Abstract
The gram-positive soil bacterium Corynebacterium glutamicum, a major amino acid-producing microorganism in biotechnology, is equipped with several osmoregulated uptake systems for compatible solutes, which is relevant for the physiological response to osmotic stress. The most significant carrier, BetP, is instantly activated in response to an increasing cytoplasmic K+ concentration. Importantly, it is also activated by chill stress independent of osmotic stress. We show that the activation of BetP by both osmotic stress and chill stress is altered in C. glutamicum cells grown at and adapted to low temperatures. BetP from cold-adapted cells is less sensitive to osmotic stress. In order to become susceptible for chill activation, cold-adapted cells in addition needed a certain amount of osmotic stimulation, indicating that there is cross talk of these two types of stimuli at the level of BetP activity. We further correlated the change in BetP regulation properties in cells grown at different temperatures to changes in the lipid composition of the plasma membrane. For this purpose, the glycerophospholipidome of C. glutamicum grown at different temperatures was analyzed by mass spectrometry using quantitative multiple precursor ion scanning. The molecular composition of glycerophospholipids was strongly affected by the growth temperature. The modulating influence of membrane lipid composition on BetP function was further corroborated by studying the influence of artificial modulation of membrane dynamics by local anesthetics and the lack of a possible influence of internally accumulated betaine on BetP activity.
Modulation of transport protein activity by external stimuli is an important aspect of cellular adaptation to osmotic, acid, base, heat, or chill stress. The compatible solute carrier BetP of Corynebacterium glutamicum is a transport system that is subjected to regulation both at the level of transcription and at the level of activity (34, 46). Two stimuli relevant for BetP activation are known; these two stimuli, hyperosmotic stress (46) and chill stress (38), act at both levels of regulation. Whereas the molecular mechanisms of the hyperosmotic stress response have been elucidated in vitro and in vivo, so far the effect of chill stress has been analyzed only at the level of activity modulation in intact cells (38).
An increase in the level of cytoplasmic K+ is a major stimulus for BetP activation in response to hyperosmotic stress. The contribution of the C-terminal domain of BetP and the influence of the membrane surface charge have been analyzed in detail (39, 48). More recently, chill stress was identified as an activating stimulus for BetP activity (38). Maximum activation of BetP could be achieved either by osmotic stimulation at any temperature or directly by low temperatures (around 10 to 15°C) in the absence of osmotic stress. Arguably, there may be a protein-specific, intrinsically determined maximal activity of betaine uptake catalyzed by BetP, and this maximal activity might be stimulated independently by chill or osmotic stress. It is therefore conceivable that for any (osmo)regulated carrier protein active and inactive conformations might occur (58). An external stimulus, represented by hyperosmotic stress, would then shift the transporter from the inactive state to the active state. In the case of osmotic stimulation, this concept has been elaborated for three osmoregulated proteins, ProP from Escherichia coli, OpuA from Lactococcus lactis, and BetP from C. glutamicum (11, 36, 41, 58). For osmotic stimulation, it was possible to assign specific physical stimuli to this general concept, not only for BetP, where K+ was identified as the major stimulus, but also for ProP (43) and OpuA (52).
The physiologic responses to chill stress have been studied in several bacteria, including E. coli (40), Bacillus subtilis (1, 2, 12, 13, 23, 25, 53, 54), and Listeria monocytogenes (3, 6, 22, 30, 49, 55). Several types of stress response have been described; these include synthesis of cold shock proteins (18), synthesis of branched-chain and unsaturated fatty acids (24), and uptake and synthesis of compatible solutes (3, 21, 22, 55). Detailed data are available at the biochemical level for L. monocytogenes, where the primary betaine transporter, Gbu, was found to be directly activated by exposure to low temperatures (22, 49). Furthermore, in L. monocytogenes, evidence for a correlation between osmotic stress, chill stress, and the physical state of the membrane was obtained (22).
In the case of C. glutamicum, which harbors three osmoregulated carrier proteins belonging to the betaine-choline-carnitine family of transporters, only BetP has been shown to respond to chill stress at the level of protein activity (38). BetP was activated at low temperatures without noticeable perturbation of the cytoplasmic K+ concentration. In this case, a major role of the membrane lipid composition was indicated since BetP synthesized in E. coli membranes was not activated by chill stress. Nevertheless, the nature of the physical stimulus related to chill stress remains enigmatic both for Gbu from L. monocytogenes and for BetP from C. glutamicum.
The aim of this work was to identify a physical stimulus(i) related to chill activation of BetP that differs from the stimuli responsible for the osmodependent activation (38). We found a close correlation between both the osmoregulatory and chill stress-responsive properties of BetP and the molecular composition of membrane lipids.
MATERIALS AND METHODS
Chemicals and synthesis of compounds.
Synthetic glycerophospholipids (see below) were obtained from Avanti Polar Lipids, Inc., Alabaster, AL. All chemicals were analytical grade. Enzymatic synthesis of labeled glycine betaine was performed by the method of Landfald and Strom (32) and has been described previously (38).
Bacterial strains, plasmids, and growth conditions.
E. coli DH5αmcr cells (Table 1) were grown at 37°C in LB medium supplemented with carbenicillin (50 μg/ml). betP gene expression was induced in exponentially growing cells by addition of 200 μg anhydrotetracycline/liter of culture. For homologous expression of betP in C. glutamicum, strain DHPF, which is deficient in the uptake of compatible solutes, was used (50). In this case, the betP gene was under the control of the Ptac promoter of plasmid pXMJ19-betP-C252T (47). For transport assays, analysis of the glycerophospholipids, and internal betaine determination, C. glutamicum cells were grown in brain heart infusion medium (Difco) at 30°C to late exponential phase (optical density at 600 nm [OD600, 8). Subsequently, these cells were used to inoculate a fresh medium (OD600, 1) in which betP expression was induced by 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at various growth temperatures (10 to 40°C). When an OD600 of 8 was reached, cells were centrifuged and then washed in buffer containing 50 mM potassium phosphate (pH 7.5) and 50 mM NaCl.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and/or characteristics | Source or reference |
|---|---|---|
| C. glutamicum strains | ||
| ATCC 13032 | Wild type | American Type Culture Collection |
| DHPFa | ATCC 13032 ΔbetP ΔectP ΔlcoP ΔproP ΔputP | 50 |
| Cgl ΔotsA ΔtreS ΔtreY | ATCC 13032 ΔotsA ΔtreS ΔtreY | 56 |
| E. coli DH5αmcr | endA1 supE44 thi-1 λ recA1 gzrA96 relA1 deoR Δ(lacZYA-argF)U169 φ80dlacZΔM15 mcrA Δ(mrr hsdRMS mcrBC) | Grant et al., 1990 |
| Plasmids | ||
| pAcl1 | pASK-IBA5-betP-C252T, Apr | 46 |
| pXMJ19 | ptac lacIq, Cmr, E. coli-C. glutamicum shuttle vector | 28 |
| pXMJ19-betP-C252T | pXMJ19 encoding BetP C252T | 48 |
Wild-type derivative.
Purification and reconstitution of Strep-BetP.
Strep-BetP was purified as previously described (45). From a standard culture, we routinely obtained 200 μg of highly pure BetP/liter of cell culture. For reconstitution, liposomes (5 mg glycerophospholipids/ml) prepared from glycerophospholipids of E. coli polar lipid extract (Avanti Polar Lipids, Inc., Alabaster, AL) were preformed by extrusion (13 cycles) through polycarbonate filters (pore size, 400 nm), as described previously (47). The liposomes were titrated by stepwise addition of 20% (vol/vol) Triton X-100. The insertion of detergent into the liposomes was followed by measurement of the turbidity at 540 nm. Upon saturation with detergent, the liposomes were mixed with solubilized BetP at a lipid/protein ratio of 30:1 (wt/wt). The mixture was incubated for 30 min at room temperature. For removal of detergent, Bio-Beads prewashed with H2O were added at a Bio-Bead/Triton X-100 ratio of 5 (wt/wt) and a Bio-Bead/dodecyl maltoside ratio of 10 (wt/wt). Bio-Beads were added four times, and the mixture was gently stirred at room temperature for 1 h. The conditions for the third treatment were different from these incubation conditions; in this case a double amount of Bio-Beads was added and the incubation time was 16 h at 4°C. Finally, the proteoliposomes were centrifuged and washed twice with 100 mM potassium phosphate (pH 7.5) before they were frozen in liquid nitrogen and stored at −80°C.
