YcfN is responsible for the observed in vivo phosphorylation of AdoCbi.
MATERIALS AND METHODS
Microbiological techniques. (i) Bacteria, culture media, and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. All serovar Typhimurium strains carry a null allele of the metE gene that encodes the cobalamin (Cbl)-independent methionine synthase (MetE) enzyme. metE strains require exogenous methionine to grow. Alternatively, a metE strain can use the Cbl-dependent methionine synthase (MetH) enzyme to methylate homocysteine when Cbl is available (25). Serovar Typhimurium strains were cultured in nutrient broth (NB; Difco), while Escherichia coli strains were cultured in lysogeny broth (3, 4). Plasmids were maintained by the addition of ampicillin (100 μg/ml), chloramphenicol (20 μg/ml), kanamycin (50 μg/ml), or tetracycline (20 μg/ml) to NB. No-carbon essential (NCE) medium (28) supplemented with glycerol (30 mM) and MgSO4 (1 mM) was used to grow cells under nutrient-defined conditions. Solid media contained 15 g of Bacto agar (Difco) per liter. Corrinoids were added to media at 15 nM. Cyanocobyric acid was a gift from Paul Renz (Institut für Biologische Chemie und Ernahrungswissenschaft, Universität-Hohenheim, Stuttgart, Germany); dicyanocobinamide [(CN)2Cbi] and cyanocobalamin (CNCbl) were purchased from Sigma.
TABLE 1.
Strains and plasmids used in these studies
| Strain or plasmid | Relevant genotype and/or description | Reference or source |
|---|---|---|
| Strains | ||
| Salmonella entericaa | ||
| TR6583 (formerly SA2929) | metE205 ara-9 | K. Sanderson via J. Roth |
| Derivatives of TR6583 | ||
| JE7127 | ΔcobUST ycfN121::Tn10d(tet+)/pCOBY35 | |
| JE7178 | ΔcobUST272/pCOBY35 | Laboratory collection |
| JE8249 | ΔcobU1315 | |
| JE8268 | ΔcobU1315 ΔycfN112 | |
| JE8308 | ΔcobU1315/pSU39 | |
| JE8309 | ΔcobU1315/pCOBY38 | |
| JE8310 | ΔcobU1315 ΔycfN112/pJO52 | |
| JE8312 | ΔcobU1315 ΔycfN112/pCOBY38 | |
| JE8313 | ΔcobU1315 ΔycfN112/pCLK740 | |
| JE8314 | ΔcobU1315 ΔycfN112/pCOBY38 pET-16b | |
| JE8315 | ΔcobU1315 ΔycfN112/pCOBY38 pCLK740 | |
| JE8407 | ΔcobU1315 ΔycfN112/pCLK740 pTara | |
| JE8408 | ΔcobU1315 ΔycfN112/pET-16b pTara | |
| Escherichia coli | ||
| BL21(DE3) | B F−ompT hsdS (rB− mB−) dcm gal λ(DE3) | Invitrogen |
| Plasmids | ||
| pBAD33 | Cloning vector; cat+ | 14 |
| pCLK740 | E. coli ycfN+ cloned into plasmid pET-16b | 18 |
| pCOBA17 | cobA+ in pET-15b; encodes His6-CobA | 9 |
| pCOBY35 | S. enterica cobST+Methanosarcina mazei cobY+ (cobYST+) cloned into plasmid pBAD33 | Laboratory collection |
| pCOBY38 | M. mazei cobY+ cloned into plasmid pSU39 | 31 |
| pET-16b | N-terminal His10 tag vector; bla+ | Novagen |
| pGP1-2 | T7 RNA polymerase+kan+ | 24 |
| pJO52 | cobU+ cloned into plasmid pT7-7 | 19 |
| pSU39 | Cloning vector; kan+ | 2 |
| pT7-7 | Cloning vector; bla+ | 24 |
| Ptara | T7 RNA polymerase+cat+ | 34 |
All strains are derivatives of S. enterica serovar Typhimurium strain LT2. Unless otherwise stated, strains were constructed for this study.
Genetics and recombinant DNA techniques. (i) Transposon mutagenesis.
Phage P22 HT105/1 int-201 (21, 22) was grown on a pool of ∼100,000 serovar Typhimurium strains, each carrying one transposition-defective Tn10d(tet+) element (30) in the chromosome. The resulting phage lysate was used as a donor to transduce strain JE7178 (ΔcobUST/pcobYST+) to tetracycline resistance (Tcr) on nutrient agar-tetracycline plates. Tcr transductants were screened by replica printing for the loss of the ability to salvage (CN)2Cbi.
