Abstract
It had been suggested that the flagella of enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli (EHEC) might contribute to host colonization. In this study, we set out to investigate the adhesive properties of H7 and H6 flagella. We studied the abilities of EHEC EDL933 (O157:H7) and EPEC E2348/69 (O127:H6) flagella to bind to bovine mucus, host proteins such as mucins, and extracellular matrix proteins. Through several approaches, we found that H6 and H7 flagella and their flagellin monomers bind to mucins I and II and to freshly isolated bovine mucus. A genetic approach showed that EHEC and EPEC fliC deletion mutants were significantly less adherent to bovine intestinal tissue than the parental wild-type strains. In addition, we found that EPEC bacteria and H6 flagella, but not EHEC, bound largely, in a dose-dependent manner, to collagen and to a lesser extent to laminin and fibronectin. We also report that EHEC O157:H7 strains agglutinate rabbit red blood cells via their flagella, a heretofore unknown phenotype in this pathogroup. Collectively, our data demonstrate that the H6 and H7 flagella possess adhesive properties, particularly the ability to bind mucins, that may contribute to colonization of mucosal surfaces.
Enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli (EHEC) are both causes of diarrheal disease and death worldwide (47). EPEC infections are an important source of potentially fatal diarrhea in infants (47). EHEC causes nonbloody and bloody (hemorrhagic colitis) diarrhea (57). A key virulence factor that distinguishes EHEC from EPEC is the production of Shiga toxin (Stx). Stx is produced by EHEC in the colon and later travels to the kidney, where it triggers the hemolytic uremic syndrome (38, 50, 64). Both of these organisms produce an outer membrane protein called intimin, which potentiates a tight attachment to host epithelial cells, leading to the loss of brush border microvilli and thus creating a histopathology known as the attaching and effacing (AE) lesion (25, 44). Thus, EPEC and EHEC are collectively called AE E. coli (AEEC). The genetic elements responsible for the production of AE lesions are carried in a pathogenicity island called the locus of enterocyte effacement (41). However, in EPEC and EHEC there are other genes outside the locus-of-enterocyte-effacement region that take part in the establishment of these organisms in the gut and contribute to bacterial virulence (5, 17, 21, 24).
Analysis of the genomic sequences of EPEC and EHEC reveals the presence of numerous putative fimbrial operons; however, only a few of them have been characterized, and thus, their function remains to be elucidated. The bundle-forming pilus of EPEC is known to mediate localized adherence (21). Other factors, such as the EspA fiber and flagella, have also been proposed to mediate nonintimate adhesion of EPEC (22, 30). As for EHEC O157:H7, bacterial components such as the outer membrane protein OmpA, long polar fimbriae, and lipopolysaccharide have been suggested to mediate host colonization (27, 52, 63). It has also been shown that the flagella of EHEC O157:H7 isolates play a role in persistent colonization of chicks (4). However, to this point it is still unknown as to how EHEC colonizes the human or bovine gut.
Flagella and motility are critical elements in the virulence strategies of many bacterial pathogens. For example, for Vibrio cholerae, Salmonella enterica, Campylobacter jejuni, and Helicobacter pylori the presence of flagella and motility are required for host colonization and induction of inflammation (1, 3, 12, 45, 68). Flagella have also been shown to play a role in biofilm formation in Stenotrophomonas maltophilia, E. coli, and Aeromonas (10, 29, 67). The adhesive properties of bacterial flagella have been further supported in studies demonstrating that the flagella of Pseudomonas aeruginosa and Clostridium difficile promote adherence to mucus (2, 62). A clinical E. coli strain (O25:H1) associated with bacteremia and meningitis was observed to bind plasminogen, a glycoprotein abundant in human plasma and intracellular fluids, via its flagella (33).
The adhesive properties of flagella most likely lie within their molecular structures. Flagella are composed of several thousand copies of flagellin subunits (40). Flagellins of enterobacteria contain highly conserved sequences in the amino and carboxyl termini, while their middle regions are significantly variable (54). The conserved end regions remain hidden in the polymeric structure, whereas the hypervariable middle region is exposed on the flagellum (54). The flagella of EHEC EDL933 O157:H7 and EPEC E2348/69 O127:H6 portray high sequence similarity, 93% in the amino termini and 92% in the carboxy termini; however, the middle hypervariable region remains significantly different. While the hypervariable region provides antigenic differences in diverse flagellins and contributes to the unique adhesive properties of flagella in distinct serotypes, the conserved region of flagellins is responsible for inducing synthesis of proinflammatory molecules in host cells via recognition of Toll-like receptor 5 (19, 43, 69).
Mucins are high-molecular-mass (200- to 2,000-kDa) glycoproteins that are composed of a peptide backbone linked to carbohydrates and have been shown to act as receptors for bacterial adhesins promoting adherence, as in the case of P. aeruginosa (2). Mucins secreted by specialized epithelial cells form a mucosal surface that acts as a first line of defense against infectious agents (48). Consequently, it is not surprising that previous reports have shown mucins to reduce adhesion to epithelial cells by EPEC (35, 39, 60). Like mucins, the extracellular matrix (ECM) proteins laminin, collagen, and fibronectin have been demonstrated to be receptors for many bacterial pathogens, such as E. coli, S. enterica, Haemophilus influenzae, Neisseria meningitidis, and Staphylococcus aureus (13, 16, 31, 47). In this study, we report new adhesive attributes of EPEC H6 and EHEC H7 flagella. Our data support the notion that the adherence mechanisms of these pathogens are multifactorial, involving fimbrial and nonfimbrial adhesins, and that flagella, in addition to driving motility, play a role in the interaction of these organisms with host cells.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and antibodies.
We employed prototype EPEC strain E2348/69, which belongs to the classical serotype O127:H6, and EHEC strain EDL933, which belongs to the O157:H7 serotype. Increased amounts of flagellum production were observed in these strains when they were grown on 1% tryptone (pH 7.2) agar plates. In consequence, bacterial strains were grown routinely on 1% tryptone agar plates overnight at 37°C. fliC mutants were grown in media supplemented with 0.01% kanamycin. Antisera against EHEC H7 and EPEC H6 flagella were raised by immunization of chickens with purified flagella (Lampire, Pipersville, PA).
Construction of bacterial mutants.
