Abstract
Traditional culture of Leishmania spp. is labor intensive and has poor sensitivity. We evaluated a microculture method for the diagnosis of cutaneous leishmaniasis in consecutive patients presenting to the Leishmaniasis Clinic at the Instituto de Medicina Tropical Alexander von Humboldt, Peru, for evaluation of skin lesions. Lesion aspirates were cultured in duplicate and parallel in traditional culture tubes containing modified Novy-MacNeal-Nicolle (NNN) medium or Roswell Park Memorial Institute medium 1640 with 10% fetal bovine serum (10% RPMI) and in 70-μl capillary tubes containing a mixture of lesion aspirate and 10% RPMI. For sensitivity analysis, the consensus standard was considered to be a positive result in any two of the following four tests: Giemsa-stained lesion smear, culture, kinetoplast DNA PCR, or leishmanin skin test. The outcome measures were sensitivity and time to culture positivity. Forty-five patients with 62 skin lesions were enrolled in the study, of which 53 lesions fulfilled the consensus criteria for a final diagnosis of cutaneous leishmaniasis. Of these 53 lesions, 39 were culture positive: 38 in capillary tubes, 29 in traditional culture tubes with modified NNN medium, and 19 in traditional culture tubes with 10% RPMI medium. The sensitivity of microculture was 71.7%, versus 54.7% for traditional culture with NNN (P, 0.038) and 35.8% with 10% RPMI (P, <0.001). The mean times to culture positivity were 4.2 days by microculture, 5.2 days in NNN, and 6 days in 10% RPMI (P, 0.009). We have demonstrated that microculture is a more sensitive and time-efficient means of isolating Leishmania parasites from cutaneous lesions than traditional culture.
Cutaneous leishmaniasis (CL) is a major health problem in tropical and subtropical countries, affecting 1 to 1.5 million people annually (14). Peru is one of the top 10 countries contributing to the worldwide burden of CL (16). In Peru, the causative agent of CL is predominantly Leishmania (Viannia) braziliensis, though other related New World species, such as Leishmania (Viannia) peruviana, Leishmania (Viannia) guyanensis, Leishmania (Viannia) lainsoni, and Leishmania (Leishmania) amazonensis, can be isolated as well (5, 24). Mucocutaneous leishmaniasis is mainly due to L. (V.) braziliensis, though it has been attributed to other species, including L. (V.) guyanensis (34) and L. (L.) amazonensis (24) as well. Thus, unlike Old World CL which does not progress to mucosal involvement, New World CL, caused by species other than Leishmania mexicana, necessitates treatment (8). However, due to the toxicity associated with standard pentavalent antimonial therapy for New World CL, treatment initiation follows definitive diagnosis and treatment is not administered empirically. Thus, timely and definitive diagnosis is important for the appropriate management of CL. Definitive diagnosis of CL depends on the demonstration of parasites by direct microscopic examination of tissue aspirates, smears, or biopsies or culture of these specimens (16, 27, 35, 36). Traditional culture methods have sensitivities in the range of 40 to 75% (2, 4, 6), though combined with direct exam, this increases to 85% (8, 27, 31, 36).
Molecular techniques such as PCR are promising, with diagnostic sensitivities on the order of 95% in acute lesions, but at present, their practical utility in clinical and field settings is limited by their high costs and infrastructural requirements and the lack of personnel trained to use them (27, 36). While PCR-based detection of parasites in specimens is generally regarded as the most sensitive method for the diagnosis of CL and mucosal leishmaniasis (13, 15, 30, 38, 40), it is rarely the only test that produces positive results (30, 40). Thus, culture-based testing may be a more practical approach, particularly in underresourced settings. In addition to being more economical and accessible, cultivation has the advantage of isolating organisms for drug susceptibility testing, speciation, and genotyping, which are important in countries where infection with L. (V.) braziliensis can lead to mucosal disease and where diverse Leishmania species coexist.
Traditional culture methods consist of biphasic culture systems—blood agar with a liquid overlay that is sampled periodically throughout the incubation for the presence of motile promastigotes. Prolonged incubation (15 to 30 days) is often required (2), as are large numbers of amastigotes in the culture inoculum (22, 29), due to the relatively large volume of liquid overlay which must be sampled repeatedly. A newly developed microculture method (2) consisting of 70-μl capillary tubes and single-phase liquid medium has the advantages of being less costly, due to the smaller volume of medium required and a 10-fold cost reduction between traditional culture tubes and capillary tubes, easier to prepare and use, and more sensitive, even when the parasite burden is low (1, 2, 17, 18). This method, however, has been tested only in Old World species of Leishmania and has yet to be validated in Latin America (1, 2, 17, 18), where difficult-to-culture species such as L. (V.) braziliensis are endemic.
