Abstract
Prostate tissues from patients with prostate cancer and benign prostatic hyperplasia (BPH) frequently contain histological inflammation, and a proportion of these patients show evidence of Propionibacterium acnes infection in the prostate gland. We developed a multicolor fluorescent in situ hybridization (FISH) assay targeting P. acnes 23S rRNA along with a 14-kb region of the P. acnes genome. This assay was used to analyze prostate tissues from patients with prostate cancer and BPH. P. acnes infection of the prostate gland was demonstrated in prostatic tissue in 5 of 10 randomly selected prostate cancer patients. FISH analysis and confocal laser microscopy imaging revealed intracellular localization and stromal biofilm-like aggregates as common forms of P. acnes infection in prostate tissues from both prostate cancer and BPH patients. A sequential analysis of prostate tissue from individual patients suggested that P. acnes can persist for up to 6 years in the prostate gland. These results indicate that P. acnes can establish a persistent infection in the prostate gland. Further study is needed to clarify the link between this bacterium and prostatic inflammation which may contribute to the development of BPH and prostate cancer.
Clinical benign prostatic hyperplasia (BPH) occurs in half of all men by the age of 80 years (20), while prostate cancer is a leading cause of morbidity and death among men in the United States and Western Europe (13). Chronic or recurrent inflammation has been implicated in the development and progression of both BPH (20) and prostate cancer (9, 24, 25, 30), and various degrees of histological inflammation are seen in the majority of prostate tissue specimens examined. The possibility of an infectious etiology for this prostatic inflammation has been raised in the context of both BPH (20) and prostate cancer, although the accumulation of a larger amount of evidence supports this hypothesis for the latter. For example, epidemiological studies have reported an increased risk of prostate cancer in patients with a history of sexually transmitted infections (29, 31, 32). The genetic signatures of bacteria were found in prostate tissues from 21 of 107 patients with prostate cancer (21). Moreover, a positive correlation between prostatic inflammation and the detection of bacterial 16S rRNA gene sequences in tissue specimens obtained by radical prostatectomy has been reported (16).
Recently, a single bacterial species, Propionibacterium acnes, has been shown to infect a considerable proportion (35%) of prostate glands removed at radical prostatectomy (6). Moreover, the presence of P. acnes was strongly correlated with histological inflammation, suggesting that this bacterium might be linked to cancer development. The possible involvement of P. acnes in prostate pathology was further highlighted by the detection of P. acnes 16S rRNA gene sequences in BPH tissues, with a positive association between the detection of P. acnes DNA and subsequent prostate cancer development reported (1). We have developed a multicolor fluorescent in situ hybridization (FISH) assay in order to localize P. acnes in the prostate tissue of patients with prostate cancer and BPH and to search for evidence of persistent or recurrent P. acnes infection in the prostate gland.
MATERIALS AND METHODS
Prostate tissue samples.
FISH analysis was performed with fixed, paraffin-embedded specimens obtained from patients with prostate cancer (radical prostatectomy or needle biopsy samples) or BPH (tissues obtained during transurethral resection of the prostate [TURP]). The assay was optimized by using the following positive control tissues: 4 specimens obtained by radical prostatectomy previously shown to be culture positive for P. acnes (6) and 10 tissue specimens obtained by TURP, previously shown to be positive for P. acnes 16S rRNA gene sequences, selected from patients who had subsequently been diagnosed with prostate cancer (1). We then analyzed 10 specimens obtained by prostatectomy randomly selected from patients who underwent surgery at the University Hospital of Northern Sweden, Umeå, Sweden, in 2006. All radical prostatectomy patients but one (including the four culture-positive controls) had presented with elevated prostate-specific antigen levels (greater than 4 ng/ml) and had cancer diagnosed by needle biopsy, and none had acute urinary retention or clinical symptoms of bacterial prostatitis. Finally, to search for evidence of persistent or recurrent P. acnes infection in the prostate gland, we identified 10 patients with a prostate tissue sample positive for P. acnes by FISH and a second sample available (taken between 3 months and 10 years prior to or subsequent to the time that the positive specimen was taken). The 10 pairs of matched samples analyzed were obtained by prostatectomy with a preceding needle biopsy (three specimens) or by TURP with follow-up specimens obtained by prostatectomy (two specimens) or needle biopsy (five specimens). When several specimens obtained by TURP or biopsy were available, the first one obtained chronologically was selected.
Multicolor FISH assay.
