Abstract
Although human rhinoviruses (HRVs) are common causes of respiratory illness, their molecular epidemiology has been poorly investigated. Despite the recent findings of new HRV genotypes, their clinical disease spectrum and phylogenetic positions were not fully understood. In this study, 203 prospectively collected nasopharyngeal aspirates (NPAs), negative for common respiratory viruses (83 were human bocavirus [HBoV] positive and 120 HBoV negative), from hospitalized children during a 1-year period were subjected to reverse transcription-PCR for HRV. HRV was detected in 14 NPAs positive and 12 NPAs negative for HBoV. Upon VP4 gene analysis, 5 of these 26 HRV strains were found to belong to HRV-A while 21 belonged to a genetic clade probably representing a previously undetected HRV species, HRV-C, that is phylogenetically distinct from the two known HRV species, HRV-A and HRV-B. The VP4 sequences of these HRV-C strains were closely related to the newly identified HRV strains from the United States and Australia. Febrile wheeze or asthma was the most common presentation (76%) of HRV-C infection, which peaked in fall and winter. Complete genome sequencing of three HRV-C strains revealed that HRV-C represents an additional HRV species, with features distinct from HRV-A and HRV-B. Analysis of VP1 of HRV-C revealed major deletions in regions important for neutralization in other HRVs, which may be signs of a distinct species, while within-clade amino acid variation in potentially antigenic regions may indicate the existence of different serotypes among HRV-C strains. A newly identified HRV species, HRV-C, is circulating worldwide and is an important cause of febrile wheeze and asthmatic exacerbations in children requiring hospitalization.
Since a substantial proportion of respiratory tract infections remain undiagnosed (20, 29), research has been conducted to identify novel causative agents. In the past few years, several novel respiratory viruses, including human metapneumovirus (38), severe acute respiratory syndrome coronavirus (SARS-CoV) (24), human CoV NL63 (HCoV-NL63) (7, 39), CoV-HKU1 (17, 42, 43), and human bocavirus (HBoV) have been identified (1).
Human rhinoviruses (HRVs) are the most common etiological agents of upper respiratory tract infections (URTIs), or common colds. They are small, nonenveloped, single-stranded, positive-sense, RNA viruses that represent one of the nine genera belonging to the family Picornaviridae. Most HRVs have a relatively low optimal temperature for growth (33°C), which may reflect their adaptation to the human nasopharynx and association with URTIs. However, they are increasingly shown to be associated with more severe illnesses, such as pneumonia and exacerbations of asthma (12, 22, 36). Since laboratory diagnosis of RV infection is generally not available in clinical virology laboratories, their impact on health is often ignored.
HRVs consist of more than 100 immunologically distinct serotypes, which have been shown to correlate with VP1 gene sequences (23). HRVs have been classified according to several parameters, including receptor specificity, antiviral susceptibility, and nucleotide sequence homologies. For the latter, phylogenetic analysis of VP4/VP2 sequences has shown that all but one HRV serotype belonged to two different species, HRV-A (comprising 74 serotypes) and HRV-B (comprising 25 serotypes). The same topologies were also observed upon the subsequent analysis of the VP1, partial 2A, and 3D-coding regions (13, 18, 30, 31). The only exception was HRV87, which was found to belong to the human enterovirus D (HEV-D) species that has both RV and EV features (3, 31). A recent phylogenetic study using 12 HRV-A and HRV-B complete genomes suggested that HRV-B and HEV diverged from their last common ancestor after their separation from HRV-A (35).
During the winter of 2004, a new RV genotype was identified in respiratory samples of patients from New York with influenza-like illness by using MassTag PCR (15). Using PCR for picornaviruses, a previously undescribed HRV strain, HRV-QPM, was also identified in infants with bronchiolitis from Queensland hospitals (21). During our recent study on HBoV infections in hospitalized children (16), we also identified the presence of HRV sequences that did not cluster with either HRV-A or HRV-B species. In this study, we examined the presence of HRVs in nasopharyngeal aspirates (NPAs) of hospitalized children and described the clinical features of patients with HRV infections. VP4 sequence analysis demonstrated the presence of 21 strains belonging to a separate genetic cluster, clade C, distinct from HRV-A and HRV-B. The complete genome sequences of the HRV strains from three individual samples were determined and compared to those of known HRVs with available genome sequences. Based on the results, we propose that these novel HRV strains belong to a previously undetected HRV species, HRV-C.
MATERIALS AND METHODS
Patients and microbiological methods.
All NPAs in this study were collected from hospitalized children (<18 years of age) in two hospitals in Hong Kong during a 1-year period (November 2004 to October 2005) and tested negative for influenza A and B viruses, parainfluenza virus types 1, 2, and 3, respiratory syncytial virus (RSV), and adenovirus by direct immunofluorescence and for human metapneumovirus, HCoV-229E, HCoV-OC43, HCoV-NL63, and CoV-HKU1 by reverse transcription (RT)-PCR (17, 25, 41, 42). Eighty-three NPAs positive for HBoV in our previous study and 120 NPAs negative for HBoV (10 NPAs were randomly selected per month) were subjected to RT-PCR for HRV. PCR for HBoV was performed as described previously (1, 16). The clinical features, laboratory results, and outcome of illnesses in patients positive for HRV were analyzed.
RT-PCR for HRV and sequencing.
Viral RNA was extracted from NPAs by using a QIAamp viral RNA mini kit (QIAgen, Hilden, Germany). RT was performed using random hexamers and a SuperScript II kit (Invitrogen, San Diego, CA) as described previously (42, 43). PCR for HRV was performed using protocols described previously, using conserved primers covering the VP4 region (31). The amplified products were detected by agarose gel electrophoresis. Both strands of the PCR products were sequenced twice with an ABI prism 3700 DNA analyzer (Applied Biosystems, Foster City, CA), using the PCR primers. The nucleotide sequences of the VP4 regions were compared to those of HRV-A and HRV-B strains with sequences available in GenBank. Phylogenetic tree construction was performed using the neighbor-joining method with GrowTree using Kimura's two-parameter correction, with bootstrap values calculated from 1,000 trees (Genetics Computer Group, Inc.).