(ii) Strain construction: construction of a ΔcobU ΔycfN strain of serovar Typhimurium.
A chromosomal in-frame deletion of the cobU gene was constructed in strain TR6583 as described previously (11). For this purpose, we used primers OL25 (5′-ATG ATG ATT CTG GTG ACG GGC GGG GCA CGT AGT GGT AAA AGC CGT CAT GCT GTG TAG GCT GGA GCT GCT TC-3′) and OL26 (5′-TCA TTT AAT TTT GAC TCC AAT ACC TGA GAC TAC CAG CCA GAC CTC ATC CGC CAT ATG AAT ATC CTC CTT AG-3′); the resulting strain was JE8249 (ΔcobU). An in-frame deletion of the ycfN gene was constructed in strain JE8249 by using primers OL27 (5′-GTG CGG TCC AAC AAC AAT AAT CCC TTA ACG CGC GAC GAG ATC CTG TCG CGC GTG TAG GCT GGA GCT GCT TC-3′) and OL28 (5′-TTA TCC TTT CAT ACG TAG CTG GCG CCA GGT TTC ATC GGC CAG CCT GAT AAA CAT ATG AAT ATC CTC CTT AG-3′); the resulting strain was JE8268 (ΔcobU ΔycfN). Both in-frame deletions were verified by DNA sequencing using BigDye protocols (ABI PRISM). Reaction mixtures were resolved by the University of Wisconsin—Madison Biotechnology Center.
(iii) Complementation of AdoCbi kinase activity.
Plasmids pCLK740 (ycfN+) and pCOBY38 (cobY+) were electroporated into strain JE8268 (ΔcobU ΔycfN) to assess (CN)2Cbi salvaging; the resulting strain was JE8315. Plasmid pET-16b was used as a negative control. Plasmids pET-16b and pCOBY38 were electroporated into strain JE8268, yielding strain JE8314. Isolated colonies of strains tested were inoculated into NB and incubated at 37°C overnight (∼16 h). A 5-μl aliquot of the overnight culture was diluted into 195 μl of NCE medium as described above and incubated at 37°C with shaking in a 96-well microtiter plate for 32 h using an ELx808 Ultra microplate reader (Bio-Tek Instruments).
Biochemical techniques. (i) Protein overproduction and purification. (a) His6-CobA protein.
A 100-ml volume of lysogeny broth supplemented with ampicillin was inoculated with a fresh transformant of E. coli strain BL21(DE3) (Novagen) carrying plasmid pCOBA17 (cobA+) (9) and incubated at 37°C with shaking at 200 rpm for approximately 18 h. Cells were harvested by centrifugation at 4°C at 15,000 × g for 15 min using a JA-25.50 rotor in an Avanti J-25I Beckman/Coulter refrigerated centrifuge and resuspended in 5 ml of lysis buffer (His-Bind binding buffer [Novagen] containing protease inhibitor phenylmethylsulfonyl fluoride [1 mM] and BugBuster [Novagen]). After a 20-min incubation at room temperature with occasional mixing, cell extract was obtained by centrifugation at 4°C at 43,000 × g for 30 min as described above.
Particulates were removed from the cell extract by filtration through a 0.2-μm filter, and His6-tagged CobA protein (His6-CobA) was purified over a His-Bind Quick 900 cartridge (Novagen) equilibrated with binding buffer according to the manufacturer's protocol. EDTA was added to the eluted protein to a final concentration of 5 mM to remove excess nickel.
Protein concentration was determined using the Bradford method (7), and the protein was concentrated to 1 to 2 mg/ml using a Centriprep centrifugal filter unit with an Ultracel YM-10 membrane (Millipore). Protein was transferred to SnakeSkin dialysis tubing with a 10,000-Da molecular-mass cutoff (Pierce) and dialyzed against a series of five 750-ml buffer changes: (i) Tris hydrochloride buffer (Tris-HCl [50 mM, pH 8.0, at 4°C]) containing EDTA (10 mM) for approximately 12 h, (ii) Tris-HCl buffer (50 mM, pH 8.0, at 4°C) containing EDTA (5 mM) for 2 h, and (iii) Tris-HCl buffer (50 mM, pH 8.0, at 4°C) containing NaCl (100 mM) for 2 h. The third dialysis step was performed thrice. Dithiothreitol (DTT; 1 mM) and glycerol (10% [vol/vol] final concentration) were added to the protein, and the final protein concentration was determined using the Bradford method (7). Two-hundred-microliter samples of dialyzed His6-CobA protein (>95% homogeneous [data not shown]) were stored at −80°C until use.