Preparation of liposomes from synthetic glycerophospholipids.
1,2-Dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (phosphatidylglycerol [PG] 18:1-18:1), 1,2-dipalmitoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (PG 16:0-16:0), and 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (PG 16:0-18:1) were dissolved in chloroform and dried under a vacuum before they were suspended in 100 mM potassium phosphate (pH 7.5) by agitation for 24 h. Subsequently, liposomes were prepared as described above, snap frozen in liquid nitrogen, and stored at −80°C.
Variation of the lipid composition of proteoliposomes.
To vary the membrane lipid composition in the liposome system, proteoliposomes made from an E. coli lipid extract were fused with liposomes composed of synthetic PG species. The fraction of synthetic lipids varied from 33 to 66 mol% of the total lipid content. For this purpose, thawed proteoliposomes were mixed with either PG 18:1-18:1, PG 16:0-18:1, or PG 16:0-16:0 or with C. glutamicum lipid liposomes at the ratios indicated below and extruded 13 times before they were frozen in liquid nitrogen. After gentle thawing, the mixture was kept for 45 min at 20°C and extruded 13 times trough a polycarbonate filter (pore size, 400 nm) before the proteoliposomes were used for transport assays.
Transport assays with proteoliposomes.
Uptake of [14C]glycine betaine in proteoliposomes was determined essentially as described previously (45). Briefly, after thawing, proteoliposomes were extruded 13 times through a polycarbonate filter (pore size, 400 nm) in 100 mM potassium phosphate (pH 7.5), collected by centrifugation, and resuspended in the extrusion buffer to obtain a lipid concentration of ∼60 mg/ml. Normally, the proteoliposomes had a protein concentration between 0.8 and 1.0 mg/ml. An appropriate amount of proteoliposomes (containing ∼2 to 2.5 μg of BetP) was diluted 200-fold into potassium-free buffer (20 mM sodium Pi [pH 7.5], 25 mM NaCl) containing 15 μM [14C]glycine betaine and 1 μM valinomycin to create an outwardly directed K+ diffusion potential. To establish hyperosmotic conditions, sorbitol or proline was added to the external buffer. After different time intervals (up to 20 s after the addition of the liposomes), samples were taken and filtered rapidly through 0.22-μm GS nitrocellulose filters (Millipore Corp., Eschborn, Germany). The filters were washed with 100 mM LiCl, and the radioactivity was determined by liquid scintillation counting.
Transport assays with C. glutamicum cells.
For determination of [14C]glycine betaine uptake in C. glutamicum cells expressing betP from pXMJ19, cells were grown and washed in buffer containing 50 mM potassium phosphate (pH 7.5) and 50 mM NaCl. After suspension in the same buffer, cells were energized with 10 mM glucose and incubated on ice. After incubation at various osmolalities and temperatures for 3 min, the reaction was started by addition of 250 μM [14C]glycine betaine. At certain time intervals (0.25 to 1.25 min), samples were filtered through glass fiber filters (GF; Schleicher & Schuell GmbH, Dassel, Germany) and washed twice with 2.5 ml of 100 mM LiCl. The radioactivity on the filters was determined by liquid scintillation counting.
High-performance liquid chromatography determination of betaine concentration in C. glutamicum cells.
For measurement of the internal betaine concentration in C. glutamicum DHPF(pbetPC252T), cells were cultivated in brain heart infusion medium and washed in cold buffer containing 50 mM potassium phosphate (pH 7.5) and 50 mM NaCl. The internal solutes were released by treatment of the cells with 0.1% cetyltrimethylammonium bromide. The supernatant was diluted 1:1 with 80% (vol/vol) acetonitrile and loaded onto a reversed-phase column (Grom-Sil 100 Amino 1-PR; 125 by 4 mm; 3 μm; Grom, Herrenberg, Germany). For isocratic elution, 80% (vol/vol) acetonitrile at a flow rate of 1 ml/min was used. Betaine was detected with an refraction index detector (RI-71; Shodex, Tokyo, Japan).
Lipid extraction.
Cells were pelleted and washed in cold buffer containing 50 mM potassium phosphate (pH 7.5) and 50 mM NaCl. Cell membranes were isolated, purified (45), and extracted using a modified Bligh-Dyer method (8). Cell membranes were incubated with methanol-chloroform (2:1, vol/vol) at 4°C overnight and subsequently filtered. The organic phase was isolated and washed with diethyl ether, a dextran gel slurry (Sephadex G-25 medium), and twice with chloroform. The extract was filtered and dried in a vacuum centrifuge, and samples were stored under nitrogen at −20°C until they were analyzed.
Mass spectrometric lipid analysis.
Total lipid extracts of C. glutamicum were subjected to quantitative lipid analysis as previously described (20). The concentrations of total lipid extracts and synthetic lipid standards were determined by phosphate analysis (44). Prior to the analysis, total lipid extracts were diluted to obtain a total phosphate concentration of 3 μM in methanol containing 0.05% (vol/vol) methylamine. A mixture of internal standards was spiked into the analyte at the following concentrations: 0.10 μM cardiolipin 14:0/14:0/14:0/14:0; 0.15 μM phosphatidic acid (PA) 17:0/17:0; 0.10 μM PG 17:0/17:0, and 0.36 μM phosphatidylinositol (PI) 17:0/17:0. The quantitative lipid analysis was performed by multiple precursor ion scanning in negative-ion mode as previously described (20), using a hybrid QSTAR Pulsar i quadrupole time of flight mass spectrometer (MDS Sciex, Concord, Canada) equipped with a robotic nanoflow ion source (NanoMate HD system; Advion Biosciences, Inc., Ithaca, NY). Twenty-one precursor ion spectra for structure-specific fragment ions produced by collision-induced dissociation of molecular anions of glycerophospholipids were acquired. A list of specific fragment ions was compiled from precomputed m/z values for head group-derived fragment ions and acyl anions of all plausible fatty acid moieties having a total number of carbon atoms ranging from 10 to 20 with either zero, one, or two double bonds. Automated processing of acquired spectra and identification and quantification of detected molecular glycerophospholipid species were performed by using the Lipid Profiler software (MDS Sciex) as previously described (20).
RESULTS
Activity regulation of BetP by chill and osmotic stresses in cells grown at different temperatures.
We recently demonstrated that the compatible solute transporter BetP from C. glutamicum is activated by both hyperosmotic stress and chill stress (38). In this previous study we analyzed direct effects of chill stress on BetP in intact C. glutamicum cells grown under optimum conditions at 30°C and compared the chill stress response to the osmoresponsive BetP activation. In those experiments, cells were directly shifted to lower temperatures in the presence or absence of osmotic stress, and the instant activation of BetP was monitored. Several aspects of the chill stress response, however, remained unresolved. It was not determined what the exact activating stimulus is and whether chill and osmotic stimuli affect BetP activity in the same way.
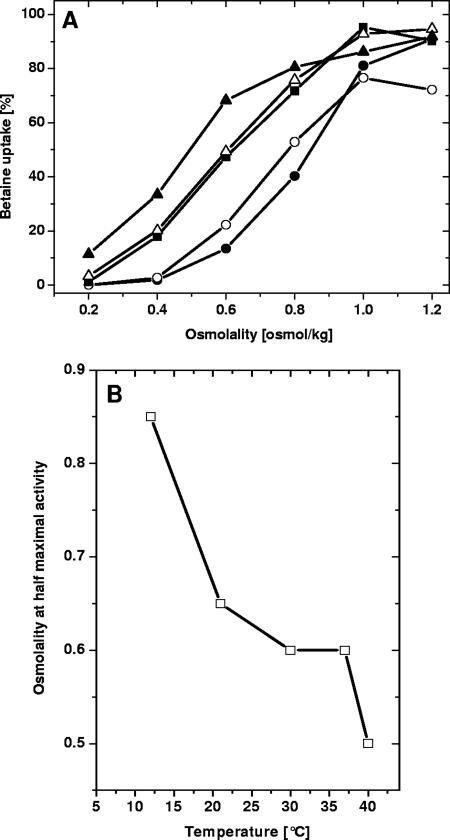
To elucidate molecular mechanisms of chill activation and adaptation, we subjected cells to long-term exposure to temperatures ranging from 10 to 40°C during growth. Temperatures below 10°C were not used because of the very low growth rates. We first investigated cells adapted to different temperatures during growth for osmotic stimulation of BetP in the osmolality range from 0.20 osmol/kg (osmolality of the original medium) to 1.2 osmol/kg (after addition of NaCl) at 30°C (Fig. 1A). Both the maximum rate of glycine betaine uptake and the osmolality required for maximal stimulation were similar for all cells. The sensitivity of BetP to osmotic stress, characterized by an osmolality value required for BetP stimulation up to one-half the maximal activity, was dependent on the growth temperature; it increased from ca. 0.50 osmol/kg with growth at 40°C to 0.85 osmol/kg with growth at 12°C (Fig. 1B).