Complete genome sequencing and genome analysis of HRV-C.
Since sequence analysis suggested the presence of a previously undetected HRV species in 21 NPAs, the complete genomes of the HRV strains from three of these NPAs were amplified and sequenced using the strategy described in our previous publications (16, 42). The RNA was converted to cDNA by a combined random priming and oligo(dT) priming strategy. The cDNA was amplified by degenerate primers designed by multiple alignment of the genomes of HRV-A and HRV-B strains available in GenBank and additional primers designed from the results of the first and subsequent rounds of sequencing. These primer sequences are available on request. The terminal sequences were confirmed by rapid amplification of cDNA ends using a 5′/3′ rapid amplification of cDNA ends kit (Roche, Mannheim, Germany). The nucleotide and the deduced amino acid sequences of the single open reading frame in each genome were compared to those of HRV-A and HRV-B strains with sequences available in GenBank. Phylogenetic tree construction was performed using the neighbor-joining method with GrowTree using Kimura's two-parameter correction, with bootstrap values calculated from 1,000 trees (Genetics Computer Group, Inc.).
Viral cultures.
Attempts to isolate HRV-C were made by inoculating RT-PCR-positive specimens to human cell lines, including Hep2, RD, HeLa, MRC-5, WI-38, FRhK-4, and Vero E6 cells. Viral replication was monitored by observation for cytopathic effects and RT-PCR.
Nucleotide sequence accession numbers.
The complete genome sequences of the three strains of HRV-C have been deposited in the GenBank sequence database under accession no. EF582385 to EF582387.
RESULTS
Detection of HRV in NPAs from pediatric patients.
Among the 83 NPAs (ratio of male to female patients, 1.5:1; age, 2.4 ± 1.7 [mean ± standard deviation] years) that were positive for HBoV, 14 (16.9%) were positive for HRV by RT-PCR. Among the 120 NPAs (ratio of male to female patients, 1.3:1, age, 3.0 ± 3.2 [mean ± standard deviation] years) that were negative for HBoV, 12 (10%) were positive for HRV by RT-PCR. These 26 NPAs positive for HRV were from 25 patients. Most patients were infants or young children (median age, 1 year; range, 7 days to 8 years). Fourteen were males and 11 were females. The clinical characteristics of these 25 children are summarized in Table 1. All patients survived.
TABLE 1.
Clinical characteristics of the 26 cases of HRV infections
| Sample no. | Mo | Sexb | Age | Underlying disease | Diagnosis | HRV species | Codetection of HBoV |
|---|---|---|---|---|---|---|---|
| 1 | November | F | 3 yr | None | URTI | A | Yes |
| 2 | November | M | 13 mo | History of febrile wheeze | Pneumonia, febrile wheeze | C | Yes |
| 3 | November | M | 17 mo | History of febrile wheeze | Febrile wheeze | C | Yes |
| 4 | November | F | 2 yr | History of febrile wheeze | Febrile wheeze | A | Yes |
| 5 | November | F | 7 yr | None | URTI | C | No |
| 6 | December | M | 11 mo | None | Febrile wheeze | C | Yes |
| 7 | December | M | 2 yr | History of febrile wheeze | Febrile wheeze | C | Yes |
| 8 | December | F | 5 yr | Asthma, allergic rhinitis | Pneumonia, asthma | C | No |
| 9 | December | M | 1 yr | History of febrile wheeze | Febrile wheeze | C | No |
| 10 | January | F | 3 yr | Asthma | Asthma | C | Yes |
| 11 | January | M | 1 yr | History of febrile wheeze | Febrile wheeze | C | Yes |
| 12 | January | F | 2 yr | History of febrile wheeze | Pneumonia, asthma | C | Yes |
| 13a | January | M | 1 yr | Short gut syndrome, bronchopulmonary dysplasia | Febrile wheeze | C | Yes |
| 14 | January | M | 7 day | None | URTI | C | No |
| 15 | January | M | 10 mo | Bronchopulmonary dysplasia, intraventricular hemorrhage | URTI, otitis media, gastroenteritis | C | No |
| 16 | January | F | 1 yr | History of febrile wheeze, congenital heart disease | Febrile wheeze | C | Yes |
| 17 | February | F | 1 yr | None | Pneumonia, urinary tract infection | A | No |
| 18a | February | M | 1 yr | Short gut syndrome, bronchopulmonary dysplasia | Febrile wheeze | C | Yes |
| 19 | March | M | 8 yr | Allergic rhinitis | Acute sinusitis, asthma | A | No |
| 20 | April | M | 1 yr | Short gut syndrome, patent ductus arteriosus | Febrile wheeze | A | No |
| 21 | August | M | 7 mo | None | Febrile wheeze | C | No |
| 22 | October | M | 2 yr | History of febrile wheeze | Pneumonia, febrile wheeze | C | Yes |
| 23 | October | M | 5 yr | Asthma | Asthma | C | Yes |
| 24 | October | F | 2 yr | None | URTI, gastroenteritis | C | No |
| 25 | October | F | 4 mo | None | URTI | C | No |
| 26 | October | F | 1 yr | Bronchopulmonary dysplasia, congenital heart disease, intraventricular hemorrhage | Febrile wheeze | C | No |
The two samples were from the same patient collected 2 weeks apart.
F, female; M, male.