(b) CobU protein.
CobU protein was overproduced and purified as described previously (27), with the following modifications. Cells from a 4-liter culture of the overproducing strain were lysed using a French press operating at 138 × 103 kPa. After the addition of ammonium sulfate (9.6% saturation at 4°C; UltraPure; ICN Biomedicals), we used fast protein liquid chromatography (ÁKTA explorer; GE Healthcare) to isolate CobU protein from the clarified cell extract in two additional steps. Step 1, hydrophobic interaction chromatography, was performed as follows. Cell extract was applied to two 5-ml HiTrap Phenyl (high-sub) FastFlow (high substitution) columns (GE Healthcare) connected in tandem and equilibrated with buffer A [Tris-HCl buffer (0.1 M, pH 8.0, at 4°C) containing DTT (10 mM), glycerol (10%, vol/vol), and (NH4)2SO4 (9.6%, wt/vol)]. Protein was eluted off the columns at a flow rate of 2 ml per min using a linear gradient to 100% buffer B (Tris-HCl buffer [0.1 M Tris, pH 8.0, at 4°C] containing DTT [10 mM]). CobU was found in the flowthrough fractions. Step 2, dye ligand chromatography, was performed as follows. CobU-containing fractions from the phenyl column purification step were pooled, dialyzed against buffer C (Tris-HCl buffer [0.1 M, pH 8.0, at 4°C] containing DTT [10 mM] and MgCl2 [5 mM]), and applied to a 5-ml HiTrap Blue HP column (GE Healthcare) equilibrated with buffer C. Protein was eluted at a flow rate of 1 ml per min with a linear gradient to 100% buffer D (buffer C containing NaCl [2 M]). CobU was found in the flowthrough fractions.
CobU-containing fractions were pooled and concentrated in a Centricon Plus-80 device with an Ultracel PL membrane (Millipore). Concentrated CobU protein was dialyzed against HEPES (10 mM, pH 7.5) containing NaCl (100 mM) and DTT (10 mM). Protein concentration was determined by the Bradford method (7). CobU protein (>95% homogeneous [data not shown]) was flash frozen in liquid N2 and stored at −80°C until use.
(c) His10-YcfN.
The E. coli YcfN protein was overproduced fused to an amino-terminal decahistidine (His10) tag. The synthesis of His10-YcfN protein was directed by plasmid pCLK740 (kindly provided by T. Begley) in serovar Typhimurium strain JE8407 [ΔcobU ΔycfN/pCLK740 (ycfN+)/pTara (T7 rpo+)]. One-hundred-milliliter cultures were grown at 37°C to an optical density at 600 nm (OD600) of ∼0.5, and IPTG (isopropyl-β-d-thiogalactopyranoside) was added to 0.5 mM to induce ycfN+ expression; incubation at 37°C continued for ∼16 h after IPTG induction. Cells were pelleted and resuspended in 5 ml of Tris-HCl buffer (0.1 M, pH 8.0, at 37°C) containing phenylmethylsulfonyl fluoride (1 mM). Cells were placed on ice and lysed by sonication for 2 min (5-s pulse followed by 10 s of cooling) in a model 550 sonic dismembrator (Fisher). The His10-YcfN protein was observed only in the insoluble fraction as a band that migrated with an approximate mass of 34 kDa and was estimated by densitometry to constitute 5% of the total protein.
(ii) Assessment of protein purity.
Protein concentration was determined by the Bradford method (7). Purity was assessed by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (16), followed by staining with Coomassie brilliant blue (20). Protein purity was established by band densitometry using a computer-controlled Fotodyne imaging system with Foto/Analyst version 5.00 software (Fotodyne Incorporated) for image acquisition and TotalLab version 2005 software (Nonlinear Dynamics) for analysis.
(iii) Synthesis of corrinoid substrates. (a) AdoCbi.
AdoCbi was prepared enzymatically as reported previously (19), except that 1 mg of purified His6-CobA enzyme was added to the reaction mixture in lieu of crude cell extract. AdoCbi was purified from the reaction mixture under red light, using a Sep-Pak C18 cartridge (Waters) preequilibrated with double-distilled water (ddH2O). The sample was eluted with 6 ml of 100% methanol and dried under a vacuum in a SpeedVac concentrator (Thermo Savant).