The EPEC E2348/69 (O127:H6) fliC mutant (AGT01) and AGT01 complemented with plasmid harboring fliC (AGT02) were available from a previous study (22). The EHEC EDL933 (O157:H7) fliC mutant was constructed as described earlier (7). Briefly, the chromosomal fliC gene in EDL933 was replaced with a kanamycin antibiotic resistance gene amplified from pKD4 by using forward primer G72 (AATATAGGATAACGAATCATGGCACAAGTCATTAATACCAACTGTAGGCTGGAGCTGCTTCG) and reverse primer G73 (TTAATCAGGTTACAACGATTAACCCTGCAGCAGAGAC AGAACCATATGAATATCCTCCTTA). The amplified gene segment was electroporated into bacterial cells carrying the λ Red recombinase plasmid (pKD46). Mutants were grown on selective media, followed by verification of the fliC mutation via PCR utilizing primers G94 (TCCCAGCGATGAAATACTTGC) and G95 (GAGTTATCGGCATGATTATCC).
Purification of flagella.
Bacteria grown on tryptone agar were harvested, and the flagella were detached by shearing them three times in an Omnimixer (Dupont Sorvall, Newton, CT) (22). The bacteria were then separated by centrifugation at 9,000 × g for 20 min, and the supernatant was centrifuged at 12,000 × g for 30 min to remove outer membranes and bacterial debris. To precipitate the flagella, solid ammonium sulfate was added to the supernatant until 50% saturation was reached. The flagella were recovered by centrifugation, extensively dialyzed against distilled water, and then loaded onto a cesium chloride-1% sarcosyl gradient (density, 1.2 g/ml) (22). The gradient was centrifuged at 230,000 × g for 18 h at 18°C, forming an opaque, thick band in the middle of the gradient. The putative flagellum band was pulled out and extensively dialyzed against water and phosphate-buffered saline (PBS). To break down flagella into flagellin monomers, the flagella were treated with 1% sodium dodecyl sulfate (SDS) at 37°C for 30 min and then boiled for 5 min.
TEM.
For transmission electron microscopy (TEM), bacteria grown overnight on 1% tryptone agar plates were suspended in sterile distilled water, applied onto carbon-Formvar copper grids, and then negatively stained with 1% phosphotungstic acid (pH 7.4) before being viewed under a CM12 Philips TEM (22).
SDS-PAGE and Western blotting.
Confirmation of the presence of purified flagella was done by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie blue staining (32). Purified H7 and H6 flagella were resuspended in sample buffer, heated at 100°C for 5 min, and loaded onto 16% polyacrylamide gels. After electrophoresis, flagellin proteins were visualized with a 0.25% Coomassie blue solution. To determine ECM binding, purified H6 and H7 flagella were electrophoresed in 12% denaturing polyacrylamide gels. The proteins were transferred onto polyvinylidene difluoride membranes, and the immobilized flagellins were incubated with 5 μg/ml each of the ECM proteins collagen, laminin, fibronectin, and vitronectin and then reacted with primary antibodies against the individual ECM proteins followed by secondary anti-rabbit or anti-mouse immunoglobulin G (IgG) conjugated to horseradish peroxidase (Sigma). The blots were developed with ECL Plus Western blotting detection reagents (GE Biosciences).
Mucus preparations.
Isolation and preparation of crude mucus from bovine colon, obtained from the Meat Science Laboratory, University of Arizona (Tucson, AZ), was carried out as previously described (36). The colon was cut into sections and split open with a scalpel, followed by flushing with HEPES-Hanks buffer (pH 7.4) to remove debris. The mucosal surface of each section was gently scraped with a microscope slide into the buffer. The mucosal scrapings from each section were pooled and then centrifuged twice at 27,000 × g for 15 min to remove solids. The resulting supernatant, containing crude intestinal mucus, was used in binding assays with flagella and flagellins.
Adherence to cow intestinal explants.
Intestinal tissue from a cow was cut into 8-by-8-mm squares (0.8 g of weight). After being washed thoroughly with PBS, the tissues were incubated for 6 h with 10 μl of 106 bacteria in 1 ml Dulbecco's modified Eagle's medium. After infection, unbound bacteria were removed by washing and the adherent bacteria were detached by vortexing them for 10 min with glass beads. Tenfold serial dilutions were plated onto MacConkey agar to obtain CFU. The results shown are the means for three experiments performed in triplicate. Statistical analysis was performed using Student's t test.
Immunodot blotting.
Serial dilutions of mucin I or II (Sigma) (1,000 to 0.01 μg/ml) and mucus (1:10 to 1:10,000) were applied onto a nitrocellulose membrane in a vacuum. After being blocked with 1% bovine serum albumin (BSA), the nitrocellulose membrane was incubated with H7 or H6 flagella or flagellins (1 μg/ml) to enable binding and then probed with primary antibody raised against H7 or H6 flagella in chickens. Subsequently, the membrane was reacted with secondary antibody conjugated to horseradish peroxidase against chicken IgG (Sigma). The colony blot was developed with ECL Plus Western blotting detection reagents (GE Biosciences).
Immunofluorescence.
Mucins I and II and bovine mucus were immobilized onto glass coverslips and fixed with 2% formalin and then blocked with 1% BSA. The control slide was precoated with BSA rather than mucin. One hundred microliters of 1 μg/ml purified H7 flagella was incubated with mucins for 30 min at room temperature, followed by treatment with primary chicken antibodies against H7 flagellin (Lampire) and secondary goat anti-chicken IgG antibodies conjugated to Alexa Fluor 594 (Invitrogen). The coverslips were mounted and visualized with an Axio Imager 1.0 Zeiss microscope.
Binding assay.
To further confirm the binding of flagella to mucins, equal concentrations of purified flagella and mucin (0.2 mg/ml) were incubated overnight in sodium phosphate dibasic buffer (pH 7.8) at 4°C. The proteins were applied onto a molecular exclusion chromatography column (Sephadex G-100; Sigma) and eluted with sodium phosphate dibasic buffer (pH 7.8). The optical densities (ODs) of the resulting elutions were read at 280 nm, and the two peaks were depolymerized in a 12% SDS-PAGE gel and then stained with Coomassie blue.
Bacterial binding to ECM proteins.
One hundred microliters each of the ECM proteins collagen, fibronectin, laminin, and vitronectin (Sigma) at 1 μg/ml was immobilized onto a glass coverslip and fixed with 2% formalin. After a wash with PBS-Tween (PBST), 50 μl of bacterial cultures grown to an OD600 of 1.1 was applied in 1 ml of PBS to the substrate-coated coverslips and incubated for 3 h. The coverslips were washed three times with PBST, fixed with methanol, and stained with Giemsa (Sigma) before observation by light microscopy.
ELISA-based binding of flagella to host proteins.