Phylogenetic analysis of the VP4 genes from these 26 NPAs showed that 5 belonged to HRV-A species and 21 belonged to a distinct genetic cluster, clade C, with <63% nucleotide identities to known HRV-A strains and <61% identities to known HRV-B strains (Fig. 1A). The sequences of the latter 21 strains also possessed 62% to 83% nucleotide identity to an unclassified HRV strain, 003-HRV NY, from New York, the United States (GenBank accession no. DQ875929), and 65% to 89% identity to another HRV strain, HRV-QPM, from Queensland, Australia (GenBank accession no. EF186077). 003-HRV NY and related strains were detected in patients with influenza-like illness, whereas HRV-QPM was identified in infants with bronchiolitis (15, 21). The results suggested that these novel HRV strains are closely related and represent a previously undetected HRV species, proposed to be named HRV-C.
FIG. 1.
(A) Phylogenetic tree of the VP4 region of the 26 HRV strains from NPAs. (A) Two hundred nine nucleotide positions in each VP4 region were included in the analysis. The scale bar indicates the estimated number of substitutions per 50 bases. HRV NY strains were from New York, and strain HRV-QPM was from Queensland. The GenBank accession numbers of the previously published sequences are as follows: 003-HRV NY, DQ875929; 1085-HRV NY, DQ875925; HRV-QPM, EF186077; HRV1B, D00239; HRV2, X02316; HRV16, L24917; HRV39, AY751783; HRV89, A10937; HRV3, AY016403; HRV14, NC_001490; HRV42, AY016404; HRV84, AY040240; CAV16 (coxsackievirus A16), NC_001612; CAV21 (coxsackievirus A21), NC_001428; CAV24 (coxsackievirus A24), D90457; Echo1 (echovirus 1), AF029859; Echo2 (echovirus 2), AF465518; EV70, NC_001430; EV71, DQ452074; EV73, AF241359; and PV1 (poliovirus 1), V01150. (B) Seasonality of HRV and distribution of HBoV codetections.
Clinical characteristics of HRV-C infections.
Of the 21 children with HRV-C infections, 15 had underlying diseases, with respiratory diseases being the most common (Table 1). Fever was present in all patients, and coryzal symptoms, such as cough and rhinorrhea, were common. Three (14%) children had URTI as the sole diagnosis, and two (10%) had gastroenteritis in addition to coryzal symptoms. Four (19%) had pneumonia. Wheezing or asthmatic exacerbations were common (16 [76%] of 21 cases), especially in those with a history of febrile wheeze or asthma. Two patients' illnesses were complicated by acute nonsuppurative otitis media and acute sinusitis, respectively. Twelve of these 21 children had HBoV codetected in their NPAs. However, no difference in disease severity was observed between those with and without HBoV codetection. Interestingly, two separate NPAs (samples 13 and 18), both positive for HBoV, collected 2 weeks apart from the same patient during the same admission were found to possess two different strains of HRV-C (Table 1 and Fig. 1A). Except for one case detected in August, all HRV-C infections were detected from October to February (Fig. 1B).
Complete genome analysis of HRV-C.
Since phylogenetic analysis of the VP4 region suggested the presence of a previously undetected species, HRV-C, in 21 of the 26 samples, the complete genomes of three HRV-C strains from three patients (HRV-C 024, HRV-C 025, and HRV-C 026) were amplified and sequenced. The genome of HRV-C was relatively small compared to other reported genomes of Picornaviridae as a result of a number of deletions, with 7,086 to 7,114 nucleotides and G+C content of 42 to 43% [excluding the 3′ poly(A) tract]. The genome organization was the same as those of HRV-A and HRV-B, comprising a 5′ noncoding region (5′-NCR) of 611 to 616 nucleotides, a single open reading frame of 6,429 to 6,459 nucleotides encoding a single polyprotein of 2,142 to 2,152 amino acids, and a 3′-NCR of 39 to 49 nucleotides prior to a polyadenylated tract.
The predicted amino acid sequence of the polyprotein showed 51% identity to that of HRV-A and 48% to that of HRV-B. This precursor polyprotein is believed to be translated in the host cell cytoplasm and processed by virus-encoded proteases to yield the mature viral proteins. The arrangement of these resulting proteins is the same as that in HRV-A and HRV-B (Table 2). The capsid-coding region (P1) encodes the capsid gene products VP4, VP2, VP3, and VP1, while the P2 region encodes 2A protease, 2B, and 2C, and the P3 region encodes 3A, VPg (small genome-linked protein) 3B, 3C protease, and 3D (RNA-dependent RNA polymerase). Comparison with HRV-A and HRV-B strains with available complete genome sequences allowed the prediction of putative protease cleavage sites which were largely similar to those in other HRVs (Table 3). However, a distinct alignment-predicted cleavage site was noted between VP4 and VP2, where a methionine-serine pair was present in all three HRV-C strains and HRV-QPM, compared to a glutamine-serine pair observed in HRV-A and an asparagine-serine pair in HRV-B. This methionine-serine pair has only been described in picornaviruses other than RVs (2). Since this site was not cleaved by the viral proteases and the mechanism of cleavage remains unknown, further studies are required to determine the significance of such a change from hydrophilic residues to a hydrophobic residue.
TABLE 2.