We evaluated herein the microculture method by comparing it to traditional culture for the isolation of Leishmania parasites from cutaneous lesions of patients presenting to a specialized leishmaniasis clinic in Lima, Peru.
MATERIALS AND METHODS
Study site.
The study was conducted at the Leishmaniasis Clinic of the Instituto de Medicina Tropical Alexander Von Humboldt in Lima, Peru, between February and April 2007, following institutional review board approval. The Institute houses a large outpatient clinic for the diagnosis and management of tegumentary leishmaniasis, with an average of 20 to 30 new cases diagnosed per month.
Study population.
Consecutive patients presenting to the Leishmaniasis Clinic for the evaluation of skin lesions were approached to participate in this study and screened for eligibility criteria. We included patients who were referred to the Leishmaniasis Clinic for suspected CL, had a clinical indication for skin scraping or aspirate, and were able to give verbal informed consent for the diagnostic procedure. We excluded patients with intercurrent bacterial or fungal superinfection of the ulcer and those undergoing active treatment for CL.
Sampling and culture of lesion aspirates.
Skin lesions were cleansed with topical antiseptic and aspirated in duplicate by inserting a 20-gauge needle with a syringe containing 0.6 ml of sterile phosphate-buffered saline with 1,000 U/ml penicillin and 0.3 mg/ml streptomycin into the outer border and base of the lesion and vigorously aspirating tissue fluid as the syringe was rotated. Aspirated fluid was divided evenly in a biosafety cabinet under sterile conditions and inoculated in parallel and duplicate as follows: (i) 250 μl into 16-by-110-mm flat-sided tissue culture tubes (Nalge Nunc International, Rochester, NY) containing either 3.0 ml modified NNN (Novy-MacNeal-Nicolle) medium (blood agar base, DIFCO catalog no. 245400) with 15% defibrinated rabbit blood or 3.0 ml Roswell Park Memorial Institute medium 1640 (RPMI 1640; Invitrogen Corp., Carlsbad, CA) supplemented with l-glutamine, 10% fetal bovine serum, 2 mM NaHCO3 and pH adjusted to 7.3 (10% RPMI) or (ii) 50 μl of a 1:1 mixture of aspirate and 10% RPMI into sterile, nonheparinized 1-by-75-mm capillary tubes (Chase Scientific Glass, Rockwood, TN). For the inoculation of capillary tubes, 200 μl of aspirate was first mixed with 200 μl 10% RPMI in a sterile Eppendorf tube. Following the inoculation, the capillary tubes were sealed with commercially available capillary tube sealant (Fisher Scientific, Ottawa, ON). The remainder of the sample was stored at −20°C for qualitative PCR testing. The cultures were labeled with the patient's unique identifier and the date of collection, incubated vertically at 22 to 26°C under standard atmospheric conditions, and independently examined by two different investigators every 1 to 2 days under an inverted microscope at 200× magnification. The cultures were incubated and examined for 21 days before being considered negative. Positive-control cultures were inoculated as described for study specimens once weekly. Uninoculated negative controls were also set up once weekly.
Smears.
Smears were obtained in order to quantify the amastigote burden in the lesion. After cleansing with topical antiseptic, lesion material was scraped from the ulcer base and border using a sterile lancet and spread on a glass slide. The slides were air dried, fixed in methanol, and stained with Giemsa. The slides were examined under light microscopy, and amastigotes were quantitated according to the method of Chulay and Bryceson (10) (density scoring: grade 0, no amastigotes per high-power field [hpf]; grade 1, 1 to 10 amastigotes per 1,000 hpfs; grade 2, 1 to 10 amastigotes per 100 hpfs; grade 3, 1 to 10 amastigotes per 10 hpfs; grade 4, 1 to 10 amastigotes per hpf; grade 5, 10 to 100 amastigotes per hpf; grade 6, >100 amastigotes per hpf). The lancets were stored at −20°C in 1.5-ml Eppendorf tubes containing 700 μl 100% ethanol for qualitative PCR testing.
Isolation of kinetoplastid DNA (kDNA) from aspirates and lancets.
