Abstract
A highly reproducible and discriminative typing system is essential for better understanding of the epidemiology of Penicillium marneffei, the most important thermal dimorphic fungus causing respiratory, skin, and systemic mycosis in Southeast Asia. The sequences of 11 housekeeping genes were identical among 10 strains of P. marneffei, but those of MP1 and its 13 homologues, a novel superfamily of mannoproteins in the subdivision Pezizomycotina of Ascomycetes, mostly species of Penicillium and Aspergillus, showed significant variations. Therefore, a multilocus sequence typing (MLST) system for P. marneffei was constructed using MP1 (549 bp) and the four of its homologues (MPLP4 [337 bp], MPLP7 [347 bp], MPLP10 [546 bp], and MPLP13 [422 bp]) that showed the greatest variations. Among the 2,201 bp of the five loci, 183 polymorphic sites were observed in 44 strains of P. marneffei. The median number of alleles at each locus was five (range, 5 [MPLP4, MPLP7, and MPLP13] to 15 [MPLP10]). Four of the five genes had nonsynonymous substitution/synonymous substitution (dn/ds) ratios of >1. A total of 35 different sequence types (STs) were assigned to the 44 P. marneffei isolates, with 28 of the 35 STs identified only once. The discriminatory power was 0.9884. MP1 and its homologues were better than housekeeping genes for MLST in P. marneffei. Due to their more rapid evolutionary rates, lineage-specific genes may be better candidates than housekeeping genes for sequence-based typing, especially in microbes that evolve slowly or have evolved recently.
Penicillium marneffei is the most important thermal dimorphic fungus causing respiratory, skin, and systemic mycosis in Southeast Asia (17, 31, 39, 45). P. marneffei was first discovered in Chinese bamboo rats, Rhizomys sinensis. Subsequently, it was also recovered from other species of bamboo rats in the Rhizomyinae subfamily, including hoary bamboo rats (Rhizomys pruinosus), large bamboo rats (Rhizomys sumatrensis), and lesser bay bamboo rats (Cannomys badius) (8, 11). After the discovery of P. marneffei in 1956, only 18 cases of human disease were reported until 1985 (10). The appearance of the human immunodeficiency virus (HIV) pandemic, especially in Southeast Asian countries, saw the emergence of the infection as an important opportunistic mycosis in HIV-positive patients. About 8% of AIDS patients in Hong Kong are infected with P. marneffei (24). In northern Thailand, penicilliosis is the third-most-common indicator disease of AIDS, following tuberculosis and cryptococcosis (31). Besides in HIV-positive patients, P. marneffei infections have been reported in other immunocompromised patients, such as transplant recipients, patients with systemic lupus erythematosus, and patients on corticosteroid therapy (23, 37, 40, 43). Despite its medical importance, the ecology, epidemiology, and mode of transmission of P. marneffei remain largely unknown.
A highly reproducible and discriminative typing system is essential for a better understanding of the epidemiology of P. marneffei. Trewatcharegon et al. have used pulsed-field gel electrophoresis (PFGE) and Fisher et al. have used multilocus microsatellite typing for typing P. marneffei (13, 14, 36). However, due to experimental variations, PFGE patterns are difficult to compare among different laboratories. As multilocus sequence typing (MLST) is well known to be highly reproducible and discriminative for bacteria and has also been used for a number of fungal pathogens, we started to develop such a typing system for P. marneffei 3 years ago. Recently, Lasker has published a nucleotide sequence-based system for typing P. marneffei (21). In this article, we report the difficulty in using housekeeping genes and the subsequent success in using lineage-specific loci—a set of homologous genes belonging to a novel mannoprotein superfamily—for MLST in P. marneffei. The potential application of using genes of higher lineage specificity with accelerated evolutionary rates for typing fungal pathogens is also discussed.
MATERIALS AND METHODS
P. marneffei strains.
Forty-four nonduplicated strains of P. marneffei collected from five hospitals in Hong Kong were used in this study (Table 1). All strains were identified by a combination of cultural and microscopic characteristics. When cultured on Sabouraud dextrose agar at 25°C, the colonies were mycelial in nature, were greenish-yellow in color, and produced a diffusible red pigment. Microscopically, the mycelia bore numerous penicilli, characteristic of other Penicillium species. When cultured at 37°C, yeast-like colonies were formed without pigment production. A single colony of each strain grown on Sabouraud dextrose agar at 37°C was inoculated into yeast-peptone broth and incubated in a shaker at 37°C for three days. Ten microliters of each P. marneffei culture was used for DNA extraction.
TABLE 1.
