Abstract
Many filamentous cyanobacteria are motile by gliding, which requires attachment to a surface. There are two main theories to explain the mechanism of gliding. According to the first, the filament is pushed forward by small waves that pass along the cell surface. In the second, gliding is powered by the extrusion of slime through pores surrounding each cell septum. We have previously shown that the cell walls of several motile cyanobacteria possess an array of parallel fibrils between the peptidoglycan and the outer membrane and have speculated that the function of this array may be to generate surface waves to power gliding. Here, we report on a study of the cell surface topography of two morphologically different filamentous cyanobacteria, using field emission gun scanning electron microscopy (FEGSEM) and atomic force microscopy (AFM). FEGSEM and AFM images of Oscillatoria sp. strain A2 confirmed the presence of an array of fibrils, visible as parallel corrugations on the cell surface. These corrugations were also visualized by AFM scanning of fully hydrated filaments under liquid; this has not been achieved before for filamentous bacteria. FEGSEM images of Nostoc punctiforme revealed a highly convoluted, not parallel, fibrillar array. We conclude that an array of parallel fibrils, beneath the outer membrane of Oscillatoria, may function in the generation of thrust in gliding motility. The array of convoluted fibrils in N. punctiforme may have an alternative function, perhaps connected with the increase in outer membrane surface area resulting from the presence of the fibrils.
Cyanobacteria are phototrophic prokaryotes that vary in morphology from unicellular strains to filamentous forms capable of complex cellular differentiation (1, 2). Many cyanobacteria are motile. Some unicellular Synechococcus strains are capable of swimming by a mechanism that does not employ flagella (28) in which thrust is generated by an extracellular protein, SwmA (6, 19), but most motile cyanobacteria employ gliding, which requires attachment to a surface. The unicellular Synechocystis sp. strain PCC 6803 moves by a form of gliding often referred to as twitching motility, which is powered by type IV pili on the cell surface (4, 5, 30). In filamentous strains, in which forward movement is often accompanied by rotation of the filament about its long axis (10), the mechanism of gliding is unknown. Halfen and Castenholz proposed a model for gliding (11) based on the results of their own electron microscopy (EM) studies of motile filamentous cyanobacteria and on the theories of Jarosch (16). They speculated that the motor for gliding in the filamentous Oscillatoriaceae might be provided by proteinaceous contractile fibrils, 6 to 9 nm in diameter, located between the peptidoglycan layer and the outer membrane (OM) and that rotation of the filaments during gliding might be a result of the helical arrangement of the fibrils. An ultrastructural examination of the cell walls of four gliding filamentous cyanobacteria by Hoiczyk and Baumeister (12) failed to identify the fibrillar structures described by Halfen and Castenholz (10, 11). However, they did observe a tetragonal S-layer external to the outer membrane and, above this, an array of helically arranged surface fibrils, 10 to 12 nm in diameter, with a regular spacing of 14 nm. The fibrils were later shown to consist of a single calcium-binding protein that was given the name oscillin (13). Hoiczyk and Baumeister concluded that the oscillin fibrils served as a screw thread guiding the rotation of the trichome, which was powered by the extrusion of slime from junctional pores that form a ring around the filament on either side of each cell septum (13, 14). They were also able to show that spontaneous, nonmotile mutants of Phormidium uncinatum failed to produce extracellular slime and lacked the S-layer and oscillin fibrils, implying that one, or more, of these components was essential for motility.
