Abstract
We report two cases of suture-related keratitis following penetrating keratoplasty. In both cases, Corynebacterium macginleyi was isolated from corneal specimens. Scanning electron microscopy revealed that corynebacteria could aggregate and form a biofilm. The MICs of sulbenicillin and fluoroquinolones were high for both isolates. Our findings show that C. macginleyi can cause keratitis with biofilm formation.
CASE REPORT
Case 1.
A 74-year-old woman underwent penetrating keratoplasty for a corneal opacity. Postoperatively, she was treated with topical corticosteroids (0.1% dexamethasone) and 0.3% gatifloxacin four times daily, and her recovery was uneventful. Four months later, she visited us with a complaint of blurred vision in her right eye. Slit-lamp biomicroscopy revealed an epithelial defect and a moderate degree of stromal infiltration, along with a loose corneal suture thread. We scraped over the surface of the suppurative area of the cornea and removed the loose corneal suture thread. Direct microscopy and bacterial culture of the corneal scraping were performed. The direct microscopy of the corneal scraping demonstrated the presence of gram-positive rods, and confluent growth of corynebacteria occurred after 48 h of incubation at 37°C in a 5% CO2-enriched atmosphere on Columbia agar plates supplemented with 5% sheep blood (SBA). Colonies were grayish translucent and less than 0.5 mm in diameter. We considered corynebacteria to be the causative agent of the keratitis. We stopped the topical corticosteroids and 0.3% gatifloxacin and started treatment with topical 0.3% tobramycin and 0.5% cefmenoxime every hour. The corneal lesion responded to these agents promptly, and the corneal infiltration healed within 1 week.
Case 2.
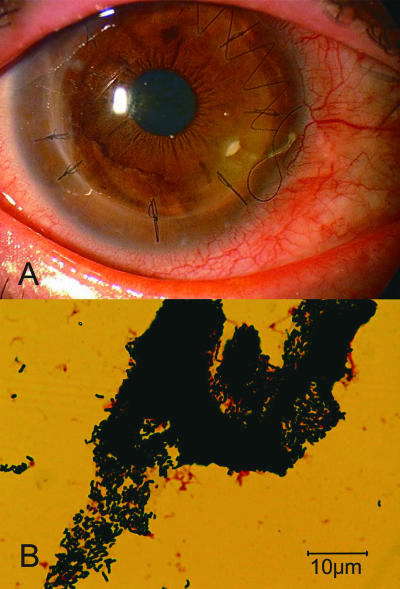
A 49-year-old man underwent penetrating keratoplasty for bullous keratopathy caused by a birth injury. Postoperatively, he was treated with topical corticosteroids (0.1% dexamethasone) and 0.5% levofloxacin four times daily, and his recovery was uneventful. The antibiotic eye drops were stopped 1 year after surgery. When he visited us 3 years after the surgery, slit-lamp biomicroscopy revealed an epithelial defect and a corneal plaque with a loose corneal suture thread (Fig. 1A). We removed the loose corneal suture thread and performed direct microscopy and bacterial culture of the removed corneal plaque. Direct microscopy demonstrated the presence of numerous gram-positive rods (Fig. 1B), and a large number of small colonies (<0.5 mm in diameter after 48 h of incubation) were observed on SBA. We diagnosed keratitis caused by corynebacteria, stopped the topical corticosteroids, and initiated treatment with topical 0.3% tobramycin and 0.3% gatifloxacin every hour. The epithelial defect and corneal plaque disappeared within 1 week.
FIG. 1.
Case 2. (A) Photograph of the cornea showing corneal plaque with the loose suture thread. (B) Photograph of a gram-stained specimen from the corneal scraping showing an aggregation of gram-positive rods.
Bacteriological findings.
The isolates (EC009 in case 1 and EC010 in case 2) were suspected of being lipophilic corynebacteria because small colonies (<0.5 mm in diameter) were found after 48 h of incubation on SBA. In order to identify corynebacteria, biochemical testing and molecular genetic methods were performed. The commercial API Coryne system was used together with the API Coryne database 2.0 (6) according to the instructions of the manufacturer (bioMerieux, Marcy l'Etoile, France). In the API Coryne system, both EC009 and EC010 produced the numerical pattern 5-1-0-0-3-0-5, by which the API Coryne database identified them as Corynebacterium macginleyi with 99.5% probability. Furthermore, the complete 16S rRNA (∼1.5 kb) and the partial rpoB genes were amplified with previously described primers (11, 12). Primers were as follows: for 16S rRNA, 8UA (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1485B (5′-TACGGTTACCTTGTTACGAC-3′), and for rpoB, C2700F (5′-CGWATGAACATYGGBCAGGT-3′) and C3130R (5′-TCCATYTCRCCRAARCGCTG-3′). The DNA sequences were compared to published sequences retrieved from the GenBank database (National Center for Biotechnology Information, National Library of Medicine, Bethesda, MD). CLUSTAL W software, originally described by Thompson et al. (20), was used to align the sequences, calculate percentages of similarity, and construct a phylogenetic tree. Both the 16S rRNA gene sequences obtained from the clinical isolates had 99.2% similarity to sequences from both C. macginleyi CIP 104099 (accession number X80499) and C. accolens CIP 104783 (accession number AJ439346). The rpoB sequences of both isolates showed 97.3 and 91.6% similarity to the sequences from C. macginleyi CIP 104099 (accession number AY492276) and C. accolens CIP 104783 (accession number AY492242), respectively. Our clinical isolates were identified as C. macginleyi by the 16S rRNA and rpoB sequences, along with the result from the API Coryne system.