Purified H6 and H7 flagella (∼1 ng/well) were coated onto enzyme-linked immunosorbent assay (ELISA) plates in carbonate buffer (pH 9.8) at 4°C for 18 h. The plates were washed and blocked for 1 h with 3% BSA in PBST. After the wash, 10-fold dilutions of matrix proteins (0.01 to 100 μg/ml) were added in quadruplicate for 1 h and washed with PBST, followed by 1 h of incubation with antibodies against the individual anti-ECM proteins collagen, laminin, fibronectin, and vitronectin (diluted 1:5,000). After being washed, the plates were incubated for 1 h with anti-rabbit or anti-mouse IgG-alkaline phosphatase conjugate (1:10,000) before addition of the phosphatase substrate. Wells with no flagella were used as negative controls. The plates were read at OD405 in an ELISA multiscan reader.
HA assays.
Red blood cells (RBC) obtained from rabbit, horse, sheep, and bovine samples (Lampire) were assayed for agglutination by EPEC and EHEC strains and purified flagella in the presence of 1% d-mannose as previously described (9, 20). Hemagglutination (HA) assays were performed with 96-well, round-bottom microtiter plates. Bacteria were adjusted to 108 cells per ml in PBS. Twofold serial dilutions of the bacteria or purified flagella (∼1 mg/ml) were incubated with 1% RBC suspensions and incubated on ice for 2 h. HA was recorded when a pellet of RBC was observed in the well containing only PBS and RBC. The highest dilution showing HA was considered 1 HA unit.
For HA inhibition tests, twofold serial dilutions of anti-H7, anti-H6, and anti-lipopolysaccharide antibodies were incubated with equal volumes of 1 HA unit of purified flagella followed by 1% rabbit RBC. In addition, HA inhibition tests were performed with twofold serial dilutions of different substrates, including mucins I and II, N-acetylglucosamine, N-acetylgalactosamine, chondroitin sulfate, sialoganglioside GM1, asialoganglioside GM1, monoganglioside GM1, collagen, laminin, and fibronectin stocks at 1 mg/ml (Sigma).
RESULTS
H6 and H7 flagella bind to mucins I and II.
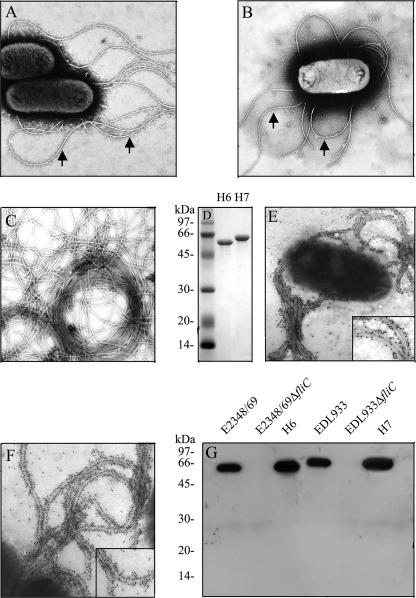
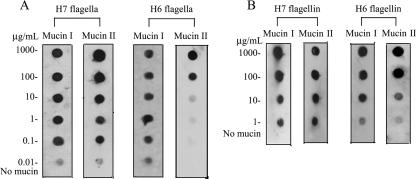
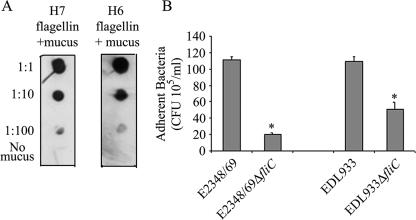
It has been reported that the FliD cap protein of the flagella of P. aeruginosa binds to mucin, a mechanism that could be important in colonization of epithelial surfaces in the airways (2). We previously demonstrated that flagella mediate adherence of motile EPEC strains to cultured epithelial cells (22), suggesting that EPEC flagella possess adhesive domains. We wanted to know if H6 or H7 flagella bound mucins. First, we purified flagella from wild-type strains EPEC E2348/69 (O127:H6) and EHEC EDL933 (O157:H7) (Fig. 1A and B) as indicated in Materials and Methods. Second, the presence of flagella was confirmed by TEM (Fig. 1C), and its purity was analyzed by SDS-PAGE under denaturing conditions. The flagella of both organisms migrated with apparent molecular masses of 60 kDa (Fig. 1D). To test the reactivities of the anti-flagellum antibodies, we performed immunogold labeling with whole bacterial cells of EPEC (Fig. 1E) and EHEC (Fig. 1F), which showed flagella decorated with gold particles and no reactivity with the bacterial cell. To further demonstrate the specificities of the antibodies employed against H6 and H7 flagella, a Western blot was conducted with the parental wild-type EPEC and EHEC strains and their fliC mutants (Fig. 1G). A band corresponding to flagella was observed only with the purified flagella and in the wild-type strains. Next, purified H7 and H6 flagella or flagellins were used in an immunodot assay to test their affinities for immobilized mucins I and II. We observed that both flagella types (Fig. 2A) and their flagellin monomers (Fig. 2B) bound to mucins in a dose-dependent manner; however, H7 flagella showed more affinity for mucin II than H6 flagella, whereas both flagellum types seemed to have similar affinities for mucin I (Fig. 2). Nonetheless, these data collectively suggest that both H7 and H6 flagella carry binding sites for mucins I and II. Next, we determined whether AEEC flagella possessed affinity for bovine mucus via dot blotting. Interestingly, we observed that both flagellum types (data not shown) and flagellin monomers (Fig. 3A) were capable of binding bovine mucus. Subsequently, we performed adherence assays utilizing bovine intestinal tissue to determine whether the presence of flagella would affect the level of bacterial binding. As expected, fliC mutants of EHEC O157:H7 and EPEC O127:H6 showed significant reductions (P = 0.0009 and P = 0.016, respectively) in adherence to bovine tissue compared to the wild-type strains (Fig. 3B).
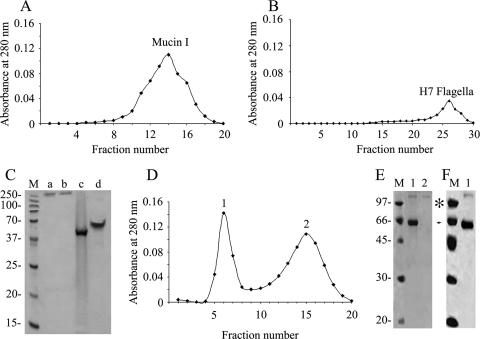
FIG. 1.
Purification of EHEC H7 flagella. A transmission electron micrograph of EPEC E2348/69 (O127:H6) (A) and EHEC EDL933 (O157:H7) (B) grown in 1% tryptone expressing flagella (arrows) is shown. (C) Transmission electron micrograph of purified flagella from EDL933. (D) SDS-PAGE analysis of purified H7 and H6 in 16% SDS-PAGE gel. Both flagella are composed of an approximately 60-kDa subunit. Immunogold labeling of EPEC H6 (E) and EHEC H7 (F) flagella is shown. The inset is a high magnification of the decorated flagella. (G) Western blot analysis of whole bacterial cells by use of antibodies against H6 or H7 flagella demonstrating the specificity of antibodies utilized. Molecular mass markers (in kDa) are indicated on the left.
