Abstract
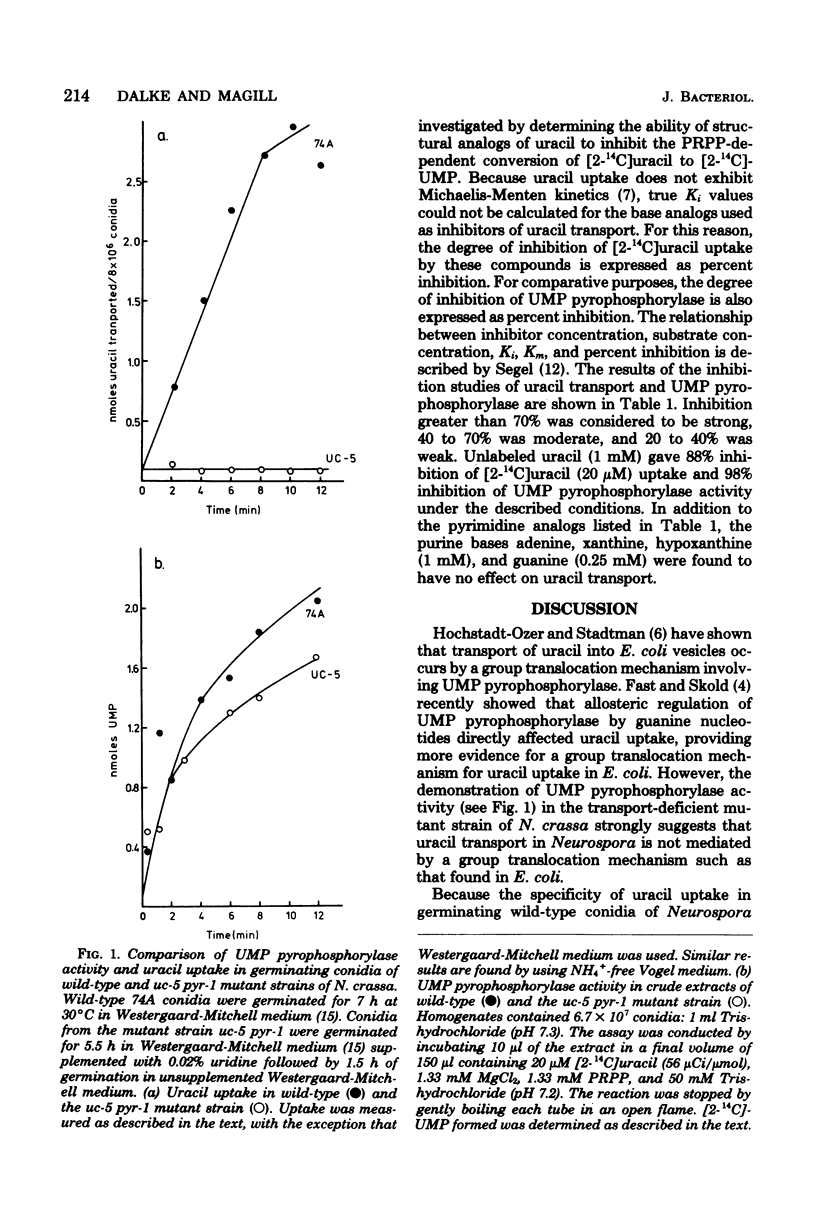
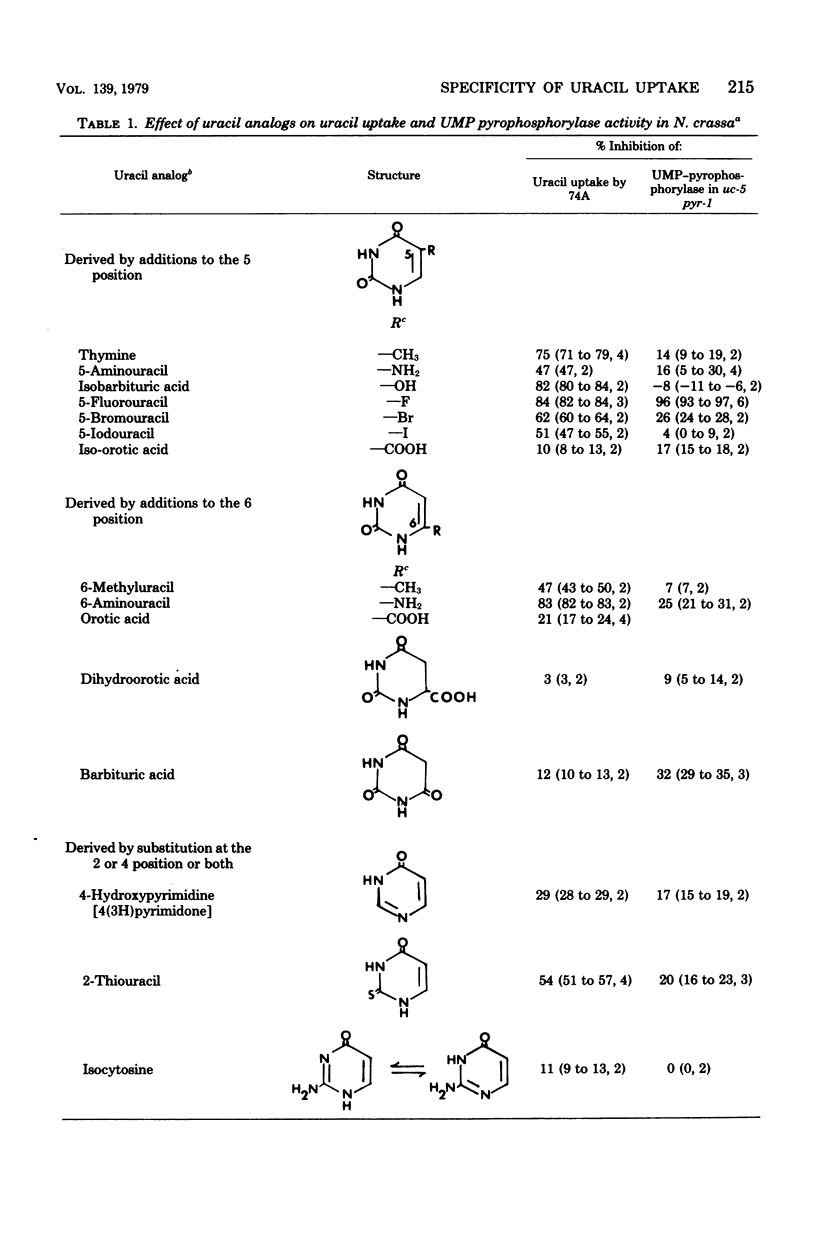
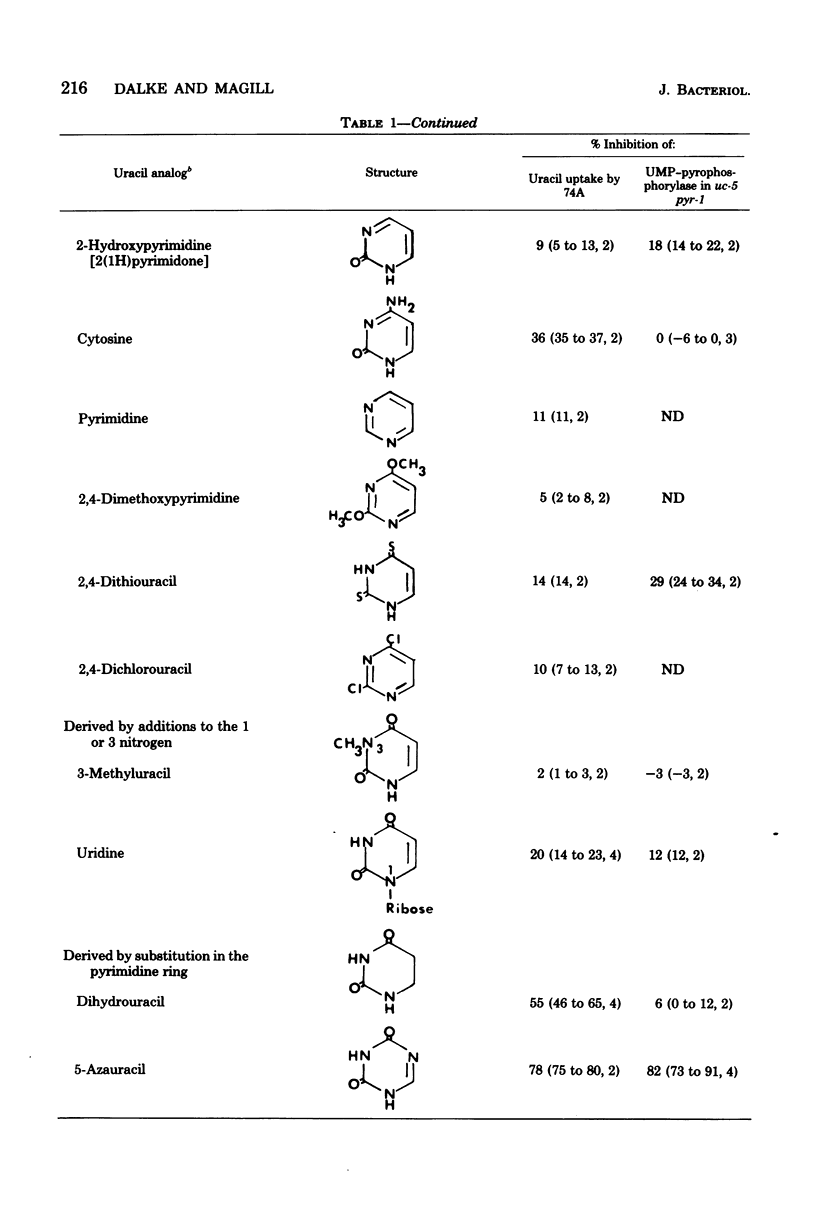
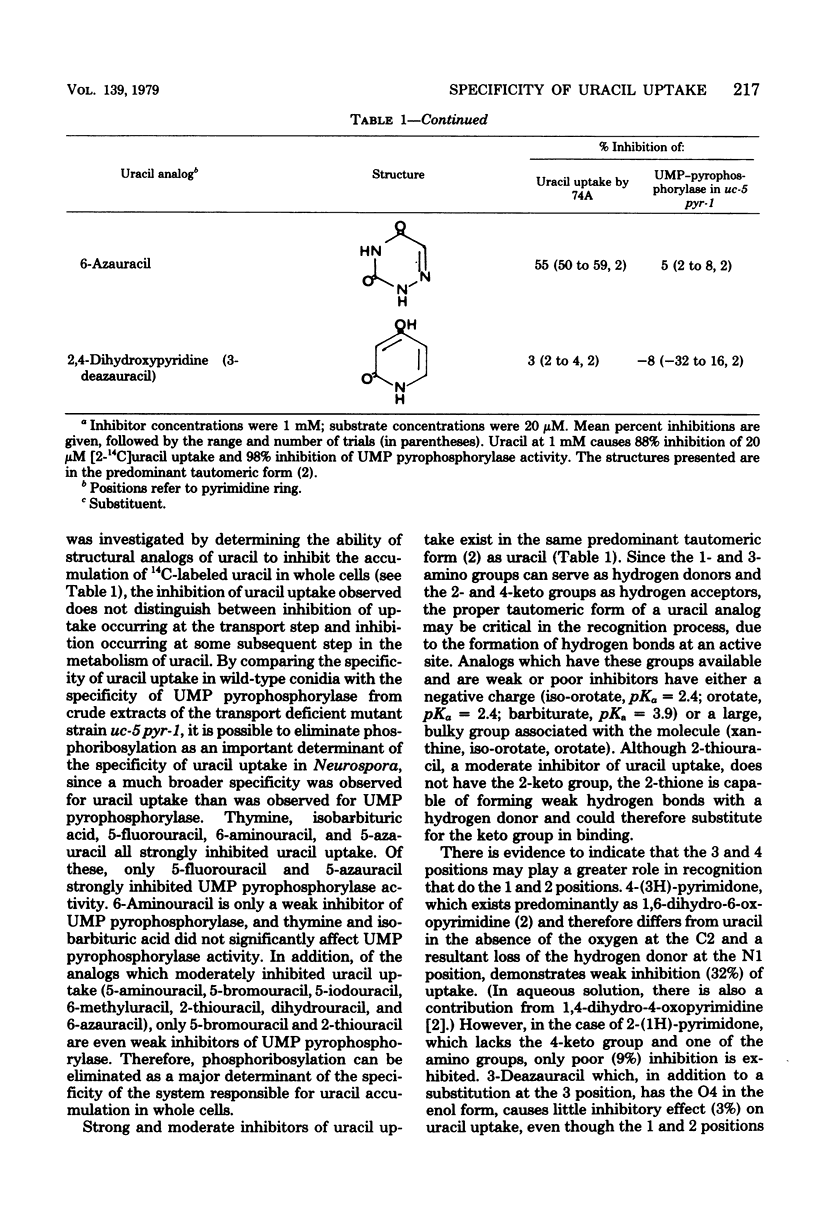
The specificity of uracil uptake was investigated in germinating wild-type conidia of Neurospora crassa. From comparative inhibition studies, several generalizations concerning the specificity of uracil uptake can be made. (i) The tautomeric forms of uracil analogs is an important determinant of recognition by the uptake system. (ii) Substituents at the 5 position of the pyrimidine ring may impose steric constraints on binding. (iii) The presence of a negative charge results in the loss of recognition. (iv) The double bond between the 5 and 6 carbons appears to be important for recognition. (v) Purine bases do not inhibit uracil uptake. Crude extracts of the transport-deficient mutant strain uc-5 pyr-1 were shown to have uridine 5'-monophosphate pyrophosphorylase activity comparable to that of the wild-type strain, suggesting that uracil uptake in Neurospora does not occur