Abstract
The Firmicutes Thermoanaerobacter sulfurigignens and Thermoanaerobacterium thermosulfurigenes convert thiosulfate, forming sulfur globules inside and outside cells. X-ray absorption near-edge structure analysis revealed that the sulfur consisted mainly of sulfur chains with organic end groups similar to sulfur formed in purple sulfur bacteria, suggesting the possibility that the process of sulfur globule formation by bacteria is an ancient feature.
The geochemical cycling of sulfur species plays an important role in energy generation and supports microbial communities in sulfur- and sulfide-rich environments (6, 25). Thiosulfate (S2O32−) is known to be one of the important products of biological oxidation or chemical oxidation of sulfides and plays a key role in the sulfur cycle in sediments (4, 11, 12). Thiosulfate can be oxidized to sulfate, disproportionated to sulfate and sulfide, reduced to sulfide under anaerobic conditions, and regarded as a widely used electron donor and acceptor for many microorganisms (1, 13). Recently, the thermophilic organism Thermoanaerobacter sulfurigignens, isolated from SO2-emitting and sulfur-accumulating volcanic White Island (New Zealand), was described to convert up to 1 M thiosulfate to elemental sulfur and to tolerate sulfite up to 90 mM (15). The conversion of thiosulfate only to elemental sulfur instead to sulfide is no longer a taxonomic discriminating feature for distinguishing the Firmicutes genera Thermoanaerobacterium and Thermoanaerobacter (14, 18).
The formation of elemental sulfur (S°) is ecologically important for several groups of microorganisms (7), and the chemical nature of the formed sulfur has been analyzed for a variety of bacteria (9, 10, 17, 19, 20, 21, 29, 30). However, most of the studies of the sulfur analysis focused on mesophilic phototrophic sulfur bacteria (5, 23). Thus, little information was available on the properties of sulfur globules produced in thermophilic chemoheterotrophic anaerobic Firmicutes.
Biologically produced sulfur can be stored inside and/or outside a cell, and the sulfur stored inside a cell exhibits properties different from that of sulfur stored outside a cell (15, 21). Previous works showed that the chemical natures of the sulfur and the surface properties of sulfur globules vary and differ in different groups of bacteria (20, 21). For example, sulfur in bacterial sulfur globules is liquid and rather amorphous compared to that in pure elemental sulfur (10) and shows low density and hydrophilicity (9, 17, 28, 29). So far, it has not been unequivocally demonstrated whether the sulfur produced by members of the thermophilic anaerobic Firmicutes is formed inside, outside, or both inside and outside cells and, subsequently, whether its location has an effect on the chemical structure and sulfur differentiation of the sulfur globules.