FIG. 2.
Binding of H6 and H7 flagella and flagellin monomers to mucins I and II. Different concentrations of mucins I and II were immobilized onto nitrocellulose membranes and then incubated with purified H7 or H6 flagellum filaments (A) or flagellin monomers (B). Note the dose-dependent binding of flagella and flagellins to mucins.
FIG. 3.
AEEC flagella mediate binding to cow intestinal mucus. (A) H7 and H6 flagellins binding to 10-fold serial dilutions of crude bovine mucus immobilized onto nitrocellulose membranes. (B) Quantification of bacteria adherent to bovine intestinal tissue, demonstrating the difference in adherence between the wild-type strains and their respective aflagellate fliC mutants. The results shown represent the averages for three separate experiments. *, P < 0.05.
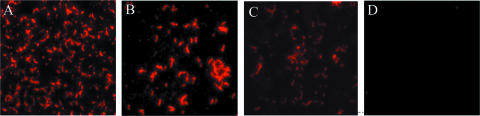
Other undertaken approaches, such as immunofluorescence and molecular exclusion chromatography, further demonstrated the interaction between mucins and AEEC flagella (Fig. 4 and 5). Since similar results were obtained for H6 and H7 flagella and to avoid redundancy, only the data obtained for H7 flagella are shown. We observed binding of native H7 flagella to mucins I (Fig. 4A) and II (Fig. 4B) and bovine mucus (Fig. 4C) immobilized onto glass coverslips. Coverslips precoated with albumin instead of mucin were used as negative controls, and as expected, no flagellum binding was observed (Fig. 4D). In addition, the binding between flagella H7 and mucins was analyzed by molecular exclusion chromatography. First, the mucins and flagella were separately subjected to chromatography to determine the elution pattern of each protein. Mucins eluted between fractions 10 and 19, while flagella eluted later between fractions 22 and 29 (Fig. 5A and B). These fractions were run in a 12% SDS-PAGE gel (Fig. 5C). With their dilution patterns established, H7 and mucin I or II were mixed at equimolar concentrations (0.2 mg/ml) and incubated overnight, after which the complexed and single proteins were separated by molecular exclusion chromatography using a Sephadex column. Fractions (500 μl) of eluted proteins were collected, and absorbance was read at OD280. Two protein peaks were obtained (Fig. 5D), of which the early peak corresponded to the H7 flagellum-mucin I complex while the second peak contained unbound mucin I alone, as shown by SDS-PAGE (Fig. 5E). Similar results were obtained for H7 flagella and mucin II (data not shown). This experiment was repeated with a higher concentration of H7 flagella (0.5 mg/ml), and in this case, only one elution peak, which contained all of the mucin I bound to H7 flagella, was observed (Fig. 5F). Collectively, these are compelling data that reveal the affinities of EHEC H7 and EPEC H6 flagella for mucus and mucins I and II and strongly suggest that this property could be of biological relevance in the host gut mucosa.
FIG. 4.
Demonstration of binding of H7 flagella to mucins and bovine mucus. Purified H7 flagella were incubated with mucins I (A) and II (B) and bovine mucus (C) immobilized onto glass coverslips. Flagella were stained by immunofluorescence using primary antibodies against flagella from chicken and secondary goat anti-chicken antibodies conjugated to Alexa Fluor 594 (red). The control slide was precoated with albumin only (D).
FIG. 5.
Molecular exclusion chromatography of mucin I and H7 flagella. Elution patterns of mucin I (A) and H7 flagella (B) are shown. (C) SDS-PAGE Coomassie staining of protein peaks obtained from the elution of mucin I (lane a), mucin II (lane b), H6 flagella (lane c), and H7 flagella (lane d). (D) Protein elution pattern after interaction of H7 flagella and mucin I. Note the presence of two peaks, where peak 1 is mucin I bound to H7 flagella (0.2 mg/ml) and peak 2 is only mucin I. (E) SDS-PAGE Coomassie staining of peak 1 (lane 1) and peak 2 (lane 2). (F) SDS-PAGE Coomassie staining of mucin I bound to H7 flagella (0.5 mg/ml) (lane 1). Note that all mucin was bound by flagella. M, mass standards (kDa). An asterisk indicates mucin I, and an arrowhead indicates H7 flagella.
EPEC E2348/69 binds to immobilized ECM proteins via flagella.
Bacterial adhesion to epithelial and subepithelial tissue is an important event in colonization of the host intestine and other tissues. ECM proteins such as fibronectin, laminin, collagen, and vitronectin have been shown to serve as receptors for various bacterial pathogens. For example, staphylococci and streptococci bind fibronectin, N. gonorrhoeae binds vitronectin, and uropathogenic E. coli binds type IV collagen (23, 26, 66). We sought to determine whether EPEC E2348/69 (O127:H6) or EHEC EDL933 (O157:H7) would bind to ECM proteins. We found that E2348/69 bound more to collagen than to laminin and fibronectin, while no binding to vitronectin was noted. In contrast to E2348/69, EDL933 did not show affinity for any of the immobilized ECM proteins (data not shown).
To corroborate these results and to provide genetic evidence of the role of EPEC H6 flagella in ECM recognition, the E2348/69 fliC mutant AGT01 and the complemented strain AGT02 (22) were tested for their abilities to bind to ECM proteins immobilized onto glass coverslips. In contrast to the wild-type, AGT01 showed only a few bacteria binding to any of the immobilized ECM proteins, suggesting that the decrease in adherence was due to the loss of flagella. Binding to ECM proteins was restored in the complemented fliC mutant strain AGT02 (data not shown).