FIG. 1.
Osmotic stimulation of BetP in C. glutamicum DHPF(pXMJ19-betP-C252T) upon cultivation at different temperatures. (A) Osmotic activation in cells grown at 12°C (•), 21°C (○), 30°C (▪), 37°C (▵), and 40°C (▴). The relative betaine uptake was measured at 30°C following stimulation by addition of NaCl; 0.2 osmol/kg was the osmolality of the basic incubation buffer. (B) Dependence of osmotic sensitivity (i.e., the osmolality at which one-half the maximum betaine uptake rate was reached [see panel A]) on growth temperature.
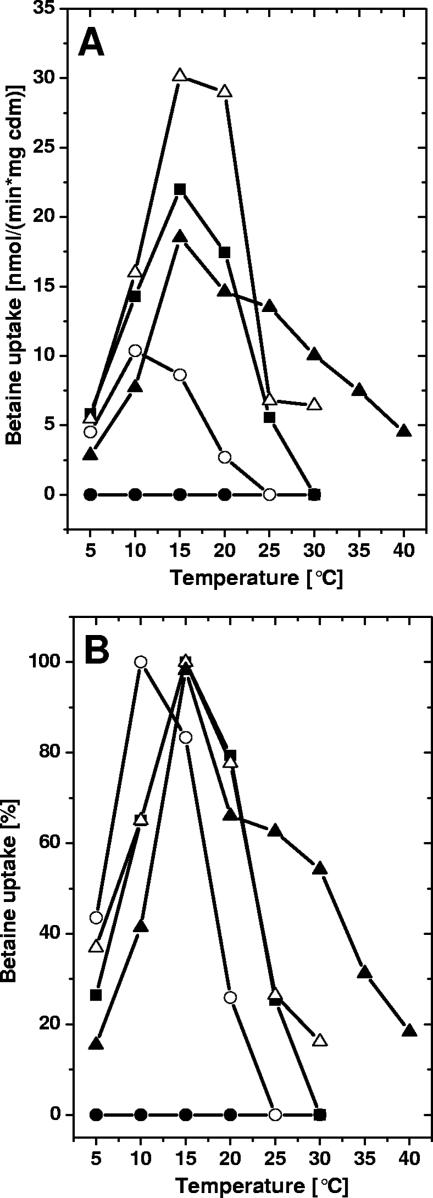
In a similar way we tested how chill activation of BetP was influenced by the growth temperature. Cells grown at the temperatures shown in Fig. 1 were harvested, and the temperature dependence of uptake activity in media with low osmotic strength (0.20 osmol/kg) was analyzed as previously described (38). The data in Fig. 2A show that there was a strong dependence of the pattern of chill activation of BetP on the growth history of the cells. The chill activation previously described for cells grown at 30°C (the optimum growth temperature of C. glutamicum) was more pronounced in cells grown at 37°C, was severely restricted in cells grown at 21°C, and was completely absent in cells grown at 12°C. Except for cells cultured at 40°C, in which the regulation pattern might have been altered, possibly because of reduced vitality of cells at this temperature, chill activation was reduced after adaptation to lower growth temperatures. Interestingly, Figure 2A shows not only a strong dependence of the extent of chill activation on the growth history of C. glutamicum but also a shift in the optimum activation temperature and the course of temperature influence. For a detailed analysis, the data in Fig. 2A were replotted as relative values (Fig. 2B). Obviously, lower growth temperatures shifted the optimum for chill activation to lower temperatures, whereas growth at 12°C completely abolished chill activation. Consequently, a higher growth temperature sensitized BetP to stimulation at low temperatures without changing its activation properties at high temperatures.
FIG. 2.
Betaine uptake in C. glutamicum DHPF(pXMJ19-betP-C252T) cells cultivated at different temperatures. (A) Temperature dependence of betaine uptake in the absence of osmotic stimulation (i.e., 0.2 osmol/kg). Cells were grown at 12°C (•), 21°C (○), 30°C (▪), 37°C (▵), and 40°C (▴), and the uptake was measured as described in the legend to Fig. 1. cdm, cell dry mass. (B) Relative betaine uptake. The same data that were used for panel A were used, but the uptake values were normalized to the maximum uptake rate for each sample of cells except for the cells grown at 12°C.
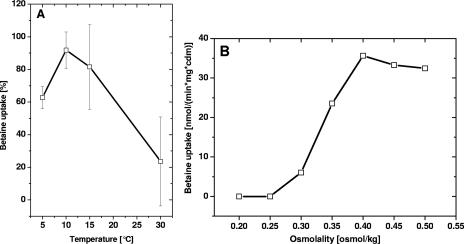
It was previously demonstrated that chill stimulation and osmotic stimulation are functionally linked (38); i.e., maximal BetP activity was observed at low temperatures and BetP activity could not be further enhanced by osmotic stress. In order to test whether the absence of chill activation in cells grown at low temperatures might be related to the synergy of the two different stress responses, we repeated the experiment whose results are shown in Fig. 2A with elevated osmolality, i.e., in buffer with 0.60 osmol/kg. This osmolyte concentration was sufficient to stimulate BetP by about 15% for cells cultured at 12°C (Fig. 1A), without causing full activation. Surprisingly, with elevated external osmolality, efficient chill activation of BetP with a maximum effect at around 10 to 15°C was observed (Fig. 3A). To study this further, we measured betaine uptake by BetP at 5, 10, 15 (optimum temperature for chill activation), and 30°C using cells grown at 12°C with various osmolalities (0.2 to 0.5 osmol/kg) during the activation experiment (Fig. 3B). It turned out that an osmolality of around 0.40 osmol/kg was the basic requirement for chill stimulation of BetP in cells grown at low temperature, indicating that BetP is able to integrate the two different stimuli. It should be noted that a comparable experiment was previously described; however, it was carried out with cells grown at 30°C (38). Under these conditions, no influence of increasing osmotic stress was observed, since BetP was already maximally chill stimulated at 15°C in these cells. These results indicate that there is close cross talk between chill stress and osmotic stress. In cells grown at 30°C, maximal activation of BetP could be obtained at low temperature at low osmolality, although in cells grown at 12°C (38; this study) osmotic preactivation was required for chill stimulation.
FIG. 3.
(A) Temperature dependence of betaine uptake in C. glutamicum DHPF(pXMJ19-betP-C252T) cells cultivated at 12°C in buffer with elevated osmolality (0.6 osmol/kg). Betaine uptake was measured at the indicated temperatures. (B) Dependence of betaine uptake in C. glutamicum DHPF(pXMJ19-betP-C252T) cells cultivated at 12°C and measured at 15°C with various buffer osmolalities. cdm, cell dry mass.
As a control, using full osmotic stimulation conditions and cells grown at different temperatures, we tested whether a “normal” Arrhenius behavior was conserved for BetP activation. This analysis proved that the general (nonregulatory) temperature dependence of BetP, typical of all enzymatic and transport reactions, was more or less unchanged (results not shown) (38).
Glycerophospholipidome of C. glutamicum at different growth temperatures.
Next, we investigated whether specific differences in the membrane lipid composition correlated with the temperature-dependent regulatory properties of betaine uptake by BetP. The C. glutamicum lipidome comprises mycolic acids (17) and three glycerophospholipid classes, PG, PI, and cardiolipin (CL) (17).
Mycolic acids are primary constituents of the cell wall, whereas the glycerophospholipids constitute the plasma membrane. Individual molecular glycerophospholipid species within each lipid class are composed of unique fatty acid moieties attached to the sn-1 and sn-2 positions of the glycerophosphate backbone via ester linkages. Previous studies demonstrated that C. glutamicum primarily synthesizes palmitic acid (16:0) and oleic acid (18:1) (17). Furthermore, minor amounts of biosynthetic intermediate phosphatidic acid and diacylglycerol species, as well phosphatidylinositol mannosides (26, 31), were observed. Here we performed a molecular characterization analysis of the C. glutamicum lipidome using a novel mass spectrometric methodology that provides sensitive, specific, and quantitative analysis of individual glycerophospholipid species by direct infusion of total lipid extracts (20).