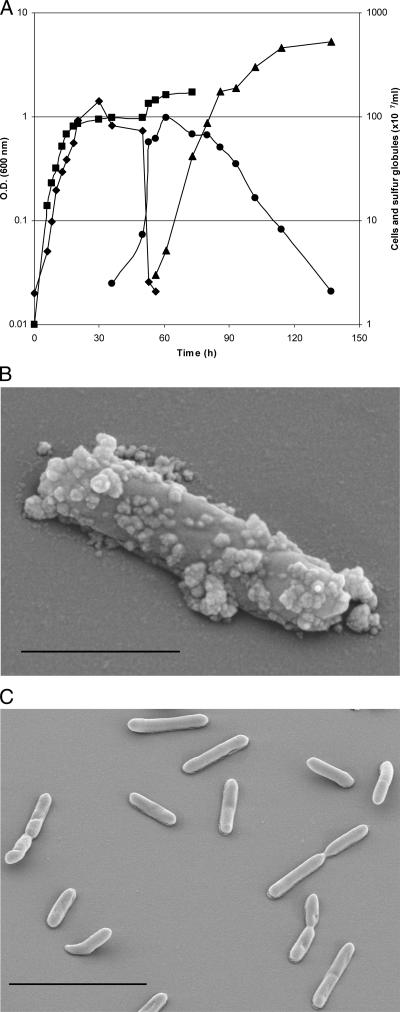
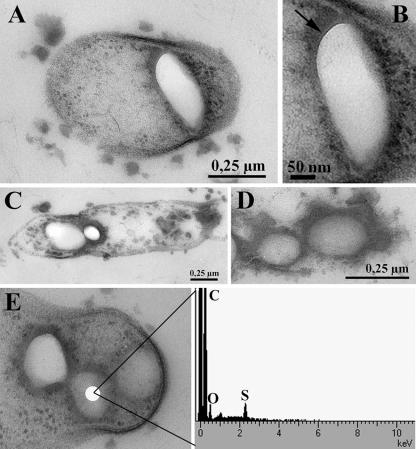
Both Thermoanaerobacter sulfurigignens JW/SL-NZ826T and Thermoanaerobacterium thermosulfurigenes 4BT were cultured heterotrophically in the presence of various concentrations of thiosulfate (between 10 and 500 mM) as a possible electron acceptor. The culture medium contained 0.5% (wt/vol) glucose as a carbon source supplemented with 0.1% (wt/vol) yeast extract, and the pH was adjusted to 6.5 (15). Thiosulfate solution was prepared anaerobically using the modified Hungate technique (16) and sterilized separately. The sulfur globules were produced as early as the mid-exponential growth phase but mainly at the end of the exponential growth phase and during the stationary phase. Since the appearance of the majority of extracellular sulfur globules was correlated with a decline in cell numbers, it was assumed that the extracellular sulfur globules were due mainly to cell lysis (Fig. 1A). However, scanning electron micrographs revealed that small sulfur globules were also produced outside the cells when they were grown with thiosulfate, which, however, were absent when the cells were grown without thiosulfate (Fig. 1B and C). The morphology of sulfur globules was identified by transmission electron microscopy by examining ultrathin sections (Fig. 2A to E). Energy-dispersive X-ray analysis confirmed that both types of globules contained sulfur (Fig. 2E). The intracellular sulfur globules appeared to be enclosed by a membrane (not further characterized), as revealed by transmission electron microscopy (Fig. 2B). However, the observed structure could also be due to the mixtures of organic sulfanes with hydrophilic end groups (30).
FIG. 1.
Production of sulfur globules by T. sulfurigignens JW/SL-NZ826T. (A) Measurement of the release of sulfur globules into the medium during a growth cycle. ▪, optical density (O.D.) of the culture; ♦, cells without internal sulfur globules; •, cells with internal sulfur globules; ▴, free sulfur globules in the medium. (B and C) Electron micrographs of a bacterium grown in the presence of 50 mM thiosulfate producing sulfur globules outside the cell (B) and of cells grown in the absence of thiosulfate (C). Bars, 1 μm (B) and 5 μm (C).
FIG. 2.
Electron micrographs of Thermoanaerobacter sulfurigignens JW/SL-NZ826T showing a sulfur globule inside the cell (A), the membrane around the sulfur globule (B), a cell containing sulfur globules in the process of lysis (C), sulfur globules in the culture from lysed cells (D), and the results of energy dispersive X-ray analysis indicating that the globules inside the cell contain sulfur (E).