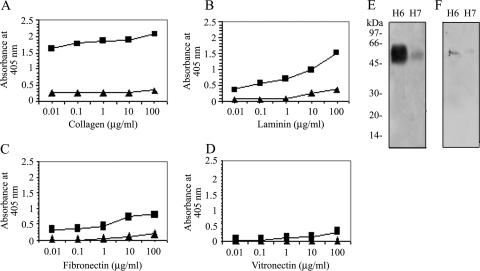
Next, we investigated whether purified H6 flagella of EPEC and H7 flagella of EHEC were directly responsible for the displayed affinity for ECM proteins. An ELISA-based assay in which immobilized H6 or H7 flagella (1 ng/well) were incubated with 10-fold dilutions of collagen, fibronectin, laminin, or vitronectin proteins between 0.01 and 100 μg/ml was performed. Binding to ECM proteins was probed with antibodies against individual ECM proteins. In line with the observations above, H7 flagella showed nearly no affinity for any of the ECM proteins tested. In contrast, H6 flagella bound in a dose-dependent manner to collagen and with less affinity to laminin and fibronectin (Fig. 6A, B, and C). The level of binding to collagen was approximately double that of binding to laminin and fibronectin, suggesting that H6 has a higher affinity for collagen than for the other ECMs. In agreement with our previous observations, vitronectin was not recognized by any of the flagellum types (Fig. 6D). The strong correlation between the results obtained with bacteria and purified flagella suggest that, under the conditions tested, H6 but not H7 flagella mediate binding to ECM proteins. To further confirm the flagellum-ECM protein association, a Far-Western experiment was performed. Purified H6 and H7 flagella were electrophoresed and transferred onto a polyvinylidene difluoride membrane and then incubated with ECM proteins collagen, laminin, fibronectin, and vitronectin at 5 μg/ml each. Among these ECMs, only collagen and laminin bound to native H6 flagellin whereas H7 bound weakly to collagen and laminin (Fig. 6E and F). In sum, it appears that H6 flagella have binding sites for ECM proteins, while H7 flagella have almost no selectivity for ECM proteins.
FIG. 6.
Dose-dependent binding of purified H6 and H7 flagella to ECM proteins. Purified H6 (▪) and H7 (▴) flagella (1 ng/well) were immobilized onto 96-well plates and incubated with 10-fold dilutions of collagen (A), laminin (B), fibronectin (C), and vitronectin (D). Binding was quantified by ELISA at an absorbance of 405 nm. The data are a representative of three identical experiments performed in quadruplicate. H6 flagella showed affinity for collagen, laminin, and fibronectin, whereas H7 did not have an affinity for any of these ECM proteins tested. For panels E and F, immobilized flagellins were reacted with collagen or laminin. The binding was detected with anti-collagen (α-collagen) and anti-laminin (α-laminin) antibodies. Note the strong affinity of H6 flagellin for collagen. Molecular mass markers (in kDa) are indicated on the left.
EHEC O157:H7 strains agglutinate rabbit RBC.
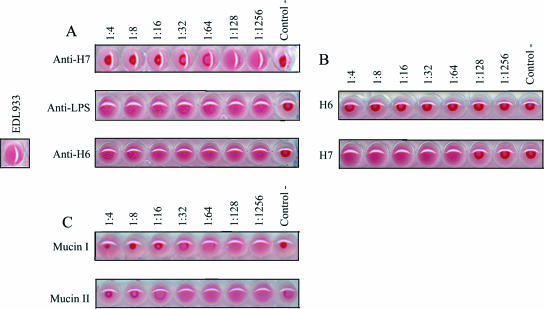
The abilities of bacterial pathogens to agglutinate human and animal RBC correlate with the presence of pili, as in the case of the colonization factors of enterotoxigenic E. coli (15) and flagella in Vibrio fischeri (42). We sought to determine whether EPEC and EHEC strains showed HA of animal RBC. Mannose-resistant HA was observed only between EHEC EDL933 (O157:H7) bacteria and rabbit RBC (Fig. 7A), while EPEC E2348/69 (O127:H6) did not cause HA of any of the blood types tested (data not shown). To support this notion, we tested eight EHEC O157:H7 strains [275F1, 85-170, 23380-85, 34(4)AKAN F4, 93-111, 37(1), 279F1, and 278F2] from our laboratory collection for agglutination of RBC and confirmed this heretofore unknown property of EHEC. We also noted that for the same eight EHEC O157:H7 strains, HA of rabbit RBC was inhibited in a dose-response manner with antibodies against H7 flagella (Fig. 7A) but not with antibodies against H6 flagella or O157 lipopolysaccharide (Fig. 7A), strongly suggesting that the observed HA reaction was specific and due to H7 flagella. We tested two EHEC strains carrying a null mutation of the flagellin fliC gene for HA of rabbit RBC. The isogenic fliC mutants showed decreases in HA titer compared to the wild-type strains (data not shown), confirming that H7 flagella play a role in HA.
FIG. 7.
HA assays. (A) EHEC EDL933 (O157:H7) hemagglutinates rabbit RBC (positive control). For inhibition of HA, whole bacteria were incubated with rabbit RBC, followed by the addition of twofold serial dilutions of anti-H7, anti-O157, or anti-H6 antibodies. (B) HA mediated by purified H7 and not H6 flagella. (C) Inhibition of HA by mucins I and II. Twofold serial dilutions of mucins were incubated with rabbit RBC and 1 HA unit of purified H7 flagella. A dose-dependent inhibition of H7-mediated HA by mucins was observed. For all HA assays, wells containing RBC with PBS were used as negative controls.
H7 flagella are hemagglutinins.
We wanted to know if purified EHEC O157:H7 flagella agglutinated rabbit RBC. We performed HA assays with twofold serial dilutions of purified H7 with 1% rabbit RBC. RBC alone were used as a negative control. As expected from the results obtained with whole bacteria, we observed that H7 but not H6 flagella caused agglutination of rabbit RBC (Fig. 7B). Based on these results, we conclude that H7 flagella of EHEC have the ability to bind and agglutinate rabbit RBC.
In addition, we were also interested in studying other molecules that might be acting as receptor analogues for EHEC H7 and EPEC H6 flagella. Thus, we performed HA assays to test mucins I and II, laminin, fibronectin, collagen, N-acetyl-d-galactosamine, N-acetyl-d-glucosamine, chondroitin sulfate, sialoganglioside GM1, asialoganglioside GM1, and monoganglioside GM1 as possible receptors of flagella, since they have been shown to act as receptors for several bacterial hemagglutinins (6, 9, 18, 37, 56, 59, 65). The results revealed that only mucins I and II (Fig. 7C) inhibited HA mediated by H7 flagella, confirming the interaction between H7 flagella and mucins.
DISCUSSION
Bacterial colonization is a multifactorial process that includes the participation of distinct adhesins. Flagella not only contribute to bacterial motility but also are involved in biofilm formation, binding to host proteins, adherence, invasion, and colonization of host cells (2, 10, 22, 34, 51, 53). It was recently suggested that the flagella of EPEC contribute to adherence to cultured epithelial cells (22); however, the mechanism of adherence via flagella is not clear. A study by Lahteenmaki et al. demonstrated that flagella of a bacteremia- and meningitis-associated E. coli strain bind plasminogen (33). Tasteyre et al. reported that FliC and FliD of C. difficile mediated attachment to the mucus layer of the intestine (62). In this study, we sought to examine the adhesive properties of EHEC H7 and EPEC H6 flagella.
