Abstract
The Aer receptor guides Escherichia coli to specific oxygen and energy-generating niches. The input sensor in Aer is a flavin adenine dinucleotide-binding PAS domain, which is separated from a HAMP/signaling output domain by two membrane-spanning segments that flank a short (four-amino-acid) periplasmic loop. In this study, we determined the overall membrane organization of Aer by introducing combinations of residues that allowed us to differentiate intradimeric collisions from interdimeric collisions. Collisions between proximal residues in the membrane anchor were exclusively intra- or interdimeric but, with one exception, not both. Cross-linking profiles were consistent, with a rigid rather than flexible periplasmic loop and a tilted TM2 helix that crossed TM2′ at residue V197C, near the center of the lipid bilayer. The periplasmic loop formed a stable neighborhood that (i) included a maximum of three Aer dimers, (ii) did not swap neighbors over time, and (iii) appeared to be constrained by interactions in the cytosolic signaling domain.
The aerotaxis (oxygen-seeking) receptor Aer senses environmental oxygen levels indirectly by sensing changes in the redox state of its flavin adenine dinucleotide (FAD) cofactor (12). Unlike conventional Escherichia coli chemoreceptors, Aer (i) has a cytosolic, FAD-binding PAS domain, (ii) exhibits methylation-independent adaptation (6, 25), (iii) lacks a periplasmic ligand-binding domain (5, 31), and (iv) attaches to the membrane through two membrane-spanning segments that flank a four-amino-acid periplasmic loop (2). Conventional chemoreceptor dimers have a periplasmic ligand-binding domain that is connected to the cytosolic signaling domain through two transmembrane (TM) segments (TM1 and TM2) per monomer. These pairs of TM segments form a four-helix bundle, with cognate TM1 and TM1′ segments closer together than cognate TM2 and TM2′ segments (13, 18, 30). In response to chemoattractants, which bind asymmetrically to a homodimer, the TM2 (or TM2′) segment moves downward 1 to 2 Å towards the cytosol (see reference 13 and references therein). This movement propagates to a conserved signaling domain some 200 Å away (38). The C-terminal signaling mechanism is likely shared by the Aer receptor despite its unique features, because it exhibits several hallmarks of conventional chemoreceptors, including a HAMP domain (23), a conserved signaling domain (5, 31), formation of dimers (24), and control of flagellar rotation through the chemotaxis two-component system (7, 15, 17, 33, 39).
Although the ligand-binding and signaling unit of chemoreceptors is a dimer, receptors organize into larger squads consisting of trimers of dimers (1, 21). These complexes may be either homogeneous or mixed, and their purpose may be to amplify the signal emanating from one dimer (1, 8, 11, 28). This organization also occurs in Aer, and, given the low copy number of Aer, it is likely that nearly all Aer dimers in wild-type cells form mixed trimers of dimers (14). This arrangement not only helps amplify Aer signals but also appears to rescue several signaling defects in Aer (14).
Previously, we measured disulfide cross-linking between 48 introduced cysteines to determine the basic membrane topology and boundaries of the Aer receptor (2). In that study, cysteine disulfide scanning revealed sparse cross-linking between monomers in the TM segments and no obvious periodicity that would be expected for helix-helix interactions. In the current study, we found a reasonable explanation for the previous data by differentiating intradimeric collisional faces from interdimeric collisional faces. At expression levels of Aer that were approximately the same as the sum of the levels for the other chemoreceptors (50-fold above Aer chromosomal levels), we identified cysteine replacements in the periplasmic/membrane region of Aer that collided exclusively within dimers and others that collided exclusively between Aer dimers. We expanded the analysis to study the overall organization of the Aer receptor. The periplasmic/membrane anchor region formed a stable neighborhood that included as many as, but not more than, three dimers. However, the stability of this region was controlled by cytosolic interactions in the signaling domain, as Aer truncations missing this region had increased degrees of freedom.
MATERIALS AND METHODS
Bacterial strains and plasmids.
BT3312 (aer tsr) (32) and BT3388 (aer tsr tar tap trg) (40) are derivatives of E. coli strain RP437, which is wild type for aerotaxis (29). Plasmid pMB1 was derived from pGH1 and expresses a cysteineless Aer protein (2). Plasmid pGH1 expresses wild-type Aer and was derived from pTrc99A, an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible expression vector (31).
Single cysteine replacements in Aer were made by site-directed mutagenesis using pMB1 as described previously (2). When necessary, these products were used as templates to create double- and triple-cysteine replacements. All cysteine replacement mutants were inoculated onto semisolid succinate motility plates containing 100 μg ml−1 ampicillin to assess aerotactic behavior as described previously (12). C-terminal truncations in Aer were created in pDA1 (Table 1) using PCR with a forward primer complementary to the SphI site of pTrc99A nucleotide 2962 (pTrcSphIF) (36). This primer was paired with reverse primers Aer285SphIR (36), Aer240SphIR, and Aer220SphIR (not shown) individually to amplify fragments of aer and introduce a stop codon (UAA) after residues 285, 240, and 220, respectively, followed by an engineered SphI site. PCR products were digested with SphI and ligated to the 2.64-kb SphI fragment of pDA1 to make the corresponding pDA2, pDA3, and pDA4 plasmids (Table 1). BT3312 and/or BT3388 was transformed with these plasmids, and expression was confirmed by Western blot analysis using antisera against His6-Aer2-166 (32). The introduced mutations were confirmed by DNA sequencing. Although FAD binding was not measured for truncated peptides, we previously determined that His6-Aer2-285 bound FAD, whereas His6-Aer2-231 did not (16). These data are consistent with the finding that all Aer peptides truncated prior to residue 260 do not bind FAD (4).