(b) AdoCbi-P.
All synthesis and purification steps were performed under red light. AdoCbi was resuspended in 10 ml of Tris-HCl buffer (100 mM, pH 8.5, at 25°C) containing MgCl2 (25 mM). ATP (4 mM) and CobU protein (3 mg) were added to the reaction mixture, which was incubated at 37°C for 2 h. AdoCbi-P was resolved from AdoCbi by high-performance liquid chromatography (HPLC) using a System Gold HPLC system (Beckman/Coulter) equipped with an Alltima HP C18 AQ 5-μm column (150 by 4.6 mm) (Alltech). We employed System II for corrinoid separation (described elsewhere) without modifications (5).
(iv) In vitro assay for ATP:AdoCbi kinase activity.
The reaction was performed under red light to avoid photolysis of the C—Co bond of the AdoCbi substrate. Reaction mixtures contained Tris-HCl buffer (0.1 M, pH 8.0, at 37°C) containing MgCl2 (5 mM), ATP (2 mM), His10-YcfN-enriched cell extract or cell extract obtained from cells carrying the empty cloning vector (25 μg), and AdoCbi (0.2 mM). The final volume of the reaction mixture was 20 μl. Reaction mixtures were incubated at 37°C for 16 h. Reactions were stopped by the addition of 2.5 μl of KCN (100 mM) to derivatize the adenosylated compounds to their dicyanated forms, followed by heating at 80°C for 10 min. Reaction mixtures were centrifuged for 1 min at 14,000 × g in a Beckman Coulter Microfuge 18 centrifuge to remove denatured protein. Formation of (CN)2Cbi-phosphate [(CN)2Cbi-P] was detected using the bioassay described below.
(v) Bioassay for detection of (CN)2Cbi-P.
A bioassay was used to detect the presence of (CN)2Cbi-P in reaction mixtures. Strain JE8309 [ΔcobU ΔycfN/pCOBY38 (cobY+)] was used as an indicator strain. Cells in 1 ml of an overnight NB culture were washed three times with sterile saline and resuspended in 1 ml of sterile saline. A 400-μl aliquot of saline-washed cells (1 ml) was added to 5 ml of molten 0.7% (wt/vol) agar and overlaid on NCE agar plates supplemented with glucose (11 mM). A 5-μl aliquot of the in vitro reaction mixture was spotted onto the overlay; equal volumes of 7.5 μM CNCbl and (CN)2Cbi-P (authentic standards) were spotted as positive controls. Growth was assessed after incubation at 37°C for 16 h.
(vi) MS.
The remaining product of the in vitro Cbi kinase reaction mixture (15 μl) was loaded onto a Sep-Pak C18 cartridge (Waters) preequilibrated with ddH2O. Purified corrinoids were eluted off the resin with 2.5 ml of 100% (vol/vol) methanol, and the sample was dried under a vacuum in a SpeedVac concentrator (Thermo Savant). The dried sample was resuspended in 15 μl of ddH2O; a 5-μl sample was removed and analyzed by mass spectrometry (MS) at the Mass Spectrometry Facility of the University of Wisconsin—Madison Biotechnology Center. The remainder of the mixture was treated with shrimp alkaline phosphatase (SAP; Fermentas) according to the manufacturer's instructions, loaded onto a preequilibrated Sep-Pak C18 cartridge (Waters), and eluted and dried as described above. The dried sample was analyzed by MS. Mass spectra were obtained using a Bruker Daltronics (Billerica, MA) BILFLEX III matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) MS.
RESULTS AND DISCUSSION
Cbi salvaging is abolished in a strain lacking cobU and ycfN functions.
As mentioned above, the archaeal CobY protein has the guanylyltransferase activity associated with CobU but lacks the kinase activity required to salvage Cbi (26). In the absence of CobU, serovar Typhimurium cannot convert the precursor AdoCby or AdoCbi to AdoCbl (Fig. 1). Consistent with previous results, a serovar Typhimurium ΔcobU strain carrying plasmid pCOBY38 (cobY+) (JE8309) efficiently salvaged Cby (Table 2). Unexpectedly, strain JE8309 also salvaged (CN)2Cbi, albeit less efficiently than strain TR6583 (cobU+) (Fig. 2). Because CobY does not have AdoCbi kinase activity and its substrate is AdoCbi-P (26), the ability of the strain to salvage (CN)2Cbi suggested the existence of an alternate AdoCbi kinase in serovar Typhimurium. To identify the alternate AdoCbi kinase, we performed transposon mutagenesis on strain JE7178 (ΔcobUST/pcobYST+), using the method described above. We isolated one tetracycline-resistant (Tcr) strain that failed to salvage (CN)2Cbi. The location of the mini-Tn10 element was determined by sequencing the DNA flanking the insertion as described previously (10) and matching the DNA sequence to a region of the serovar Typhimurium genome (1). This search showed that the transposon in strain JE7127 [ΔcobUST ycfN121:Tn10d(tet+)/pcobYST+] was located within the ycfN gene.