We undertook several approaches to determine the interaction of AEEC flagella and flagellins with mucins I and II, all of which led us to conclude that these flagellum types possess affinity for these molecules. The interaction of mucins with native flagella strongly suggests that the tertiary and quaternary conformations of flagellins either carry exposed receptors for mucins or are simply trapped by mucins. Additionally, the fact that flagella bind to mucus isolated from bovine intestine further demonstrates the importance of this interaction. This may have relevance as to how these organisms colonize the gut or as to how they are eliminated from the gastrointestinal tract.
Mucins are high-molecular-mass glycoproteins that are composed of a peptide backbone linked to carbohydrates. In the gastrointestinal tract, specialized epithelial cells (i.e., goblet cells) express mucins, forming a mucosal surface. This surface is positioned strategically between the apical intestinal epithelial membrane and the intestinal lumen, thus acting as a first line of defense against infectious agents (48). Consequently, it is not surprising that previous reports have shown mucins to reduce adhesion to epithelial cells by EPEC (35, 60). Mucin I has also been proposed to act as a barrier against bacterial pathogens, as demonstrated in human breast milk and the female reproductive tracts of mice (11, 58), and is recognized as a receptor for FliD, the flagellar cap protein of P. aeruginosa, which colonizes inside the airways of cystic fibrosis patients (2). Furthermore, Mack et al. hypothesized that through the induction or addition of mucin II and other intestinal mucins, adherence of AEEC could be abolished (39). Thus, it is reasonable to assume that mucins I and II serve a protective role by trapping bacteria via binding to their flagella. This could have a beneficial affect on the innate immune system of the gut by restricting the motility of microbes and prompting their removal by peristaltic flow action. Conversely, bacterial binding to mucus could favor intestinal colonization. This was suggested from our results demonstrating that the presence of flagella was important in bacterial adherence to bovine tissue. Mutations in the fliC genes of both EPEC and EHEC strains showed significant decreases in adherence, underlining the importance of flagella in the interaction with mucins.
We found that EPEC bound to ECM proteins collagen, laminin, and fibronectin via flagella. Vitronectin was not a binding substrate for EPEC. In contrast, the fliC mutant AGT01 did not bind to these ECM proteins. These results correlate with the finding that purified H6 flagella bind ECM proteins in the following order of increasing affinity: fibronectin, laminin, and collagen. On the other hand, purified H7 flagella showed almost no affinity for any of the ECM proteins tested. In all, these findings suggest that EPEC H6 flagella, but not EHEC H7 flagella, possess binding sites for most ECM proteins. The differences between EPEC and EHEC flagella in their affinities for ECM molecules are likely due to differences in the hypervariable regions of the flagellin subunits (54). The abilities of enteric pathogens to bind ECM proteins could contribute to host colonization when the intestinal barrier is disrupted.
In the gut, cells and ECM constituting the epithelial tissue form barriers to prevent microorganisms from penetrating these tissues. Bacteria have evolved mechanisms for breaking these epithelial barriers so as to benefit from nutrients found in deeper tissue and evade the immune system (8, 16). Epithelial cells infected with EPEC show disruption of tight junctions, an event mediated by the type III secreted protein EspF (46). Therefore, one can speculate that EPEC might have evolved mechanisms for breaking tight junctions that lead to flagellum-mediated binding to ECM in the basal lamina.
We observed that EHEC O157:H7 strains agglutinate rabbit RBC via their flagella. While this is a well-known pilus-mediated property of enterotoxigenic E. coli and uropathogenic E. coli, this is to our knowledge the first report on the HA of RBC by EHEC O157:H7 (14, 61). While a significant reduction in HA was observed in the EHEC fliC mutant compared to what was observed in the wild-type strain, it is clear that in addition to flagella other hemagglutinins that mediate HA exist. It is possible that the absence of flagella decreases steric hindrance caused by flagella, allowing other components on the bacterial surface to interact with RBC, resulting in their agglutination.
Consistent with these results, purified H7 flagella, but not H6 flagella, caused HA in a dose-dependent manner. Our HA data strongly suggest that EHEC H7 flagella have hemagglutinating properties. Several chemicals were subsequently tested as putative receptors for H7 flagella. Among a variety of substrates, which included carbohydrates, proteins, and glycoproteins, only mucins I and II inhibited HA mediated by H7 flagella. These data correlate with our previous experiments demonstrating the interaction between H7 flagella and these host proteins.
The interaction of AEEC with host epithelial cells is a multifactorial process involving fimbrial and nonfimbrial adhesins (21, 25, 28, 49, 55). The adhesive properties of H6 and H7 flagella highlighted here, particularly the binding to mucus and mucins, may be biologically relevant within the context of colonization of the host.
Acknowledgments
Aysen Erdem and Fabiola Avelino contributed equally to this work. We thank Helen Jost for donation of bovine colon and mucus and the Arizona Hispanic Center of Excellence for support.
This work was supported by NIH grants AI60211 and AI66012 to J.A.G.
Footnotes
Published ahead of print on 10 August 2007.