FISH analysis was performed with one whole-mount tissue section from each prostatic lobe (left and right) for positive control specimens obtained by prostatectomy, one whole-mount tissue section from a single lobe for randomly selected specimens obtained by prostatectomy, and a total tissue area of approximately 2 cm2 for samples obtained by TURP. The tissue sections were dewaxed in xylene, rehydrated in ethanol series, and then enzymatically treated as described previously (35), with minor modifications. The probes used in this study were as follows: a 6-carboxyfluorescein (FAM)-tagged eubacterial EUB338 probe, a Cy3-tagged nonsense EUB338 probe (2), and a Cy3.5-tagged probe specific for P. acnes 23S rRNA (5′GAGTGTGTGAACCGATCATGTAGTAGGCAA3′; designed for this study). The probes were obtained from DNA Technology A/S, Århus, Denmark. In addition, four probes (size range, 5 to 6 kb) overlapping a 14,313-bp segment of the P. acnes genome were generated as follows: P. acnes (isolate CCUG 41530, type 1A) was grown in brain heart infusion broth and the bacterial DNA was purified with a QIAamp DNA blood mini kit (QIAGEN, Hilden, Germany). A set of four primer pairs based on the published genome sequence of P. acnes strain KPA171202 (5) was designed by using the web-based PCR Suite program (Erasmus University, Rotterdam, The Netherlands; http://www2.eur.nl/fgg/kgen/primer/). The primer pairs were primers 1L (CCAAAGACAATTCTGGGAAGATTACAG; positions 320829 to 320855) and 1R (ATACAACTTCGGCCTTTACACTACAGC; positions 326085 to 326059), primers 2L (AAAATGTGAAGTGTGAGGTGATTCTGC; positions 325533 to 325559) and 2R (ATTAAGGTGGAATGGTTCTACTGGTTG; positions 331266 to 331240), primers 3L (ATAATGAGGATGGTGCAGACCAGTAG; positions 330317 to 330342) and 3R (GATGGAGGAAATCACATATAGCGAGAC; positions 335700 to 335674), and primers 4L (GAGTGGATCTTCTTCGTCAACGTC; positions 335119 to 335142) and 4R (AGAACTCGTCCTTCTTCAACTTCCAAC; positions 341090 to 341064). Four cDNA fragments were synthesized by using Elongase enzyme mix (Invitrogen) and were subsequently cloned by using a Topo XL PCR cloning kit (Invitrogen). Plasmids were isolated by use of the QIAGEN midi kit. The libraries were directly labeled with diethylaminocoumarin-dUTP (DEC) (Perkin-Elmer, Boston, MA) by using a nick translation kit (Vysis Inc., Des Plaines, IL). The probe cocktail, which contained 100 to 120 ng of each probe, was resuspended in 10 μl of buffer (50% formamide, 2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 10% dextran sulfate, 0.1% sodium dodecyl sulfate, 1× Denhardt's solution, 40 mM sodium phosphate, pH 7) and was incubated on the slide at 75°C for 5 min, followed by overnight incubation at 37°C. After the slides were washed with 2% SSC containing 0.3% Nonidet P-40 (Biochem), the slides were stained with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma, Israel). The slides were then mounted with antifade solution (Citi-fluor, England) and read with an Axioplan 2 imaging microscope (Carl Zeiss). For selected samples, confocal laser scanning microscopic imaging was performed with a Leica DM RXE microscope attached to a TCS SP2 AOBS confocal system and high-resolution objectives (Leica Microsystems, Exton, PA). Stacks of images with an image size of 512 by 512 pixels were obtained, and a step size of 0.40 μm was performed.
In vitro study.
P. acnes (prostate isolate, type 1B [6]) was cultivated at 37°C in brain heart infusion broth supplemented with 5% horse serum in tubes without agitation, thus creating a microaerobic environment. A cell monolayer of normal prostate epithelial cell line RWPE-1 (ATCC CRL 11609) was infected with P. acnes (ratio, 1:50), and after 24 h of infection, the cells were examined microscopically.
RESULTS
Optimization of P. acnes-specific FISH.