Prior to DNA extraction, frozen aspirates were incubated at room temperature with 500 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA) for 5 min to remove traces of blood and other contaminants. Subsequently, samples were centrifuged at 8,050 × g for 10 min and the supernatant was discarded. Tissue traces were removed from stored lancets using sterile micropipette tips and transferred to microcentrifuge tubes. The kDNA isolation was performed with the phenol-chloroform-isoamyl alcohol method as previously described (33).
PCR.
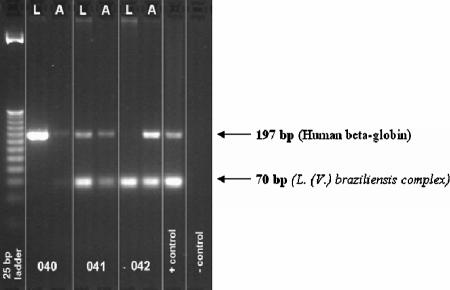
PCR was performed using a HotStar Taq DNA polymerase kit (QIAGEN, Lima, Peru). The final volume of the reaction mixture was 25 μl. PCR conditions were as follows: 95°C for 15 min, followed by 35 cycles of denaturation at 94°C for 30 s, primer annealing at 55°C for 30 s, extension at 72°C for 30 s, and a final extension step at 72°C for 10 min (iCycler iQ; Bio-Rad). Two pairs of primers were employed for this reaction. The first, specific for Leishmania subgenus Viannia, generated a product 70 bp long (23) with the following sequences: (MP1-L [fwd]) 5′-TACTCCCCGACATGCCTCTG-3′ and (MP3-H [rev]) 5′-GAACGGGGTTTCTGTATGC-3′. The sequences of the control primers, which amplify a region of the human beta hemoglobin gene, were (HBBL [fwd]) 5′-GGCAGACTTCTCCTCAGGAGTC-3′ and (HBBR [rev]) 5′-CTTAGACCTCACCCTGTGGAGC-3′. These primers generated a product with a length of 197 bp. PCR products were visualized on 4% agarose gels (Promega, BioMOL, Lima, Peru) and stained with ethidium bromide.
Consensus standard.
We defined a lesion to be due to Leishmania when any two of four tests were positive, where “tests” refers to the leishmanin skin test (which measures delayed-type hypersensitivity reactions to intradermal injection of a suspension of killed promastigotes), lesion smear, culture, and PCR. These four tests served as the consensus or “gold” standard against which each individual diagnostic test was compared.
Sample size calculation.
Based on the results in the existing literature (27, 32, 35-37), we estimated the overall sensitivity of traditional culture methods to be 50% and the mean number of days to positivity for all lesion samples to be 15 ± 10 (mean ± standard deviation) days for traditional culture, versus 4.6 ± 1.5 days for microculture. In order to detect a 50% increase in sensitivity with microculture (sensitivity of 75%) and a significant difference in time to positivity, assuming a distribution phase of 0.05 and a power of 80%, 62 lesions were required per group. For the sensitivity analysis, the aforementioned consensus standard was applied, and the unit of analysis was the lesion. Because multiple, anatomically distinct lesions in a patient's skin result from different bites (with the exception of “sporotrichoid” L. [V.] braziliensis infection), lesions were assumed to not be correlated within patients.
Statistical analysis.
Descriptive statistics (mean, standard deviation, median, and range) were calculated for continuous variables, and differences were compared using one-tailed t-testing or, in the case of ordinally transformed, nonnormally distributed variables, the Mann-Whitney rank sum test. Differences in time to culture positivity were compared between groups by using one-way analysis of variance. Categorical variables were quantitated by proportions, and differences between the groups were compared using Yates' corrected chi-square analysis. Differences in sensitivities were compared using the z-test. Statistical analyses were performed using SigmaStat 2.03 software (SPSS Inc., Chicago, IL). The level of significance was set as a P of <0.05.
RESULTS
Forty-five patients with 62 skin lesions were enrolled in the study; 23 were males and 22 females. The median age was 26 years (range, 7 months to 87 years), and the median duration of exposure in the risk area was 48 months (range, 1 day to 87 years). Work in agriculture and residence in a region of endemicity were the principal risk occupations (31% and 29%, respectively). Students and tourists accounted for only 7% each of the cohort. The median duration of the lesions was 6.7 months (range, 3 weeks to 9 years). Twenty patients (44%) presented with multiple lesions, with a median number of lesions per patient of 1 (range, 1 to 9). Cutaneous and mucosal involvement was present in three study participants. The majority of skin lesions were ulcers (82%), with fewer lesions having a nodular or verrucous presentation (11% and 6%, respectively). The lesions were located primarily on the lower extremity (39%), face (32%), or upper extremity (21%).