TABLE 2.
Doubling times of ΔcobU mutantsa
| Strain | Relevant genotype | Doubling time (min) |
|---|---|---|
| TR6583 | cobU+ycfN+ | 52 ± 2 |
| JE8308 | ΔcobU/pSU39 | NG |
| JE8310 | ΔcobU ΔycfN/pcobU+ | 51 ± 1 |
| JE8309 | ΔcobU/pcobY+ | 54 ± 2 |
Doubling times of cultures grown in NCE glycerol medium supplemented with 15 nM Cby. Doubling times were calculated graphically from semilog plots of OD595 as a function of time and are shown as arithmetic means ± standard errors of the means (n = 3). NG, no growth. pSU39 is the empty vector control. pCOBY38 is pcobY+ (cobY+ cloned into pSU39). pJO52 is pcobU+ (cobU+ cloned into pT7-7).
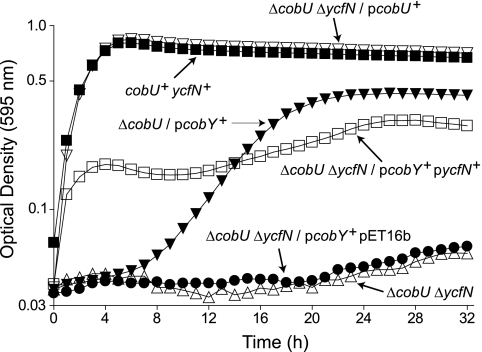
FIG. 2.
YcfN allows a ΔcobU mutant to salvage Cbi. Results are shown for Cbl-dependent growth of serovar Typhimurium strains in NCE glycerol medium containing (CN)2Cbi (15 nM) at 37°C. Strains used are methionine auxotrophs that must synthesize Cbl to satisfy their requirement for methionine. Growth is reported as OD595 as a function of time. Strains are indicated by their relative genotypes. Strains used were TR6583 (cobU+ ycfN+), JE8268 (ΔcobU ΔycfN), JE8309 (ΔcobU/pcobY+), JE8310 (ΔcobU ΔycfN/pcobU+), JE8314 [(ΔcobU ΔycfN/pcobY+ pET16b; empty-vector control)], and JE8315 (ΔcobU ΔycfN/pcobY+ pycfN+).
The chromosomal organization of S. enterica suggests that ycfN is in an operon. To confirm that the inability of strain JE7127 to salvage (CN)2Cbi was due to the inactivation of ycfN and not due to polar effects of the mini-Tn10 insertion, an in-frame deletion of the ycfN gene was generated in strain JE8249 (ΔcobU), yielding strain JE8268 (ΔcobU ΔycfN). The latter strain failed to salvage (CN)2Cbi (Fig. 2). Introduction of both cobY and the E. coli ycfN+ gene into strain JE8268 (yielding strain JE8315 [ΔcobU ΔycfN/pcobY+ pycfN+]) resulted in a substantial restoration of (CN)2Cbi salvaging, whereas introduction of cobY and the empty cloning vector (yielding strain JE8314 [ΔcobU ΔycfN/pcobY+ pET-16b]) did not (Fig. 2). Taken together, these results showed that, in a ΔcobU strain, the inactivation or deletion of ycfN was sufficient to abrogate the ability of serovar Typhimurium to salvage (CN)2Cbi and suggested that YcfN was the alternate AdoCbi kinase.
In vitro evidence that the YcfN protein has AdoCbi kinase activity.