The FISH methodology can be used to detect a pathogen within a tissue without destroying the tissue morphology. The major obstacles encountered when FISH is applied to the detection of P. acnes are due to the low sensitivity of this bacterium to lysozyme (38) and proteinase K (O. Alexeyev, unpublished data). These are the enzymes commonly used to make bacterial walls penetrable for probes. We found that use of achromopeptidase, an enzyme known to be lytic for gram-positive anaerobes (11), helped circumvent the problem. The specificity of the P. acnes-specific 23S rRNA binding and the specificities of the four overlapping long probes were established as follows: staining was detectable in a smear of cultured P. acnes and in tissue samples from the four specimens that were obtained by radical prostatectomy and that were previously shown to be culture positive for P. acnes (6) (Fig. 1); but no signal was detected in a blood smear, an Escherichia coli culture smear, or breast cancer tissue or brain tissue specimens. As a control for specificity during screening of the prostate tissues, only signals which were positive with all three probes (the probe for 23S rRNA, the long probes, and the EUB338 probe) were considered positive for P. acnes. It is interesting to note that all positive signals seen in prostate tissue met this criterion. No signal was observed with the nonsense EUB338 probe in any sample.
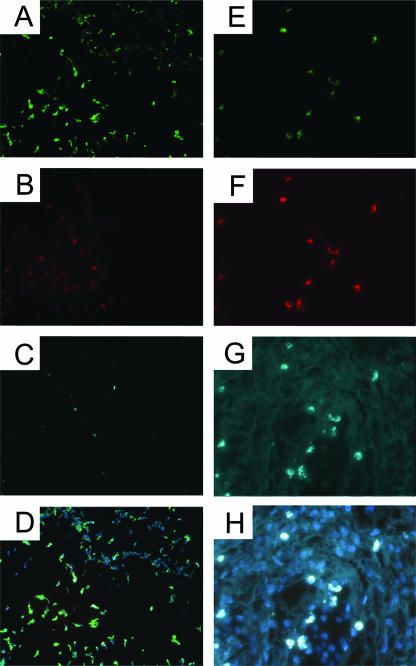
FIG. 1.
Specificity of FISH for P. acnes. The positive controls for FISH were a smear of cultured P. acnes (A to D) and P. acnes culture-positive prostate tissue (E to H). The panels show the results obtained with the FAM-labeled eubacterial EUB338 probe (A and E), the Cy3.5-labeled P. acnes-specific 23S rRNA-binding probe (B and F), DEC-labeled P. acnes specific long probes (C and G), and an overlay of all three probes plus DAPI staining (D and H). P. acnes cells are seen as intracellular forms in the tissue. Magnifications, ×400.
Forms of P. acnes infection in prostate tissue.
Two major forms of P. acnes infection could be demonstrated in prostate tissues: intracellular bacteria (most likely within macrophages) and dense stromal bacterial aggregates which were incompatible with intracellular forms in both size and shape (Fig. 2). The intracellular forms accounted for approximately 80% of the positive signals, while the remainder occurred as aggregates or, occasionally, as individual colonies. The intracellular and aggregate forms of infection were confirmed by confocal laser scanning microscopic image analysis (Fig. 3), which showed that the aggregates consisted of organized, evenly spaced signals apparently embedded within a diffusely stained matrix, which may be suggestive of biofilm formation. The possibility of biofilm-based infection was supported by the results of the in vitro study, where a type IB prostatic P. acnes isolate formed biofilm-like conglomerates attached to the cocultured cells of a prostate epithelial cell line (Fig. 3F).
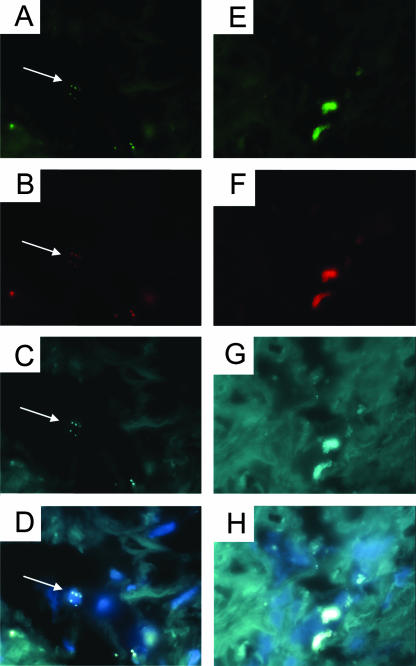
FIG. 2.
FISH images showing intracellular (A to D) and biofilm-like aggregate (E to H) forms of P. acnes infection in prostate tissue. The panels show the results obtained with the FAM-labeled eubacterial EUB338 probe (A and E), the Cy3.5-labeled P. acnes-specific 23S rRNA-binding probe (B and F), DEC-labeled P. acnes-specific long probes (C and G), and an overlay of all three probes plus DAPI staining (D and H). Arrows show the positions of intracellular bacteria. Magnifications, ×1,000.