TABLE 1.
Summary of plasmids and data
| Plasmid | Properties | Cross-linked productsa | Source or reference |
|---|---|---|---|
| pTrc99A | IPTG-inducible ptrc expression vector | None | Pharmacia |
| pGH1 | pTrc99A Aer+[1-506] | M2 | 31 |
| pMB1 | pGH1 Aer[C193S/C293A/C253A] [Cys-less] | None | 2 |
| pDA1 | pMB1 Aer[A184C/V187C] | M2, M4, M6 | This study |
| pDA2 | pDA1 Aer1-285 | M2, M3, M4, M5, M6 | This study |
| pDA3 | pDA1 Aer1-240 | M2, M3, M4, M5 | This study |
| pDA4 | pDA1 Aer1-220 | M2, M3, M4, M5 | This study |
| pDA5 | pMB1 Aer[A184C/V260C] | M2 | This study |
| pDA6 | pMB1 Aer[V187C/V260C] | M2, M4, M6 | This study |
| pDA7 | pMB1 Aer[I191C/A184C] | M2, M4, M6 | This study |
| pDA8 | pMB1 Aer[I191C/V187C] | M2 | This study |
| pDA9 | pMB1 Aer[I191C/V260C] | M2, M4, M6 | This study |
| pDA10 | pMB1 Aer[I191C/V197C] | M2, M4, M6 | This study |
| pDA11 | pMB1 Aer[V197C/V187C] | M2, M4, M6 | This study |
| pDA12 | pMB1 Aer[V197C/V260C] | M2 | This study |
| pDA13 | pMB1 Aer[V168C/A184C] | M2, M4, M6 | This study |
| pDA14 | pMB1 Aer[V168C/V187C] | M2 | This study |
| pDA15 | pMB1 Aer[A185C/I191C] | M2, M4, M6 | This study |
| pDA16 | pMB1 Aer[A185C/V260C] | M2, M4, M6 | This study |
| pDA17 | pMB1 Aer[P186C/I191C] | M2, M4, M6 | This study |
| pDA18 | pMB1 Aer[P186C/V260C] | M2 | This study |
| pDA19 | pMB1 Aer[A184C/A377C] | M2, M4, M6 | This study |
| pDA20 | pMB1 Aer[A184C/A379C] | M2, M4, M6 | This study |
| pDA21 | pMB1 Aer[V187C/A379C] | M2, M4, M6 | This study |
| pDA22 | pMB1 Aer[A184C/G380C]b | M2, M4, M6 | This study |
| pDA23 | pMB1 Aer[A184C/E381C] | M2, M4, M6 | This study |
| pDA24 | pMB1 Aer[A362C/A403C] | M2, M4, M6 | This study |
| pDA25 | pMB1 Aer[A184C/V187C/A377C] | M2, M4, M6 | This study |
| pDA26 | pMB1 Aer[A184C/V187C/A379C] | M2, M4, M6 | This study |
| pDA27 | pMB1 Aer[A184C/V187C/A362C] | M2, M4, M6 | This study |
| pDA28 | pMB1 Aer[A184C/V187C/E381C] | M2, M4, M6 | This study |
| pDA29 | pMB1 Aer[A184C/V187C/A403C] | M2, M4, M6 | This study |
CuPhe-mediated patterns on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. M2, dimers; M3, trimers; M4, tetramers; M5, pentamers; M6, hexamers.
This Aer mutant did not mediate aerotaxis.
In vivo cross-linking using copper phenanthroline.
For in vivo cross-linking, 80 μl of cells was mixed with 80 μl of 0.6 mM Cu(II)-(1,10-phenanthroline)3 (CuPhe), incubated at the desired temperature (4, 23, or 30°C) for various time intervals (from 1 to 30 min), and quenched with 40 μl of 5× stop solution (2). Samples were boiled for 5 min and placed on ice. For the zero-time control, water was added in place of CuPhe and immediately quenched with stop solution. To confirm that free sulfhydryls were properly blocked by the stop solution, a parallel control was treated with 2.5 mM N-ethylmaleimide (NEM) (Sigma, St. Louis, MO) 10 min before the addition of CuPhe. Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotted as described previously (32). Aer was identified using antisera against Aer2-166 under conditions where aer strains yielded no bands (2).
RESULTS
Interactions between periplasmic loops from different dimers.
We recently reported that the membrane anchor of Aer has two TM segments (TM1 [residues 164 to 183] and TM2 [residues 188 to 205]) that flank a short periplasmic loop (residues 184 to 187) (2). From the previous study, it appeared likely that the periplasmic loop was flexible and dynamic, because each periplasmic cysteine replacement cross-linked with its cognate residue at 4°C (2), and “intradimeric” collisions between four consecutive residues could not have occurred unless the loop was highly flexible. However, it was also possible that one or more of these residues cross-linked with an introduced cysteine from a different dimer. In this case the loop might not need to be flexible for all residues to cross-link. To differentiate intradimeric collisions from interdimeric collisions in the periplasm, we chose a second residue in Aer that is cytosolic and on the dimer interface and could therefore be used as a reference for intradimeric collisions. The cysteine replacement V260C is the strongest cross-linker in the Aer segment between the TM2/HAMP boundary (residue 206) and residue 290 in the signaling region of Aer (K. Watts, unpublished observation). In cysteine scanning studies, oxidized V260C cross-links rapidly in vivo and maps to the interface within a dimer (K. Watts, unpublished data).