REFERENCES
- 1.Allen-Vercoe, E., and M. J. Woodward. 1999. The role of flagella, but not fimbriae, in the adherence of Salmonella enterica serotype Enteritidis to chick gut explant. J. Med. Microbiol. 48:771-780. [DOI] [PubMed] [Google Scholar]
- 2.Arora, S. K., B. W. Ritchings, E. C. Almira, S. Lory, and R. Ramphal. 1998. The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect. Immun. 66:1000-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attridge, S. R., and D. Rowley. 1983. The role of the flagellum in the adherence of Vibrio cholerae. J. Infect. Dis. 147:864-872. [DOI] [PubMed] [Google Scholar]
- 4.Best, A., R. M. La Ragione, A. R. Sayers, and M. J. Woodward. 2005. Role for flagella but not intimin in the persistent infection of the gastrointestinal tissues of specific-pathogen-free chicks by Shiga toxin-negative Escherichia coli O157:H7. Infect. Immun. 73:1836-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campellone, K. G., D. Robbins, and J. M. Leong. 2004. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev. Cell 7:217-228. [DOI] [PubMed] [Google Scholar]
- 6.Castaneda-Roldan, E. I., S. Ouahrani-Bettache, Z. Saldana, F. Avelino, M. A. Rendon, J. Dornand, and J. A. Giron. 2006. Characterization of SP41, a surface protein of Brucella associated with adherence and invasion of host epithelial cells. Cell. Microbiol. 8:1877-1887. [DOI] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bentzmann, S., A. Tristan, J. Etienne, N. Brousse, F. Vandenesch, and G. Lina. 2004. Staphylococcus aureus isolates associated with necrotizing pneumonia bind to basement membrane type I and IV collagens and laminin. J. Infect. Dis. 190:1506-1515. [DOI] [PubMed] [Google Scholar]
- 9.del Rocha-Gracia, R. C., E. I. Castaneda-Roldan, S. Giono-Cerezo, and J. A. Giron. 2002. Brucella sp. bind to sialic acid residues on human and animal red blood cells. FEMS Microbiol. Lett. 213:219-224. [DOI] [PubMed] [Google Scholar]
- 10.de Oliveira-Garcia, D., M. Dall'Agnol, M. Rosales, A. C. Azzuz, M. B. Martinez, and J. A. Giron. 2002. Characterization of flagella produced by clinical strains of Stenotrophomonas maltophilia. Emerg Infect. Dis. 8:918-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSouza, M. M., G. A. Surveyor, R. E. Price, J. Julian, R. Kardon, X. Zhou, S. Gendler, J. Hilkens, and D. D. Carson. 1999. MUC1/episialin: a critical barrier in the female reproductive tract. J. Reprod. Immunol. 45:127-158. [DOI] [PubMed] [Google Scholar]
- 12.Eaton, K. A., S. Suerbaum, C. Josenhans, and S. Krakowka. 1996. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect. Immun. 64:2445-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberhard, T., R. Virkola, T. Korhonen, G. Kronvall, and M. Ullberg. 1998. Binding to human extracellular matrix by Neisseria meningitidis. Infect. Immun. 66:1791-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, D. G., D. J. Evans, Jr., and W. Tjoa. 1977. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect. Immun. 18:330-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans, D. J., Jr., D. G. Evans, and H. L. DuPont. 1979. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect. Immun. 23:336-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fink, D. L., B. A. Green, and J. W. St Geme III. 2002. The Haemophilus influenzae Hap autotransporter binds to fibronectin, laminin, and collagen IV. Infect. Immun. 70:4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garmendia, J., A. D. Phillips, M. F. Carlier, Y. Chong, S. Schuller, O. Marches, S. Dahan, E. Oswald, R. K. Shaw, S. Knutton, and G. Frankel. 2004. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell. Microbiol. 6:1167-1183. [DOI] [PubMed] [Google Scholar]
- 18.Geuijen, C. A., R. J. Willems, and F. R. Mooi. 1996. The major fimbrial subunit of Bordetella pertussis binds to sulfated sugars. Infect. Immun. 64:2657-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giron, J. A. 2005. Role of flagella in mucosal colonization, p. 213-236. In J. P. Nataro (ed.), Colonization of mucosal surfaces. ASM Press, Washington, DC.
- 20.Girón, J. A., A. S. Ho, and G. K. Schoolnik. 1993. Characterization of fimbriae produced by enteropathogenic Escherichia coli. J. Bacteriol. 175:7391-7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girón, J. A., A. S. Ho, and G. K. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710-713. [DOI] [PubMed] [Google Scholar]
- 22.Girón, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Duarte, O. G., M. Dehio, C. A. Guzman, G. S. Chhatwal, C. Dehio, and T. F. Meyer. 1997. Binding of vitronectin to Opa-expressing Neisseria gonorrhoeae mediates invasion of HeLa cells. Infect. Immun. 65:3857-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez-Duarte, O. G., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joh, D., E. R. Wann, B. Kreikemeyer, P. Speziale, and M. Hook. 1999. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18:211-223. [DOI] [PubMed] [Google Scholar]
- 27.Jordan, D. M., N. Cornick, A. G. Torres, E. A. Dean-Nystrom, J. B. Kaper, and H. W. Moon. 2004. Long polar fimbriae contribute to colonization by Escherichia coli O157:H7 in vivo. Infect. Immun. 72:6168-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 29.Kirov, S. M., M. Castrisios, and J. G. Shaw. 2004. Aeromonas flagella (polar and lateral) are enterocyte adhesins that contribute to biofilm formation on surfaces. Infect. Immun. 72:1939-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kukkonen, M., T. Raunio, R. Virkola, K. Lahteenmaki, P. H. Makela, P. Klemm, S. Clegg, and T. K. Korhonen. 1993. Basement membrane carbohydrate as a target for bacterial adhesion: binding of type I fimbriae of Salmonella enterica and Escherichia coli to laminin. Mol. Microbiol. 7:229-237. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 33.Lahteenmaki, K., B. Westerlund, P. Kuusela, and T. K. Korhonen. 1993. Immobilization of plasminogen on Escherichia coli flagella. FEMS Microbiol. Lett. 106:309-314. [DOI] [PubMed] [Google Scholar]
- 34.La Ragione, R. M., W. A. Cooley, P. Velge, M. A. Jepson, and M. J. Woodward. 2003. Membrane ruffling and invasion of human and avian cell lines is reduced for aflagellate mutants of Salmonella enterica serotype Enteritidis. Int. J. Med. Microbiol. 293:261-272. [DOI] [PubMed] [Google Scholar]
- 35.Larson, M. A., S. H. Wei, A. Weber, D. R. Mack, and T. L. McDonald. 2003. Human serum amyloid A3 peptide enhances intestinal MUC3 expression and inhibits EPEC adherence. Biochem. Biophys. Res. Commun. 300:531-540. [DOI] [PubMed] [Google Scholar]
- 36.Laux, D. C., E. F. McSweegan, T. J. Williams, E. A. Wadolkowski, and P. S. Cohen. 1986. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12(K88ab). Infect. Immun. 52: 18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, K. K., H. B. Sheth, W. Y. Wong, R. Sherburne, W. Paranchych, R. S. Hodges, C. A. Lingwood, H. Krivan, and R. T. Irvin. 1994. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol. Microbiol. 11:705-713. [DOI] [PubMed] [Google Scholar]
- 38.Lingwood, C. A. 1994. Verotoxin-binding in human renal sections. Nephron 66:21-28. [DOI] [PubMed] [Google Scholar]
- 39.Mack, D. R., S. Michail, S. Wei, L. McDougall, and M. A. Hollingsworth. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276:G941-G950. [DOI] [PubMed] [Google Scholar]
- 40.Macnab, R. M. 2003. How bacteria assemble flagella. Annu. Rev. Microbiol. 57:77-100. [DOI] [PubMed] [Google Scholar]
- 41.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millikan, D. S., and E. G. Ruby. 2002. Alterations in Vibrio fischeri motility correlate with a delay in symbiosis initiation and are associated with additional symbiotic colonization defects. Appl. Environ. Microbiol. 68:2519-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyamoto, Y., M. Iimura, J. B. Kaper, A. G. Torres, and M. F. Kagnoff. 2006. Role of Shiga toxin versus H7 flagellin in enterohaemorrhagic Escherichia coli signalling of human colon epithelium in vivo. Cell. Microbiol. 8:869-879. [DOI] [PubMed] [Google Scholar]
- 44.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morooka, T., A. Umeda, and K. Amako. 1985. Motility as an intestinal colonization factor for Campylobacter jejuni. J. Gen. Microbiol. 131:1973-1980. [DOI] [PubMed] [Google Scholar]
- 46.Muza-Moons, M. M., E. E. Schneeberger, and G. A. Hecht. 2004. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell. Microbiol. 6:783-793. [DOI] [PubMed] [Google Scholar]
- 47.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neutra, M. R., and J. F. Forstner. 1987. Gastrointestinal mucus:synthesis, secretion, and function, p. 975-1009. In L. R. Johnson (ed.), Physiology of the gastrointestinal tract, 2nd ed. Raven Press, New York, NY.