We performed in vitro AdoCbi kinase assays to show that YcfN phosphorylated AdoCbi directly. YcfN protein was overproduced in strain JE8407 (ΔcobU ΔycfN) as an N-terminal His10 fusion protein (His10-YcfN), which, as reported earlier, was mainly insoluble (data not shown) (18). We used affinity chromatography in an attempt to isolate soluble His10-YcfN protein. Cells from a 1-liter culture of strain JE8407 [ΔcobU ΔycfN/pCLK740 (ycfN+)/pTara (T7 rpo+)] were lysed as described above, and the soluble fraction was passed over a His-Bind Quick 900 cartridge (Novagen) according to the manufacturer's protocol. The eluted fraction was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by silver staining. We were unable to detect soluble His10-YcfN protein (data not shown.) The eluted fraction also did not have AdoCbi kinase activity in vitro.
These results suggest that the amino terminus of the fusion protein may not be surface exposed, rendering the protein unable to bind to the affinity column, or that the purification process abrogated the protein's activity. Therefore, for our assays, we used His10-YcfN-enriched, unresolved cell extracts.
Reaction mixtures included ATP, AdoCbi, MgCl2, and either His10-YcfN-enriched extract or cell extract obtained from a strain carrying the empty cloning vector (pET-16b) used to overexpress ycfN. The reaction was allowed to proceed for 16 h at 37°C. The presence of AdoCbi-P in the reaction products was detected with a bioassay that used strain JE8309 (ΔcobU ΔycfN/pcobY+) as an indicator. Strain JE8309 is a methionine auxotroph that synthesizes methionine via the Cbl-dependent methionine synthase (MetH) enzyme. Because serovar Typhimurium requires very few (<100) molecules of Cbl per cell to satisfy its requirement for methionine (6), the bioassay is an extremely sensitive measure of Cbl synthesis. Cell growth around the site of application indicated synthesis of Cbl from AdoCbi-P through the pathway described in Fig. 1. Authentic CNCbl (vitamin B12) and HPLC-purified (CN)2Cbi-P were used as positive controls. The product of the His10-YcfN-containing reaction supported growth, while the empty-vector control did not (Fig. 3A, inset).
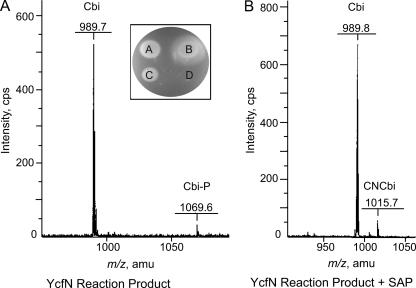
FIG. 3.
The product of the YcfN reaction is Cbi-phosphate. Shown are the MALDI-TOF mass spectra and the results of bioassays used to identify the product of the in vitro YcfN reaction. (A) YcfN reaction product. Signals with m/z values of 989.7 and 1069.6 were consistent with the molecular masses of Cbi (990.1) and Cbi-P (1069.1), respectively. (Inset) Five microliters of the YcfN reaction product or 5 μl of 75 μM HPLC-purified corrinoids was used in the bioassay. A, HPLC-purified (CN)2Cbi-P; B, authentic (CN)Cbl (vitamin B12); C, YcfN reaction product; D, empty-vector control reaction product. (B) YcfN reaction product treated with SAP. Signals with m/z values of 989.8 and 1015.7 were consistent with the molecular masses of Cbi without (990.1) and with (1016.1) an upper cyano ligand, respectively. amu, atomic mass units.
The YcfN reaction product was cyanated, purified, and submitted to the Mass Spectrometry (MS) Facility at the University of Wisconsin—Madison Biotechnology Center for analysis. The MALDI-TOF mass spectrum of the YcfN reaction product contained a molecular ion signal with an m/z of 1069.6 atomic mass units, consistent with Cbi-P lacking an upper ligand (Fig. 3A). Loss of the upper ligand is frequently, but not always, observed during MS analysis. After treatment with SAP and subsequent repurification, the molecular ion signal shifted to an m/z of 1015.7 atomic mass units, consistent with Cbi with an upper cyano ligand (Fig. 3B). Together, the bioassay and MS data confirmed that YcfN phosphorylated AdoCbi in vitro.