In a previous study of several motile filamentous cyanobacteria, we demonstrated the presence of a parallel array of 25- to 30-nm diameter fibrils, situated between the peptidoglycan and the OM (3); these fibrils are much wider than those reported by Halfen and Castenholz (11) and Hoiczyck and Baumeister (12, 13). The OM was seen to penetrate between the rows of fibrils, and as a consequence the outer surface of the cells appeared corrugated rather than smooth. To our knowledge, this unique OM topography has not been reported previously in any gram-negative bacteria. Here, we describe the results of a survey of the cell surface topography of several filamentous cyanobacteria by atomic force microscopy (AFM) and field emission gun scanning electron microscopy (FEGSEM). These techniques have confirmed our earlier observations and demonstrated that such fibrillar arrays are not limited to the genus Oscillatoria but are found in a modified version in the very different cyanobacteria of the genus Nostoc. We have also shown that it is possible to obtain high-resolution AFM scans under liquid of fully hydrated filamentous cyanobacteria. These AFM scans under liquid are the first such scans obtained for filamentous bacteria and, indeed, some of the few obtained for any bacteria.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Nostoc punctiforme ATCC 29133 was grown in BG11o liquid medium (23) supplemented with 17.6 mM NaNO3. Cultures were incubated at 30°C on an orbital shaker, at a rate of 120 rpm, under constant white light (15 μmol of photons m−2 s−1). Oscillatoria sp. strain A2, isolated previously by us (3), was grown on agar plates consisting of BG11o medium containing 17.6 mM NaNO3 and solidified with 1.5% (wt/vol) purified agar (Oxoid). Plate cultures were incubated at 30°C under constant white light (8 μmol of photons m−2 s−1).
FEGSEM.
Samples were fixed with 2.5% (vol/vol) glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) for 2.5 h. Fixed cells were washed twice, for 30 min each time, in 0.1 M phosphate buffer, and postfixed with 1.0% (wt/vol) osmium tetroxide in 0.1 M phosphate buffer. The cells were then dehydrated by 30-min washes in a graded ethanol series of 20% (vol/vol), 40%, 60%, and 80% and a final two washes in 100%. Samples were critical-point dried, mounted on aluminum pin stubs, and coated with either a 15- to 20-nm layer of gold or a 5-nm layer of platinum-palladium. Gold coating was done using an Emscope SC500 sputter coating unit, and platinum-palladium coating was done using an Agar Scientific high-resolution sputter coater fitted with an Agar Scientific thickness monitor. Samples were examined with an LEO 1530 series FEGSEM instrument operating at 3 kV.
AFM imaging.
Cells were imaged in air and in liquid. Cells imaged in air were transferred to a glass microscope slide and left to air dry for 3 min prior to imaging. Cells imaged in liquid were immobilized by being partially embedded in dental wax (Agar Scientific) in the following way. A thin layer of melted dental wax was spread on the surface of a glass slide, and cyanobacteria was spread on another slide. The two slides were immediately pressed together; once the wax hardened, the slides were separated, and the embedded cyanobacteria were covered with BG11o medium supplemented with 17.6 mM NaNO3 for at least 30 min before imaging.
AFM imaging was performed with a Bioscope AFM equipped with a Nanoscope IV controller (Veeco Instruments, California) operated using the tapping mode. For tapping in air and in fluid, the tips employed were, respectively, NCH Pointprobe tips and NP-S tips (Veeco Instruments) used at a frequency of 27 kHz.
RESULTS
FEGSEM and dry AFM imaging of Oscillatoria strain A2 and N. punctiforme.
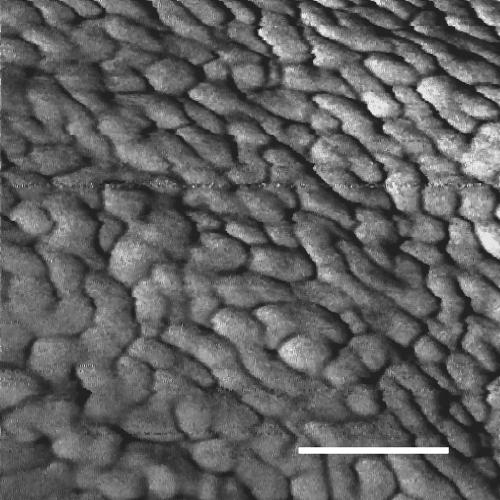
In FEGSEM images of Oscillatoria strain A2, the parallel nature of the fibrillar array was clearly visible, as was the pitch of the array in relation to the long axis of the filament (Fig. 1). This pitch, which is approximately 30°, correlates with the rotation of the filament as it glides. AFM images obtained by scanning filaments transferred from agar to a glass slide (Fig. 2 and 3) confirmed the parallel nature of the array, but there were differences from the FEGSEM images. Some disruption of the regularity of the array was apparent, with some fibrils appearing to be broken or lying in directions other than the direction of most of the array (Fig. 2 and 3).