by a group translocation mechanism involving phosphoribosylation. Specificity studies of uridine 5'-monophosphate pyrophosphorylase indicated that phosphoribosylation was not an important determinant of the specificity of uracil uptake.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin R. D., Oliver J. M. Membrane transport of purine and pyrimidine bases and nucleosides in animal cells. Int Rev Cytol. 1975;42:287–336. doi: 10.1016/s0074-7696(08)60983-3. [DOI] [PubMed] [Google Scholar]
- Caroline D. F. Pyrimidine synthesis in Neurospora crassa: gene-enzyme relationships. J Bacteriol. 1969 Dec;100(3):1371–1377. doi: 10.1128/jb.100.3.1371-1377.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast R., Sköld O. Biochemical mechanism of uracil uptake regulation in Escherichia coli B. Allosteric effects on uracil phosphoribosyltransferase under stringent conditions. J Biol Chem. 1977 Nov 10;252(21):7620–7624. [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. II. Adenine phosphoribosyltransferase in isolated membrane preparations and its role in transport of adenine across the membrane. J Biol Chem. 1971 Sep 10;246(17):5304–5311. [PubMed] [Google Scholar]
- Hochstadt J. The role of the membrane in the utilization of nucleic acid precursors. CRC Crit Rev Biochem. 1974 Mar;2(2):259–310. doi: 10.3109/10409237409105449. [DOI] [PubMed] [Google Scholar]
- Magill J. M., Edwards E. S., Sabina R. L., Magill C. W. Depression of uracil uptake by ammonium in Neurospora crassa. J Bacteriol. 1976 Sep;127(3):1265–1269. doi: 10.1128/jb.127.3.1265-1269.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas P. W., Uglem G. L., Read C. P. The influx of purines and pyrimidines across the brush border of Hymenolepis diminuta. Parasitology. 1973 Jun;66(3):525–538. doi: 10.1017/s0031182000046072. [DOI] [PubMed] [Google Scholar]
- Polak A., Grenson M. Evidence for a common transport system for cytosine, adenine and hypoxanthine in Saccharomyces cerevisiae and Candida albicans. Eur J Biochem. 1973 Jan 15;32(2):276–282. doi: 10.1111/j.1432-1033.1973.tb02608.x. [DOI] [PubMed] [Google Scholar]
- Roy-Burman S., Visser D. W. Transport of purines and deoxyadenosine in Escherichia coli. J Biol Chem. 1975 Dec 25;250(24):9270–9275. [PubMed] [Google Scholar]
- SCHANKER L. S., JEFFREY J. J., TOCCO D. J. INTERACTION OF PURINES WITH THE PYRIMIDINE TRANSPORT PROCESS OF THE SMALL INTESTINE. Biochem Pharmacol. 1963 Sep;12:1047–1053. doi: 10.1016/0006-2952(63)90028-5. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Magill J. M., Magill C. W. Regulation of hypoxanthine transport in Neurospora crassa. J Bacteriol. 1976 Nov;128(2):598–603. doi: 10.1128/jb.128.2.598-603.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. G., Mitchell H. K. Mutants affecting thymidine metabolism in Neurospora crassa. J Bacteriol. 1969 Oct;100(1):383–389. doi: 10.1128/jb.100.1.383-389.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka J. M., Plagemann P. G. Purine and pyrimidine transport by cultured Novikoff cells. Specificities and mechanism of transport and relationship to phosphoribosylation. J Biol Chem. 1975 Aug 10;250(15):5756–5767. [PubMed] [Google Scholar]