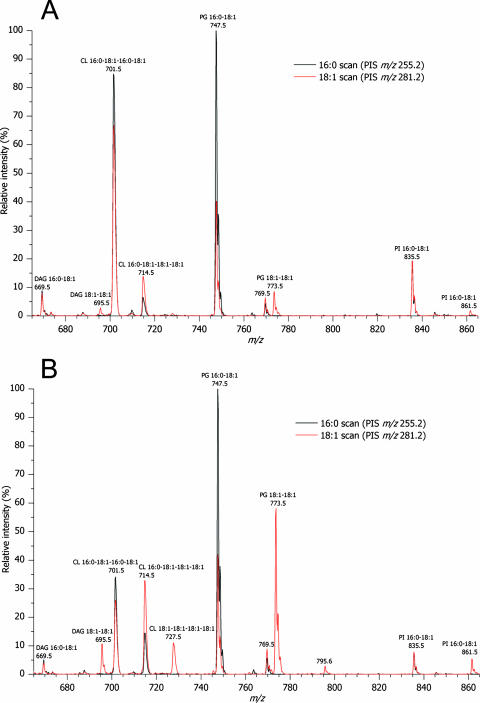
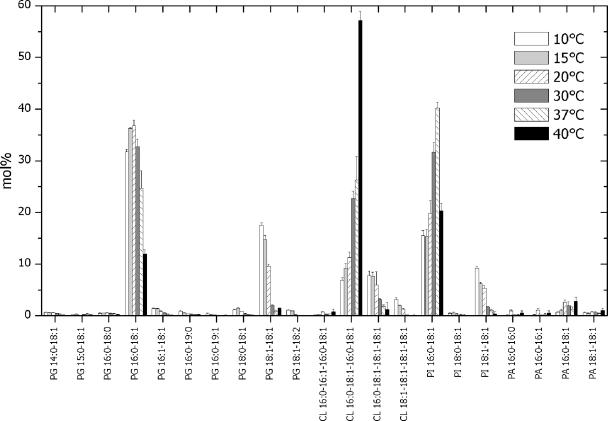
To correlate the membrane lipid composition to altered regulatory properties of BetP, we analyzed the molecular composition of glycerophospholipids from C. glutamicum cultured in the temperature range from 10 to 40°C. C. glutamicum cell membranes were isolated and subjected to lipid extraction. Total lipid extracts were spiked with defined amounts of synthetic lipid standards and analyzed by multiple precursor ion scanning (20). As expected, the most abundant molecular ions were detected in precursor ion scans corresponding to acyl anions of palmitic acid (16:0) and oleic acid (18:1) (Fig. 4). Automated interpretation of all acquired precursor ion spectra by Lipid Profiler software identified and quantified 23 individual glycerophospholipid species in the total lipid extracts (Fig. 5). In addition, we identified two low-abundance diacylglycerol species, which were not quantified due lack of an internal standard. At every growth temperature, the C. glutamicum membrane lipidome was composed of six major lipid species, PG 16:0-18:1, PG 18:1-18:1, PI 16:0-18:1, PI 18:1-18:1, CL 16:0-18:1-16:0-18:1, and CL 16:0-18:1-18:1-18:1 (Fig. 5), and a few low-abundance species. The content was dependent on the growth temperature (Fig. 5). Three unexpected low-abundance PG species were identified that had fatty acid moieties containing an odd number of carbon atoms, PG 15:0-18:1, PG 16:0-19:0, and PG 16:0-19:1. We noted that multiple precursor ion scanning analysis produced a detailed lipidomic data set, which lent itself to several user-defined interpretations (20). Hence, using the data set outlined in Fig. 5, we deduced the overall fatty acid composition (typically obtained by gas chromatography-mass spectrometry) (Fig. 6) and lipid class composition (typically obtained by thin-layer chromatography) (Fig. 7).
FIG. 4.
Representative spectra obtained by lipid species-specific multiple precursor ion scanning analysis. Lipid extracts of membranes from C. glutamicum cultured at 15°C (A) and 30°C (B) were analyzed by multiple precursor ion scanning as described in Materials and Methods. The overlays of precursor ion scans (PIS) for palmitic acid (16:0) (PIS m/z 255.2) and oleic acid (18:1) (PIS m/z 281.2) show detected lipid species annotated by their fatty acid moieties. For clarity, only 2 of 21 precursor ion spectra acquired are shown. Identified lipid species were quantified by spiking the total lipid extracts with known amounts of synthetic glycerophospholipid standards (one for each lipid class) having fatty acid 17:0 moieties (for PG and PI) and fatty acid 14:0 moieties (for CL) (data not shown). DAG, diacylglycerol.
FIG. 5.
Lipidome of C. glutamicum. To correlate the membrane lipid composition to altered regulatory properties of BetP, we analyzed the molecular composition of glycerophospholipids from C. glutamicum cultured in the temperature interval from 10 to 40°C. C. glutamicum cell membranes were isolated and subjected to lipid extraction. Total lipid extracts were spiked with defined amounts of synthetic lipid standards and analyzed by multiple precursor ion scanning (20). As expected, the most abundant molecular ions were detected in precursor ion scans corresponding to acyl anions of palmitic acid (16:0) and oleic acid (18:1) (see Fig. 4). The automated interpretation of all acquired precursor ion spectra by Lipid Profiler software identified and quantified 23 individual glycerophospholipid species in the total lipid extracts (Fig. 5). In addition, we identified two low-abundance diacylglycerol species, which were not quantified due lack of an internal standard. At every growth temperature, the C. glutamicum membrane lipidome was composed of six major lipid species, PG 16:0-18:1, PG 18:1-18:1, PI 16:0-18:1, PI 18:1-18:1, CL 16:0-18:1-16:0-18:1, and CL 16:0-18:1-18:1-18:1, and a few low-abundance species. The content was dependent on the growth temperature. Three unexpected low-abundance PG species were identified that had fatty acid moieties containing an odd number of carbon atoms; these species were PG 15:0-18:1, PG 16:0-19:0, and PG 16:0-19:1. We noted that multiple precursor ion scanning analysis produced a detailed lipidome data set, which lent itself to several user-defined interpretations (20). Hence, using the data set shown in this figure, we deduced the overall fatty acid composition (typically obtained by gas chromatography-mass spectrometry) (see Fig. 6) and lipid class composition (typically obtained by thin-layer chromatography) (see Fig. 7).
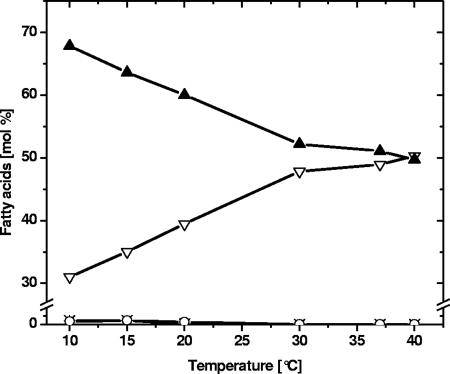
FIG. 6.
Fatty acid composition of C. glutamicum membranes as a function of growth temperature. C. glutamicum was cultured at the indicated temperatures and harvested in late exponential phase, and this was followed by isolation of cell membranes and lipid extraction. Total lipid extracts were analyzed by quantitative multiple precursor ion scanning analysis as outlined in Materials and Methods. The total fatty acid composition of all quantified molecular PG, PI, and CL species (see Fig. 5) was estimated and converted to mol% of palmitic acid (16:0) (▿), palmitoleic acid (16:1) (×), stearic acid (18:0) (○), and oleic acid (18:1) (▴). The data were derived from the averages of four replicate analyses. The same result was independently obtained by gas chromatography-mass spectrometry analysis (data not shown).
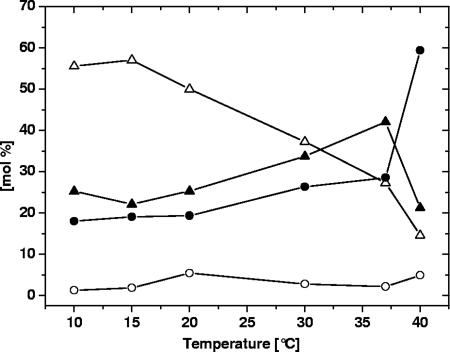
FIG. 7.