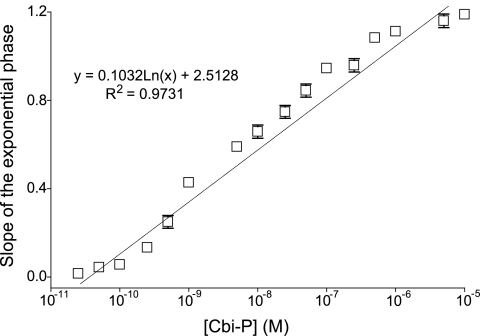
We used a bioassay to quantify the amount of AdoCbi-P synthesized by YcfN in vitro. For this purpose, we grew strain JE8315 in minimal medium containing various concentrations of (CN)2Cbi-P, and the kinetics of growth was monitored at 595 nm as a function of time (data not shown). The slopes of the exponential phase of the growth curves were plotted against the concentrations of (CN)2Cbi-P in the medium, and a best-fit line was drawn (Fig. 4). Using the equation of this line, we determined the concentration of (CN)2Cbi-P in the reaction mixture that contained His10-YcfN and all the other substrates needed to phosphorylate AdoCbi. Using the above-described bioassay, we estimated the concentration of (CN)2Cbi-P in the reaction mixture to be 370 pM ± 28 pM (average for three determinations).
FIG. 4.
Growth rate of the ΔcobU ΔycfN/pcobY+ mutant correlates with Cbi-phosphate concentration in the medium. Growth in NCE glycerol medium supplemented with various concentrations of Cbi-P was monitored at 595 nm. Slopes of the exponential phase growth rates were calculated graphically from semilog plots of OD595 as a function of time. Slopes are shown as a function of concentration of Cbi-P in the medium (n = 3). Equation of the best-fit line and the coefficient of determination (R2 value) were found using Microsoft Excel.
In summary, we have identified the YcfN protein as a nonspecific AdoCbi kinase enzyme. This activity of YcfN is not associated with AdoCbl biosynthesis, but as shown, it allows a CobU-deficient strain of serovar Typhimurium to synthesize enough AdoCbl from Cbi so that the cell can grow. YcfN is required for the phosphorylation of thiamine in the thiamine-salvaging pathway of E. coli. Given the obvious structural differences between thiamine and Cbi, we speculate that the corrin ring of Cbi does not interact with YcfN and that most likely the active site is close to the surface of the enzyme.
Acknowledgments
This work was supported by PHS grant R01-GM40313 to J.C.E.-S.
We thank T. Begley (Cornell University) for the plasmid encoding the E. coli His10-YcfN protein and P. Renz for his gift of cyanocobyric acid.
Footnotes
Published ahead of print on 10 August 2007.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Bartolomé, B., Y. Jubete, E. Martínez, and F. de la Cruz. 1991. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102:75-78. [DOI] [PubMed] [Google Scholar]
- 3.Bertani, G. 2004. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J. Bacteriol. 186:595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanche, F., D. Thibaut, M. Couder, and J. C. Muller. 1990. Identification and quantitation of corrinoid precursors of cobalamin from Pseudomonas denitrificans by high-performance liquid chromatography. Anal. Biochem. 189:24-29. [DOI] [PubMed] [Google Scholar]
- 6.Bradbeer, C. 1982. Cobalamin transport in microorganisms, p. 31-56. In D. Dolphin (ed.), B12, vol. 2. John Wiley & Sons, New York, NY. [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-255. [DOI] [PubMed] [Google Scholar]
- 8.Brushaber, K. R., G. A. O'Toole, and J. C. Escalante-Semerena. 1998. CobD, a novel enzyme with L-threonine-O-3-phosphate decarboxylase activity, is responsible for the synthesis of (R)-1-amino-2-propanol O-2-phosphate, a proposed new intermediate in cobalamin biosynthesis in Salmonella typhimurium LT2. J. Biol. Chem. 273:2684-2691. [DOI] [PubMed] [Google Scholar]
- 9.Buan, N. R., and J. C. Escalante-Semerena. 2005. Computer-assisted docking of flavodoxin with the ATP:Co(I)rrinoid adenosyltransferase (CobA) enzyme reveals residues critical for protein-protein interactions but not for catalysis. J. Biol. Chem. 280:40948-40956. [DOI] [PubMed] [Google Scholar]
- 10.Caetano-Anolles, G. 1993. Amplifying DNA with arbitrary oligonucleotide primers. PCR Methods Appl. 3:85-94. [DOI] [PubMed] [Google Scholar]