With use of the consensus standard (at least two of four tests positive), 53 lesion samples fulfilled the criteria for a diagnosis of CL. Sixty lesion samples were positive by at least one test, 48 were positive by three or more tests, and 24 were positive by all four tests.
Culture. (i) Sensitivity.
Of the 53 lesion samples that were positive by at least two of four diagnostic tests, 39 were culture positive: 38 by microculture, 29 in traditional culture tubes with modified NNN medium, and 19 in traditional culture tubes with 10% RPMI medium. The overall sensitivity of microculture was 71.7%, while traditional culture had a sensitivity of 54.7% with NNN medium (P, 0.038) and 35.8% with 10% RPMI medium (P, <0.001) (Table 1). The sensitivity of the pooled culture methods was 73.6% (Table 1). When the individual patient was used as the unit of analysis, the sensitivities, 73.7%, 55.3%, and 34.2% in microculture, NNN, and 10% RPMI, respectively, did not change appreciably from the per-lesion analysis and remained statistically significant.
TABLE 1.
Analysis of four diagnostic tests used in the evaluation of 62 lesions suspected to be CLa
| Assay | No. of positive samples | No. of negative samples | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| LSTb | 43 | 17 | 84.3 | 100 | 100 | 52.9 |
| Smear | 45 | 17 | 84.9 | 100 | 100 | 52.9 |
| kDNA PCR | 57 | 5 | 94.3 | 22.2 | 87.7 | 40 |
| All cultures | 39 | 23 | 73.6 | 100 | 100 | 39.1 |
| MCM | 38 | 24 | 71.7 | 100 | 100 | 37.5 |
| TCM NNN | 29 | 33 | 54.7 | 100 | 100 | 27.3 |
| TCM RPMI | 19 | 43 | 35.8 | 100 | 100 | 20.9 |
LST, leishmanin skin test; MCM, microculture method; TCM, traditional culture method; NPV, negative predictive value; PPV, positive predictive value.
Two individuals did not undergo leishmanin skin testing.
(ii) Time to positivity.
The mean time to culture positivity was 4.2 ± 0.2 days by microculture (range, 2 to 10 days), 5.2 ± 0.4 days by traditional culture with NNN medium (range, 3 to 14 days), and 6 ± 0.8 days by traditional culture with 10% RPMI (range, 3 to 17 days) (P, 0.009) (Table 2). When the individual patient was used as the unit of analysis, the mean times to culture positivity, 4.4 days, 5.4 days, and 6.9 days in microculture, NNN, and 10% RPMI, respectively, did not change significantly from the per-lesion analysis and remained statistically significant.
TABLE 2.
Culture results based on medium and method
| Culture method | Time to positivity (days)
|
||
|---|---|---|---|
| Mean ± SD | Median | Range | |
| Microculture with 10% RPMI | 4.2a ± 0.2 | 3 | 2-10 |
| Traditional culture with NNN | 5.2 ± 0.4 | 5 | 3-14 |
| Traditional culture with 10% RPMI | 6.0 ± 0.8 | 5 | 3-17 |
P, 0.009 by one-way analysis of variance.
Lesions that were culture positive tended to be younger in age than lesions that were culture negative (4.7 months versus 12.4 months, respectively), though this trend did not achieve statistical significance (P, 0.099). There was no discordance in the results of positive and negative control cultures, nor was there evidence of bacterial contamination in any of the control or study specimens. The agreement between assessors was 100%.
Smears.
Forty-five lesions were positive by Giemsa-stained smear, yielding a sensitivity of 84.9% (Table 1). In those lesions that were smear positive, the mean smear amastigote density was grade 2.4 (median, 2). In those lesions that fulfilled the consensus standard diagnostic criteria, the median smear density was higher in those that were also culture positive (density grade 3) than in those that were culture negative (density grade 1) (P, 0.001).
PCR.
Fifty-seven lesion samples were positive by PCR (Fig. 1), yielding a sensitivity of 94.3% (Table 1). However, only 50 of those positive by PCR fulfilled the consensus standard diagnostic criteria, and so, for 7 lesions, PCR was the only positive test. Compared to the consensus standard, the specificity of PCR was 22% (Table 1).