Glycerophospholipid class composition of C. glutamicum as a function of growth temperature. Total lipid extracts were analyzed by quantitative multiple precursor ion scanning as described in Material and Methods. The total mol% of all quantified PG species (▵), CL species (•), PI species (▴), and PA species (○) was determined. The data were derived from the averages of four replicate analyses.
The emulated fatty acid composition demonstrated that palmitic acid (16:0) and oleic acid (18:1) accounted for approximately 95% of all glycerophospholipid fatty acid moieties. In addition, minor amounts of palmitoleic acid (16:1), stearic acid (18:0), myristic acid (14:0), and other fatty acids (15:0, 19:0, and 19:1) were found (Fig. 5 and 6). The deduced fatty acid compositions were independently verified by lipid hydrolysis followed by gas chromatography-mass spectrometry analysis (data not shown). The fraction of unsaturated and saturated fatty acid moieties, in this case essentially the fraction of oleic acid (18:1) and palmitic acid (16:0), varied in a strikingly linear fashion, with the unsaturated/saturated ratios decreasing from more than 2:1 at lower growth temperatures to 1:1 at the optimum growth temperature (30°C) and then remaining unchanged. Interestingly, further in-depth analysis of the composition of fatty acid moieties in each lipid class (PG, CL, and PI) showed no significant systematic differences at any growth temperature (data not shown). This indicated that PG, CL, and PI lipid species are derived from a common biosynthetic intermediate (PA) and that C. glutamicum is devoid of any lipase activities mediating extensive fatty acid remodeling.
At every growth temperature, 95% of the glycerophospholipidome of C. glutamicum was composed of PG, PI, and CL class lipid species (Fig. 7). At temperatures below 20°C the C. glutamicum glycerophospholipidome comprised approximately 50 mol% PG species, 25 mol% PI species, 19 mol% CL species, and less than 5 mol% PA species (Fig. 7). Increasing the growth temperature to either 30 or 37°C reduced the relative content of PG species by approximately 2-fold, and there was concomitant 1.5-fold increase in the abundance of CL species and PI species. Interestingly, culturing C. glutamicum at 40°C had a pleiotropic impact on the lipid class composition: compared to the composition after growth at 37°C, we observed a further twofold reduction in the level of PG species, an unexpected twofold reduction in the level of PI species, and a twofold increase in the level of CL species.
Since the three major glycerophospholipid classes of C. glutamicum carry negatively charged phosphate groups, we speculated that the net surface charge (and thus the surface polarity) of the membrane is preserved in the range from 10 to 37°C. With the exception of a growth temperature of 40°C, which is at the borderline of temperatures for viability of C. glutamicum, a shift from the dominating PG species at low temperatures towards PI and CL class species at 37°C was observed.
BetP activity in proteoliposomes.
Since the temperature-dependent regulatory properties of BetP and the membrane lipid composition of C. glutamicum might be functionally connected, we designed a series of in vitro chill activation experiments with proteoliposomes having various lipid compositions. A reconstituted system for BetP for studying the influence of osmotic stress had been developed previously (45). BetP did not show any chill activation in proteoliposomes made from E. coli lipids, which agrees with the results of our previous studies with intact E. coli cells (38). It was not possible, however, to obtain functionally reconstituted BetP-containing proteoliposomes from C. glutamicum lipid extracts, since such proteoliposomes were permeable to ions, making establishment of an electrochemical K+ potential impossible, which is a prerequisite for active betaine transport (45). It was also impossible to create proteoliposomes from synthetic lipids in which BetP showed chill activation, although BetP was fully functional, most probably because it was not possible experimentally to exactly mirror C. glutamicum lipids in both head group and fatty acid composition, in part because of a lack of availability or high cost (synthetic PI lipids).
Impact of artificial modulation of the physical state of the membrane.
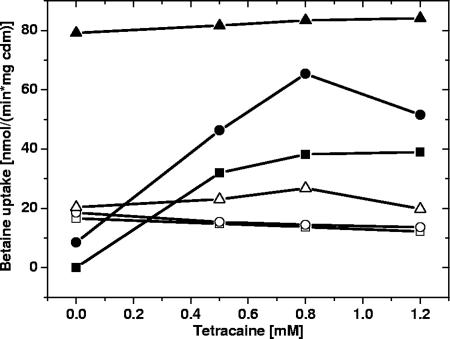
The observed correlation between regulatory properties of BetP and the membrane lipid composition indicates that the membrane composition has a modulating influence on BetP activity. This is not surprising in view of the described impact of lipids on the activation by osmotic stress (45, 48). Since it was not possible to prove this concept with proteoliposomes, we used intact cells of C. glutamicum in the presence of tetracaine, which perturbs the physical state of membranes and is often used to study transport protein regulation (e.g., BetP from C. glutamicum [45, 46] and OpuA from L. lactis [7, 52]).
For these experiments, C. glutamicum cells grown at 30°C were incubated with increasing concentrations of tetracaine at 10°C (chill stress) and at 30°C (optimum temperature) in the presence of different levels of osmotic stress (i.e., different concentrations of NaCl added to the medium) (Fig. 8). We observed that BetP could be stimulated by tetracaine, but only when it had not been already activated by other stimuli. In cases of chill stimulation (10°C) or hyperosmotic activation (high NaCl concentration), BetP could not be further activated by tetracaine. This indicates that there is a reciprocal relationship between the three factors activating BetP, osmotic stress, chill stress, and direct modulation of membrane dynamics by tetracaine addition or by a change in the membrane lipid composition.
FIG. 8.
Betaine uptake by BetP in response to the addition of tetracaine. C. glutamicum DHPF(pXMJ19-betP-C252T) was cultivated at 30°C, and the assay temperature was varied. The basic buffer was 50 mM potassium phosphate (pH 7.5) at an osmolality of 0.2 osmol/kg. Either the assay temperature was 30°C and 50 mM (▪), 100 mM (•), or 400 mM NaCl (▴) was added, or the assay was carried out at 10°C with addition of 50 mM (□), 100 mM (○), or 400 mM NaCl (▵). cdm, cell dry mass.
Possible influence of cytosolic betaine accumulation.
Accumulation of compatible solutes in bacterial cells has been observed not only in response to hyperosmotic stress but also as a compensatory response to chill stress in several organisms (5, 11, 13, 30, 33). During early exponential growth at low temperature a similar response was also observed in C. glutamicum (unpublished observations). Consequently, the observed alteration in the regulatory behavior of BetP could, in principle, be attributed to a greatly altered cytosolic solute content. It is interesting that BetP has previously been shown to respond to changes in the cytosolic concentration of betaine in its functional properties (10).
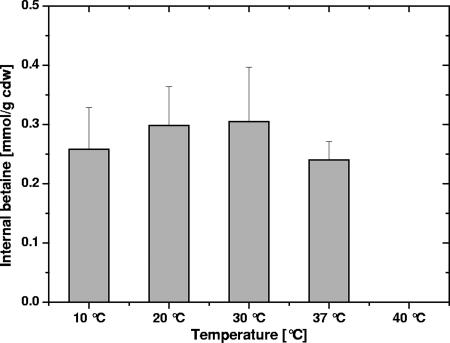
Note that before being used in the uptake experiments described here, C. glutamicum cells were washed in hypoosmotic buffer solutions in order to standardize the internal content of compatible solutes. Under the experimental conditions used, only betaine was present at significant concentrations, since the proline level was low in the absence of hyperosmotic stress (unpublished results) and ectoine, the third relevant compatible solute of C. glutamicum, was not present. Figure 9 shows that the internal concentration of betaine was independent of the growth temperature under the experimental conditions used (i.e., after application of a hypoosmotic shock).
FIG. 9.
Internal levels of betaine in C. glutamicum cells grown at different temperatures. Cells were washed in hypoosmotic buffer, and betaine was measured as outlined in Material and Methods. At 40°C, no internal betaine was detected. cdw, cell dry weight.