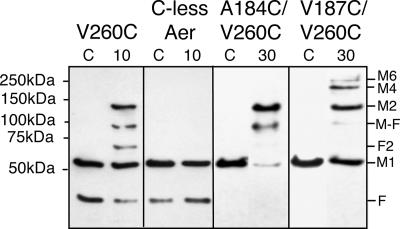
Using cystosolic V260C as a reference for intradimeric cross-linking, we paired this residue with each of the four cysteine replacements residing in the periplasmic loop. We first tested the double-cysteine replacements Aer-A184C/V260C and Aer-V187C/V260C, since residues A184C and V187C are the two strongest cross-linkers in the periplasmic loop (2). We reasoned that dimers would form exclusively if both V260C and the periplasmic cysteine cross-linked within a dimer but that larger oligomers would form if the periplasmic cysteine was able to cross-link between dimers. We expressed Aer-A184C/V260C and Aer-V187C/V260C in BT3312 (aer tsr) and verified that they orchestrated normal aerotaxis on succinate motility plates. We then conducted disulfide cross-linking in whole cells with the oxidant CuPhe. As shown in Fig. 1, Aer-A184C/V260C formed dimers exclusively, whereas Aer-V187C/V260C formed dimers and multimers when cells were incubated for 30 min with CuPhe. If either A184C or V260C were able to cross-link interdimerically, it is likely that we would have observed multimers on Western blots, as both residues are strong cross-linkers. Our interpretation is that both A184C and V260C cross-link exclusively within a dimer. In contrast, Aer-V187C/V260C formed multimers, indicating that V187C must be able to cross-link between dimers. At this point, however, we could not exclude the possibility that V187C was also cross-linking within a dimer.
FIG. 1.
Periplasmic cysteine replacement A184C, but not V187C, collided exclusively within an Aer dimer. Both periplasmic residues (A184C and V187C) were individually paired with V260C, which is on the cytosolic dimer interface. Aer-A184C/V260C formed only dimers, while Aer-V187C/V260C formed multimers. Plasmid-bearing BT3312 cells were incubated with 0.3 mM CuPhe at 23°C for 10 min (lane 10) or 30 min (lane 30) before quenching with 2.5 mM (final concentration) NEM. Untreated cells were quenched immediately as a zero-time control (lane C). Samples were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels under nonreducing conditions and Western blotted as described in Materials and Methods. Abbreviations: F, an endogenously formed proteolytic fragment of Aer (24); M1, full-length Aer monomer; F2, fragment dimer, which does not form in Aer that lacks cysteines (C-less Aer); M-F, dimer between a monomer and proteolytic fragment F; M2, Aer dimer; M4, Aer tetramer; M6, Aer hexamer.
To determine the specificity of the other two periplasmic cysteine replacements that formed disulfide bonds, we paired A185C and P186C with V260C. Whereas Aer-P186C/V260C formed dimers exclusively, Aer-A185C/V260 formed a small amount (<10%) of multimers (data not shown). This indicated that A185C, but not P186C, could collide and cross-link to some extent with adjacent dimers.
Organization of the TM domain.
The finding that periplasmic loop residues V187C and A185C can cross-link between dimers provided a basis for interpreting the previous cross-linking data for the membrane anchor region (2). In the previous study, TM2 cysteine residues I191C and V197C were the only cysteines introduced within TM2 that cross-linked between cognate residues (2). This indicated that cognate TM2-TM2′ helices do not form parallel neighboring helices, because cross-linking would have occurred with a characteristic periodicity (22, 27). Instead, TM2 and TM2′ helices may be tilted relative to one another and intersect at V197C. If so, residues I191C and V197C would not cross-link within the same dimer.
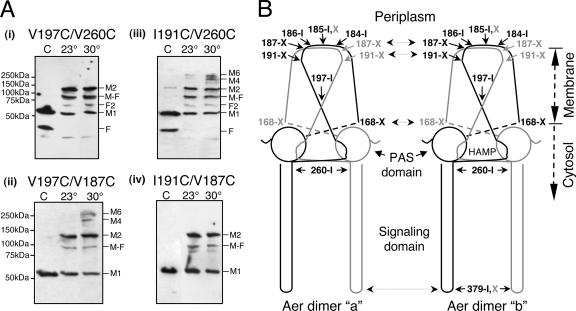
To test this model, we performed two sets of experiments with double-cysteine replacements pairing either TM2 residue V197C (in one set) or residue I191C (in the other set) with (i) a residue at the dimer interface (periplasmic residue A184C or proximal signaling residue V260C) or (ii) a residue capable of cross-linking between dimers (periplasmic residue V187C). These modified Aer proteins mediated normal behavior when they were expressed in BT3312 (aer tsr) cells and assayed on succinate motility plates. The cells expressing the Aer mutant proteins were subjected to oxidative cross-linking at 23°C and also at 30°C for 30 min. The higher temperature and longer incubation time increased the number of collisions and therefore the probability of interdimeric cross-linking; approximately 1 in 105 cysteine-cysteine collisions are trapped as a disulfide bond (10).
Figure 2A shows the cross-linking results for four representative double-cysteine combinations in Aer. Their approximate positions, as well as those of other introduced cysteines, are mapped in Fig. 2B. Dimers formed exclusively (Fig. 2A, panel i) with oxidized Aer-V197C/V260C (or Aer-V197/A184C [data not shown]), indicating that V197C cross-linked to the same monomer as A184C and V260C (i.e., within a dimer). Consistent with these results, Aer-V197C/V187C formed higher-order multimers (Fig. 2A, panel ii), as one would expect if V197C cross-linked within a dimer and V187C were able to cross-link between dimers.
FIG. 2.