Characteristics of P. marneffei strains used in the present studya
| Strain | Yr of isolation | Origin
|
Patient
|
ST | BURST group | Allelic profile | ||
|---|---|---|---|---|---|---|---|---|
| Country | Type of clinical specimen | Sex/age (yr) | HIV status | |||||
| PM1 | NA | Hong Kong | Blood | M/2 | − | ST-1 | Singleton | 1,1,1,1,1 |
| PM2 | 1994 | Hong Kong | LN biopsy | M/34 | + | ST-2 | 1 | 2,2,1,1,1 |
| PM3 | 1994 | Hong Kong | Blood | M/51 | + | ST-3 | Singleton | 3,1,2,2,4 |
| PM4 | 1993 | Philippines | Blood | F/30 | − | ST-2 | 1 | 2,2,1,1,1 |
| PM5 | NA | Hong Kong | BAL fluid | M/23 | − | ST-4 | 2 | 3,2,2,12,1 |
| PM6 | NA | Hong Kong | Blood | M/NA | + | ST-5 | 3 | 4,1,2,4,1 |
| PM7 | NA | Hong Kong | Blood | M/NA | + | ST-6 | 2 | 3,2,1,9,1 |
| PM8 | NA | Hong Kong | Blood | M/NA | + | ST-6 | 2 | 3,2,1,9,1 |
| PM9 | NA | Hong Kong | Bone marrow | F/21 | − | ST-7 | 1 | 4,1,1,13,1 |
| PM10 | NA | Hong Kong | Bone marrow | M/3 | − | ST-8 | Singleton | 10,1,1,14,1 |
| PM11 | 1998 | Hong Kong | Pleural fluid | F/65 | − | ST-9 | 1 | 3,1,1,2,1 |
| PM12 | NA | Hong Kong | Blood | NA | NA | ST-1 | Singleton | 1,1,1,1,1 |
| PM13 | 1996 | Hong Kong | Blood | F/45 | − | ST-10 | Singleton | 7,2,2,7,1 |
| PM14 | 1996 | Hong Kong | Blood | M/52 | + | ST-11 | Singleton | 7,2,1,4,4 |
| PM15 | NA | Hong Kong | Blood | NA | NA | ST-7 | 1 | 4,1,1,13,1 |
| PM16 | NA | Hong Kong | Blood | NA | NA | ST-7 | 1 | 4,1,1,13,1 |
| PM17 | 1998 | Hong Kong | Sputum | M/60 | − | ST-1 | Singleton | 1,1,1,1,1 |
| PM18 | 1999 | Thailand | Blood | F/22 | NA | ST-12 | Singleton | 5,3,3,3,1 |
| PM19 | 1999 | Hong Kong | Blood | M/35 | + | ST-13 | Singleton | 2,2,1,2,2 |
| PM20 | 1999 | Thailand | Blood | F/26 | + | ST-14 | 3 | 6,2,2,4,1 |
| PM21 | 1999 | Hong Kong | Blood | M/39 | + | ST-13 | Singleton | 2,2,1,2,2 |
| PM22 | 1999 | Hong Kong | Blood | M/73 | − | ST-15 | 1b | 4,1,1,2,1 |
| PM23 | 2000 | Hong Kong | Blood | M/63 | + | ST-16 | 3 | 6,2,2,1,1 |
| PM24 | 2001 | Hong Kong | Blood | M/39 | + | ST-17 | Singleton | 2,1,2,8,1 |
| PM25 | 2002 | Hong Kong | Blood | M/47 | + | ST-18 | Singleton | 3,2,1,2,3 |
| PM26 | 2003 | Philippines | Blood | F/35 | + | ST-19 | 1 | 4,2,1,2,1 |
| PM27 | 2004 | Hong Kong | Blood | M/25 | + | ST-20 | 1 | 4,2,1,1,1 |
| PM28 | 2004 | Hong Kong | Blood | M/57 | − | ST-21 | Singleton | 3,1,4,9,4 |
| PM29 | 2005 | Hong Kong | Blood | F/45 | + | ST-22 | Singleton | 7,4,1,10,3 |
| PM30 | 2005 | Hong Kong | Blood | M/33 | + | ST-14 | 3 | 6,2,2,4,1 |
| PM31 | NA | Hong Kong | Skin biopsy | F/38 | − | ST-23 | 4 | 3,2,4,7,1 |
| PM32 | 2005 | Hong Kong | Blood | F/40 | + | ST-24 | Singleton | 4,1,1,4,4 |
| PM33 | 2005 | Vietnam | Blood | M/22 | + | ST-25 | Singleton | 8,2,2,5,1 |
| PM34 | 2004 | Hong Kong | Blood | M/48 | + | ST-26 | Singleton | 9,5,2,11,5 |
| PM35 | 2005 | Hong Kong | Blood | M/41 | NA | ST-27 | 2 | 3,2,1,6,1 |
| PM36 | 2005 | Hong Kong | Blood | M/48 | + | ST-28 | 3 | 4,2,2,4,1 |
| PM37 | 2005 | Hong Kong | Blood | F/48 | − | ST-29 | 2b | 3,2,2,6,1 |
| PM38 | 2006 | Hong Kong | Blood | M/46 | + | ST-30 | 2 | 3,2,2,10,1 |
| PM39 | 2006 | Hong Kong | Blood | F/52 | − | ST-31 | Singleton | 11,2,1,4,1 |
| PM40 | 2006 | Hong Kong | Blood | M/37 | + | ST-15 | 1b | 4,1,1,2,1 |
| PM41 | 2006 | Vietnam | Blood | M/30 | + | ST-32 | Singleton | 10,2,2,15,1 |
| PM42 | 2006 | Vietnam | Blood | M/34 | + | ST-33 | 4 | 3,2,4,1,1 |
| PM43 | 2006 | China | Blood | M/35 | + | ST-34 | 3 | 4,1,2,1,1 |
| PM44 | 2006 | Philippines | Blood | F/44 | + | ST-35 | 3 | 6,2,5,4,1 |
NA, not available; LN, lymph node; BAL, bronchoalveolar lavage; M, male; F, female.
Ancestral type in BURST analysis.
DNA extraction.
DNA extraction was performed by using a DNeasy plant mini kit according to the manufacturer's instructions (QIAGEN, Hilden, Germany). The extracted DNA was eluted in 50 μl of AE buffer (QIAGEN, Hilden, Germany), the resultant mixture was diluted 10 times, and 1 μl of the diluted extract was used for PCR.
PCR amplification and sequencing.