DISCUSSION
The activity of the compatible solute transporter BetP was previously shown to be regulated by hyperosmotic stress and by chill stress (35, 36, 38). Activation of BetP by chill stress turned out to result in the same maximal activity that was observed with osmotic stress (38); however, it was shown that the mechanism of action was different. The major osmorelated stimulus, the change in the cytoplasmic K+ concentration, did not influence chill activation. In the present work, a different aspect of chill activation of BetP was studied, namely, its response to adaptive effects with different growth temperatures. We found that in spite of the fact that chill stress and osmotic stress are different in physical nature, these two stimuli seem to cross talk at the level of BetP activity. When cells were grown at different temperatures, the sensitivity to hyperosmotic challenge varied greatly, whereas the maximum activity of fully stimulated BetP did not change significantly. Since neither the physical nature of BetP nor its maximum transport activity directly related to the amount of BetP was significantly changed, we deduced that another factor depending on the growth history of the cells is altered in C. glutamicum cells growing at different temperatures and in turn influences the regulatory pattern of BetP.
We are aware of the fact that membrane protein topology may depend on membrane lipid composition under particular conditions (9, 59). Since the response of BetP to osmotic stress, which depends on the location of the terminal domains of BetP with respect to the membrane leaflet (46), was changed in terms of sensitivity but remained unchanged in terms of the basic regulatory pattern, it seems unlikely that the topology of BetP is influenced under these conditions.
The presence of a so-far-unrecognized factor responsible for BetP activation in response to chill stress became even more evident when we tested the temperature-dependent regulation pattern of BetP using cells grown at different temperatures. The typical chill activation of BetP, as described previously (38), seemed to be lost in cells cultured at low temperature, whereas the “normal” Arrhenius-type response to increasing temperature with full osmotic stimulation was unchanged. Growth at lower temperatures made activation of BetP by both osmotic stress and chill stress more difficult. As a result, BetP in cells grown at low temperature could be activated by chill stress only in the presence of a higher basic level of osmotic (pre)activation. The fact that this has not been observed previously with C. glutamicum cells grown at the optimum temperature (30°C) is explained by the fact that the osmolality of the basic buffer (0.2 osmol/kg) used in these experiments was high enough for preactiviation.
Our results indicate that the sensitivity of BetP to external stimuli can be modified by temperature-dependent alteration of the membrane lipid composition. To pinpoint the molecular mechanism, we charted the molecular composition of the C. glutamicum glycerophospholipidome at various growth temperatures by applying quantitative and molecular lipid species-specific multiple precursor ion scanning technology. By acquiring a comprehensive lipidome data set by multiple precursor ion scanning analysis (20), we identified and quantified molecular lipid species and deduced the global changes in fatty acid and lipid class composition caused by the different growth temperatures. Specifically, we found that oleic acid (18:1) and palmitic acid (16:0) are the major fatty acid moieties in the C. glutamicum glycerophospholipidome. The relative abundance of these acids depends on the growth temperature between 10 and 30°C (optimum temperature) in a linear manner, whereas no significant changes were observed at temperatures above 30°C. The growth temperature-affected alterations in the fatty acid composition of the plasma membrane change the physical state of the membrane (27). A number of related observations have been reported previously. Besides alteration of the fatty acid composition, adaptation to temperature also results in changes in saturation and branching (4, 37, 54). Bacillus cereus strains respond to a decrease in growth temperature from 37 to 15°C with an increase in the ratio of anteiso-branched fatty acids to iso-branched fatty acids, as well as in the ratio of unsaturated fatty acids to saturated fatty acids (24). E. coli cells respond to chill stress by increasing the fraction of unsaturated fatty acids (15), whereas in L. monocytogenes the content of anteiso-C15:0 fatty acids is increased (37, 60).
Interestingly, the lipid class composition also varied significantly in cells grown at different temperatures; PG species were most abundant at lower temperatures, while the levels of PI species and CL species were elevated at higher growth temperatures. It is noteworthy that at 40°C the levels of the CL class species were highly elevated and the levels of PG and PI class species were reduced. It should be noted that the high negative surface charge of C. glutamicum membranes was maintained at temperatures between 10 and 37°C. The relative levels of abundance of the fatty acid moieties oleic acid (18:1) and palmitic acid (16:0) were identical with respect to the three major lipid classes monitored; i.e., there was no preference of a particular fatty acid for a particular head group structure at any growth temperature studied. Consequently, at the optimum growth temperature, 30°C, the contents (based on the number of molecules) of PG 16:0-18:1, PI 16:0-18:1, and CL 16:0-18:1-16:0-18:1 were nearly equal, whereas at low temperatures PG 16:0-18:1 and PG 18:1-18:1 were the dominating species. Note that mycolic acids did not interfere with the quantification of glycerophospholipids of the C. glutamicum plasma membrane. First, the mycolic acids in C. glutamicum have a mass (or m/z value) less than that of the monitored glycerophospholipid species. Second, the multiple precursor ion scanning analysis was not compromised by mycolic acid species since they released molecular fragment ions different than those of the glycerophospholipid species.
In contrast to changes in the fatty acid composition, changes in the content of glycerophospholipid classes do not seem to be a widespread temperature adaptation mechanism in bacteria. Yersinia pseudotuberculosis was found to respond to decreasing growth temperatures by an increase in phosphatidylethanolamine and a decrease in CL (4). In contrast, in membranes of B. cereus, which predominantly consist of CL, the content of PG and phosphatidylethanolamine was further decreased when a culture was shifted to low temperature (24).
It is therefore conceivable that chill activation of BetP is influenced by the membrane lipid composition. This interpretation is in line with the findings that (i) the extent and nature of chill activation are correlated to the membrane lipid composition and (ii) chill activation does not depend on the presence of the C-terminal domain of BetP or on internal K+, the trigger responsible for osmotic activation of BetP (38). Thus, chill activation was interpreted to be a consequence of protein-lipid interactions. There are additional arguments for the hypothesis that the membrane lipid composition has a regulatory influence on BetP activity. (i) The changed membrane lipid composition also influences the response of BetP to osmotic stress, leading to decreased sensitivity of cells grown at low temperatures. (ii) The influence of a membrane-active compound, tetracaine, indicates that there is a direct interaction of BetP via modulation of membrane dynamics, as also demonstrated previously (45). It should be noted that the modulation of BetP activity by tetracaine is not simply a nonspecific effect since the closely related osmoregulated carrier EctP from C. glutamicum was shown not to be influenced by tetracaine (50) and is, interestingly, also not chill activated (38). (iii) Previous investigations indicated that downregulation of BetP activity in response to the shift into an adapted (low-activity) state may be triggered by changes in the properties of the surrounding membrane (10).
Unequivocal correlation of the change in glycerophospholipid species composition with the altered regulatory behavior of BetP requires demonstration of this effect in an artificial system (proteoliposomes). We did, however, not succeed in measuring chill activation in proteoliposomes. First, it was not possible to obtain functionally active BetP proteoliposomes using lipids from lipid extracts of C. glutamicum. On the one hand, it was not possible to fully separate the cell wall and the plasma membrane in C. glutamicum due to its complex cell wall structure (42). We eliminated a major possible source of problems for doing this by using a mycolic acid-deficient strain of C. glutamicum (51, 56) for lipid extraction, which lacks the putatively interfering presence of these compounds. Also, the presence of a large number of porin proteins has to be taken into account (16), which could render the proteoliposomes permeable. On the other hand, it was not possible to prepare proteoliposomes from synthetic lipids which exactly mimic the endogenous C. glutamicum plasma membrane composition with respect to fatty acids and head group structures.
As chill activation could not be observed in the proteoliposome system, a possible additional proteinaceous factor responsible for chill activation of BetP could not be ruled out completely. Experiments are under way to perturb the lipid metabolism of C. glutamicum, thus directly altering the plasma membrane composition under in vivo conditions. On the other hand, the results presented here unequivocally support the hypothesis that chill activation is not an intrinsic quality of the BetP protein itself but depends on the input of external stimuli, most probably a change in membrane lipid composition or dynamics. This has fundamental consequences for understanding BetP activation. It is generally accepted that osmotic stimulation may be related to changes in the properties of the membrane (57). In the case of BetP this means that, besides the well-documented influence of K+ on BetP activity mediated via the C-terminal domain (46, 47, 48), osmotic stress-related membrane changes may additionally be involved in BetP activation in response to a hyperosmotic shift.