Mapping inter- and intradimeric collisional faces in Aer dimers. (A) Representative Western blots demonstrating the strategy used to differentiate intradimeric cross-linking from interdimeric cross-linking. Intact cells expressing Aer proteins with introduced cysteine residues were treated with CuPhe for 30 min at 23°C (lane 23°) or 30°C (lane 30°) as described in the text. Lane C contained the control. (Panel i) Exclusive dimer formation with oxidized Aer-V197C/V260C. (Panel ii) Multimer formation at 30°C with oxidized Aer-V197C/V187C. (Panel iii) Multimer formation with oxidized Aer-I191C/V260C. (Panel iv) Exclusive dimer formation with oxidized Aer-I191C/V187C. Abbreviations are defined in the legend to Fig. 1. (B) Cartoon of two unfolded and flattened Aer dimers summarizing collisional faces deduced from disulfides formed from introduced cysteine residues. Abbreviations: I, exclusive intradimeric cross-linkers; X, exclusive extradimeric cross-linkers; I,X, intra- and extradimeric cross-linker. All interpretations are based on the initial assumption that V260C in the cytosolic region cross-links within dimers.
We paired I191C, the other cross-linking residue in the TM2 region, with residue V260C (or A184C), which resides at the dimer interface. Aer-I191C/V260C (or Aer-I191C/A184C [data not shown]) cross-linked to form multimers (Fig. 2A, panel iii), indicating that I191C (unlike V197C) can cross-link between dimers. In contrast, Aer-I191C/V187C formed dimers but no higher-order multimers (Fig. 2A, panel iv), indicating that both I191C and V187C cross-link exclusively between dimers. From these data, we inferred that TM2 residues I191C and V197C have different collisional faces. This conclusion was further supported by pairing I191C with V197C; Aer-I191C/V197C formed multimers (data not shown), like Aer-V197C/V187C (Fig. 2A, panel ii), as expected. Among the periplasmic loop residues, residue A185C was unique. It formed multimers when it was paired with the interdimeric cross-linker I191C, indicating that it could cross-link both within and between dimers (Table 1).
Using the same strategy, we determined that residue V168C, which is near the cytosolic boundary of TM1, forms interdimeric cross-links. Both Aer-V168C/A184C and Aer-V168C/V260C formed multimers, but Aer-V168C/V187C formed dimers exclusively (Table 1).
Periplasmic neighborhood can form stable hexamers.
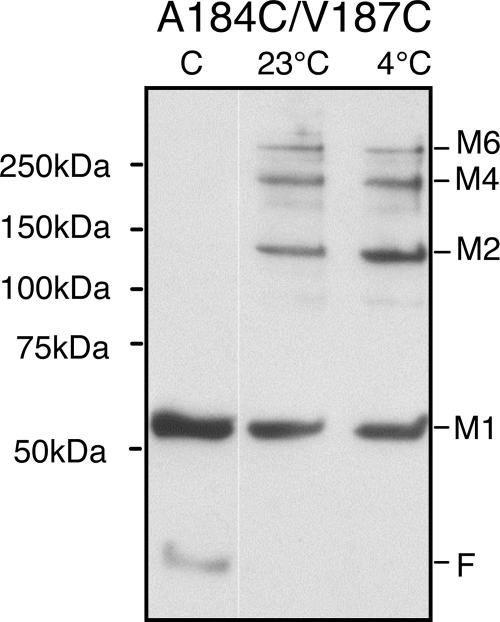
Although the collisional specificity of residues in the periplasmic loop was consistent with a structured loop, it was possible that some flexibility was still required for intra- or interdimeric contacts. If so, disulfide bond formation within dimers might prevent subsequent cross-linking between dimers (or vice versa). To address this question, we introduced two cysteine replacements that reside on opposite faces of the periplasmic loop. We chose residues A184C and V187C because they are the strongest cross-linkers in the periplasmic loop and they collide exclusively with different monomers. We expressed Aer-A184C/V187C in BT3312 (aer tsr), confirmed that it orchestrated normal aerotaxis on succinate motility plates, and initiated disulfide cross-linking in whole cells with CuPhe. As shown in Fig. 3, Aer monomers cross-linked as dimers, tetramers, and hexamers, indicating that neither of the periplasmic disulfide bonds hindered cross-linking of the other. Notably, multimers were trapped both at 23°C and at 4°C, which is below the lipid phase transition temperature in E. coli (26). We assumed that lateral mobility in the membrane was inhibited at 4°C, since several cysteine replacements that cross-link at 23°C do not cross-link at 4°C (2). The percentages of total Aer trapped in different forms at 23 and 4°C were similar (Aer1-506[A184C/V187C] in Fig. 4C). A 25-min time course at 23°C (not shown) indicated that the reaction was complete at 1 min, suggesting that the local neighborhood was relatively stable and dimers were not swapping neighbors.
FIG. 3.
Periplasmic loop forms a neighborhood of hexamers: Western blot showing disulfide trapping of Aer monomers containing the two cysteine replacements, A184C and V187C, located in the periplasmic loop. Intact cells expressing the mutant protein were incubated without CuPhe (control) (lane C), with 0.3 mM CuPhe at 23°C for 10 min, or with 0.3 mM CuPhe at 4°C for 20 min before quenching with 2.5 mM (final concentration) NEM. Abbreviations are defined in the legend to Fig. 1.