Comparison of genomic features and amino acid identities between the predicted proteins of HRV-C 024 and the corresponding proteins of HRV-C 025 and HRV-C 026 and of other members of Picornaviridae
| Picornavirus species and straina | Genome features
|
Pairwise amino acid identity (%)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (no. of bases) | G+C content | VP4 | VP2 | VP3 | VP1 | 2A | 2B | 2C | 3A | 3B | 3C | 3D | |
| HRV-A (HRV89) | 7,152 | 0.39 | 62 | 63 | 44 | 45 | 56 | 53 | 49 | 42 | 45 | 46 | 57 |
| HRV-B (HRV14) | 7,212 | 0.41 | 50 | 61 | 40 | 36 | 36 | 51 | 47 | 30 | 47 | 48 | 62 |
| HRV-C 024 | 7,099 | 0.43 | |||||||||||
| HRV-C 025 | 7,114 | 0.42 | 82 | 79 | 65 | 60 | 69 | 66 | 70 | 57 | 45 | 73 | 69 |
| HRV-C 026 | 7,086 | 0.43 | 76 | 81 | 64 | 65 | 66 | 58 | 66 | 64 | 59 | 69 | 68 |
| HEV-A (EV71) | 7,410 | 0.48 | 55 | 49 | 40 | 37 | 41 | 41 | 47 | 31 | 54 | 45 | 57 |
| HEV-B (EV73) | 7,411 | 0.48 | 62 | 53 | 46 | 40 | 39 | 50 | 45 | 33 | 54 | 45 | 58 |
| HEV-C (CAV21) | 7,401 | 0.45 | 60 | 54 | 43 | 41 | 40 | 50 | 43 | 29 | 40 | 43 | 57 |
| HEV-D (EV70) | 7,390 | 0.43 | 67 | 49 | 39 | 37 | 43 | 48 | 45 | 31 | 54 | 49 | 59 |
| Poliovirus (PV1) | 7,441 | 0.46 | 64 | 51 | 43 | 40 | 42 | 50 | 46 | 26 | 40 | 44 | 57 |
HRV89, GenBank accession no. A10937; HRV14, GenBank accession no. NC_001490; EV71, GenBank accession no. DQ452074; EV73, GenBank accession no. AF241359; CAV21 (human coxsackievirus A21), GenBank accession no. NC_001428; EV70, GenBank accession no. NC_001430; PV1 (human poliovirus strain Sabin 1), GenBank accession no. V01150.
TABLE 3.
Putative proteolytic cleavage sites within HRV-C polyprotein and those within the polyproteins of HRV-A and HRV-B strains with available complete genome sequences
| Protein junction | Amino acid residuesa at cleavage site in:
|
|||||
|---|---|---|---|---|---|---|
| HRV-C 024 | HRV-C 025 | HRV-C 026 | HRV-QPM | HRV-Ab | HRV-Bc | |
| VP4/VP2 | M/S | M/S | M/S | M/S | Q/S | N/S |
| VP2/VP3 | Q/G | Q/G | Q/G | Q/G | Q,E/G | Q/G |
| VP3/VP1 | Q/N | Q/N | Q/N | Q/N | Q/N | E/G |
| VP1/2A | A/G | A/G | L/G | L/G | A,F,V,Y/G | Y/G |
| 2A/2B | Q/G | Q/G | Q/G | Q/G | Q/G | Q/G |
| 2B/2C | Q/G | Q/S | Q/G | Q/S | E,Q/S | Q/A,S |
| 2C/3A | Q/G | Q/G | Q/G | Q/G | Q/G | Q/G |
| 3A/Vpg | Q/G | Q/G | Q/G | Q/G | Q/G | Q/G |
| Vpg/3C | Q/G | Q/G | Q/G | Q/G | Q/G | Q/G |
| 3C/3D | Q/G | Q/G | Q/G | Q/G | Q/G | Q/G |
Unique amino acid residues at cleavage sites observed in HRV-C are in bold.
Conserved amino acid residues at cleavage sites based on multiple alignment of 26 HRV-A sequences from GenBank with the following accession numbers: HRV30, DQ473512; HRV55, DQ473511; HRV75, DQ473510; HRV28, DQ473508; HRV53, DQ473507; HRV46, DQ473506; HRV36, DQ473505; HRV88, DQ473504; HRV7, DQ473503; HRV76, DQ473502; HRV34, DQ473501; HRV59, DQ473500; HRV44, DQ473499; HRV10, DQ473498; HRV23, DQ473497; HRV49, DQ473496; HRV38, DQ473495; HRV74, DQ473494; HRV15, DQ473493; HRV73, DQ473492; HRV41, DQ473491; HRV39, AY751783; HRV16, L24917; HRV89, A10937; HRV1B, D00239; and HRV2, X02316.
Comparison of the deduced amino acid sequences of the predicted individual cleaved proteins from HRV-C indicated that the least homology to the two known HRV species was found in 3A (42% identity to HRV-A and 30% identity to HRV-B) and the greatest in VP2 (63% identity to HRV-A and 62% identity to HRV-B) (Table 2). However, major insertions and deletions were mainly observed in VP1 compared to VP1 of HRV-A and HRV-B. Phylogenetic analysis of the 5′-NCR, VP1, 3C, and 3D showed that the three HRV-C strains, together with the HRV-QPM strain from Queensland, formed a separate clade distinct from HRV-A and HRV-B, although they were more closely related to HRV-A (Fig. 2). Most of the other regions of the polyprotein displayed similar topologies upon phylogenetic analysis. Although the three HRV-C strains formed a distinct cluster away from HRV-A and HRV-B in most genes, they exhibited significant sequence variations among themselves, with <70% amino acid identity in VP1, suggesting the possibility of the existence of different serotypes (Table 2). Within the 3D region, the YGDD motif thought to be a common nucleic acid recognition site and the downstream YGL, FLKR, and SIRWT motifs shared by HRV-A, HRV-B, and EVs were present (4, 30).
FIG. 2.
Phylogenetic trees of the 5′-NCR, VP1, 3C, and 3D regions of three HRV-C strains. The analysis included 793, 1,004, 549, and 1,394 nucleotide positions in each 5′-NCR, VP1, 3C, and 3D region, respectively. The scale bars indicate the estimated number of substitutions per 50 bases. PV, poliovirus.
Conserved motifs and functional domains in VP1 of HRV-C.