When studying three possible stimuli of BetP (osmotic stress, chill stress, and tetracaine), we found that there seems to be a protein-specific, intrinsically defined maximum value for BetP activity, independent of by which trigger it is achieved. This is in line with the concept of a two-state mechanism for this type of carriers (41, 58). Activation of BetP is interpreted as a shift of BetP from an inactive state to the active state, and all possible triggering parameters may contribute to this shift to different extents. Based on this work, we suggest a modification of the model. We assume that the lipid species surrounding BetP directly influences the balance between the inactive state and the active state of BetP. Consequently, a lipid composition resulting from growth at low temperature favors the inactive state of BetP and vice versa. Experimentally, this shift is observed in terms of different sensitivity of BetP to the major stimulus, the increase in the internal K+ level. Consequently, a lower level of the K+ trigger is needed if BetP is already partially shifted to the active state in cells grown at a higher temperature.
Furthermore, direct BetP activation by chill stress is interpreted by assuming that there is a second stimulus input into BetP directly via the membrane. Adaptation to high temperatures, resulting in a changed membrane composition, shifts the balance towards the active form of BetP, and an instant chill stress (i.e., a sudden decrease in the membrane dynamics) leads to activation of BetP. Thus, the two different regulatory influences assigned to BetP's lipid environment can be combined conceptually. These results also indicate that the different stimuli, osmotic stress and instant chill stress, as well as addition of tetracaine, may feed into the same pathway of intramolecular signal transduction, which has not been elucidated yet.
Finally, the impact of these results on biotechnology seems obvious. Growth of C. glutamicum at a high temperature (40°C) leads to a composition of the plasma membrane significantly different from that obtained at a very similar temperature, 37°C. The significantly changed activation pattern in cells adapted to the high growth temperature indicates that ambient temperatures around 25°C are sensed in terms of chill stress by these cells. It is known that growth of C. glutamicum at an elevated temperature around 40°C leads to spontaneous glutamate excretion, which is thought to be triggered by alterations in the cell envelope (14, 19, 29). We observed severely perturbed lipid composition of the C. glutamicum plasma membrane after growth under these conditions. It seems attractive to assume that there is a correlation between this fact and the onset of amino acid excretion.
Acknowledgments
We thank Ching-Ju Tsai and Christine Ziegler (Max Planck Institute of Biophysics, Frankfurt, Germany) for providing purified C. glutamicum lipids for liposome preparation and Marlene Stein (group of E. Galinski, University of Bonn) for quantification of betaine. We are grateful to Eva Duchoslav (MDS Sciex) for expert advice on Lipid Profiler software and to Christoph Thiele (Max Planck Institute for Cell Biology and Genetics, Dresden, Germany) for providing synthetic PI 17:0/17:0.
This work was supported by grants from the Deutsche Forschungsgemeinschaft to S.M. and R.K. (grant Mo 892/1-2) and to A.S. (grant SFB-TR 13, project D1).
Footnotes
Published ahead of print on 10 August 2007.
REFERENCES
- 1.Aguilar, P. S., J. E. Cronan, and D. de Mendoza. 1998. A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase. J. Bacteriol. 190:2194-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar, P. S., A. M. Hernandez-Arriaga, L. E. Cybulski, A. C. Erazo, and D. de Mendoza. 2001. Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 20:1681-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelidis, A. S., L. T. Smith, L. M. Hoffman, and G. M. Smith. 2002. Identification of OpuC as a chill-activated and osmotically activated carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 68:2644-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakholdina, S. I., N. M. Sanina, I. N. Krasikova, O. B. Popova, and T. F. Solov'eva. 2004. The impact of abiotic factors (temperature and glucose) on physicochemical properties of lipids from Yersinia pseudotuberculosis. Biochemistry 86:875-881. [DOI] [PubMed] [Google Scholar]
- 5.Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants of Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. [DOI] [PubMed] [Google Scholar]
- 6.Bayles, D. O., B. A. Annous, and B. J. Wilkinson. 1996. Cold stress proteins induced in Listeria monocytogenes in response to temperature downshock and growth at low temperatures. Appl. Environ. Microbiol. 62:1116-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biemans-Oldehinkel, E., N. A. Mahmood, and B. Poolman. 2006. A sensor for intracellular ionic strength. Proc. Natl. Acad. Sci. USA 103:10624-10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bligh, E. G., and D. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 9.Bogdanov, M., P. N. Heacock, and W. Dowhan. 2002. A polytopic membrane protein displays a reversible topology dependent on membrane lipid composition. EMBO J. 21:2107-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botzenhardt, J., S. Morbach, and R. Krämer. 2004. Activity regulation of the betaine transporter BetP of Corynebacterium glutamicum in response to osmotic compensation. Biochim. Biophys. Acta 1667:229-240. [DOI] [PubMed] [Google Scholar]
- 11.Bremer, E., and R. Krämer. 2000. Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes in bacteria, p. 79-97. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, DC.
- 12.Brigulla, M., T. Hoffman, A. Krisp, A. Völker, E. Bremer, and U. Völker. 2003. Chill induction of the SigB-dependent general stress response in Bacillus subtilis and its contribution to low-temperature adaptation. J. Bacteriol. 195:4305-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budde, I., L. Steil, C. Scharf, U. Volker, and E. Bremer. 2006. Adaptation of Bacillus subtilis to growth at low temperature: a combined transcriptomic and proteomic appraisal. Microbiology 152:831-853. [DOI] [PubMed] [Google Scholar]
- 14.Burkovski, A., and R. Krämer. 2002. Bacterial amino acid transport proteins: occurrence, functions, and significance for biotechnological applications. App. Microbiol. Biotechnol. 58:265-274. [DOI] [PubMed] [Google Scholar]
- 15.Casadei, M. A., Mañas, P., Niven, G., Needs, E., and B. M. Mackey. 2002. Role of membrane fluidity in pressure resistance of Escherichia coli NCTC 8164. Appl. Environ Microbiol 68:5965-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa-Riu, N., E. Maier, A. Burkovski, R. Kramer, F. Lottspeich, and R. Benz. 2003. Identification of an anion-specific channel in the cell wall of the Gram-positive bacterium Corynebacterium glutamicum. Mol. Microbiol. 50:1295-1308. [DOI] [PubMed] [Google Scholar]
- 17.Daffé, M. 2005. The cell envelope of corynebacteria, p. 121-148. In L. Eggeling and M. Bott (ed.), Handbook of Corynebacterium glutamicum. Taylor & Francis, Boca Raton, FL.
- 18.Derzelle, S., B. Hallet, T. Ferain, J. Delcour, and P. Hols. 2003. Improved adaptation to cold-shock, stationary-phase, and freezing stresses in Lactobacillus plantarum overproducing cold-shock proteins. Appl. Environ. Microbiol. 69:4285-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eggeling, L., K. Krumbach, and H. Sahm. 2001. l-Glutamate efflux with Corynebacterium glutamicum: why is penicillin treatment or Tween addition doing the same? J. Mol. Miccrobiol. Biotechnol. 3:67-68. [PubMed] [Google Scholar]
- 20.Ejsing, C. S., E. Duchoslav, J. Sampaio, K. Simons, R. Bonner, C. Thiele, K. Ekroos, and A. Shevchenko. 2006. Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal. Chem. 78:6202-6214. [DOI] [PubMed] [Google Scholar]
- 21.Gerhardt, P. N. M., L. T. Smith, and G. M. Smith. 1996. Sodium-driven, osmotically activated glycine betaine transport in Listeria monocytogenes membrane vesicles. J. Bacteriol. 178:6105-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhard, P. N. M., L. T. Smith, and G. M. Smith. 2000. Osmotic and chill activation of glycine betaine porter II in Listeria monocytogenes membrane vesicles. J. Bacteriol. 192:2544-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graumann, P. L., and M. A. Marahiel. 1999. Cold shock response in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 1:203-209. [PubMed] [Google Scholar]
- 24.Haque, M. A., and N. J. Russell. 2004. Strains of Bacillus cereus vary in the phenotypic adaptation of their membrane lipid composition in response to low water activity, reduced temperature and growth in rice starch. Microbiology 150:1397-1404. [DOI] [PubMed] [Google Scholar]
- 25.Hecker, M., W. Schumann, and U. Völker. 1996. Heat-shock and general stress response in Bacillus subtilis. Mol. Microbiol. 19:417-428. [DOI] [PubMed] [Google Scholar]
- 26.Hoischen, C., and R. Krämer. 1990. Membrane alteration is necessary but not sufficient for effective glutamate secretion in Corynebacterium glutamicum. J. Bacteriol. 172:3409-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson M. B., and J. E. Cronan, Jr. 1978. An estimate of the minimum amount of fluid lipid required for the growth of Escherichia coli. Biochim. Biophys. Acta 512:472-479. [DOI] [PubMed] [Google Scholar]
- 28.Jakoby, M., R. Krämer, and A. Burkovski. 1999. Nitrogen regulation in Corynebacterium glutamicum: isolation of genes involved and biochemical characterization of corresponding proteins. FEMS Microbiol. Lett. 173:303-310. [DOI] [PubMed] [Google Scholar]
- 29.Kimura, E. 2005. l-Glutamate production, p. 439-464. In L. Eggeling and M. Bott (ed.), Handbook of Corynebacterium glutamicum. Taylor & Francis, Boca Raton, FL.