In the first part of the study, the extracted DNA of 10 of the 44 strains of P. marneffei was used as the templates for the amplification of 11 housekeeping genes and 14 lineage-specific genes. The 11 housekeeping genes were mannose phosphate isomerase (MPI), plasma membrane H+ ATPase (PM-ATPase), pyruvate kinase (PK), glutamate dehydrogenase (GDH), phosphoglucomutase (PGM), ribonucleoside-diphosphate reductase (rNDP reductase), glutamate synthase precursor (GltS precursor), ribonucleotide reductase (RNR), transcription factor PacC, carbon catabolic repressor protein (CCR), and DNA topoisomerase II (TOP2). MPI and PM-ATPase were used in the MLST scheme of Candida albicans; PK, GDH, and PGM were used in the multilocus enzyme electrophoresis scheme of C. albicans; CCR and TOP2 were known housekeeping genes of Penicillium verruculosum and Talaromyces flavus, respectively; and rNDP reductase, GltS, RNR, and PacC were genes found in our random exploration of the P. marneffei genome project (29, 30, 33, 47). None of these seven genes was the same as the four housekeeping genes chosen for the recently published nucleotide sequence-based system for typing P. marneffei (21). The 14 lineage-specific genes include MP1 and its homologues that belong to a novel mannoprotein superfamily. In the second part of the study, the extracted DNA of all the 44 strains of P. marneffei was used as the templates for the amplification of 5 of the 14 gene loci of the MP1 homologues (MP1, MPLP4, MPLP7, MPLP10, and MPLP13). The primers used for PCR are listed in Table 2. The primers were designed by multiple alignments of the 14 homologues so that they were specific to each MP1 homologue.
TABLE 2.
Primers for amplification and sequencing of 11 housekeeping genes and MP1 homologues in P. marneffei
| Gene product or locus | Primers
|
Amplicon size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| Housekeeping genes | |||
| MPI | LPW1920, 5′-AAYAGCTAYGAYTGGGGTA-3′ | LPW1921, 5′-GGYTTGTGGTTGTCATCTG-3′ | 539 |
| PM-ATPase | LPW1924, 5′-CAGTCCGCCATCACTGGTG-3′ | LPW1925, 5′-CTCACGRATRGAGAGCTGGT-3′ | 516 |
| PK | LPW1949, 5′-ACTCCGTYGAGAARATCAATG-3′ | LPW1950, 5′-ATGTTGCCRTTGTTVAGGCA-3′ | 679 |
| GDH | LPW1951, 5′-CAACTCYGCYCTHGGTCC-3′ | LPW1952, 5′-TTGSWGTCGGARAGYGAGAC-3′ | 660 |
| PGM | LPW1953, 5′-GTCACCAAYAAGATCTACGA-3′ | LPW1954, 5′-TAGTCGTARCGMGTGAAGAA-3′ | 863 |
| rNDP reductase | LPW2018, 5′-ACMTACTCYCTBCTSATTGA-3′ | LPW2019, 5′-TTCTCVAASAAGTTSGTCT-3′ | 620 |
| GltS precursor | LPW2020, 5′-CACGTKATCAACTTCTTCTAC-3′ | LPW2021, 5′-ACCRCGGAAGWAGCAGGTA-3′ | 630 |
| RNR | LPW2076, 5′-GAGACMTACTCYCTBCTSAT-3′ | LPW2077, 5′-CACCRGCCTTCTGGTAGT-3′ | 652 |
| PacC | LPW2083, 5′-GCHGATGAYTCGGTSCT-3′ | LPW2084, 5′-TCATTGGDGGDARATGRTA-3′ | 572 |
| CCR | LPW2154, 5′-TGCWCRAARCGBTTCAGTCG-3′ | LPW2155, 5′-GAGTCGTGRGAGAADGTAGG-3′ | 575 |
| TOP2 | LPW2156, 5′-AYGGTTWCGGTGCBAAGC-3′ | LPW2157, 5′-ATYTGRTCNGCRATGTAGTT-3′ | 523 |
| MP1 homologues | |||
| MP1 | LPW2562, 5′-CGTTAATCAACATGAAGTTC-3′ | LPW4800, 5′-GCTCAATATCAACATTAAACT-3′ | 657 |
| MPLP4 | LPW4801, 5′-GACAAAATTGACCGCAGT-3′ | LPW4802, 5′-TCCTTGATATACCGATCAAA-3′ | 398 |
| MPLP7 | LPW4803, 5′-GATTCAGAAATTCCGACG-3′ | LPW2736, 5′-GCTCTAAGCTGCACCAGTG-3′ | 415 |
| MPLP10 | LPW4804, 5′-CGTAGGGTTTTTGCGAAT-3′ | LPW4805, 5′-TGTCGTCCAACTATCACAAA-3′ | 613 |
| MPLP13 | LPW3510, 5′-AAGCAACCTACAGCACCTTA-3′ | LPW3512, 5′-TCGAAATCTATAGAAGCTATC-3′ | 491 |
The PCR mixture (100 μl) contained denatured P. marneffei DNA, PCR buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl, 2 mM MgCl2, and 0.01% gelatin), 200 μM of each deoxynucleoside triphosphate, and 2.5 U Taq polymerase (Perkin-Elmer Cetus, Norwalk, CT). The sample was amplified in 40 cycles of 95°C for 1 min, 50°C for 1 min, and 72°C for 1 min with a final extension at 72°C for 10 min in an automated thermal cycler (Perkin-Elmer Cetus, Gouda, The Netherlands). Ten microliters of each amplified product was electrophoresed in 2% (wt/vol) agarose gel with a molecular size marker (φX174 HaeIII digest; Fermentas, Canada) in parallel. Electrophoresis in Tris-borate-EDTA buffer was performed at 100 V for 1 h. The gel was stained with ethidium bromide (0.5 μg/ml) for 15 min, rinsed, and photographed under UV light illumination.