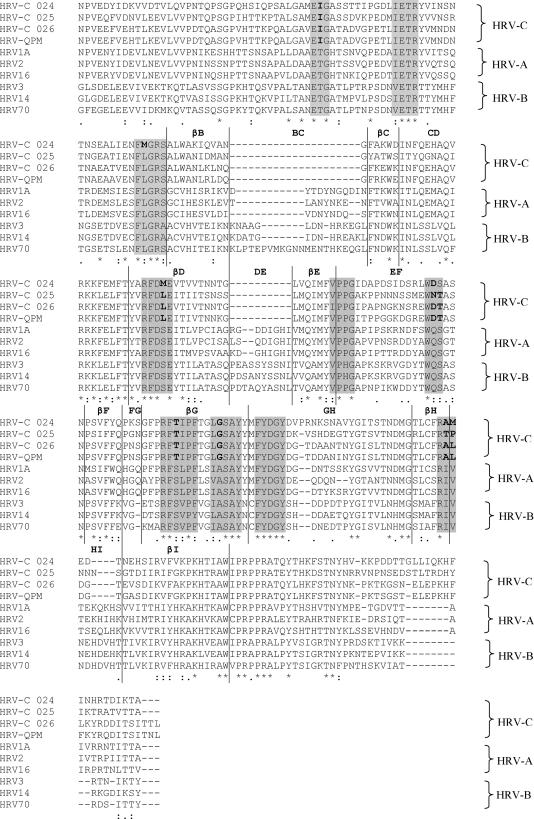
The capsid of HRV is composed of the three larger proteins VP1, VP2, and VP3, whereas the smaller VP4 is located inside the capsid shell. In particular, VP1 is the largest and most surface-exposed protein and contains most of the motifs important for interaction with neutralizing antibodies, cellular receptors, and antiviral agents (18, 28). Therefore, amino acids and motifs conserved within the VP1 of HRV-C in comparison to those in HRV-A and HRV-B were examined. Alignment of VP1 of the three HRV-C strains and HRV-QPM and those of prototype strains of HRV-A and HRV-B showed that the most striking differences between HRV-C and the other two species were found in the BC and DE loops, where deletions have occurred (Fig. 3). Since these two loops have been shown by X-ray crystallographic analysis to be located at the viral surface and harbor major neutralization antigens in other HRVs (27, 32), HRV-C may have evolved under serological selection by deleting these antigenic epitopes. Among the 10 amino acid motifs previously reported to be conserved between HRV-A and HRV-B (14), six were found to have undergone amino acid substitutions in HRV-C (Fig. 3).
FIG. 3.
Comparison of the deduced amino acid sequences of VP1 in the three strains of HRV-C and HRV-QPM to those of HRV-A and HRV-B strains. The 10 motifs conserved between HRV-A and HRV-B, based on X-ray crystallographic analysis described previously, are shaded. Amino acid substitutions within the corresponding motifs in HRV-C are in bold. The putative secondary structures are marked above the sequence alignment. *, amino acid residues fully conserved across the RVs; :, strongly conserved residues; ., weakly conserved residues.
Despite the antigenic diversity of HRVs, they utilize only two main receptors. All HRV serotypes except HRV87 bind to either intercellular adhesion molecule 1 (ICAM-1) (major receptor, used by 90% of known HRV serotypes) or low-density lipoprotein receptor (LDL-R) (minor receptor) as their receptor (10, 37). HRV87, which represents a unique serotype belonging to EV-D, may share the same receptor, decay accelerating factor, with EV 70 and several echovirus serotypes (3). While the major (ICAM-1) receptor-binding serotypes were distributed among both HRV-A and HRV-B, minor (LDL-R) receptor-binding serotypes were found exclusively in the HRV-A species (18). On the basis of interactions between ICAM-1 and the canyon floors of HRV14 and HRV16, a set of nonlinear, species-specific, potentially ICAM-1-interacting amino acid residues in VP1 and carboxy-terminal VP3 that are conserved among the major (ICAM-1) receptor group have been identified previously (14). When the corresponding amino acid residues, based on amino acid sequence alignment, in HRV-C were studied, only five of the seven and four of the nine conserved residues were observed between HRV-C and the major receptor serotypes of HRV-A and HRV-B, respectively (Table 4). As for the VLDL or minor receptor group of HRVs, it has previously been reported that Lys224 was essential for interaction with the minor receptor protein (40). This Lys224 was not found in VP1 of HRV-C and HRV-QPM. Most of the amino acids identified in the contact region between echovirus 7 and decay accelerating factor were also not present (9). Therefore, HRV-C may either utilize different receptors or use very different contacting amino acids for interaction with the same receptors as HRV-A and HRV-B. Since attempts to stably passage HRV-C in cell lines were unsuccessful, which could be related to the lack of a susceptible cell line or the quality of the stored specimens, further studies are required to elucidate the receptor for HRV-C.
TABLE 4.
Potentially ICAM-1-interacting amino acid residues in VP1 and carboxy-terminal VP3 conserved among the major receptor serotypes of HRV-A and HRV-B and the corresponding amino acid residues in minor receptor serotypes and HRV-C
| Rhinovirus group and receptor serotype | Conserved amino acid(s) at indicated position
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3179 | 3180 | 3181 | 3182 | 1148 | 1151 | 1213 | |||
| HRV-A major receptor | T | P | D | A/I/K/M/N/S/T | G | A/F/K/I/L/T/V | A/D/H | ||
| HRV-A minor receptor | A/S | D/K/N/P/R/S | D/G/N | E/K/P/T | G | I/V | D/N/R | ||
| HRV-Ca | K/R | A/K | D | N | G | A/P | D | ||
| 3177 | 3178 | 3179 | 3180 | 3226 | 1155 | 1159 | 1206 | 1217 | |
| HRV-B major receptor | D | K/P | D | T | Q/S | P | N | H | V |
| HRV-Ca | K/R | A/K | D | N | P | P | A/P | G/K/V | L/S/V |
Amino acid residues conserved among the three HRV-C strains and HRV-QPM.
Apart from being important for antibody recognition and receptor binding, VP1 also contains a hydrophobic pocket into which small-molecule antiviral compounds, such as pleconaril, bind and inhibit capsid functions. Analysis of the amino acids within the drug-binding pocket has previously demonstrated correlation with pleconaril susceptibility (18). While all HRV-A strains reported to date were susceptible to pleconaril, the presence of Phe152 and Leu191 in HRV-B strains was found to be “diagnostic” of naturally occurring resistance to pleconaril, although Thr191 was also found in strains with higher 50% effective concentrations of pleconaril. While Phe152 was present in VP1 in two of the three HRV-C strains and HRV-QPM, all three HRV-C strains and HRV-QPM possessed a Thr191 (21). However, experimental data are necessary to determine if HRV-C is resistant to pleconaril.