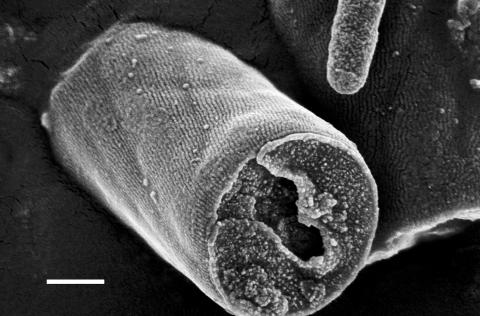
FIG. 1.
FEGSEM image of Oscillatoria sp. strain A2. The original filament has fragmented to release the single cell visible in the center of the micrograph. The fibrillar array is clearly visible as parallel corrugations on the cell surface, which run at an angle to the long axis of the filament. Part of a small, contaminating bacterium can be seen at the upper right of the micrograph. Scale bar, 500 nm.
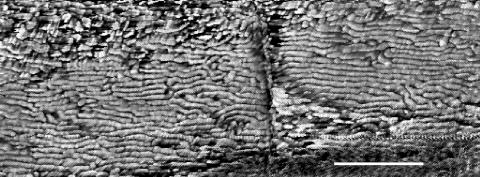
FIG. 2.
AFM image of Oscillatoria sp. strain A2. The scan was performed on a sample that had been transferred from an agar plate to a glass slide and was, consequently, dehydrated when scanned. Parts of two cells are visible, with a cell septum running vertically down the center of the micrograph. Scale bar, 500 nm.
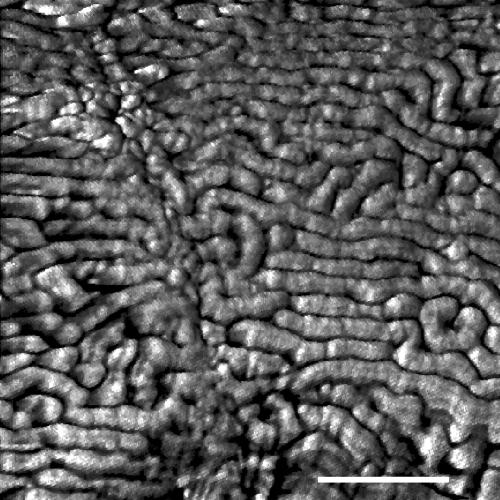
FIG. 3.
AFM image of Oscillatoria sp. strain A2. The scan was performed on a sample transferred from an agar plate to a glass slide. Parts of two cells are visible, with a cell septum running from the top left to the bottom middle of the micrograph. Scale bar, 200 nm.
FEGSEM images of N. punctiforme revealed corrugations with the same diameter as those of Oscillatoria strain A2 but with a very different arrangement, forming not a parallel array but a highly convoluted one (Fig. 4). The fibrils were also visible in AFM images although, as with Oscillatoria strain A2, disruption of the regularity of the array was apparent, with the fibrils appearing mostly as short fragments of relatively uniform length (Fig. 5).
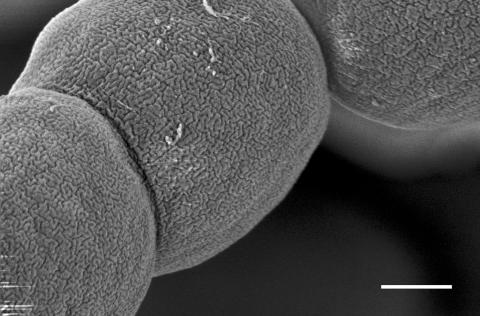
FIG. 4.
FEGSEM image of N. punctiforme. Parts of three cells can be seen, the surfaces of which are highly convoluted as a consequence of the fibrillar array beneath the OM. Note the difference between this convoluted array and the parallel array seen in Oscillatoria strain A2 (Fig. 1). Scale bar, 500 nm.
FIG. 5.