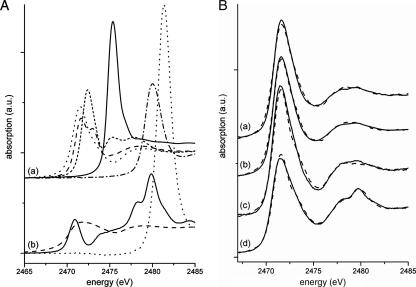
X-ray absorption near-edge structure (XANES) spectroscopy, a powerful nondestructive tool for probing sulfur species in biological samples in situ (3, 20, 21, 23, 24), was used to analyze the sulfur species of globules formed in species of two genera from the phylum Firmicutes, Thermoanaerobacter sulfurigignens JW/SL-NZ826T and Thermoanaerobacterium thermosulfurigenes 4BT (14, 15, 27). XANES spectroscopy allowed us to use directly cultured bacteria in liquid media and to determine the valence of excited S atoms, the lengths of sulfur chains, and the type of the chemical bond in the second coordination shell of the excited sulfur atom (e.g., C-C single, double, or triple bonds) (24). Sulfur globules of Thermoanaerobacter sulfurigignens JW/SL-NZ826T and Thermoanaerobacterium thermosulfurigenes 4BT were prepared according to the procedure of Schmidt et al. (28) and Brune (2), with modifications. The cells were disintegrated by ultrasonication, the sulfur globules were separated by centrifugation, and the supernatant was removed with a pipette for analysis. Samples were prepared for XANES spectroscopy using the modified procedure of Prange et al. (21) (see the supplemental material for details). For the quantitative analysis of the spectra obtained from both bacteria, a wide variety of reference compounds of different sulfur species (representatives for a given class of an atomic environment) were measured and their respective relevances were tested (Fig. 3A). XANES spectra were recorded at the DCM beamline at the CAMD, Baton Rouge, LA, and analyzed quantitatively as described previously (8). The errors of the percentages of contribution of sulfur species (Table 1) were estimated to be less than ±10% (absolute value) (21, 22). The S K-edge XANES spectra with their accompanying WinXAS fits (26) for Thermoanaerobacter sulfurigignens (Fig. 3B, spectra a and b) and Thermoanaerobacterium thermosulfurigenes (Fig. 3B, spectra c and d) revealed that the sulfur in cells of these bacteria consisted mainly of sulfur chains (∼80% had the structure R-Sn-R, and ∼15 to 18% of minor substances had the sulfur structure C-S-H/C-S-S-C) (Table 1). Only small amounts of highly oxidized sulfur species such as sulfoxides and sulfonates were detected. The other tested reference species (S8 rings, sulfate, thiosulfate), whose presence could be expected among the sulfur species since, e.g., S8 ring sulfur is thermodynamically the most stable form of S° at ambient temperatures (20), were completely absent as determined with the fitting routine except in a residual thiosulfate-containing T. thermosulfurigenes culture sample taken 2 days after thiosulfate addition. The data presented here indicated that the sulfur species produced by T. sulfurigignens and T. thermosulfurigenes were comparable. The sulfur existed mainly as sulfur chains with, presumably, an additional “organic compound” present in the form of mono- or bis-organyl sulfanes.
FIG. 3.
Sulfur K-edge XANES spectra. (A) Spectra of the reference compounds which were used for fitting the bacterial spectrum. (a) Polymeric sulfur (dotted line) (cf. Table 1), oxidized glutathione (line of long dashes), reduced glutathione (line of short dashes), dimethyl sulfoxide (solid line), and cysteic acid (line of dots and dashes); (b) S8 rings (line of long dashes), sodium thiosulfate (solid line), and zinc sulfate (dotted line). a.u., arbitrary units. (B) Spectra of Thermoanaerobacter sulfurigignens (solid lines) and accompanying WinXAS fits (dashed lines). (a) T. sulfurigignens fed with 50 mM thiosulfate; (b) T. sulfurigignens fed with 500 mM thiosulfate; (c) Thermoanaerobacterium thermosulfurigenes fed with thiosulfate (4 days after thiosulfate addition); (d) T. thermosulfurigenes fed with thiosulfate (2 days after thiosulfate addition).
TABLE 1.
Results of fitting the sulfur K-edge XANES spectra of Thermoanaerobacterium thermosulfurigenes and Thermoanaerobacter sulfurigignens to the sum of the reference spectra
| Sample | % Contributed by sulfur speciesa:
|
|||||
|---|---|---|---|---|---|---|
| Thiosulfate | Glutathione (reduced) | Glutathione (oxidized) | Polymeric sulfur | Dimethyl sulfoxide | Cysteic acid | |
| Thermoanaerobacter sulfurigignens fed 50 mM thiosulfate | — | 10 | 5 | 83 | — | 1 |
| Thermoanaerobacter sulfurigignens fed 500 mM thiosulfate | — | 8 | 10 | 79 | 1.6 | 1.3 |
| Thermoanaerobacterium thermosulfurigenes (2 days after thiosulfate addition) | 10 | 13 | 15 | 50 | — | — |
| Thermoanaerobacterium thermosulfurigenes (4 days after thiosulfate addition) | — | 3 | 24 | 71 | — | — |
With percentages of contribution by different sulfur species in the sulfur analysis, errors were less than ±10%. —, the contribution was <1%.