DISCUSSION
We report the clinical features and complete genome characterization of a distinct HRV genetic cluster, HRV-C, detected in NPAs from children with acute respiratory tract infections. Among the 26 HRV strains identified, 21 were found upon VP4 sequence analysis to belong to a separate cluster distinct from HRV-A and HRV-B, suggesting that they represent an additional HRV species. HRV strains recently reported in GenBank by two different groups of scientists, from the United States and Australia, respectively, were also found to fall into the same cluster upon phylogenetic analysis (Fig. 1A). These data suggest that HRV-C is circulating worldwide and is associated with acute respiratory infections.
Complete genome analysis reveals that HRV-C probably represents a previously undetected HRV species. In the New York study, only the VP4 and VP1 regions were sequenced, without further characterization of the virus (15). In the study from Queensland, the polyprotein gene sequence was determined in one of the HRV-QPM strains. Based on phylogenetic analysis of the VP regions, the authors concluded that HRV-QPM strains were most closely related to, but distinct from, HRV-A and classified them as HRV-A2 (21). To better characterize this potentially distinct HRV species, we performed complete genome sequencing on three HRV-C strains and carried out comparative analysis with available HRV genomes. Although the genome organization of HRV-C concurs with that of HRV-A and HRV-B, the genome size of HRV-C was relatively small, which may reflect its different phylogenetic position. Similar to known HRVs, the HRV-C genome tended to be A+U rich (Table 2) and had a marked preponderance of A+U in the third codon position (61.6% to 67%) compared to the occurrence in EVs (11). The polyprotein of HRV-C possessed equally low amino acid identities to those of HRV-A (51%) and HRV-B (48%). While the P1 region of HRV-C possessed higher amino acid identity to that of HRV-A than that of HRV-B, the P3 region possessed higher amino acid identity to that of HRV-B than that of HRV-A (Table 2). Phylogenetic analysis of the 5′-NCR and the predicted proteins revealed that HRV-C strains formed a distinct cluster away from HRV-A and HRV-B, indicating that they represent a previously undetected species within the HRV genus.
HRV-C exhibits additional genomic features that are distinct from HRV-A and HRV-B. A putative cleavage site different from that of HRV-A and HRV-B was identified between VP4 and VP2. Upon further analysis of the VP1 region, major insertions and deletions were observed compared to VP1 of HRV-A and HRV-B, especially in regions that were important for neutralization in other HRVs. Motifs conserved between VP1 of HRV-A and HRV-B were also frequently found to contain amino acid substitutions in HRV-C. HRV-C has apparently never been detected in cell culture, as HRV-A and HRV-B were both first grown in tissue cultures. These data suggest that HRV-C may be made up of new serotypes, but this would need to be confirmed by neutralization assays using antisera on culture isolates when available. Further studies on cytopathic effect and antiviral and acid sensitivity assays should also be performed to better characterize HRV-C. Nevertheless, the present data suggest that it is more appropriate to classify HRV-C as a separate species than a subspecies of HRV-A.
HRV-C appears to be an important cause of febrile wheeze and asthmatic exacerbation in children requiring hospitalization. The new HRV genotypes detected in the New York study were from patients with influenza-like illness (15). However, no additional demographic or clinical data were provided. In the Queensland study, 5 of the 17 strains were identified from infants with bronchiolitis, while two were from adults of whom one had exacerbation of chronic obstructive pulmonary disease (21). In this study, HRV-C was found among 21 (10.3%) of 203 NPAs from children hospitalized for acute respiratory illness. Febrile wheeze or asthma was the most common presentation (76%) among those with HRV-C infections, especially in children with a history of wheeze/asthma and chronic lung diseases. This is in line with previous studies that demonstrated associations between RV and asthmatic exacerbations (22, 26). Our results suggest that HRV-C may contribute to a significant proportion of febrile wheeze in infants and young children under 2 years of age and asthmatic exacerbations in older children. HRV-C infections appear to peak in fall and winter. Since only samples from children were included in the present study, further studies are required to determine the epidemiology and clinical disease spectrum of HRV-C infections in adults.
Since HRVs are often considered to be of little health impact and clinical significance, the possible existence of novel species and relative importance of the different species were poorly investigated. Moreover, genomic studies on HRVs have been limited in the past, with only five HRV-A and one HRV-B genomes published before 2007 until a recent report of 12 HRV-A and HRV-B genomes (5, 6, 8, 11, 19, 33, 34, 35). Recently, increasing numbers of reports have implicated HRVs in more severe URTIs and lower respiratory tract infections in children, the elderly, and immunocompromised adults (12, 22, 36). In a population-based surveillance study on RV-associated hospitalizations in the United States, 156 (26%) of 592 children hospitalized with respiratory symptoms or fever were found to be RV positive, causing 5 hospitalizations/1,000 children <5 years old (22). This detection rate was even higher than for RSV (20%) in their study population, in contrast to the historical belief that RSV is the most important virus associated with hospitalizations due to acute respiratory illness in young children. However, no genotyping was performed to determine the species responsible for the infections. In the New York study, among the 13 HRVs with VP4 regions successfully sequenced, two belonged to HRV-A, three belonged to HRV-B, and eight were novel strains belonging to HRV-C (15). In the present study, 13% (26/203) of NPAs from children hospitalized for respiratory tract infections were positive for HRVs. This suggests that HRVs are important causes of hospitalizations in children in our locality, despite the fact that HRVs often cause mild respiratory illness that often does not result in hospitalizations, which also explains the relatively low detection rate in the present study. Although our PCR assay would have allowed the detection of HRV-B, none of the 203 samples were positive for HRV-B. As the sensitivities of the present PCR towards the three HRV species were not studied, it remains to be determined if HRV-C was the most prevalent species in our population. There was no apparent difference between the clinical manifestations of HRV-A and HRV-C infections in the present study. However, given the small number of patients with HRV-A infections in this study, further epidemiological studies are also required to compare the clinical significance of the different species of HRVs.