The MICs of various antimicrobial agents used in ophthalmic solutions for these isolates and the type strain (GTC3120 from the Gifu Type Culture Collection) were determined by the microtiter broth dilution method by following specific guidelines from the Clinical and Laboratory Standards Institute (1). All samples were cultured with Mueller-Hinton medium containing 3% lysed horse blood and incubated at 35°C in an ambient atmosphere for 24 and 48 h. We estimated the MICs for both isolates and the type strain because breakpoints have not been established for corynebacteria (1). The MICs of most antimicrobial agents for the type strain were low, while the MICs of sulbenicillin and fluoroquinolones for the clinical isolates were high (Table 1).
TABLE 1.
Antibiotic susceptibilities of C. macginleyi strains
| Antibiotic | MIC (μg/ml)a for:
|
||
|---|---|---|---|
| EC009b | EC010c | Type straind | |
| Sulbenicillin | 16 | 16 | 4 |
| Cefmenoxime | 0.5 | 0.25 | ≤0.13 |
| Tobramycin | ≤0.13 | ≤0.13 | ≤0.13 |
| Erythromycin | 2 | ≤0.13 | ≤0.13 |
| Vancomycin | 0.5 | 0.5 | 0.5 |
| Ofloxacin | >128 | 128 | 0.25 |
| Norfloxacin | 128 | 16 | 0.5 |
| Ciprofloxacin | 128 | 8 | ≤0.13 |
| Levofloxacin | >128 | 64 | ≤0.13 |
| Gatifloxacin | 32 | 8 | ≤0.13 |
Determined using the broth microdilution method with Mueller-Hinton medium containing 3% lysed horse blood.
EC009 was isolated in case 1.
EC010 was isolated in case 2.
The type strain (GTC3120) was obtained from the Gifu Type Culture Collection.
SEM of the suture threads.
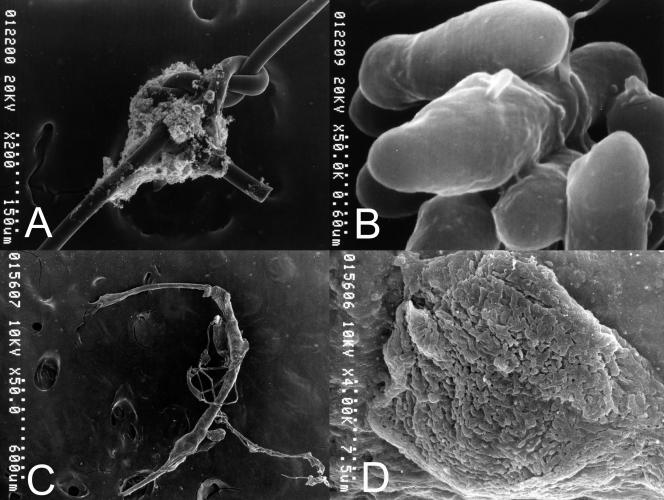
The suture threads removed in cases 1 and 2 were prefixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 2 h, washed with cacodylate buffer, immersed in 1% tannic acid in aqueous solution for 1 h, washed with cacodylate buffer, postfixed with 2% osmium tetroxide in cacodylate buffer for 2 h, washed again with distilled water, dehydrated in a graded ethanol series, and dried using the critical-point drying method. The sutures were observed under a scanning electron microscope (model S-800; Hitachi, Tokyo, Japan). In case 1, the scanning electron microscopy (SEM) findings showed numerous bacilli on the suture knot, with a matrix connecting the bacilli, and the bacilli appeared to form a biofilm on the surface (Fig. 2A and B). In case 2, the SEM findings showed some agents covering the suture, and high magnification confirmed that these agents were a substantial gathering of bacilli (Fig. 2C and D).
FIG. 2.