AFM image of N. punctiforme. The scan was performed on a sample transferred from agar to a glass slide and was, consequently, dehydrated when scanned. The convolutions seen in Fig. 4 appear disrupted in places. Scale bar, 100 nm.
AFM scanning of Oscillatoria strain A2 under liquid.
The initial AFM scans of Oscillatoria strain A2 were performed on filaments transferred from agar to the surface of a glass slide for scanning. Although initially fully hydrated, these filaments would have dried rapidly upon transfer to the glass slide and so would have been dehydrated when scanned. To examine the surface topography of fully hydrated samples, further AFM scans were performed under liquid.
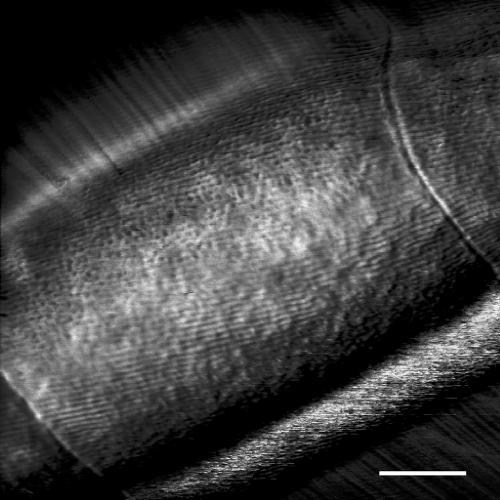
The effect of dehydration on filament diameter is apparent from height measurements made by AFM scanning of filaments in dry and hydrated states; the height of a filament of Oscillatoria strain A2 scanned under dry conditions was approximately 0.9 μm, whereas that of one scanned under liquid was 3 μm (data not shown). The parallel fibrillar array was seen in AFM scans obtained under growth medium, and the cell septa were clearly visible as depressions in the surface (Fig. 6). Despite this dip in the OM at the septum, there appeared to be continuity of each fibril either side of the septum, and this was observed previously in transmission EM images of negatively stained, crushed filaments of another Oscillatoria strain (3). In these fully hydrated filaments, the fibrillar array showed the same regularity apparent in the FEGSEM images (Fig. 1), with none of the disruption seen in the dry AFM scans (Fig. 2 and 3). This implies that drying of the filament causes some disturbance to the fibrils. This is more evident in the case of N. punctiforme since the AFM (Fig. 5) and FEGSEM (Fig. 4) images show even greater differences.
FIG. 6.
AFM image of Oscillatoria sp. strain A2. The scan was performed under liquid on a sample immobilized in dental wax. Cell septa can be seen at the bottom left and top right of the micrograph. The fibrillar array shows the same highly regular arrangement seen in FEGSEM images (Fig. 1); the partial disruption of the fibrils apparent in the AFM scans of dry samples (Fig. 2 and 3) is not seen in this fully hydrated sample. Scale bar, 500 nm.
DISCUSSION
AFM scanning under liquid.
AFM scanning under liquid offers great potential for studying the topology and physicochemical properties of the bacterial cell surface under physiological conditions, but this approach has been hindered by the need to immobilize the sample to avoid its becoming detached by the scanning tip (7, 8). A recent study of the gliding, unicellular bacterium Myxococcus xanthus produced good AFM images of cells dried onto glass slides but was unable to generate clear images of cells under liquid because of cell movement caused by the scanning AFM tip. However, it was possible to determine cell wall elasticity of such cells by measuring force curves (22). Some unicellular bacteria can be immobilized by trapping the cells in the pores of a polymer membrane with a pore size comparable to the cell diameter (26). However, this approach cannot be used with filamentous bacteria such as the cyanobacterial strains examined in the present study. Instead, we have immobilized filaments in dental wax, such that small areas of cell surface remain uncovered and are suitable for scanning. This has enabled us to obtain the first AFM scans under liquid of filamentous bacteria (Fig. 6).
Possible function of the fibrillar array.