Acknowledgments
We are grateful for the generous support of Hui Hoy and Hui Ming in the genomic sequencing platform.
This work is partly supported by a Research Grant Council grant, the University development fund, an HKU special research achievement award, and an outstanding young researcher award from The University of Hong Kong, the Croucher senior medical research fellowship 2006-2007, and the HKSAR research fund for the control of infectious diseases of the Health, Welfare, and Food Bureau.
Footnotes
Published ahead of print on 5 September 2007.
REFERENCES
- 1.Allander, T., M. T. Tammi, M. Eriksson, A. Bjerkner, A. Tiveljung-Lindell, and B. Andersson. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. USA 102:12891-12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blom, N., J. Hansen, D. Blaas, and S. Brunak. 1996. Cleavage site analysis in picornaviral polyproteins: discovering cellular targets by neural networks. Protein Sci. 5:2203-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomqvist, S., C. Savolainen, L. Raman, M. Roivainen, and T. Hovi. 2002. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J. Clin. Microbiol. 40:4218-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, B., M. S. Oberste, K. Maher, and M. A. Pallansch. 2003. Complete genomic sequencing shows that polioviruses and members of human enterovirus species C are closely related in the noncapsid coding region. J. Virol. 77:8973-8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callahan, P. L., S. Mizutani, and R. J. Colonno. 1985. Molecular cloning and complete sequence determination of RNA genome of human rhinovirus type 14. Proc. Natl. Acad. Sci. USA 82:732-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duechler, M., T. Skern, W. Sommergruber, C. Neubauer, P. Gruendler, I. Fogy, D. Blaas, and E. Kuechler. 1987. Evolutionary relationships within the human rhinovirus genus: comparison of serotypes 89, 2, and 14. Proc. Natl. Acad. Sci. USA 84:2605-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fouchier, R. A., N. G. Hartwig, T. M. Bestebroer, B. Niemeyer, J. C. de Jong, J. H. Simon, and A. D. Osterhaus. 2004. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. USA 101:6212-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris, J. R., and V. R. Racaniello. 2005. Amino acid changes in proteins 2B and 3A mediate rhinovirus type 39 growth in mouse cells. J. Virol. 79:5363-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He, Y., F. Lin, P. R. Chipman, C. M. Bator, T. S. Baker, M. Shoham, R. J. Kuhn, M. E. Medof, and M. G. Rossmann. 2002. Structure of decay-accelerating factor bound to echovirus 7: a virus-receptor complex. Proc. Natl. Acad. Sci. USA 99:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofer, F., M. Gruenberger, H. Kowalski, H. Machat, M. Huettinger, E. Kuechler, and D. Blaas. 1994. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. USA 91:1839-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes, P. J., C. North, C. H. Jellis, P. D. Minor, and G. Stanway. 1988. The nucleotide sequence of human rhinovirus 1B: molecular relationships within the rhinovirus genus. J. Gen. Virol. 69:49-58. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser, L., J. D. Aubert, J. C. Pache, C. Deffernez, T. Rochat, J. Garbino, W. Wunderli, P. Meylan, S. Yerly, L. Perrin, I. Letovanec, L. Nicod, C. Tapparel, and P. M. Soccal. 2006. Chronic rhinoviral infection in lung transplant recipients. Am. J. Respir. Crit. Care Med. 174:1392-1399. [DOI] [PubMed] [Google Scholar]
- 13.Laine, P., C. Savolainen, S. Blomqvist, and T. Hovi. 2005. Phylogenetic analysis of human rhinovirus capsid protein VP1 and 2A protease coding sequences confirms shared genus-like relationships with human enteroviruses. J. Gen. Virol. 86:697-706. [DOI] [PubMed] [Google Scholar]
- 14.Laine, P., S. Blomqvist, C. Savolainen, K. Andries, and T. Hovi. 2006. Alignment of capsid protein VP1 sequences of all human rhinovirus prototype strains: conserved motifs and functional domains. J. Gen. Virol. 87:129-138. [DOI] [PubMed] [Google Scholar]
- 15.Lamson, D., N. Renwick, V. Kapoor, Z. Liu, G. Palacios, J. Ju, A. Dean, K. St. George, T. Briese, and W. I. Lipkin. 2006. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004-2005. J. Infect. Dis. 194:1398-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau, S. K. P., C. C. Y. Yip, T. L. Que, R. A. Lee, R. K. H. Au-Yeung, B. Zhou, L. Y. So, Y. L. Lau, K. H. Chan, P. C. Y. Woo, and K. Y. Yuen. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J. Infect. Dis., in press. [DOI] [PMC free article] [PubMed]
- 17.Lau, S. K. P., P. C. Y. Woo, C. C. Y. Yip, H. Tse, H.-W. Tsoi, V. C. C. Cheng, P. Lee, B. S. F. Tang, C. H. Y. Cheung, R. A. Lee, L.-Y. So, Y.-L. Lau, K.-H. Chan, and K.-Y. Yuen. 2006. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J. Clin. Microbiol. 44:2063-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledford, R. M., N. R. Patel, T. M. Demenczuk, A. Watanyar, T. Herbertz, M. S. Collett, and D. C. Pevear. 2004. VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J. Virol. 78:3663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, W. M., W. Wang, and R. R. Rueckert. 1995. Complete sequence of the RNA genome of human rhinovirus 16, a clinically useful common cold virus belonging to the ICAM-1 receptor group. Virus Genes 9:177-181. [DOI] [PubMed] [Google Scholar]