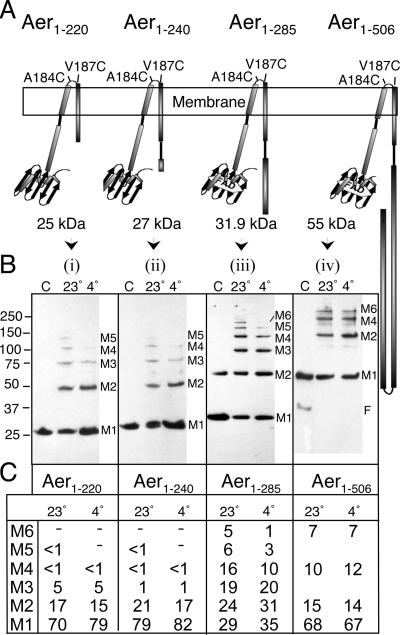
FIG. 4.
Cytosolic signaling domain limits the degrees of freedom in the periplasmic neighborhood. (A) Cartoons representing the Aer-A184C/V187C truncations used for this analysis. (B) Western blots showing the distribution of multimers formed from truncations in response to incubation with 0.3 mM CuPhe for 10 min at 23°C (lane 23°) and for 20 min at 4°C (lane 4°). Lane C contained control cells not treated with CuPhe. (C) Percentage of total Aer-A184C/V187C trapped in each multimeric state for each construct. The numbers in M1 to M6 indicate the number of monomers trapped in each multimeric state. A dash indicates that the fraction of Aer in the form was below the threshold for accurate quantitation.
Signaling region of Aer limits the movement of periplasmic residues.
Previous crystallographic (21) and disulfide trapping studies (34) with chemoreceptors showed that the stability of trimers of dimers is promoted by interdimeric contacts in the signaling region. To test the influence of the signaling region on periplasmic collisions in Aer, we constructed truncated derivatives of Aer-A184C/V187C (Fig. 4A). These included (i) Aer1-285[A184C/V187C], which contains all of the HAMP and proximal signaling region (23) and binds an FAD cofactor (16, 24); (ii) Aer1-240[A184C/V187C], which lacks most of the AS-2 subdomain of the HAMP region (23, 37) and probably does not bind FAD (4, 16) (see Materials and Methods); and (iii) Aer1-220[A184C/V187C], which is truncated for all of the HAMP-AS-2 region and does not bind FAD (16).
Removing the signaling region (Aer1-285[A184C/V187C]) changed the profile of the multimers so that it included trimers and pentamers (Fig. 4B, panel iii, lane 23°) in addition to dimers, tetramers, and hexamers. The odd-numbered complexes must have contained un-cross-linked A184C residues, unlike complexes from full-length Aer1-506[A184C/V187C], which ostensibly formed dimers, tetramers, and hexamers exclusively (Fig. 4B, panel iv). Thus, removing the signaling region must have increased the degrees of freedom for an Aer monomer. Larger truncations in the C terminus that removed part (Fig. 4B, panel ii) or all (Fig. 4B, panel i) of the HAMP-AS-2 region decreased the proportion of trapped multimers, particularly when trapping was measured at 4°C (Fig. 4b, panels i and ii). At 23°C, hexamers were still evident for both mutants, but the level was too low to accurately determine it. Thus, the periplasmic neighborhood in Aer appears to be constrained by the signaling domain, but productive collisions also require the HAMP domain.
Specificity of residues in the signaling loop of Aer.
Aer, like the other chemoreceptors in E. coli, forms homogeneous trimers of dimers (1, 14, 21), as well as mixed receptor trimers of dimers (1, 14). This multimeric structure is based on specific interactions in the signaling region of the cytosolic domain (1, 14, 21). At its tip (residues 378 to 383), the signaling domain has a helix-turn-helix structure (Fig. 2 and 4A). To compare the interdimeric neighbors in the signaling region of Aer with those in the periplasmic region, we first made double-cysteine replacements that paired A184C in the periplasmic region with a second introduced cysteine in the signaling region. The rationale was to fuse Aer dimers by oxidizing periplasmic residue A184C and trap productive interdimeric collisions with the other cysteine replacement in the signaling region. We made single cysteine replacements at three sites near Aer residue A379 (A377C, A379C, and E381C), which corresponds to Tar residue A387C, which is known to cross-link (3). By themselves, the constructs with single cysteine replacements in the signaling region dimerized to some extent within 10 min when they were treated with CuPhe. When each of these cytosolic cysteine replacements was paired with A184C in the periplasmic region, tetramers and hexamers were trapped by 0.3 mM CuPhe treatment (Fig. 5, left panel). Although the efficiency of oligomer formation was not equivalent, all three binary combinations showed some tetramer and hexamer formation. No cross-linking was evident in the untreated cells or in cells that had been pretreated for 10 min with 2.5 mM NEM to quench the CuPhe reaction (data not shown). Since A184C collides exclusively within a dimer, each of these residues in the signaling region must be able to collide to some extent with other dimers.
FIG. 5.
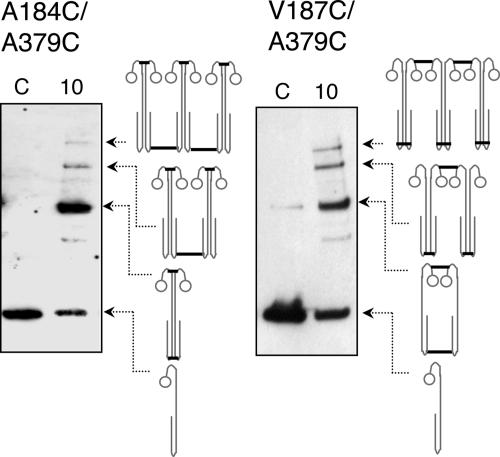
Double combinations of introduced cysteine residues in the periplasmic and cytosolic signaling region form hexamers. Periplasmic residues A184C and V187C were individually paired with A379C in the cytosolic signaling region. Whole cells expressing Aer-A184C/A379C and Aer-V187C/A379C were treated with CuPhe at 23°C as described in the legend to Fig. 1. The presence of some dimer in the control lane of Aer-V187C/A379C (right panel) is due to the air-oxidizable, periplasmic V187C (2). Thick lines in the cartoons represent the proposed intra- and interdimeric cross-links that comprise each band. (Hexamers were also trapped to different extents when A184C or V187C was paired with other cysteines in the signaling region [Table 1].)