Based on previous results, it was assumed that different sulfur species in the sulfur globules reflect the different metabolic properties and ecological niches (21). Sulfur chains in anaerobically grown cells differed from those of aerobically grown cells (21). Interestingly, the sulfur species determined in this study, formed from thiosulfate reduction by anaerobic thermophilic Firmicutes, are very similar to those formed from the oxidation of sulfide by mesophilic phototrophic sulfur bacteria (21, 22). The phototrophic sulfur bacteria (phyla Proteobacteria and Chlorobi) are regarded as evolutionarily quite distant from the family Thermoanaerobacteriaceae of the phylum Firmicutes. The atmosphere of early Earth was sulfur rich, so anoxygenic photosynthesis using reduced sulfur compounds as electron donors prevailed among purple sulfur bacteria (e.g., Chromatiaceae and Ectothiorhodospiraceae) and green sulfur bacteria (Chlorobiaceae). In this context, the hypothesis by Urich et al. (31) should be noted; based on the crystal structure of the sulfur oxygenase reductase from the thermoacidophilic archaeon “Aquifex aeolicus” and theoretical considerations, only linear sulfur and not cyclic sulfur species can serve as a substrate for this enzyme. Furthermore, Franz et al. (8) found evidence that Allochromatium vinosum uses only the sulfur chain fraction of elemental sulfur and is unable to take up cyclo-octasulfur.
The results observed in this study imply that the formation of sulfur globules in the dissimilatory uses of various sulfur compounds may be more widespread among bacteria than previously thought and existed before the distant phyla (i.e., Proteobacteria, Chlorobi, and Firmicutes) diverged. At this time, the possibility of horizontal gene transfer cannot be excluded; thus, a thorough comparative analysis awaits the availability of the corresponding genome sequences.
Supplementary Material
Acknowledgments
This study was supported in part by grant from U.S. Department of Energy (DE-FG05-95ER-20199 to J.W.), a grant from the National Science Foundation (NSF-MO 0238407), and a grant from the Fonds der Chemischen Industrie (661209 to A.P.).
We are grateful to Josef Hormes, Amitava Roy, and the staff members of CAMD for invaluable assistance and the State of Louisiana for providing the operating budget of the CAMD. We thank Ralf Steudel for kindly providing pure cyclo-octasulfur (S8 rings) and polymeric sulfur and Ina Schleicher (HZI) for technical assistance in electron microscopy.
Footnotes
Published ahead of print on 20 July 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Barrett, E., and M. A. Clark. 1987. Tetrathionate reduction and production of hydrogen sulfide from thiosulfate. Microbiol. Rev. 51:92-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brune, D. C. 1995. Isolation and characterization of sulfur globule proteins from Chromatium vinosum and Thiocapsa roseopersicina. Arch. Microbiol. 163:391-399. [DOI] [PubMed] [Google Scholar]
- 3.Chauvistré, R., J. Hormes, E. Hartmann, N. Etzenbach, R. Hosch, and J. Hahn. 1997. Sulfur K-shell photoabsorption spectroscopy of the sulfanes R-Sn-R, n = 2-4. Chem. Phys. 223:293-302. [Google Scholar]
- 4.Chen, K. Y., and J. C. Morris. 1972. Kinetics of oxidation of aqueous sulfide by O2. Environ. Sci. Technol. 6:529-537. [Google Scholar]
- 5.Dahl, C., and A. Prange. 2006. Bacterial sulfur globules: occurrence, structure and metabolism. In J. M. Shively (ed.), Bacterial inclusions, p. 21-51. Springer, New York, NY.