- 20.Macfarlane, J. T., A. Colville, A. Guion, R. M. Macfarlane, and D. H. Rose. 1993. Prospective study of aetiology and outcome of adult lower-respiratory-tract infections in the community. Lancet 341:511-514. [DOI] [PubMed] [Google Scholar]
- 21.McErlean, P., L. A. Shackelton, S. B. Lambert, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2007. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J. Clin. Virol. 39:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, E. K., X. Lu, D. D. Erdman, K. A. Poehling, Y. Zhu, M. R. Griffin, T. V. Hartert, L. J. Anderson, G. A. Weinberg, C. B. Hall, M. K. Iwane, K. M. Edwards, and the New Vaccine Surveillance Network. 2007. Rhinovirus-associated hospitalizations in young children. J. Infect. Dis. 195:773-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberste, M. S., K. Maher, D. R. Kilpatrick, and M. A. Pallansch. 1999. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 73:1941-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peiris, J. S., S. T. Lai, L. L. Poon, Y. Guan, L. Y. Yam, W. Lim, J. Nicholls, W. K. Yee, W. W. Yan, M. T. Cheung, V. C. Cheng, K. H. Chan, D. N. Tsang, R. W. Yung, T. K. Ng, K. Y. Yuen, and the SARS Study Group. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peiris, J. S., W. H. Tang, K. H. Chan, P. L. Khong, Y. Guan, Y. L. Lau, and S. S. Chiu. 2003. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg. Infect. Dis. 9:628-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawlinson, W. D., Z. Waliuzzaman, I. W. Carter, Y. C. Belessis, K. M. Gilbert, and J. R. Morton. 2003. Asthma exacerbations in children associated with rhinovirus but not human metapneumovirus infection. J. Infect. Dis. 187:1314-1318. [DOI] [PubMed] [Google Scholar]
- 27.Rossmann, M. G., E. Arnold, J. W. Erickson, E. A. Frankenberger, J. P. Griffith, H.-J. Hecht, J. E. Johnson, G. Kamer, M. Luo, A. G. Mosser, R. R. Rueckert, B. Sherry, and G. Vriend. 1985. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature 317:145-153. [DOI] [PubMed] [Google Scholar]
- 28.Rueckert, R. R. 1996. Picornaviridae: the viruses and their replication, p. 609-654. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, PA.
- 29.Ruiz, M., S. Ewig, M. A. Marcos, J. A. Martinez, F. Arancibia, J. Mensa, and A. Torres. 1999. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am. J. Respir. Crit. Care Med. 160:397-405. [DOI] [PubMed] [Google Scholar]
- 30.Savolainen, C., P. Laine, M. N. Mulders, and T. Hovi. 2004. Sequence analysis of human rhinoviruses in the RNA-dependent RNA polymerase coding region reveals large within-species variation. J. Gen. Virol. 85:2271-2277. [DOI] [PubMed] [Google Scholar]
- 31.Savolainen, C., S. Blomqvist, M. N. Mulders, and T. Hovi. 2002. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J. Gen. Virol. 83:333-340. [DOI] [PubMed] [Google Scholar]
- 32.Sherry, B., and R. Rueckert. 1985. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J. Virol. 53:137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skern, T., W. Sommergruber, D. Blaas, P. Gruendler, F. Fraundorfer, C. Pieler, I. Fogy, and E. Kuechler. 1985. Human rhinovirus 2: complete nucleotide sequence and proteolytic processing signals in the capsid protein region. Nucleic Acids Res. 13:2111-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanway, G., P. J. Hughes, R. C. Mountford, P. D. Minor, and J. W. Almond. 1984. The complete nucleotide sequence of a common cold virus: human rhinovirus 14. Nucleic Acids Res. 12:7859-7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tapparel, C., T. Junier, D. Gerlach, S. Cordey, S. Van Belle, L. Perrin, E. M. Zdobnov, and L. Kaiser. 2007. New complete genome sequences of human rhinoviruses shed light on their phylogeny and genomic features. BMC Genomics 8:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner, R. B. 2007. Rhinovirus: more than just a common cold virus. J. Infect. Dis. 195:765-766. [DOI] [PubMed] [Google Scholar]
- 37.Uncapher, C. R., C. M. DeWitt, and R. J. Colonno. 1991. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 180:814-817. [DOI] [PubMed] [Google Scholar]
- 38.van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Hoek, L., K. Pyrc, M. F. Jebbink, W. Vermeulen-Oost, R. J. Berkhout, K. C. Wolthers, P. M. Wertheim-van Dillen, J. Kaandorp, J. Spaargaren, and B. Berkhout. 2004. Identification of a new human coronavirus. Nat. Med. 10:368-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlasak, M., S. Blomqvist, T. Hovi, E. Hewat, and D. Blaas. 2003. Sequence and structure of human rhinoviruses reveal the basis of receptor discrimination. J. Virol. 77:6923-6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo, P. C. Y., S. S. S. Chiu, W. H. Seto, and M. Peiris. 1997. Cost-effectiveness of rapid diagnosis of viral respiratory tract infections in pediatric patients. J. Clin. Microbiol. 35:1579-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo, P. C. Y., S. K. P. Lau, C.-M. Chu, K.-H. Chan, H.-W. Tsoi, Y. Huang, B. H. L. Wong, R. W. S. Poon, J. J. Cai, W.-K. Luk, L. L. M. Poon, S. S. Y. Wong, Y. Guan, J. S. M. Peiris, and K.-Y. Yuen. 2005. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79:884-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo, P. C. Y., S. K. Lau, H. W. Tsoi, Y. Huang, R. W. Poon, C. M. Chu, R. A. Lee, W. K. Luk, G. K. Wong, B. H. Wong, V. C. Cheng, B. S. Tang, A. K. Wu, R. W. Yung, H. Chen, Y. Guan, K. H. Chan, and K. Y. Yuen. 2005. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J. Infect. Dis. 191:1898-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]