The PCR product was gel purified using a QIAquick PCR purification kit (QIAGEN, Hilden, Germany). Both strands of the PCR product were sequenced with an ABI prism 3700 DNA analyzer according to the manufacturer's instructions (Applied Biosystems, Foster City, CA), using the PCR primers.
Allele and ST assignment.
The nucleotide sequences of the five gene loci used for MLST in all the P. marneffei strains were aligned and compared with those of strain PM1 using ClustalX 1.83 (35). An arbitrary number was assigned to each distinct allele in a locus. Therefore, five numbers, representing the sequence type (ST), were given to each strain. Each ST was numbered in the order of identification (ST-1, ST-2, etc.).
Sequence analysis.
The proportions of nucleotide alterations that led to a change in the amino acid sequence (nonsynonymous substitution, dn) and the proportions of nucleotide alterations that did not lead to a change in the amino acid sequence (synonymous substitution, ds) were calculated with START2 (http://www.mlst.net). Construction of the dendrogram was performed with the unweighted-pair group method with arithmetic mean using MEGA 3.1, excluding insertions and deletions (20). The grouping of STs into lineages was performed with BURST. The members of a BURST lineage were defined as groups of two or more independent isolates where each had alleles at four or more loci that were identical with at least one other member of the group.
Nucleotide sequence accession numbers.
The sequences of the MP1, MPLP4, MPLP7, MPLP10, and MPLP13 genes of 44 P. marneffei isolates have been deposited in GenBank (accession no. EU002558 to EU002777).
RESULTS
Sequencing of 11 housekeeping genes in P. marneffei.
Amplification and sequencing of the 11 housekeeping genes (MPI, PM-ATPase, PK, GDH, PGM, rNDP reductase, GltS precursor, RNR, PacC, CCR, and TOP2) from 10 strains of P. marneffei showed that the nucleotide sequences of all 11 genes were identical among the 10 strains of P. marneffei.
Sequencing of gene loci of MP1 homologues in P. marneffei.
Single bands of the expected sizes were observed for each MP1 homologue amplified using the specific primers. In the first 10 strains of P. marneffei sequenced, the sequences of 4 (MPLP2, MPLP3, MPLP6, and MPLP12) of the 14 homologues were identical, but the other 10 (MP1, MPLP1, MPLP4, MPLP5, MPLP7, MPLP8, MPLP9, MPLP10, MPLP11, and MPLP13) showed variations. Among these 10 loci, 5 (MP1, MPLP4, MPLP7, MPLP10, and MPLP13) showed more variations than the others (data not shown). Therefore, only these five loci were sequenced for the other 34 strains of P. marneffei, and the sequences of these five loci were used for developing the MLST system.
Variations at the five MLST loci.
Among the 2,201 bp of the five loci, a total of 183 polymorphic sites were observed in the 44 strains of P. marneffei (Fig. 1). Allelic profiles were assigned to the 44 strains of P. marneffei (Table 1). The alleles defined for the MLST system were based on sequence lengths of between 337 bp (MPLP4) and 549 bp (MP1) (Table 3). The median number of alleles at each locus was 5 (range, 5 [MPLP4, MPLP7, and MPLP13] to 15 [MPLP10]).
FIG. 1.
Polymorphic nucleotide sites in P. marneffei MLST genes. Only the variable sites are shown. Sites that are the same as genotype 1 are shown by dots, and gaps are shown by dashes. The numbers of isolates with the same genotype are indicated in parentheses. Numbers shown vertically represent polymorphic nucleotide positions in the GenBank sequences (accession no. for MP1, EU002602; for MPLP4, EU002646; for MPLP7, EU002690; for MPLP10, EU002734; and for MPLP13, EU002558) of the five genes in strain PM1.
TABLE 3.
Characteristics of the five loci included in the P. marneffei MLST system
| Locus | Size of sequenced fragment (bp) | No. of alleles identified | % of polymorphic nucleotide sites | % G+C | dn/ds |
|---|---|---|---|---|---|
| MP1 | 549 | 11 | 23.9 | 51.3 | 0.6482 |
| MPLP4 | 337 | 5 | 1.8 | 43.3 | dn = 0.0100, ds = 0 |
| MPLP7 | 347 | 5 | 1.2 | 42.7 | dn = 0.0056, ds = 0 |
| MPLP10 | 546 | 15 | 7.0 | 42.4 | 2.4773 |
| MPLP13 | 422 | 5 | 1.0 | 44.6 | 1.3243 |
In the MP1 gene locus, the insertion of two bases (GT) at positions between 255 and 256 resulted in a premature stop codon (TAA) in the MP1 gene in genotype 11 (Fig. 1). For the 21-bp tandem repeat (positions 499 to 519 in strain PM1), 1 and 23 of the 44 P. marneffei isolates possessed three and two copies of the repeat, respectively, whereas 20 isolates had one copy of the repeat only (Fig. 1). In the MPLP4 and MPLP7 loci, single nucleotide substitutions, at positions 230 (G→A) and 196 (C→T), respectively, resulted in premature stop codons (TAG and TAA, respectively) in genotype 4 (Fig. 1). In the MPLP13 locus, a single nucleotide substitution at position 160 (G→T) in genotype 3 resulted in a premature stop codon (TAA), whereas deletions at positions 237 and 238 in genotype 4 resulted in frameshift mutation and early truncation of the protein 46 nucleotides downstream from the deletions (Fig. 1). In the MPLP10 locus, none of the mutations resulted in premature stop codons.