(A and B) Case 1. (A) Photograph from a scanning electron microscope (taken at low magnification) of the suture showing organisms attached to the suture knot. (B) Photograph from a scanning electron microscope (taken at high magnification) of the suture showing bacilli with the matrix. (C and D) Case 2. (C) Photograph from a scanning electron microscope (taken at low magnification) of the suture showing agents surrounding the suture. (D) Photograph from a scanning electron microscope (taken at high magnification) of the suture showing the aggregation of numerous bacilli.
Corynebacteria, along with Staphylococcus epidermidis and Propionibacterium acnes, constitute the major colonizers of the conjunctival sac, eyelids, and meibomian glands (8). Corynebacteria other than C. diphtheriae seem to have low virulence against the cornea (18). Therefore, corynebacteria are considered microflora if they are isolated in cases of infectious keratitis. However, several studies have found that some strains of corynebacteria cause keratitis (9, 14). C. macginleyi was recently uniquely isolated from the ocular site and found to cause conjunctivitis and endophthalmitis (4, 5, 7, 10). C. macginleyi was first identified in 1995 by Riegel et al. during investigations of lipophilic corynebacteria (17). However, it was not clear whether C. macginleyi could cause keratitis, and the factors contributing to the virulence of C. macginleyi are not well understood. In our cases, corynebacteria were considered causative agents because confluent growth occurred at the site of inoculation on culture plates and the results of the cultures were consistent with direct microscopy findings showing gram-positive pleomorphic rods.
The keratitis in both cases may have been triggered by a loose suture thread adhered to by C. macginleyi. A loose suture thread seems to be a risk factor for microbial keratitis following keratoplasty because organisms easily attach to the suture thread and migrate into the cornea. A previous study also reported that 35% of infectious keratitis cases following keratoplasty were related to sutures (2). Thus, biofilm formation on the suture seems to be one mechanism of pathogenicity in infectious keratitis. In our cases, the SEM findings revealed C. macginleyi strongly attached to the nylon suture, and an extracellular matrix appeared on the surface of the organisms. Furthermore, in case 2, we detected a plaque consisting of an aggregation of C. macginleyi. These facts imply that C. macginleyi cells can form a biofilm and aggregate and thereby cause keratitis. Mihara et al. reported that corynebacteria, which could not be identified to the species level, formed a biofilm on the cornea (16). In previous case reports, C. macginleyi was identified as the pathogen causing infections associated with intravenous and bladder catheters (3, 21), and SEM has demonstrated that corynebacteria can form biofilms on catheters (15). Kwaszewska et al. showed that 75.6% of lipophilic corynebacteria isolated as natural flora from human skin were able to form biofilms (13). Therefore, biofilm formation seems to be a factor contributing to the virulence of corynebacteria, especially C. macginleyi. However, because little is known about the mechanism of biofilm formation by corynebacteria, further investigation is required.
A previous study demonstrated that C. macginleyi isolated in cases of conjunctivitis was sensitive to fluoroquinolones (10). However, the two isolates in our cases had high levels of resistance to the fluoroquinolones levofloxacin and gatifloxacin, which are used in ophthalmology. It is likely that the long-term use of topical fluoroquinolones for prophylaxis against infection led to the appearance of fluoroquinolone-resistant C. macginleyi. Along with topical antibiotics, we applied topical corticosteroids for prophylaxis against corneal rejection. Therefore, steroids can render the cornea immunocompromised and lead to infections. When the mechanism of resistance to fluoroquinolones in corynebacteria was investigated in a previous study, a mutation in the gyrA gene of corynebacteria resulted in high MICs of the fluoroquinolones ciprofloxacin, levofloxacin, and moxifloxacin (19). Therefore, the high concentrations of fluoroquinolones in ophthalmic solutions may create an environment that selects isolates carrying a mutation of gyrA. To prevent the appearance of fluoroquinolone-resistant strains, we should avoid the unnecessary use of ophthalmic solutions containing fluoroquinolones.
In conclusion, we found that C. macginleyi can cause keratitis and that biofilm formation seems to contribute to the virulence of the bacterium. The use of topical fluoroquinolones and steroids may facilitate keratitis caused by C. macginleyi.
Nucleotide sequence accession numbers.
The nucleotide sequences of the 16S rRNA genes of EC009 and EC010 have been given GenBank accession numbers AB354691 and AB354692. The sequences of the rpoB genes of EC009 and EC010 were deposited under accession numbers AB354693 and AB354694.
Acknowledgments
We thank H. Miyamoto and S. Murakami for their technical assistance.