- 6.Douglas, S., and D. D. Douglas. 2001. Structural and geomicrobiological characteristics of a microbial community from a cold sulfide spring. Geomicrobiology 18:401-422. [Google Scholar]
- 7.Elshahed, M. S., J. M. Senko, F. Z. Najar, S. M. Kenton, B. A. Roe, T. A. Dewers, J. R. Spear, and L. R. Krumholz. 2003. Bacterial diversity and sulfur cycling in a mesophilic sulfide-rich spring. Appl. Environ. Microbiol. 69:5609-5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franz, B., H. Lichtenberg, J. Hormes, H. Modrow, C. Dahl, and A. Prange. 2007. Utilization of solid ‘elemental’ sulfur by the phototrophic purple sulfur bacterium Allochromatium vinosum: a sulfur K-edge X-ray absorption spectroscopy study. Microbiology 153:1268-1274. [DOI] [PubMed] [Google Scholar]
- 9.Guerrero, R., J. Mas, and C. Pedrós-Alió. 1984. Buoyant density changes due to intracellular content of sulfur in Chromatium warmingii and Chromatium vinosum. Arch. Microbiol. 137:350-356. [Google Scholar]
- 10.Hageage, G. J., Jr., E. D. Eanes, and R. L. Gherna. 1970. X-ray diffraction studies of the sulfur globules accumulated by Chromatium species. J. Bacteriol. 101:464-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jørgensen, B. B. 1990. A thiosulfate shunt in the sulfur cycle of marine sediments. Science 249:152-154. [DOI] [PubMed] [Google Scholar]
- 12.Jørgensen, B. B. 1990. The sulfur cycle of fresh water sediments: role of thiosulfate. Limnol. Oceanogr. 35:1329-1343. [DOI] [PubMed] [Google Scholar]
- 13.Jørgensen, B. B., and F. Bak. 1991. Pathways and microbiology of thiosulfate transformations and sulfate reduction in a marine sediment (Kattegat, Denmark). Appl. Environ. Microbiol. 57:847-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, Y. E., M. K. Jain, C. Lee, S. E. Lowe, and G. Zeikus. 1993. Taxonomic distinction of saccharolytic thermophilic anaerobes: description of Thermoanaerobacterium xylanolyticum gen. nov., sp. nov., and Thermoanaerobacterium saccharolyticum gen. nov., sp. nov.; reclassification of Thermoanaerobium brockii, Clostridium thermosulfurogenes, and Clostridium thermohydrosulfuricum E100-69 as Thermoanaerobacter brockii comb. nov, Thermoanaerobacterium thermosulfurogenes comb. nov., and Thermoanaerobacter thermohydrosulfuricus comb. nov., respectively; and transfer of Clostridium thermohydrosulfuricum 39E to Thermoanaerobacter ethanolicus. Int. J. Syst. Bacteriol. 43:41-51. [Google Scholar]
- 15.Lee, Y.-J., M. Dashti, A. Prange, F. A. Rainey, M. Rohde, W. B. Whitman, and J. Wiegel. 2007. Thermoanaerobacter sulfurigignens sp. nov., an anaerobic thermophilic bacterium reducing 1 M thiosulfate to elemental sulfur and tolerating 90 mM sulfite. Int. J. Syst. Evol. Microbiol. 57:1429-1434. [DOI] [PubMed] [Google Scholar]
- 16.Ljungdahl, L. G., and J. Wiegel. 1986. Anaerobic fermentations, p. 84-96. In A. L. Demain and N. A. Solomon (ed.), Manual of industrial microbiology and biotechnology. American Society for Microbiology, Washington, DC.
- 17.Mas, J., and H. van Gemerden. 1987. Influence of sulfur accumulation and composition of sulfur globule on cell volume and buoyant density of Chromatium vinosum. Arch. Microbiol. 146:362-369. [Google Scholar]
- 18.Onyenwoke, R., and J. Wiegel. Genus Thermoanaerobacter. In G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, in press.