- 49.Nougayrede, J. P., P. J. Fernandes, and M. S. Donnenberg. 2003. Adhesion of enteropathogenic Escherichia coli to host cells. Cell. Microbiol. 5:359-372. [DOI] [PubMed] [Google Scholar]
- 50.O'Brien, A. D., G. D. LaVeck, M. R. Thompson, and S. B. Formal. 1982. Production of Shigella dysenteriae type 1-like cytotoxin by Escherichia coli. J. Infect. Dis. 146:763-769. [DOI] [PubMed] [Google Scholar]
- 51.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 52.Paton, A. W., E. Voss, P. A. Manning, and J. C. Paton. 1998. Antibodies to lipopolysaccharide block adherence of Shiga toxin-producing Escherichia coli to human intestinal epithelial (Henle 407) cells. Microb. Pathog. 24:57-63. [DOI] [PubMed] [Google Scholar]
- 53.Pavlovskis, O. R., D. M. Rollins, R. L. Haberberger, Jr., A. E. Green, L. Habash, S. Strocko, and R. I. Walker. 1991. Significance of flagella in colonization resistance of rabbits immunized with Campylobacter spp. Infect. Immun. 59:2259-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reid, S. D., R. K. Selander, and T. S. Whittam. 1999. Sequence diversity of flagellin (fliC) alleles in pathogenic Escherichia coli. J. Bacteriol. 181:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rendon, M. A., Z. Saldana, A. L. Erdem, V. Monteiro-Neto, A. Vazquez, J. B. Kaper, J. L. Puente, and J. A. Giron. 2007. Commensal and pathogenic Escherichia coli use a common pilus adherence factor for epithelial cell colonization. Proc. Natl. Acad. Sci. USA 104:10637-10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhen, M., P. Klemm, and T. K. Korhonen. 1986. Identification of two new hemagglutinins of Escherichia coli, N-acetyl-d-glucosamine-specific fimbriae and a blood group M-specific agglutinin, by cloning the corresponding genes in Escherichia coli K-12. J. Bacteriol. 168:1234-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 58.Schroten, H., F. G. Hanisch, R. Plogmann, J. Hacker, G. Uhlenbruck, R. Nobis-Bosch, and V. Wahn. 1992. Inhibition of adhesion of S-fimbriated Escherichia coli to buccal epithelial cells by human milk fat globule membrane components: a novel aspect of the protective function of mucins in the nonimmunoglobulin fraction. Infect. Immun. 60:2893-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seignole, D., M. Mouricout, Y. Duval-Iflah, B. Quintard, and R. Julien. 1991. Adhesion of K99 fimbriated Escherichia coli to pig intestinal epithelium: correlation of adhesive and non-adhesive phenotypes with the sialoglycolipid content. J. Gen. Microbiol. 137:1591-1601. [DOI] [PubMed] [Google Scholar]
- 60.Smith, C. J., J. B. Kaper, and D. R. Mack. 1995. Intestinal mucin inhibits adhesion of human enteropathogenic Escherichia coli to HEp-2 cells. J. Pediatr. Gastroenterol. Nutr. 21:269-276. [DOI] [PubMed] [Google Scholar]
- 61.Svenson, S. B., H. Hultberg, G. Kallenius, T. K. Korhonen, R. Mollby, and J. Winberg. 1983. P-fimbriae of pyelonephritogenic Escherichia coli: identification and chemical characterization of receptors. Infection 11:61-67. [DOI] [PubMed] [Google Scholar]
- 62.Tasteyre, A., M. C. Barc, A. Collignon, H. Boureau, and T. Karjalainen. 2001. Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect. Immun. 69:7937-7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torres, A. G., and J. B. Kaper. 2003. Multiple elements controlling adherence of enterohemorrhagic Escherichia coli O157:H7 to HeLa cells. Infect. Immun. 71:4985-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uchida, H., N. Kiyokawa, H. Horie, J. Fujimoto, and T. Takeda. 1999. The detection of Shiga toxins in the kidney of a patient with hemolytic uremic syndrome. Pediatr. Res. 45:133-137. [DOI] [PubMed] [Google Scholar]
- 65.Vaisanen, V., T. K. Korhonen, M. Jokinen, C. G. Gahmberg, and C. Ehnholm. 1982. Blood group M specific haemagglutinin in pyelonephritogenic Escherichia coli. Lancet i:1192. [DOI] [PubMed] [Google Scholar]
- 66.Westerlund, B., P. Kuusela, J. Risteli, L. Risteli, T. Vartio, H. Rauvala, R. Virkola, and T. K. Korhonen. 1989. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol. Microbiol. 3:329-337. [DOI] [PubMed] [Google Scholar]
- 67.Wood, T. K., A. F. Gonzalez Barrios, M. Herzberg, and J. Lee. 2006. Motility influences biofilm architecture in Escherichia coli. Appl. Microbiol. Biotechnol. 72:361-367. [DOI] [PubMed] [Google Scholar]
- 68.Xicohtencatl-Cortes, J., S. Lyons, A. P. Chaparro, D. R. Hernandez, Z. Saldana, M. A. Ledesma, M. A. Rendón, A. T. Gewirtz, K. E. Klose, and J. A. Giron. 2006. Identification of proinflammatory flagellin proteins in supernatants of Vibrio cholerae O1 by Proteomics Analysis. Mol. Cell. Proteomics 5:2374-2383. [DOI] [PubMed] [Google Scholar]
- 69.Zhou, X., J. A. Giron, A. G. Torres, J. A. Crawford, E. Negrete, S. N. Vogel, and J. B. Kaper. 2003. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect. Immun. 71:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]