FIG. 1.
Representative agarose gel stained with ethidium bromide following PCR amplification of a conserved region of kDNA specific to Leishmania subgenus Viannia. L, lancet scraping; A, aspirate.
Leishmanin skin test.
Forty-three lesion samples were from patients with positive leishmanin skin tests, yielding a sensitivity of 84.3% (Table 1). In all patients with a positive leishmanin skin test, at least one other diagnostic test was positive. In the one patient whose final diagnosis was biopsy-confirmed sporotrichosis, the leishmanin skin test was negative.
DISCUSSION
We have demonstrated that the microculture technique offers a sensitive alternative to biphasic, traditional culture systems. Microculture demonstrated a 31% increase in sensitivity compared to traditional culture with NNN medium and a 100% increase in sensitivity compared to traditional culture with 10% RPMI medium. Traditional culture with monophasic 10% RPMI medium had the lowest sensitivity of the three methods compared in this study. RPMI 1640 medium supplemented with 10 to 20% fetal bovine serum has been shown to support amastigote-to-promastigote conversion, though with lower sensitivity than other traditional methods (1, 2, 17), possibly due to the high content of dissolved oxygen in this type of culture system. Microaerophilic conditions and high CO2 levels are permissive to amastigote-to-promastigote transformation (20, 21, 39), hence the success of the microculture system (2).
Traditional cultures of lesion aspirates, scrapings, and biopsy samples using NNN medium, Tobie's medium, Schneider's Drosophila medium, or M199 Leishmania culture medium typically have sensitivities that range from 40 to 75%, though this is somewhat species dependent, with L. (V.) braziliensis constituting one of the more difficult species to culture (32, 37). While our qualitative molecular analyses did not allow for speciation, the positive cultures obtained likely reflect a mixture of L. (V.) braziliensis and the Andean L. (V.) peruviana. Follow-up studies which investigate the species-specific culturability of Leishmania in Peru would be most informative.
In addition to the improved sensitivity, the time to culture positivity was also reduced with microculture, which afforded a 1-day incubation savings over traditional NNN culture and a 2-day incubation savings over traditional RPMI culture. Standard pentavalent antimonial therapy for New World CL is potentially toxic; thus, treatment initiation only follows definitive diagnosis and treatment is not administered empirically. Pentavalent antimonials, including sodium stibogluconate and meglumine antimoniate, have toxic effects on the bone marrow, liver, and heart and can induce cardiac arrhythmias due to prolongation of the QTc interval (8, 16). Early administration of systemic antimonial therapy in L. (V.) braziliensis endemic regions has been associated with a reduced risk of developing mucosal lesions (9, 19, 26). A rapid turnaround time for diagnostic testing in CL is therefore critical to its appropriate management.
Rapid isolation of the causative organism will also facilitate more rapid species identification in centers where these capabilities exist. Species identification in countries like Peru, where multiple species cause CL, will likely become increasingly important due to species variation in response to therapy (11). For instance, sodium stibogluconate results in a higher cure rate of cutaneous lesions caused by L. (V.) braziliensis than of those caused by L. mexicana (28), the former of which is intrinsically more sensitive to the drug (3). Interspecific variability in response to other drugs, such as amphotericin B, miltefosine, pentamidine, paromomycin, and azoles, has been noted among New World species of Leishmania as well (11). Rapid isolation and species identification may therefore inform the therapeutic decision-making process.
In our study, higher smear densities were associated with culture positivity, as has been reported previously (2, 35). Giemsa-stained lesion smear, leishmanin skin testing, and kDNA PCR all proved to have diagnostic sensitivities and specificities on the order of what has been reported previously (7, 15, 25, 31, 37, 40). While positive predictive values were high for all tests, indicating that positive tests are generally good predictors of disease, negative predictive values were poor for all assays, though highest with the leishmanin skin test and direct smear. Seven lesion aspirates from patients with suspected CL were positive only by kDNA PCR and were therefore considered negative, as they failed to fulfill the consensus criteria. While these “false positives” translated into poor specificity of the assay, it is possible that these results actually reflect the outperformance of kDNA PCR compared to the consensus standard in terms of sensitivity. As all seven patients were clinically suspected to have the disease, it is possible that kDNA PCR picked up levels of parasites below the threshold of detection for the other assays. In their comparison of several PCR assays for the diagnosis of CL, Bensoussan et al. similarly found that kDNA PCR had the greatest sensitivity, which led to several specimens being positive only by this test (7). Thus, the seven PCR-positive, consensus-negative lesion samples in our study may actually represent CL. In addition to these seven “false positives,” there were three lesion samples that were PCR negative but consensus standard positive. It is possible that the causative organism in these cases was not a member of the L. (V.) braziliensis complex to which the kDNA PCR primers were specific. Infection caused by either L. (V.) lainsoni or L. (L.) amazonensis, both endemic in Peru, would not have been picked up by the kDNA PCR employed in this study. We had no cases of false-positive leishmanin skin tests, even in the setting of sporotrichosis, which has been associated with false-positive delayed-type hypersensitivity reactions to Leishmania antigen in Latin America (12).