To determine whether the interdimeric collisions in the signaling domain corresponded to those observed for the periplasmic domain, we paired each of the cytosolic cross-linkers (A377C, A379C, and E381C) with the periplasmic interdimeric cross-linker, V187C. We reasoned that only dimers would form if the interdimeric collisions between the cysteine replacements in the signaling region were similar to those between dimers in the periplasmic region (between V187Ca and V187Cb). However, when cells expressing Aer-V187C/A379C (or V187C/A377C or V187C/E381C) were treated with CuPhe, hexamers also formed (Fig. 5, right panel), indicating that residues in the signaling region could cross-link with a different subunit than the one cross-linked with periplasmic V187C. This could mean that the signaling loop region, which included residues A377C, A379C, and E381C, was flexible enough to cross-link both within dimers (Fig. 5, right panel) and between dimers (Fig. 5, left panel). Alternatively, and less likely, the signaling region might interact with neighbors different from those in the periplasmic region.
Triple-cysteine combinations.
Since the signaling region stabilizes the periplasmic neighborhood of Aer (Fig. 4), it is likely that hexameric neighbors in the two regions are identical. However, if this were not the case, it should be possible to cross-link aggregates larger than hexamers by strategic placement of three cysteines in Aer. To test this possibility, we made ternary combinations of cysteine replacements that included replacements A184C and V187C in the periplasm and a single cysteine replacement (A377C, A379C, or E381C) in the signaling loop. If interdimeric periplasmic and cytosolic disulfide bonds were joined to different groups of hexamers, a perpetuating polypeptide complex might develop. However, in all cases, hexamers, but no higher multimers, were evident when whole cells expressing any ternary combination of cysteines were treated with the oxidant CuPhe at 23 and 30°C for 15 min (Table 1). Although no higher-order complexes were evident, it is conceivable that larger complexes formed but the percentage of multiple cross-linked aggregates was too low to be resolved on Western blots.
DISCUSSION
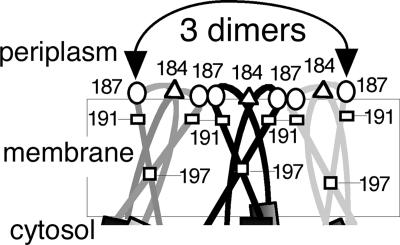
The findings from this study expand our understanding of how the membrane module of Aer is organized (2). The data are consistent with a model in which the TM2 helix of one monomer is angled relative to the TM2 helix of the cognate monomer, so that TM2 and TM2′ intersect at residue 197 near the middle of the membrane (Fig. 6). The four-residue periplasmic loop that connects TM1 and TM2 was the focal point of an Aer neighborhood that included as many as three Aer dimers (Fig. 3 and 6). Three of the four loop residues collided uniquely within or between dimers, but not both. Moreover, decreasing the flexibility of this loop by immobilizing one residue with a disulfide bond (A184C or V187C) did not prevent cross-linking at the other end of the loop (Fig. 3) (residue 184 is at the dimer interface, whereas residue 187 is at an interdimer interface [Fig. 6]). The high specificity of periplasmic cross-linking indicates that the periplasmic loop is more rigid than dynamic. Furthermore, residues A184C and V187C are close to their cross-linking partners. At 23°C, Aer-A184C/V187C formed tetramers and hexamers in a reaction that was essentially complete within 1 min. The cross-linking profile was nearly identical at 4°C (Fig. 3), a temperature well below the lipid phase transition temperature of E. coli (26).
FIG. 6.
Cartoon showing the putative arrangement of the Aer membrane anchor deduced from cysteine disulfide trapping. Although drawn as three Aer dimers, the arrangement in native E. coli would likely include one Aer dimer and two chemoreceptor dimers due to the low copy number of Aer. Cysteine replacements, A184C in the periplasmic loop (triangles) and V197C in TM2 (squares), cross-linked exclusively within a dimer; V187C in the periplasmic loop (ovals) and I191C in TM2 (rectangles) cross-linked exclusively between dimers. The arrow indicates that left-most and right-most V187C residues cross-link with each other.
The size and extent of trapped multimers formed by Aer-A184C/V187C did not change over time. This is consistent with low turnover between dimeric neighbors in the periplasmic neighborhood; if adjacent dimeric receptors had exchanged neighbors, we would have expected an increase in the fraction of cross-linked tetramers and hexamers during longer reaction periods (up to 20 min). Trimer-of-dimer stability was previously demonstrated in the signaling region, where trimer-of-dimer units combine with CheA and CheW to form a signaling team that does not exchange partners (35).
The assignment of intra- and interdimeric collisions was based on pairings with V260C, which is positioned at the dimer interface of the cytosolic, proximal signaling region. The evidence for this comes from cysteine cross-linking studies in our laboratory (K. Watts, unpublished observation) and is supported by the resolved nuclear magnetic resonance structure of a HAMP domain (19).