The possibility that the fibrillar array plays a part in providing structural rigidity to the cell wall can be discounted for two reasons. First, Oscillatoria spp. have very thick peptidoglycan layers (15) and do not require additional strengthening. Second, the fibrils are highly flexible, as can be seen in some areas of the dry AFM images in which fibrils are bent into U shapes (Fig. 3); this is in agreement with our previous transmission EM observations of negatively stained, crushed filaments from which fibrils had been liberated (3).
We have speculated previously that the 25- to 30-nm fibrillar array plays a role in gliding motility (3). The reasons for this are severalfold. First, the array is perfectly placed beneath the OM to provide and deliver the motive force for gliding, which would require an interaction between the cell surface and the substrate on which the filament rests. Second, the pitch of the fibrils in relation to the cell's long axis in cyanobacteria such as Oscillatoria strain A2 (Fig. 1) helps to explain the rotation of such cyanobacteria as they glide. But what mechanism might generate the force for gliding? Rhythmical undulations in the cell surface are one possibility; this was suggested over 30 years ago by Halfen and Castenholz (10, 11). Such undulations could originate in the fibrillar array and be transmitted through the highly flexible OM. Such a model has been subjected to a mathematical analysis by Siddiqui et al. (24). In this model transverse peristaltic waves travel the length of the cell and push exuded slime backwards, producing a force to propel the bacterium in the direction opposite to that of the traveling wave. They concluded that the model could generate sufficient force to propel the cell at a realistic speed and with a power consumption level much less than the organism's likely rate of metabolic energy generation. In such a model in which the fibrillar array generates surface waves, the slime plays a passive role. This contrasts with the model of Hoiczyk and Baumeister (14) in which the extrusion of slime through junctional pores provides the power to drive the filaments. A similar model has been proposed for adventurous motility in M. xanthus in which nozzle-like structures, similar to the junctional pores in cyanobacteria and located at the cell poles, were found to extrude slime (29). At present, experimental proof of these models is lacking although molecular dynamics simulations have recently confirmed that the compression of polymerizing slime within the nozzles could generate sufficient thrust for gliding (17).
When the outermost layer of a cell is the OM, as appears to be the case with Oscillatoria strain A2, it is easy to see how waves generated by the fibrillar array would be transmitted to the surface of the cell via the OM. However, at least some motile, filamentous cyanobacteria possess an S-layer and an oscillin layer external to the OM (13, 15). In this situation, efficient transmission of the waves would require that the S-layer and oscillin layer are sufficiently flexible to ensure propagation of the wave and its interaction with the surface to which the filament is attached. As both are monomolecular layers, it seems likely that they would have the necessary flexibility, although there is currently no proof of this. The convoluted OM topography apparent in the FEGSEM and AFM micrographs presented here cannot be seen in all cyanobacteria, not even in Oscillatoria strains very similar to strain A2, even though we can show by other means that these cyanobacteria possess fibrils (data not shown). The most likely explanation for this is the presence of an S-layer, an oscillin layer, or a polysaccharide sheath external to the OM, which masks its convolutions.
The N. punctiforme fibrillar array.
The diameter of the corrugations on the surface of N. punctiforme corresponds well with the corrugations on the Oscillatoria strain A2 cell surface and leads us to conclude that they result from an array of fibrils beneath the OM, although this has not been confirmed by transmission EM of transverse thin sections of Nostoc filaments as it has for Oscillatoria (3). If the fibrillar array provides the motive force for gliding in cyanobacteria such as Oscillatoria strain A2, why should N. punctiforme, which is motile for only a brief stage in its life cycle, the hormogonium, possess an array? In the genus Nostoc, hormogonia are short, small-celled filaments that develop in response to environmental triggers such as dilution of the growth medium or exposure to green light (1, 25). They provide the immotile vegetative filaments with a motile phase for dispersal, but they are only motile for a short period before returning to vegetative growth. The fibrillar array, unchanged in appearance from that in vegetative cells, can be seen in FEGSEM images of Nostoc hormogonia (data not shown), and so fibrils could provide the motive force for hormogonia; but we have evidence that pili are responsible for this (9). If this is the case, then what is the function of the fibrillar array in Nostoc? We can speculate that, prior to the evolution of hormogonia, Nostoc was permanently motile and employed an array of parallel fibrils of the type seen in Oscillatoria strain A2. When the ability to form hormogonia evolved, the fibrils were no longer required. But why were they retained? The answer to this may lie in the effect the fibrils have on OM surface area. When viewed in transverse section, the OM covering each fibril forms an arc. If this is assumed to be a semicircle of diameter D, then by simple geometry it is possible to calculate the increase in surface area of the OM covering the fibril, compared with a flat OM of width D without the fibril; this value is 57%. However, in N. punctiforme the fibrils are not parallel but are convoluted (Fig. 4) and, assuming that each turn of the convolution describes a semicircle, this further increases the surface area by 57%.