- 30.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komura, I., S. Yamada, S. I. Otsaka, and K. Komagata. 1975. Taxonomic significance of phospholipids on coryneform and nocardioform bacteria. J. Gen. Appl. Microbiol. 21:251-261. [Google Scholar]
- 32.Landfald, B., and A. R. Strom. 1986. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J. Bacteriol. 165:849-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendum, M. L., and L. T. Smith. 2002. Gbu glycine betaine porter and carnitine uptake in osmotically stressed Listeria monocytogenes cells. Appl. Environ. Microbiol. 68:5647-5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Möker, N., M. Brocker, S. Schaffer, R. Krämer, S. Morbach, and M. Bott. 2004. Deletion of the genes encoding the MtrA-MtrB two-component system of Corynebacterium glutamicum has a strong influence on cell morphology, antibiotics susceptibility and expression of genes involved in osmoprotection. Mol. Microbiol. 54:420-438. [DOI] [PubMed] [Google Scholar]
- 35.Morbach, S., and R. Krämer. 2005. Osmoregulation, p. 417-438. In L. Eggeling and M. Bott (ed.), Handbook of Corynebacterium glutamicum. Taylor & Francis, Boca Raton, FL.
- 36.Morbach, S., and R. Krämer. 2003. Osmoregulation and osmosensing by uptake carriers for compatible solutes in bacteria. Top. Curr. Genet. 9:155-178. [Google Scholar]
- 37.Nichols, D. S., M. R. Miller, N. W. Davies, A. Goodchild, M. Raftery, and R. Cavicchioli. 2004. Cold adaptation in the Antarctic archaeon Methanococcoides burtonii involves membrane lipid unsaturation. J. Bacteriol. 186:8508-8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Özcan, N., R. Krämer, and S. Morbach. 2005. Chill activation of compatible solute transporters in Corynebacterium glutamicum at the level of transport activity. J. Bacteriol. 187:4752-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peter, H., A. Burkovski, and R. Krämer. 1998. Osmosensing by the N- and C-terminal extensions of the glycine betaine uptake system BetP of Corynebacterium glutamicum. J. Biol. Chem. 273:2567-2574. [DOI] [PubMed] [Google Scholar]
- 40.Phadtare, S., and M. Inouye. 2004. Genome-wide transcriptional analysis of the cold shock response in wild-type and cold-sensitive, quadruple-csp-deletion strains of Escherichia coli. J. Bacteriol. 196:7007-7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poolman, B., J. J. Spitzer, and J. M. Wood. 2004. Bacterial osmosensing: roles of membrane structure and electrostatics in lipid-protein and protein-protein interactions. Biochim. Biophys. Acta 1666:88-104. [DOI] [PubMed] [Google Scholar]
- 42.Puech, V., M. Chami, A. Lemassu, M. A. Laneelle, B. Schiffler, P. Gounon, N. Bayan, R. Benz, and M. Daffé. 2001. Structure of the cell envelope of corynebacteria: importance of the non-covalently bound lipids in the formation of the cell wall permeability barrier and fracture plane. Microbiology 147:1365-1382. [DOI] [PubMed] [Google Scholar]
- 43.Racher, K. I., D. E. Culham, and J. M. Wood. 2001. Requirements for osmosensing and osmotic activation of transporter ProP from Escherichia coli. Biochemistry 40:7324-7333. [DOI] [PubMed] [Google Scholar]
- 44.Rouser, G., S. Fleischer, and A. Yamamoto. 1970. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5:494-496. [DOI] [PubMed] [Google Scholar]
- 45.Rübenhagen, R., H. Rönsch, H. Jung, R. Krämer, and S. Morbach. 2000. Osmosensor and osmoregulator properties of the betaine carrier BetP from Corynebacterium glutamicum in proteoliposomes. J. Biol. Chem. 275:735-741. [DOI] [PubMed] [Google Scholar]
- 46.Rübenhagen, R., S. Morbach, and R. Krämer. 2001. The osmoreactive betaine carrier BetP from Corynebacterium glutamicum is a sensor for cytoplasmic K+. EMBO J. 20:5412-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiller, D., R. Rübenhagen, R. Krämer, and S. Morbach. 2004. The C-terminal domain of the betaine carrier BetP of Corynebacterium glutamicum is directly involved in sensing K+ as an osmotic stimulus. Biochemistry 43:5583-5591. [DOI] [PubMed] [Google Scholar]
- 48.Schiller, D., V. Ott, R. Krämer, and S. Morbach. 2006. Influence of membrane composition on osmosensing by the betaine carrier BetP from Corynebacterium glutamicum. J. Biol. Chem. 281:7737-7746. [DOI] [PubMed] [Google Scholar]
- 49.Smith, L. T. 1996. Role of osmolytes in adaptation of osmotically stressed and chill-stressed Listeria monocytogenes grown in liquid media and on processed meat surfaces. Appl. Environ. Microbiol. 62:3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steger, R., M. Weinand, R. Krämer, and S. Morbach. 2004. LcoP, an osmoregulated betaine/ectoine uptake system from Corynebacterium glutamicum. FEBS Lett. 573:155-160. [DOI] [PubMed] [Google Scholar]
- 51.Tropis, M., X. Meniche, A. Wolf, H. Gebhardt, S. Strelkov, M. Chami, D. Schomburg, R. Krämer, S. Morbach, and M. Daffé. 2005. The crucial role of trehalose and structurally related oligosaccharides in the biosynthesis and transfer of mycolic acids in Corynebacterineae. J. Biol. Chem. 15:26573-26585. [DOI] [PubMed] [Google Scholar]
- 52.van der Heide, T., and B. Poolman. 2000. Osmoregulated ABC-transport system of Lactococcus lactis senses water stress via changes in the physical state of the membrane. Proc. Natl. Acad. Sci. USA 97:7102-7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber, M. H. W., W. Klein, L. Müller, U. M. Niess, and M. Marahiel. 2001. Role of the Bacillus subtilis fatty acid desaturase in membrane adaptation during cold shock. Mol. Microbiol. 39:1321-1329. [DOI] [PubMed] [Google Scholar]
- 54.Weber, M. H., and M. Marahiel. 2003. Bacterial cold shock responses. Sci. Prog. 86:9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wemekamp-Kamphuis, H. H., R. D. Sleator, J. A. Wouters, C. Hill, and T. Abee. 2004. Molecular and physiological analysis of the role of osmolyte transporters BetL, Gbu, and OpuC in growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 70:2912-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf, A., R. Krämer, and S. Morbach. 2003. Three pathways for trehalose metabolism in Corynebacterium glutamicum ATCC13032 and their significance in response to osmotic stress. Mol. Microbiol. 49:1119-1134. [DOI] [PubMed] [Google Scholar]
- 57.Wood, J. M. 1999. Osmosensing in bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 63:230-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wood, J. M. 2006. Osmosensing by bacteria. Sci. STKE 357:43. [DOI] [PubMed] [Google Scholar]
- 59.Xie, J., M. Bogdanov, P. Heacock, and W. Dowhan. 2006. Phosphatidylethanolamine and monoglucosyldiacylglycerol are interchangeable in supporting topogenesis and function of the polytopic membrane protein lactose permease. J. Biol. Chem. 281:19172-19178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu, K., X. Ding, M. Julotok, and B. J. Wilkinson. 2005. Exogenous isoleucine and fatty acid shortening ensure the high content of anteiso-C15:0 fatty acid required for low-temperature growth of Listeria monocytogenes. Appl. Environ Microbiol. 71:8002-8007. [DOI] [PMC free article] [PubMed] [Google Scholar]