While culture was the least-sensitive diagnostic methodology employed in this study, until such a time occurs that molecular speciation directly from cutaneous lesion aspirates and scrapings is free of current economic and logistical barriers, culture will remain a necessary diagnostic complement to other assays, such as smear and skin testing, both of which are limited by their failure to isolate organisms for further testing. Microculture has been consistently demonstrated to be more sensitive and economical—from a cost and time perspective—than traditional culture techniques used for the diagnosis of Old World species of Leishmania (1, 2, 17, 18). The work reported herein supports the utility of microculture for the isolation of Leishmania parasites from cutaneous lesions in the New World as well.
Acknowledgments
We thank Adil Allahverdiyev for his kind advice during the planning and implementation phases of this study. We also thank Eduardo Gotuzzo of the Instituto de Medicina Tropical Alexander von Humboldt for his logistical support throughout this project.
C.M.-V. contributed to study design and data collection and interpretation and was responsible for enrolling patients. G.T. contributed to study design. D.E. conducted the molecular analyses and contributed to data interpretation and the writing of the manuscript. J.A. and V.A. conducted the molecular analyses and contributed to data interpretation. A.L.-C. and D.E.L. contributed to study design and implementation and data interpretation. A.K.B. was responsible for study design and data collection, analysis, and interpretation and was primarily responsible for writing the manuscript. All authors critically appraised the manuscript.
This study was funded by the Ontario Association of Medical Laboratories.
Footnotes
Published ahead of print on 19 September 2007.
REFERENCES
- 1.Allahverdiyev, A. M., M. Bagirova, S. Uzun, D. Alabaz, N. Aksaray, E. Kocabas, and F. Koksal. 2005. The value of a new microculture method for diagnosis of visceral leishmaniasis by using bone marrow and peripheral blood. Am. J. Trop. Med. Hyg. 73:276-280. [PubMed] [Google Scholar]
- 2.Allahverdiyev, A. M., S. Uzun, M. Bagirova, M. Durdu, and H. R. Memisoglu. 2004. A sensitive new microculture method for diagnosis of cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 70:294-297. [PubMed] [Google Scholar]
- 3.Allen, S., and R. A. Neal. 1989. The in vitro susceptibility of macrophages infected with amastigotes of Leishmania spp. to pentavalent antimonial drugs and other compounds with special relevance to cutaneous isolates, p. 711-720. In D. T. Hart (ed.), Leishmaniasis. Plenum Press, New York, NY.