How might this increased surface area benefit Nostoc? For many bacteria in the natural environment, growth is limited by the availability of nutrients. Uptake of nutrients occurs via protein channels known as porins in the OM of gram-negative bacteria (20), and the transcription of specific porin genes increases under nutrient starvation (21) and in response to, for example, phosphate starvation (27) and osmotic stress (18). An increase in the number of porin molecules in the OM is therefore an important response to nutrient limitation, and so any increase in the surface area of the OM, with a concomitant increase in porins, may be beneficial. Not all the additional surface area of the OM will be accessible for nutrient uptake because, as the cleft between the fibrils decreases in width as it nears the peptidoglycan layer, the radius of a particular ion will limit its access to porins in that region of the membrane. Nevertheless, there is likely to be a selective advantage for bacteria that increase OM surface area, particularly in oligotrophic environments. It may be for this reason that Nostoc has retained its fibrils.
Acknowledgments
This work was supported by a Natural Environment Research Council Ph.D. studentship to N.R. and by Biotechnology and Biological Sciences Research Council grant 24/C13950.
We thank John Harrington for excellent technical assistance with the FEGSEM.
Footnotes
Published ahead of print on 10 August 2007.
REFERENCES
- 1.Adams, D. G. 1997. Cyanobacteria, p. 109-148. In J. A. Shapiro and M. Dworkin (ed.), Bacteria as multicellular organisms. Oxford University Press, Oxford, United Kingdom.
- 2.Adams, D. G., and P. Duggan. 1999. Heterocyst and akinete differentiation in cyanobacteria. New Phytol. 144:3-33. [Google Scholar]
- 3.Adams, D. G., D. Ashworth, and B. Nelmes. 1999. Fibrillar array in the cell wall of a gliding filamentous cyanobacterium. J. Bacteriol. 181:884-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaya, D., N. Watanabe, T. Ogawa, and A. R. Grossman. 1999. The role of an alternative sigma factor in motility and pilus formation in the cyanobacterium Synechocystis sp. strain PCC6803. Proc. Natl. Acad. Sci. USA 96:3188-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaya, D., N. R. Bianco, D. Bryant, and A. Grossman. 2000. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 37:941-951. [DOI] [PubMed] [Google Scholar]
- 6.Brahamsha, B. 1996. An abundant cell-surface polypeptide is required for swimming by the nonflagellated marine cyanobacterium Synechococcus. Proc. Natl. Acad. Sci. USA 93:6504-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufrêne, Y. F. 2003. Recent progress in the application of atomic force microscopy imaging and force spectroscopy to microbiology. Curr. Opin. Microbiol. 6:317-323. [DOI] [PubMed] [Google Scholar]
- 8.Dufrêne, Y. F. 2004. Refining our perception of bacterial surfaces with the atomic force microscope. J. Bacteriol. 186:3283-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duggan, P. S., P. Gotardello, and D. G. Adams. 2007. Molecular analysis of genes in Nostoc punctiforme involved in pilus biogenesis and plant infection. J. Bacteriol. 189:4547-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halfen, L. N. 1973. Gliding motility of Oscillatoria: ultrastructural and chemical characterization of the fibrillar layer. J. Phycol. 9:248-253. [Google Scholar]
- 11.Halfen, L. N., and R. W. Castenholz. 1971. Gliding motility in the blue-green alga Oscillatoria princeps. J. Phycol. 7:133-145. [Google Scholar]