The dn/ds ratios for the five gene loci are shown in Table 3. The relatively high dn/ds ratios for the five genes (four of the five genes have dn/ds ratios of >1) indicate that a strong positive selective pressure is present at these loci.
Relatedness of P. marneffei isolates.
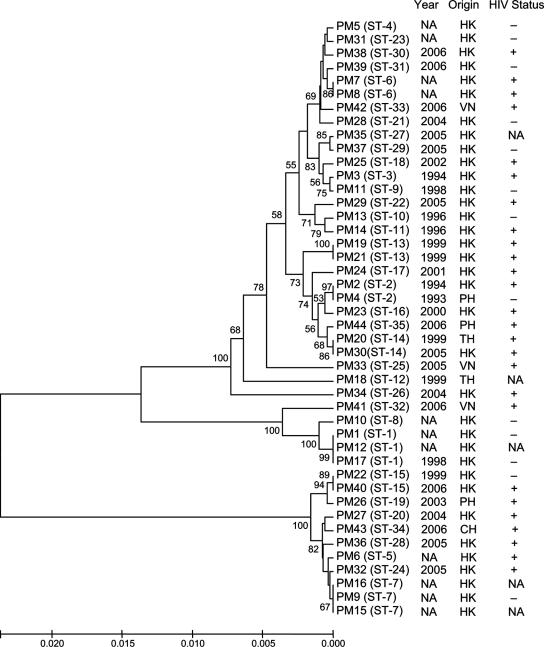
A total of 35 different STs were assigned to the 44 P. marneffei isolates, with 28 of the 35 STs identified only once (Table 1). The most common STs (ST-1 and ST-7) were identified three times in the present data set, followed by ST-2, ST-6, ST-13, ST-14, and ST-15 (two isolates each). The overall discriminatory power for the 44 isolates was 0.9884 and that for the 36 isolates from Chinese patients was 0.9857. The unweighted-pair group method with arithmetic mean was used to construct a dendrogram using the concatenated nucleotide sequences of the five gene loci of the 44 isolates (Fig. 2). BURST grouped the isolates into four lineages, with 6 STs in group 1, 5 STs in group 2, 6 STs in group 3, and 2 STs in group 4, whereas 16 STs did not belong to any of the four groups (Table 1). No relationships were observed among the 44 P. marneffei isolates, their years of isolation, or the countries of origin or HIV status of the patients (Table 1 and Fig. 2).
FIG. 2.
Phylogenetic tree showing the relationships of the 35 STs of P. marneffei in this study. The tree was inferred from concatenated nucleotide sequence data of the five MLST gene loci by the Kimura two-parameter method, and bootstrap values calculated from 1,000 trees. The analysis included 2,201 nucleotide positions in each concatenated nucleotide sequence. Only bootstrap values of >40 are shown. The scale bar shows the genetic distance estimated using the Kimura two-parameter substitution model. NA, not available; HK, Hong Kong; VN, Vietnam; PH, Philippines; TH, Thailand; CH, mainland China.
DISCUSSION
Traditionally, typing of pathogenic bacteria and fungi was carried out using various phenotypic and genotypic methods. Before the invention of MLST, PFGE of restriction enzyme-digested genomic DNA was the most widely used method because of its high discriminatory power. However, due to interlaboratory variations, the results obtained using PFGE from different laboratories cannot be easily compared. Therefore, comparison of bacterial and fungal strains from different localities cannot be performed easily, unless all strains are sent to the same laboratory for PFGE. Since the invention of MLST in 1998, this technique has been confirmed to be highly reproducible and objective, and it can be performed easily by different laboratories to type strains collected in different localities (26). In the past 10 years, MLST has been used widely for the typing of pathogenic bacteria by sequencing multiple housekeeping genes (25). For fungi, MLST has also been used for the typing of various yeasts, molds, and dimorphic fungi (1, 2, 3, 12, 16, 18, 19, 21, 22, 27, 30, 32, 33). However, in general, it has been found that MLST is less discriminative for fungi than for bacteria.
The sequences of MP1 homologues are more variable than those of housekeeping genes in P. marneffei. In 1998, we reported the cloning and characterization of MP1, which encodes an abundant, secreted, and cell wall immunogenic mannoprotein, Mp1p, from P. marneffei (5). Subsequently, we have documented that Mp1p-based enzyme-linked immunosorbent assays for antibody and antigen detection are very useful for serodiagnosis of active P. marneffei infections, and an MP1-based DNA vaccine produced a protective immune response against P. marneffei challenge in a mouse model (6, 7, 38). Recently, we discovered that genes with higher lineage specificity in Ascomycetes evolved at a much faster rate than those with lower lineage specificity (4). Therefore, genes with higher lineage specificity are potentially more useful targets than housekeeping genes for typing pathogenic fungi. MP1 homologues have so far only been found in the subdivision Pezizomycotina of Ascomycetes, mostly in species of Penicillium and Aspergillus (5, 9, 41, 46). Furthermore, Mp1p is a cell wall immunogenic protein located on the surface of P. marneffei and, hence, is subject to strong selective pressure by the immune system (5). Therefore, MP1 homologues are potentially more rapidly evolving than housekeeping genes. Recently, from the data acquired in our complete genome sequence project (42, 44), we discovered that there are more than 10 MP1 homologues in the P. marneffei genome (unpublished data). The characterization of these loci will be reported elsewhere. Therefore, we hypothesized that it would be more discriminatory if this set of gene targets were used for MLST in P. marneffei. To test this hypothesis, we sequenced 11 housekeeping genes, some of which have been used for targets of MLST in other fungal pathogens, and the MP1 homologues of P. marneffei. The results showed that the sequences of the 11 housekeeping genes were identical among the 10 strains of P. marneffei sequenced but that remarkable variations exist in the sequences of the MP1 homologues. Therefore, the MP1 homologues were used as targets for building the MLST system in P. marneffei.