- 4.Andresen, K., A. Gaafar, A. El-Hasan, A. Ismail, M. Dafalla, T. G. Theander, and A. Kharazmi. 1996. Evaluation of the polymerase chain reaction in the diagnosis of cutaneous leishmaniasis due to Leishmania major. A comparison with direct microscopy of smears and sections from lesions. Trans. R. Soc. Trop. Med. Hyg. 90:133-135. [DOI] [PubMed] [Google Scholar]
- 5.Arevalo, J., L. Ramirez, V. Adaui, M. Zimic, G. Tulliano, C. Miranda- Verastegui, M. Lazo, R. Loayza-Muro, S. D. Doncker, A. Maurer, F. Chappuis, J. C. Dujardin, and A. Llanos-Cuentas. 2007. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J. Infect. Dis. 195:1846-1851. [DOI] [PubMed] [Google Scholar]
- 6.Aviles, H., A. Belli, R. Armijos, F. Monroy, and E. Harris. 1999. PCR detection and identification of Leishmania parasites in clinical specimens in Ecuador: a comparison with direct microscopy of smears and sections from lesions. J. Parasitol. 85:181-187. [PubMed] [Google Scholar]
- 7.Bensoussan, E., A. Nasereddin, F. Jonas, L. F. Schnur, and C. L. Jaffe. 2006. Comparison of PCR assays for diagnosis of cutaneous leishmaniasis. J. Clin. Microbiol. 44:1435-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum, J., P. Desjeux, E. Schwartz, B. Beck, and C. Hatz. 2004. Treatment of cutaneous leishmaniasis among travelers. J. Antimicrob. Chemother. 53:158-166. [DOI] [PubMed] [Google Scholar]
- 9.Blum, J., T. Junghanss, and C. Hatz. 1994. Erroneous tracks in the diagnosis of cutaneous and mucocutaneous leishmaniasis. Schweiz. Rundsch. Med. Prax. 83:1025-1029. [PubMed] [Google Scholar]
- 10.Chulay, J. D., and A. D. Bryceson. 1983. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 32:475-479. [DOI] [PubMed] [Google Scholar]
- 11.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lima Barros, M. B., A. Schubach, A. C. Francesconi-do-Valle, M. C. Gutierrez-Galhardo, T. M. Schubach, F. Conceicao-Siwa, M. de Matos Salgueiro, E. Mouta-Confort, R. S. Reis, M. de Fatima Madeira, T. Cuzzi, L. P. Quintella, J. P. da Silva Passos, M. J. Conceicao, and M. C. de Almeida Marzochi. 2005. Positive Montenegro skin test among patients with sporotrichosis in Rio de Janeiro. Acta Trop. 93:41-47. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira, C. L., A. Bafica, F. Oliveira, C. B. Favali, T. Correa, L. A. Freitas, E. Nascimento, J. M. Costa, and A. Barral. 2003. Clinical utility of polymerase chain reaction-based detection of Leishmania in the diagnosis of American cutaneous leishmaniasis. Clin. Infect. Dis. 37:e149-e153. [DOI] [PubMed] [Google Scholar]
- 14.Desjeux, P. 2004. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 27:305-318. [DOI] [PubMed] [Google Scholar]
- 15.Faber, W. R., L. Oskam, T. Van Gool, N. C. Kroon, K. J. Knegt-Junk, H. Hofwegen, A. C. van der Wal, and P. A. Kager. 2003. Value of diagnostic techniques for cutaneous leishmaniasis. J. Am. Acad. Dermatol. 49:70-74. [DOI] [PubMed] [Google Scholar]
- 16.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 17.Ihalamulla, R. L., U. S. Rajapaksa, and N. D. Karunaweera. 2005. Microculture for the isolation of Leishmania parasites from cutaneous lesions—Sri Lankan experience. Ann. Trop. Med. Parasitol. 99:571-575. [DOI] [PubMed] [Google Scholar]
- 18.Ihalamulla, R. L., U. S. Rajapaksa, and N. D. Karunaweera. 2006. Microculture for the isolation of Leishmania, modified to increase efficacy: a follow-up to a previous study. Ann. Trop. Med. Parasitol. 100:87-89. [DOI] [PubMed] [Google Scholar]
- 19.Jones, T. C., W. D. Johnson, A. C. Barretto, E. Lago, R. Badaro, B. Cerf, S. G. Reed, E. M. Netto, M. S. Tada, and T. F. Franca. 1987. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J. Infect. Dis. 156:73-83. [DOI] [PubMed] [Google Scholar]
- 20.Keegan, F., and J. J. Blum. 1990. Effects of oxygen concentration on the intermediary metabolism of Leishmania major promastigotes. Mol. Biochem. Parasitol. 39:235-245. [DOI] [PubMed] [Google Scholar]
- 21.Keegan, F., and J. J. Blum. 1991. Changes in intracellular levels of fructose 2,6-bisphosphate and several glycolytic intermediates in Leishmania major promastigotes as a function of PO2. Mol. Biochem. Parasitol. 47:161-166. [DOI] [PubMed] [Google Scholar]