The relative hexameric arrangement in the membrane module was stabilized by the presence of the signaling domain (Fig. 4). Double-cysteine replacements missing the signaling domain cross-linked as trimers and pentamers in addition to tetramers and hexamers, indicating that there was an increase in the number of degrees of freedom in the periplasmic region. Some of the collisions were likely caused by lateral and/or rotational migration in the membrane, because multimer formation in the truncations decreased at 4°C (Fig. 4B and C). The fact that full-length Aer-184C/V187C cross-linking was more specific and temperature independent indicates that the cytoplasmic signaling region must tether the periplasmic/membrane region in such a way that rotational and/or lateral diffusion is inhibited. Aer truncations with larger C-terminal deletions, where most or all of the HAMP-AS-2 region was removed, showed a decrease in the percentage of cross-linked multimers. Since the HAMP domain is known to influence Aer folding and maturation (9, 16), this decrease was likely due to defects in conformational stability.
The basic topological differences between the membrane modules of Aer and the chemoreceptors are probably due to the different role that each component plays in signaling. The four-helix bundle of chemoreceptors is adapted to transmit chemotaxis signals between the periplasmic sensory-input domain and the HAMP/signaling domains in the cytosol, perpendicular to the plane of the membrane. In contrast, the signal in Aer is transmitted from the cytosolic PAS domain directly to the HAMP domain, parallel to the plane of the membrane. It was previously reported that Aer can form mixed trimers of dimers with the Tar receptor in the cytosolic signaling region (14). In that study, Aer had no preference for itself in forming these signaling teams. Whether Aer can also form hexameric arrays with the other chemoreceptors in the membrane/periplasmic region is not known, but such an association would require close contact between Aer and the chemoreceptors within the membrane module or at the periplasmic surface. It has recently been suggested that the members of a hexameric membrane array of Tar chemoreceptors rotate relative to one another during signaling, altering the packing of the array (20). If Aer were part of such an array, it is not clear how packing would be affected, since the array would need to accommodate the loss of a periplasmic sensory-input domain and the presence of a bulky cytosolic Aer-PAS domain.
Acknowledgments
We thank Kylie Watts for critical analysis and helpful discussions. We are grateful to Sheena Fry and Nathan Abraham for technical assistance.
This work was supported by grants from Loma Linda University to M. S. Johnson and from the National Institute of General Medical Sciences (grant GM29481) to B. L. Taylor.
Footnotes
Published ahead of print on 10 August 2007.
REFERENCES
- 1.Ames, P., C. A. Studdert, R. H. Reiser, and J. S. Parkinson. 2002. Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc. Natl. Acad. Sci. USA 99:7060-7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin, D. N., B. L. Taylor, and M. S. Johnson. 2006. Topology and boundaries of the aerotaxis receptor Aer in the membrane of Escherichia coli. J. Bacteriol. 186:894-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bass, R. B., and J. J. Falke. 1999. The aspartate receptor cytoplasmic domain: in situ chemical analysis of structure, mechanism and dynamics. Struct. Fold Des. 7:829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibikov, S. I., L. A. Barnes, Y. Gitin, and J. S. Parkinson. 2000. Domain organization and flavin adenine dinucleotide-binding determinants in the aerotaxis signal transducer Aer of Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5830-5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibikov, S. I., R. Biran, K. E. Rudd, and J. S. Parkinson. 1997. A signal transducer for aerotaxis in Escherichia coli. J. Bacteriol. 179:4075-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bibikov, S. I., A. C. Miller, K. K. Gosink, and J. S. Parkinson. 2004. Methylation-independent aerotaxis mediated by the Escherichia coli Aer protein. J. Bacteriol. 186:3730-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourret, R. B., J. F. Hess, K. A. Borkovich, A. A. Pakula, and M. I. Simon. 1989. Protein phosphorylation in chemotaxis and two-component regulatory systems of bacteria. J. Biol. Chem. 264:7085-7088. [PubMed] [Google Scholar]
- 8.Bray, D., M. D. Levin, and C. J. Morton-Firth. 1998. Receptor clustering as a cellular mechanism to control sensitivity. Nature 393:85-88. [DOI] [PubMed] [Google Scholar]
- 9.Buron-Barral, M., K. K. Gosink, and J. S. Parkinson. 2006. Loss- and gain-of-function mutations in the F1-HAMP region of the Escherichia coli aerotaxis transducer Aer. J. Bacteriol. 188:3477-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Careaga, C. L., and J. J. Falke. 1992. Thermal motions of surface alpha-helices in the d-galactose chemosensory receptor. Detection by disulfide trapping. J. Mol. Biol. 226:1219-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duke, T. A., and D. Bray. 1999. Heightened sensitivity of a lattice of membrane receptors. Proc. Natl. Acad. Sci. USA 96:10104-10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards, J. C., M. S. Johnson, and B. L. Taylor. 2006. Differentiation between electron transport sensing and proton motive force sensing by the Aer and Tsr receptors for aerotaxis. Mol. Microbiol. 62:823-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falke, J. J., and G. L. Hazelbauer. 2001. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem. Sci. 26:257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosink, K. K., M. del Carmen Buron-Barral, and J. S. Parkinson. 2006. Signaling interactions between the aerotaxis transducer Aer and heterologous chemoreceptors in Escherichia coli. J. Bacteriol. 188:3487-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grebe, T. W., and J. B. Stock. 1999. The histidine protein kinase superfamily. Adv. Microb. Physiol. 41:139-227. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann, S., Q. Ma, M. S. Johnson, A. V. Repik, and B. L. Taylor. 2004. PAS domain of the Aer redox sensor requires C-terminal residues for native-fold formation and flavin adenine dinucleotide binding. J. Bacteriol. 186:6782-6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess, J. F., K. Oosawa, N. Kaplan, and M. I. Simon. 1988. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell 53:79-87. [DOI] [PubMed] [Google Scholar]