Using two times the number of human isolates, we found that the present MLST system appears to be more discriminative than another MLST system recently published for molecular typing of P. marneffei (21). In the recently published article on nucleotide sequence-based analysis of P. marneffei isolates, nine gene loci were sequenced (21). Among these nine loci, five showed sequence variations. When these five loci (2,089 bp) were used for building the MLST system, four showed minimal variations. These four loci were housekeeping genes. The only locus that showed significant variation was MP1, which is in line with the results observed in the present study, that only the MP1 homologues, but not the 11 housekeeping genes, showed significant variation. In the present study, the region of the MP1 gene used was from nucleotide 57 to 626, whereas in the other study (21), the region used was from nucleotide 508 to 957, using strain PM4 as the reference. This is probably the explanation of the higher variation in the MP1 gene in the present study. The high sequence variation in 5 of the 14 MP1 homologues is probably because they are evolving rapidly, as shown by their relatively high dn/ds ratios (Table 3). These resulted in the relatively high discriminatory power in the present MLST system (0.988) compared to that in the other one (0.939) (21). In fact, with MP1 eliminated from the two MLST systems, the discriminatory power of the present MLST system is still 0.982, whereas that of the other MLST system dropped to 0.747, suggesting that the incorporation of MP1 or its homologues is important in setting up an MLST system with good discriminatory power for P. marneffei. When the same P. marneffei strain was subcultured 10 times, no difference was observed between the sequences of the five gene loci in the original strain and in the strain after 10 subcultures (data not shown). Therefore, these five loci are discriminative enough for typing but not evolving so rapidly as to mask genetic relatedness. Interestingly, a discriminatory power of only 0.8 was achieved by the MLST system of the other study when only isolates from Thailand were included (21). When only strains of Chinese patients were analyzed, the present study is only slightly more discriminatory, by 0.0221 (0.9857 versus 0.9636), than the other study. The apparent difference in the overall discriminatory powers of the MLST systems in the present study and the previous one could be due to a genuine difference in their discriminatory powers or to the difference in the collections of strains. Further studies using more isolates from other countries are required to determine whether the discriminatory power of the present MLST system is universally high for P. marneffei isolates of different origins.
Lineage-specific genes may be better candidates than housekeeping genes for sequence-based typing, especially in microbes that evolve slowly or have evolved recently. When MLST was first designed as a sequence-based typing method for bacteria, housekeeping genes were used as the targets for amplification and sequencing (26). Subsequently, most researchers who set up MLST systems for other bacteria followed this tradition. However, when MLST systems were used in fungal pathogens, it was observed that the discriminatory power was often not satisfactory when only housekeeping genes were used (1, 27, 34). On the other hand, when one or two nonhousekeeping genes were also included as targets, the discriminatory power was improved (19, 21). In fact, the same phenomenon was also observed in bacterial pathogens, and it has been shown that the same discriminatory power can be achieved by using fewer targets, hence decreasing the labor and consumable costs considerably, if nonhousekeeping genes are used (15, 28). This is in line with our recent observation that lineage-specific genes were associated with more rapid evolutionary rates (4). Hence, more mutations would be expected in these genes than in housekeeping genes, which are not lineage specific, between different strains. In P. marneffei, the small amount of variation in its housekeeping genes could be because of its slow evolutionary rate, because it has evolved as a new species recently, or both. Therefore, lineage-specific genes may be better targets for sequence-based typing systems, such as MLST, for slowly evolving or recently evolved pathogens.
Acknowledgments
This work was partly supported by a research fund for the control of infectious diseases of the Health, Welfare, and Food Bureau of the Hong Kong SAR government and research grant council grant (HKU7793/07 M), the university development fund, an outstanding young researcher award, an HKU special research achievement award, and a Croucher senior medical research fellowship, The University of Hong Kong.
Footnotes
Published ahead of print on 19 September 2007.