- 22.Lightner, L. K., J. D. Chulay, and A. D. Bryceson. 1983. Comparison of microscopy and culture in the detection of Leishmania donovani from splenic aspirates. Am. J. Trop. Med. Hyg. 32:296-299. [DOI] [PubMed] [Google Scholar]
- 23.Lopez, M., C. Orrego, M. Cangalaya, R. Inga, and J. Arevalo. 1993. Diagnosis of Leishmania via the polymerase chain reaction: a simplified procedure for field work. Am. J. Trop. Med. Hyg. 49:348-356. [DOI] [PubMed] [Google Scholar]
- 24.Lucas, C. M., E. D. Franke, M. I. Cachay, A. Tejada, M. E. Cruz, R. D. Kreutzer, D. C. Barker, S. H. McCann, and D. M. Watts. 1998. Geographic distribution and clinical description of leishmaniasis cases in Peru. Am. J. Trop. Med. Hyg. 59:312-317. [DOI] [PubMed] [Google Scholar]
- 25.Manzur, A., and A. Bari. 2006. Sensitivity of leishmanin skin test in patients of acute cutaneous leishmaniasis. Dermatol. Online J. 12:2. [PubMed] [Google Scholar]
- 26.Marsden, P. D. 1986. Mucosal leishmaniasis (“espundia” Escomel, 1911). Trans. R. Soc. Trop. Med. Hyg. 80:859-876. [DOI] [PubMed] [Google Scholar]
- 27.Murray, H. W., J. D. Berman, C. R. Davies, and N. G. Saravia. 2005. Advances in leishmaniasis. Lancet 366:1561-1577. [DOI] [PubMed] [Google Scholar]
- 28.Navin, T. R., B. A. Arana, R. E. Arana, J. D. Berman, and J. F. Chajon. 1992. Placebo-controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J. Infect. Dis. 165:528-534. [DOI] [PubMed] [Google Scholar]
- 29.Navin, T. R., F. E. Arana, A. M. de Merida, B. A. Arana, A. L. Castillo, and D. N. Silvers. 1990. Cutaneous leishmaniasis in Guatemala: comparison of diagnostic methods. Am. J. Trop. Med. Hyg. 42:36-42. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira, J. G., F. O. Novais, C. L. de Oliveira, A. C. da Cruz, Jr., L. F. Campos, A. V. da Rocha, V. Boaventura, A. Noronha, J. M. Costa, and A. Barral. 2005. Polymerase chain reaction (PCR) is highly sensitive for diagnosis of mucosal leishmaniasis. Acta Trop. 94:55-59. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez, J. R., S. Agudelo, C. Muskus, J. F. Alzate, C. Berberich, D. Barker, and I. D. Velez. 2000. Diagnosis of cutaneous leishmaniasis in Colombia: the sampling site within lesions influences the sensitivity of parasitological diagnosis. J. Clin. Microbiol. 38:3768-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero, G. A., M. Vinitius de Farias Guerra, M. Gomes Paes, and V. de Oliviera Macedo. 2001. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: clinical findings and diagnostic approach. Clin. Infect. Dis. 32:1304-1312. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Santrich, C., I. Segura, A. L. Arias, and N. G. Saravia. 1990. Mucosal disease caused by Leishmania braziliensis guyanensis. Am. J. Trop. Med. Hyg. 42:51-55. [DOI] [PubMed] [Google Scholar]
- 35.Singh, S. 2006. New developments in diagnosis of leishmaniasis. Indian J. Med. Res. 123:311-330. [PubMed] [Google Scholar]
- 36.Tavares, C. A., A. P. Fernandes, and M. N. Melo. 2003. Molecular diagnosis of leishmaniasis. Expert Rev. Mol. Diagn. 3:657-667. [DOI] [PubMed] [Google Scholar]
- 37.Tojal da Silva, A. C., E. Cupolillo, A. C. Volpini, R. Almeida, and G. A. Sierra Romero. 2006. Species diversity causing human cutaneous leishmaniasis in Rio Branco, state of Acre, Brazil. Trop. Med. Int. Health 11:1388-1398. [DOI] [PubMed] [Google Scholar]
- 38.Vega-Lopez, F. 2003. Diagnosis of cutaneous leishmaniasis. Curr. Opin. Infect. Dis. 16:97-101. [DOI] [PubMed] [Google Scholar]
- 39.Walsh, M. J., and J. J. Blum. 1992. Effects of hypoxia and acute osmotic stress on intermediary metabolism in Leishmania promastigotes. Mol. Biochem. Parasitol. 50:205-214. [DOI] [PubMed] [Google Scholar]
- 40.Weina, P. J., R. C. Neafie, G. Wortmann, M. Polhemus, and N. E. Aronson. 2004. Old World leishmaniasis; an emerging infection among deployed US military and civilian workers. Clin. Infect. Dis. 39:1674-1680. [DOI] [PubMed] [Google Scholar]