- 18.Hughson, A. G., G. F. Lee, and G. L. Hazelbauer. 1997. Analysis of protein structure in intact cells: crosslinking in vivo between introduced cysteines in the transmembrane domain of a bacterial chemoreceptor. Protein Sci. 6:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulko, M., F. Berndt, M. Gruber, J. U. Linder, V. Truffault, A. Schultz, J. Martin, J. E. Schultz, A. N. Lupas, and M. Coles. 2006. The HAMP domain structure implies helix rotation in transmembrane signaling. Cell 126:929-940. [DOI] [PubMed] [Google Scholar]
- 20.Irieda, H., M. Homma, M. Homma, and I. Kawagishi. 2006. Control of chemotactic signal gain via modulation of a pre-formed receptor array. J. Biol. Chem. 281:23880-23886. [DOI] [PubMed] [Google Scholar]
- 21.Kim, S. H., W. Wang, and K. K. Kim. 2002. Dynamic and clustering model of bacterial chemotaxis receptors: structural basis for signaling and high sensitivity. Proc. Natl. Acad. Sci. USA 99:11611-11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, G. F., G. G. Burrows, M. R. Lebert, D. P. Dutton, and G. L. Hazelbauer. 1994. Deducing the organization of a transmembrane domain by disulfide cross-linking. The bacterial chemoreceptor Trg. J. Biol. Chem. 269:29920-29927. [PubMed] [Google Scholar]
- 23.Ma, Q., M. S. Johnson, and B. L. Taylor. 2005. Genetic analysis of the HAMP domain of the Aer aerotaxis sensor localizes flavin adenine dinucleotide-binding determinants to the AS-2 helix. J. Bacteriol. 187:193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma, Q., F. Roy, S. Herrmann, B. L. Taylor, and M. S. Johnson. 2004. The Aer protein of Escherichia coli forms a homodimer independent of the signaling domain and flavin adenine dinucleotide binding. J. Bacteriol. 186:7456-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niwano, M., and B. L. Taylor. 1982. Novel sensory adaptation mechanism in bacterial chemotaxis to oxygen and phosphotransferase substrates. Proc. Natl. Acad. Sci. USA 79:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overath, P., M. Brenner, T. Gulik-Krzywicki, E. Shechter, and L. Letellier. 1975. Lipid phase transitions in cytoplasmic and outer membranes of Escherichia coli. Biochim. Biophys. Acta 389:358-369. [DOI] [PubMed] [Google Scholar]
- 27.Pakula, A. A., and M. I. Simon. 1992. Determination of transmembrane protein structure by disulfide cross-linking: the Escherichia coli Tar receptor. Proc. Natl. Acad. Sci. USA 89:4144-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parkinson, J. S., P. Ames, and C. A. Studdert. 2005. Collaborative signaling by bacterial chemoreceptors. Curr. Opin. Microbiol. 8:116-121. [DOI] [PubMed] [Google Scholar]
- 29.Parkinson, J. S., and S. E. Houts. 1982. Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J. Bacteriol. 151:106-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peach, M. L., G. L. Hazelbauer, and T. P. Lybrand. 2002. Modeling the transmembrane domain of bacterial chemoreceptors. Protein Sci. 11:912-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebbapragada, A., M. S. Johnson, G. P. Harding, A. J. Zuccarelli, H. M. Fletcher, I. B. Zhulin, and B. L. Taylor. 1997. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc. Natl. Acad. Sci. USA 94:10541-10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Repik, A., A. Rebbapragada, M. S. Johnson, J. O. Haznedar, I. B. Zhulin, and B. L. Taylor. 2000. PAS domain residues involved in signal transduction by the Aer redox sensor of Escherichia coli. Mol. Microbiol. 36:806-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowsell, E. H., J. M. Smith, A. Wolfe, and B. L. Taylor. 1995. CheA, CheW, and CheY are required for chemotaxis to oxygen and sugars of the phosphotransferase system in Escherichia coli. J. Bacteriol. 177:6011-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Studdert, C. A., and J. S. Parkinson. 2004. Crosslinking snapshots of bacterial chemoreceptor squads. Proc. Natl. Acad. Sci. USA 101:2117-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Studdert, C. A., and J. S. Parkinson. 2005. Insights into the organization and dynamics of bacterial chemoreceptor clusters through in vivo crosslinking studies. Proc. Natl. Acad. Sci. USA 102:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watts, K. J., M. S. Johnson, and B. L. Taylor. 2006. Minimal requirements for oxygen sensing by the aerotaxis receptor Aer. Mol. Microbiol. 59:1317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watts, K. J., Q. Ma, M. S. Johnson, and B. L. Taylor. 2004. Interactions between the PAS and HAMP domains of the Escherichia coli aerotaxis receptor Aer. J. Bacteriol. 186:7440-7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weis, R. M., T. Hirai, A. Chalah, M. Kessel, P. J. Peters, and S. Subramaniam. 2003. Electron microscopic analysis of membrane assemblies formed by the bacterial chemotaxis receptor Tsr. J. Bacteriol. 185:3636-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wylie, D., A. Stock, C. Y. Wong, and J. Stock. 1988. Sensory transduction in bacterial chemotaxis involves phosphotransfer between Che proteins. Biochem. Biophys. Res. Commun. 151:891-896. [DOI] [PubMed] [Google Scholar]
- 40.Yu, H. S., J. H. Saw, S. Hou, R. W. Larsen, K. J. Watts, M. S. Johnson, M. A. Zimmer, G. W. Ordal, B. L. Taylor, and M. Alam. 2002. Aerotactic responses in bacteria to photoreleased oxygen. FEMS Microbiol. Lett. 217:237-242. [DOI] [PubMed] [Google Scholar]