REFERENCES
- 1.Ayoub, M. J., J. L. Legras, R. Saliba, and C. Gaillardin. 2006. Application of multi locus sequence typing to the analysis of the biodiversity of indigenous Saccharomyces cerevisiae wine yeasts from Lebanon. J. Appl. Microbiol. 100:699-711. [DOI] [PubMed] [Google Scholar]
- 2.Balajee, S. A., J. L. Gribskov, E. Hanley, D. Nickle, and K. A. Marr. 2005. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot. Cell 4:625-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bougnoux, M. E., S. Morand, and C. d'Enfert. 2002. Usefulness of multilocus sequence typing for characterization of clinical isolates of Candida albicans. J. Clin. Microbiol. 40:1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai, J. J., P. C. Woo, S. K. Lau, D. K. Smith, and K. Y. Yuen. 2006. Accelerated evolutionary rate may be responsible for the emergence of lineage-specific genes in ascomycota. J. Mol. Evol. 63:1-11. [DOI] [PubMed] [Google Scholar]
- 5.Cao, L., C. M. Chan, C. Lee, S. S. Wong, and K. Y. Yuen. 1998. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect. Immun. 66:966-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, L., D. L. Chen, C. Lee, C. M. Chan, K. M. Chan, N. Vanittanakom, D. N. Tsang, and K. Y. Yuen. 1998. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. J. Clin. Microbiol. 36:3028-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao, L., K. M. Chan, D. Chen, N. Vanittanakom, C. Lee, C. M. Chan, T. Sirisanthana, D. N. Tsang, and K. Y. Yuen. 1999. Detection of cell wall mannoprotein Mp1p in culture supernatants of Penicillium marneffei and in sera of penicilliosis patients. J. Clin. Microbiol. 37:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chariyalertsak, S., P. Vanittanakom, K. E. Nelson, T. Sirisanthana, and N. Vanittanakom. 1996. Rhizomys sumatrensis and Cannomys badius, new natural animal hosts of Penicillium marneffei. J. Med. Vet. Mycol. 34:105-110. [PubMed] [Google Scholar]
- 9.Chong, K. T., P. C. Woo, S. K. Lau, Y. Huang, and K. Y. Yuen. 2004. AFMP2 encodes a novel immunogenic protein of the antigenic mannoprotein superfamily in Aspergillus fumigatus. J. Clin. Microbiol. 42:2287-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng, Z. L., and D. H. Connor. 1985. Progressive disseminated penicilliosis caused by Penicillium marneffei. Report of eight cases and differentiation of the causative organism from Histoplasma capsulatum. Am. J. Clin. Pathol. 84:323-327. [DOI] [PubMed] [Google Scholar]
- 11.Deng, Z. L., M. Yun, and L. Ajello. 1986. Human penicilliosis marneffei and its relation to the bamboo rat (Rhizomys pruinosus). J. Med. Vet. Mycol. 24:383-389. [DOI] [PubMed] [Google Scholar]
- 12.Dodgson, A. R., C. Pujol, D. W. Denning, D. R. Soll, and A. J. Fox. 2003. Multilocus sequence typing of Candida glabrata reveals geographically enriched clades. J. Clin. Microbiol. 41:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher, M. C., D. Aanensen, S. de Hoog, and N. Vanittanakom. 2004. Multilocus microsatellite typing system for Penicillium marneffei reveals spatially structured populations. J. Clin. Microbiol. 42:5065-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher, M. C., W. P. Hanage, S. de Hoog, E. Johnson, M. D. Smith, N. J. White, and N. Vanittanakom. 2005. Low effective dispersal of asexual genotypes in heterogeneous landscapes by the endemic pathogen Penicillium marneffei. PLoS Pathog. 1:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley, S. L., D. G. White, P. F. McDermott, R. D. Walker, B. Rhodes, P. J. Fedorka-Cray, S. Simjee, and S. Zhao. 2006. Comparison of subtyping methods for differentiating Salmonella enterica serovar Typhimurium isolates obtained from food animal sources. J. Clin. Microbiol. 44:3569-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geiser, D. M., J. I. Pitt, and J. W. Taylor. 1998. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc. Natl. Acad. Sci. USA 95:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsueh, P. R., L. J. Teng, C. C. Hung, J. H. Hsu, P. C. Yang, S. W. Ho, and K. T. Luh. 2000. Molecular evidence for strain dissemination of Penicillium marneffei: an emerging pathogen in Taiwan. J. Infect. Dis. 181:1706-1712. [DOI] [PubMed] [Google Scholar]
- 18.Kasuga, T., J. W. Taylor, and T. J. White. 1999. Phylogenetic relationships of varieties and geographical groups of the human pathogenic fungus Histoplasma capsulatum Darling. J. Clin. Microbiol. 37:653-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koufopanou, V., A. Burt, and J. W. Taylor. 1997. Concordance of gene genealogies reveals reproductive isolation in the pathogenic fungus Coccidioides immitis. Proc. Natl. Acad. Sci. USA 94:5478-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 21.Lasker, B. A. 2006. Nucleotide sequence-based analysis for determining the molecular epidemiology of Penicillium marneffei. J. Clin. Microbiol. 44:3145-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litvintseva, A. P., R. Thakur, R. Vilgalys, and T. G. Mitchell. 2006. Multilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics 172:2223-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo, C. Y., D. T. Chan, K. Y. Yuen, F. K. Li, and K. P. Cheng. 1995. Penicillium marneffei infection in a patient with SLE. Lupus 4:229-231. [DOI] [PubMed] [Google Scholar]
- 24.Low, K., and S. S. Lee. 2002. The pattern of AIDS reporting and the implications on HIV surveillance. Public Health Epidemiol. Bull. 11:41-49. [Google Scholar]