- 12.Hoiczyk, E., and W. Baumeister. 1995. Envelope structure of four gliding filamentous cyanobacteria. J. Bacteriol. 177:2387-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoiczyk, E., and W. Baumeister. 1997. Oscillin, an extracellular, Ca2+-binding glycoprotein essential for the gliding motility of cyanobacteria. Mol. Microbiol. 26:699-708. [DOI] [PubMed] [Google Scholar]
- 14.Hoiczyk, E., and W. Baumeister. 1998. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr. Biol. 8:1161-1168. [DOI] [PubMed] [Google Scholar]
- 15.Hoiczyk, E., and A. Hansel. 2000. Cyanobacterial cell walls: news from an unusual prokaryotic envelope. J. Bacteriol. 182:1191-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarosch, R. 1963. Grundlagen einer Schrauben-Mechanik des Protoplasmas. Protoplasma 57:448-500. [Google Scholar]
- 17.Jeon, J., and A. V. Dobrynin. 2005. Polymer confinement and bacterial gliding motility. Eur. Phys. J. 17:361-372. [DOI] [PubMed] [Google Scholar]
- 18.Kaeriyama, M., K. Machida, A. Kitakaze, H. Wang, Q. Lao, T. Fukamachi, H. Saito, H., and H. Kobayashi. 2006. OmpC and OmpF are required for growth under hyperosmotic stress above pH 8 in Escherichia coli. Lett. Appl. Micro. 42:195-201. [DOI] [PubMed] [Google Scholar]
- 19.McCarren, J., J. Heuser, R. Roth, N. Yamada, M. Martone, and B. Brahamsha. 2005. Inactivation of swmA results in the loss of an outer cell layer in a swimming Synechococcus strain. J. Bacteriol. 187:224-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Özkanca, R., and K. P. Flint. 2002. The effect of starvation stress on the porin protein expression of Escherichia coli in lake water. Lett. Appl. Microbiol. 35:533-537. [DOI] [PubMed] [Google Scholar]
- 22.Pelling, A. E., Y. Li, W. Shi, and J. K. Gimzewski. 2005. Nanoscale visualization and characterization of Myxococcus xanthus cells with atomic force microscopy. Proc. Natl. Acad. Sci. USA 102:6484-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rippka, R., J. Deruelles, J. B. Waterbury, M. Herdman, and R. Y. Stanier. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1-61. [Google Scholar]
- 24.Siddiqui, A. M., R. P. Burchard, and W. H. Schwarz. 2001. An undulating surface model for the motility of bacteria gliding on a layer of non-Newtonian slime. Int. J. Non-Linear Mech. 36:743-761. [Google Scholar]
- 25.Tandeau de Marsac, N. 1994. Differentiation of hormogonia and relationships with other biological processes, p. 825-842. In D. A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Amsterdam, The Netherlands.
- 26.Touhami, A., M. H. Jericho, and T. J. Beveridge. 2004. Atomic force microscopy of cell growth and division in Staphylococcus aureus. J. Bacteriol. 186:3286-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Krüger, W. M. A., L. M. S. Lery, M. R. Soares, F. S. de Neves-Manta, C. M. Batista e Silva, A. G. da Costa Neves-Ferreira, J. Perales, and P. M. Bisch. 2006. The phosphate-starvation response in Vibrio cholerae O1 and phoB mutant under proteomic analysis: disclosing functions involved in adaptation, survival and virulence. Proteomics 6:1495-1511. [DOI] [PubMed] [Google Scholar]
- 28.Waterbury, J. B., J. M. Willey, D. G. Franks, F. W. Valois, and S. W. Watson. 1985. A cyanobacterium capable of swimming motility. Science 230:74-76. [DOI] [PubMed] [Google Scholar]
- 29.Wolgemuth, C., E. Hoiczyk, D. Kaiser, and G. Oster. 2002. How myxobacteria glide. Curr. Biol. 12:369-377. [DOI] [PubMed] [Google Scholar]
- 30.Yoshihara, S., and M. Ikeuchi. 2004. Phototactic motility in the unicellular cyanobacterium Synechocystis sp. PCC6803. Photochem. Photobiol. Sci. 3:512-518. [DOI] [PubMed] [Google Scholar]