Footnotes
Published ahead of print on 3 October 2007.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2006. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. M-45A. Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PubMed]
- 2.Das, S., M. Constantinou, T. Ong, and H. R. Taylor. 2007. Microbial keratitis following corneal transplantation. Clin. Exp. Ophthalmol. 35:427-431. [DOI] [PubMed] [Google Scholar]
- 3.Dobler, G., and I. Braveny. 2003. Highly resistant Corynebacterium macginleyi as cause of intravenous catheter-related infection. Eur. J. Clin. Microbiol. Infect. Dis. 22:72-73. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer, C., J. M. Ruiz-Moreno, A. Rodriguez, J. Montero, and J. L. Alio. 2004. Postoperative Corynebacterium macginleyi endophthalmitis. J. Cataract Refract. Surg. 30:2441-2444. [DOI] [PubMed] [Google Scholar]
- 5.Funke, G., M. Pagano-Niederer, and W. Bernauer. 1998. Corynebacterium macginleyi has to date been isolated exclusively from conjunctival swabs. J. Clin. Microbiol. 36:3670-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funke, G., F. N. R. Renaud, J. Freney, and P. Riegel. 1997. Multicenter evaluation of the updated and extended API (RAPID) Coryne database 2.0. J. Clin. Microbiol. 35:3122-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giammanco, G. M., V. Di Marco, I. Priolo, A. Intrivici, F. Grimont, and P. A. Grimont. 2002. Corynebacterium macginleyi isolation from conjunctival swab in Italy. Diagn. Microbiol. Infect. Dis. 44:205-207. [DOI] [PubMed] [Google Scholar]
- 8.Hara, J., F. Yasuda, and M. Higashitsutsumi. 1997. Preoperative disinfection of the conjunctival sac in cataract surgery. Ophthalmologica 211:62-67. [DOI] [PubMed] [Google Scholar]
- 9.Heidemann, D. G., S. P. Dunn, J. A. Diskin, and T. B. Aiken. 1991. Corynebacterium striatus keratitis. Cornea 10:81-82. [PubMed] [Google Scholar]
- 10.Joussen, A. M., G. Funke, F. Joussen, and G. Herbertz. 2000. Corynebacterium macginleyi: a conjunctiva specific pathogen. Br. J. Ophthalmol. 84:1420-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khamis, A., D. Raoult, and B. La Scola. 2004. rpoB gene sequencing for identification of Corynebacterium species. J. Clin. Microbiol. 42:3925-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroki, H., H. Miyamoto, K. Fukuda, H. Iihara, Y. Kawamura, M. Ogawa, Y. Wang, T. Ezaki, and H. Taniguchi. 2007. Legionella impletisoli sp. nov. and Legionella yabuuchiae sp. nov., isolated from soils contaminated with industrial wastes in Japan. Syst. Appl. Microbiol. 30:273-279. [DOI] [PubMed] [Google Scholar]
- 13.Kwaszewska, A. K., A. Brewczynska, and E. M. Szewczyk. 2006. Hydrophobicity and biofilm formation of lipophilic skin corynebacteria. Pol. J. Microbiol. 55:189-193. [PubMed] [Google Scholar]
- 14.Li, A., and S. Lal. 2000. Corynebacterium pseudodiphtheriticum keratitis and conjunctivitis: a case report. Clin. Exp. Ophthalmol. 28:60-61. [DOI] [PubMed] [Google Scholar]
- 15.Marrie, T. J., and J. W. Costerton. 1984. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J. Clin. Microbiol. 19:687-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mihara, E., M. Shimizu, C. Touge, and Y. Inoue. 2004. Case of a large, movable bacterial concretion with biofilm formation on the ocular surface. Cornea 23:513-515. [DOI] [PubMed] [Google Scholar]
- 17.Riegel, P., R. Ruimy, D. de Briel, G. Prevost, F. Jehl, R. Christen, and H. Monteil. 1995. Genomic diversity and phylogenetic relationships among lipid-requiring diphtheroids from humans and characterization of Corynebacterium macginleyi sp. nov. Int. J. Syst. Bacteriol. 45:128-133. [DOI] [PubMed] [Google Scholar]
- 18.Rubinfeld, R. S., E. J. Cohen, J. J. Arentsen, and P. R. Laibson. 1989. Diphtheroids as ocular pathogens. Am. J. Ophthalmol. 108:251-254. [DOI] [PubMed] [Google Scholar]
- 19.Sierra, J. M., L. Martinez-Martinez, F. Vazquez, E. Giralt, and J. Vila. 2005. Relationship between mutations in the gyrA gene and quinolone resistance in clinical isolates of Corynebacterium striatum and Corynebacterium amycolatum. Antimicrob. Agents Chemother. 49:1714-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villanueva, J. L., A. Dominguez, M. J. Rios, and C. Iglesias. 2002. Corynebacterium macginleyi isolated from urine in a patient with a permanent bladder catheter. Scand. J. Infect. Dis. 34:699-700. [DOI] [PubMed] [Google Scholar]