- 25.Maiden, M. C. 2006. Multilocus sequence typing of bacteria. Annu. Rev. Microbiol. 60:561-588. [DOI] [PubMed] [Google Scholar]
- 26.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morehouse, E. A., T. Y. James, A. R. Ganley, R. Vilgalys, L. Berger, P. J. Murphy, and J. E. Longcore. 2003. Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol. Ecol. 12:395-403. [DOI] [PubMed] [Google Scholar]
- 28.Nemoy, L. L., M. Kotetishvili, J. Tigno, A. Keefer-Norris, A. D. Harris, E. N. Perencevich, J. A. Johnson, D. Torpey, A. Sulakvelidze, J. G. Morris, Jr., and O. C. Stine. 2005. Multilocus sequence typing versus pulsed-field gel electrophoresis for characterization of extended-spectrum β-lactamase-producing Escherichia coli isolates. J. Clin. Microbiol. 43:1776-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pujol, C., J. Reynes, F. Renaud, M. Raymond, M. Tibayrenc, F. J. Ayala, F. Janbon, M. Mallie, and J. M. Bastide. 1993. The yeast Candida albicans has a clonal mode of reproduction in a population of infected human immunodeficiency virus-positive patients. Proc. Natl. Acad. Sci. USA 90:9456-9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robles, J. C., L. Koreen, S. Park, and D. S. Perlin. 2004. Multilocus sequence typing is a reliable alternative method to DNA fingerprinting for discriminating among strains of Candida albicans. J. Clin. Microbiol. 42:2480-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Supparatpinyo, K., C. Khamwan, V. Baosoung, K. E. Nelson, and T. Sirisanthana. 1994. Disseminated Penicillium marneffei infection in southeast Asia. Lancet 344:110-113. [DOI] [PubMed] [Google Scholar]
- 32.Tavanti, A., A. D. Davidson, E. M. Johnson, M. C. Maiden, D. J. Shaw, N. A. Gow, and F. C. Odds. 2005. Multilocus sequence typing for differentiation of strains of Candida tropicalis. J. Clin. Microbiol. 43:5593-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavanti, A., N. A. Gow, S. Senesi, M. C. Maiden, and F. C. Odds. 2003. Optimization and validation of multilocus sequence typing for Candida albicans. J. Clin. Microbiol. 41:3765-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor, J. W., and M. C. Fisher. 2003. Fungal multilocus sequence typing—it's not just for bacteria. Curr. Opin. Microbiol. 6:351-356. [DOI] [PubMed] [Google Scholar]
- 35.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trewatcharegon, S., S. Sirisinha, A. Romsai, B. Eampokalap, R. Teanpaisan, and S. C. Chaiyaroj. 2001. Molecular typing of Penicillium marneffei isolates from Thailand by NotI macrorestriction and pulsed-field gel electrophoresis. J. Clin. Microbiol. 39:4544-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, J. L., C. C. Hung, S. C. Chang, S. C. Chueh, and M. K. La. 2003. Disseminated Penicillium marneffei infection in a renal-transplant recipient successfully treated with liposomal amphotericin B. Transplantation 76:1136-1137. [DOI] [PubMed] [Google Scholar]
- 38.Wong, L. P., P. C. Woo, A. Y. Wu, and K. Y. Yuen. 2002. DNA immunization using a secreted cell wall antigen Mp1p is protective against Penicillium marneffei infection. Vaccine 20:2878-2886. [DOI] [PubMed] [Google Scholar]
- 39.Wong, S. S., H. Siau, and K. Y. Yuen. 1999. Penicilliosis marneffei—West meets East. J. Med. Microbiol. 48:973-975. [DOI] [PubMed] [Google Scholar]
- 40.Wong, S. S., P. C. Woo, and K. Y. Yuen. 2001. Candida tropicalis and Penicillium marneffei mixed fungaemia in a patient with Waldenstrom's macroglobulinaemia. Eur. J. Clin. Microbiol. Infect. Dis. 20:132-135. [DOI] [PubMed] [Google Scholar]
- 41.Woo, P. C., K. T. Chong, A. S. Leung, S. S. Wong, S. K. Lau, and K. Y. Yuen. 2003. AFLMP1 encodes an antigenic cell wall protein in Aspergillus flavus. J. Clin. Microbiol. 41:845-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo, P. C., H. Zhen, J. J. Cai, J. Yu, S. K. Lau, J. Wang, J. L. Teng, S. S. Wong, R. H. Tse, R. Chen, H. Yang, B. Liu, and K. Y. Yuen. 2003. The mitochondrial genome of the thermal dimorphic fungus Penicillium marneffei is more closely related to those of molds than yeasts. FEBS Lett. 555:469-477. [DOI] [PubMed] [Google Scholar]
- 43.Woo, P. C., S. K. Lau, C. C. Lau, K. T. Chong, W. T. Hui, S. S. Wong, and K. Y. Yuen. 2005. Penicillium marneffei fungaemia in an allogeneic bone marrow transplant recipient. Bone Marrow Transplant. 35:831-833. [DOI] [PubMed] [Google Scholar]
- 44.Woo, P. C., K. T. Chong, H. Tse, J. J. Cai, C. C. Lau, A. C. Zhou, S. K. Lau, and K. Y. Yuen. 2006. Genomic and experimental evidence for a potential sexual cycle in the pathogenic thermal dimorphic fungus Penicillium marneffei. FEBS Lett. 580:3409-3416. [DOI] [PubMed] [Google Scholar]
- 45.Yuen, K. Y., S. S. Wong, D. N. Tsang, and P. Y. Chau. 1994. Serodiagnosis of Penicillium marneffei infection. Lancet 344:444-445. [DOI] [PubMed] [Google Scholar]
- 46.Yuen, K. Y., C. M. Chan, K. M. Chan, P. C. Woo, X. Y. Che, A. S. Leung, and L. Cao. 2001. Characterization of AFMP1: a novel target for serodiagnosis of aspergillosis. J. Clin. Microbiol. 39:3830-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuen, K. Y., G. Pascal, S. S. Wong, P. Glaser, P. C. Woo, F. Kunst, J. J. Cai, E. Y. Cheung, C. Medigue, and A. Danchin. 2003. Exploring the Penicillium marneffei genome. Arch. Microbiol. 179:339-353. [DOI] [PubMed] [Google Scholar]