- 19.Pickering, I. J., G. N. George, E. Y. Yu, D. C. Brune, C. Tuschak, J. Overmann, J. T. Beatty, and R. C. Prince. 2001. Analysis of sulfur biochemistry of sulfur bacteria using X-ray absorption spectroscopy. Biochemistry 40:8138-8145. [DOI] [PubMed] [Google Scholar]
- 20.Prange, A., I. Arzberger, C. Engemann, H. Modrow, O. Schumann, H. G. Trüper, R. Steudel, C. Dahl, and J. Hormes. 1999. In situ analysis of sulfur in the sulfur globules of phototrophic sulfur bacteria by X-ray absorption near edge spectroscopy. Biochim. Biophys. Acta 1428:446-454. [DOI] [PubMed] [Google Scholar]
- 21.Prange, A., R. Chauvistré, H. Modrow, J. Hormes, H. G. Trüper, and C. Dahl. 2002. Quantitative speciation of sulfur in bacterial sulfur globules: X-ray absorption spectroscopy reveals at least three different species of sulfur. Microbiology 148:267-276. [DOI] [PubMed] [Google Scholar]
- 22.Prange, A., B. Birzele, J. Krämer, R. Chauvistré, H. Modrow, J. Hormes, and P. Köhler. 2003. Characterization of sulfur speciation in low molecular weight subunits of glutenin after reoxidation with potassium iodate and potassium bromate at different pH values using X-ray absorption near-edge structure (XANES) spectroscopy. J. Agric. Food Chem. 51:7431-7438. [DOI] [PubMed] [Google Scholar]
- 23.Prange, A. Speciation analysis of microbiologically produced sulfur by X-ray absorption near edge structure (XANES) spectroscopy. In C. Friedrich and C. Dahl (ed.), Microbial sulfur metabolism, in press.
- 24.Prange, A., J. Hormes, and H. Modrow. X-ray absorption spectroscopy as a tool for the detection and identification of sulfur compounds in phototrophic organisms. In R. Hell, C. Dahl, T. Leustek, and D. Knaff (ed.), Sulfur metabolism in phototrophic organisms, in press.
- 25.Ravot, G., B. Ollivier, M. Magot, B. K. C. Patel, J.-L. Crolet, M.-L. Fardeau, and J.-L. Garcia. 1995. Thiosulfate reduction, an important physiological feature shared by members of the order Thermotogales. Appl. Environ. Microbiol. 61:2053-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ressler, T. 1998. WinXAS: a program for X-ray absorption spectroscopy data analysis under MS-Windows. J. Synchr. Rad. 5:118-122. [DOI] [PubMed] [Google Scholar]
- 27.Schink, B., and J. G. Zeikus. 1983. Clostridium thermohydrosulfurigenes sp. nov., a new thermophile that produces elemental sulfur from thiosulfate. J. Gen. Microbiol. 129:1149-1158. [Google Scholar]
- 28.Schmidt, G. L., G. L. Nicolson, and M. D. Kamen. 1971. Composition of the sulfur particle of Chromatium strain D. J. Bacteriol. 105:1137-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steudel, R., G. Holdt, T. Göbel, and W. Hazeu. 1987. Chromatographic separation of higher polythionates SnO62− (n = 3 . . . 22) and their detection in cultures of Thiobacillus ferrooxidans: molecular composition of bacterial sulfur secretions. Angew. Chem. Int. Ed. Engl. 26:151-153. [Google Scholar]
- 30.Steudel, R. 1989. On the nature of the ‘elemental sulfur’ (S°) reduced by sulfur-oxidizing bacteria—a model for S° globules, p. 289-303. In H. G. Schlegel and B. Bowien (ed.), Autotrophic bacteria. Science Technology Publishers, Madison, WI.
- 31.Urich, T., C. M. Gomes, A. Kletzin, and C. Frazão. 2006. X-ray structure of a self-compartmentalizing sulfur cycle metalloenzyme. Science 311:996-1000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.