- 11.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escalante-Semerena, J. C. 2007. Conversion of cobinamide into adenosylcobamide in bacteria and archaea. J. Bacteriol. 189:4555-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escalante-Semerena, J. C., S. J. Suh, and J. R. Roth. 1990. cobA function is required for both de novo cobalamin biosynthesis and assimilation of exogenous corrinoids in Salmonella typhimurium. J. Bacteriol. 172:273-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeter, R. M., B. M. Olivera, and J. R. Roth. 1984. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J. Bacteriol. 159:206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage and structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Maggio-Hall, L. A., and J. C. Escalante-Semerena. 1999. In vitro synthesis of the nucleotide loop of cobalamin by Salmonella typhimurium enzymes. Proc. Natl. Acad. Sci. USA 96:11798-11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melnick, J., E. Lis, J.-H. Park, C. Kinsland, H. Mori, T. Baba, J. Perkins, G. Schyns, O. Vassieva, A. Osterman, and T. P. Begley. 2004. Identification of the two missing bacterial genes involved in thiamine salvage: thiamine pyrophosphokinase and thiamine kinase. J. Bacteriol. 186:3660-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Toole, G. A., and J. C. Escalante-Semerena. 1995. Purification and characterization of the bifunctional CobU enzyme of Salmonella typhimurium LT2. Evidence for a CobU∼GMP intermediate. J. Biol. Chem. 270:23560-23569. [DOI] [PubMed] [Google Scholar]
- 20.Sasse, J. 1991. Detection of proteins, p. 10.6.1-10.6.8. In F. A. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 1. Wiley Interscience, New York, NY. [Google Scholar]
- 21.Schmieger, H. 1971. A method for detection of phage mutants with altered transducing ability. Mol. Gen. Genet. 110:378-381. [DOI] [PubMed] [Google Scholar]
- 22.Schmieger, H., and H. Bakhaus. 1973. The origin of DNA in transducing particles of P22 mutants with increased transduction frequencies (HT-mutants). Mol. Gen. Genet. 120:181-190. [DOI] [PubMed] [Google Scholar]
- 23.Suh, S., and J. C. Escalante-Semerena. 1995. Purification and initial characterization of the ATP:corrinoid adenosyltransferase encoded by the cobA gene of Salmonella typhimurium. J. Bacteriol. 177:921-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor, R. T., and H. Weissbach. 1973. N5-methylenetetrahydrofolate-homocysteine methyltransferases, p. 121-165. In P. D. Boyer (ed.), The enzymes, vol. 9. Academic Press, Inc., New York, NY. [Google Scholar]
- 26.Thomas, M. G., and J. C. Escalante-Semerena. 2000. Identification of an alternative nucleoside triphosphate: 5′-deoxyadenosylcobinamide phosphate nucleotidyltransferase in Methanobacterium thermoautotrophicum ΔH. J. Bacteriol. 182:4227-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson, T. B., M. G. Thomas, J. C. Escalante-Semerena, and I. Rayment. 1998. Three-dimensional structure of adenosylcobinamide kinase/adenosylcobinamide phosphate guanylyltransferase from Salmonella typhimurium determined to 2.3 A resolution. Biochemistry 37:7686-7695. [DOI] [PubMed] [Google Scholar]
- 28.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithase of Escherichia coli: partial purification, and some properties. J. Biol. Chem. 218:97-106. [PubMed] [Google Scholar]
- 29.Warren, M. J., E. Raux, H. L. Schubert, and J. C. Escalante-Semerena. 2002. The biosynthesis of adenosylcobalamin (vitamin B12). Nat. Prod. Rep. 19:390-412. [DOI] [PubMed] [Google Scholar]
- 30.Way, J. C., M. A. Davis, D. Morisato, D. E. Roberts, and N. Kleckner. 1984. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene 32:369-379. [DOI] [PubMed] [Google Scholar]
- 31.Woodson, J. D., and J. C. Escalante-Semerena. 2006. The cbiS gene of the archaeon Methanopyrus kandleri AV19 encodes a bifunctional enzyme with adenosylcobinamide amidohydrolase and α-ribazole-phosphate phosphatase activities. J. Bacteriol. 188:4227-4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodson, J. D., and J. C. Escalante-Semerena. 2004. CbiZ, an amidohydrolase enzyme required for salvaging the coenzyme B12 precursor cobinamide in archaea. Proc. Natl. Acad. Sci. USA 101:3591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodson, J. D., C. L. Zayas, and J. C. Escalante-Semerena. 2003. A new pathway for salvaging the coenzyme B12 precursor cobinamide in archaea requires cobinamide-phosphate synthase (CbiB) enzyme activity. J. Bacteriol. 185:7193-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wycuff, D. R., and K. S. Matthews. 2000. Generation of an AraC-araBAD promoter-regulated T7 expression system. Anal. Biochem. 277:67-73. [DOI] [PubMed] [Google Scholar]