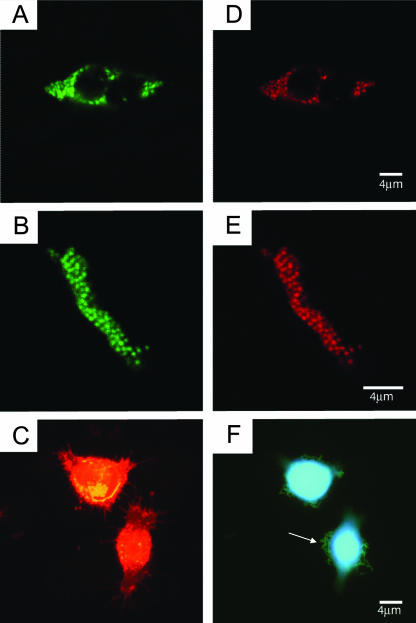
FIG. 3.
Confocal laser scanning microscopic images confirming the intracellular (A and D) and biofilm-like aggregate (B and E) forms of P. acnes infection in prostate tissue. The panels show the results obtained with the FAM-labeled eubacterial EUB338 probe (A and B) and the Cy3.5-labeled P. acnes-specific 23S rRNA-binding probe (D and E). (C and F) Attachment of a P. acnes prostatic isolate (arrow) to the surface of cultured prostate epithelial cells and the formation of biofilm-like conglomerates; (C) eukaryotic membranes stained with wheat germ agglutinin conjugated with Texas Red (Invitrogen); (F) DAPI staining of bacterial and eukaryotic DNA.
Distribution of P. acnes in prostate tissue samples.
FISH analysis confirmed the presence of P. acnes in the majority of positive control prostate tissues which were previously shown to contain P. acnes by either culture (6) or PCR (1) and also in half of the randomly selected samples obtained by radical prostatectomy for which the P. acnes infection status had not previously been determined (Table 1). While intracellular bacteria were seen in all positive samples, bacterial aggregates were detected in only a few cases (Table 1). Quantitative analysis was performed for a limited subset of each group, and the results showed that between 3 and >50 positive signals could be detected per prostate tissue specimen (Table 1). Statistical analysis was not performed due to the small numbers of cases analyzed.
TABLE 1.
Detection of P. acnes by FISH in positive control prostate tissues and randomly selected specimens from prostate cancer patients obtained by radical prostatectomy
| FISH results | No. of cases/total no. of cases tested (%)a
|
||
|---|---|---|---|
| Control prostate tissues positive for:
|
Randomly selected prostatectomy cases in which P. acnes status was unknown (n = 10) | ||
| P. acnes 16S rRNA geneb (n = 10) | P. acnes by culturec (n = 4) | ||
| Positive P. acnes signals | 7/10 (70) | 4/4 (100) | 5/10 (50) |
| Bacterial aggregates | 2/7 (29) | 0/4 (0) | 2/5 (40) |
| Intracellular bacteria | 7/7 (100) | 4/4 (100) | 5/5 (100) |
The area analyzed was approximately 2 cm2 of tissue (for positive control samples obtained by TURP) and one whole-mount tissue section of a single prostatic lobe (for specimens obtained by prostatectomy). The quantitative results were as follows: for control prostate tissues positive for the P. acnes 16S rRNA gene, five samples showed 3, 6, 7, 11, and 16 signals, respectively; for control prostate tissues positive for P. acnes by culture four samples showed 3, 10, 11, and 21 signals, respectively; and for randomly selected prostatectomy cases in which P. acnes status was unknown, two samples showed 8 and >50 signals, respectively.
Samples obtained by TURP previously shown to be PCR positive for P. acnes 16S rRNA gene (1).
Samples obtained by radical prostatectomy previously shown to be culture positive for P. acnes (6).
Analysis of the whole-mount sections obtained by radical prostatectomy showed that P. acnes was focally distributed and could be seen with equal frequency in the peripheral or transition and central zones of the prostate. Approximately 90% of the positive signals were detected in the stroma, most commonly in regions devoid of cancer (Fig. 4). Positive signals in the epithelial layer or ductal lumens were rare and mainly appeared as individual colonies. While intracellular bacteria were commonly found within clusters of inflammatory cells (Fig. 4), we seldom observed inflammatory cells in the vicinity of bacterial aggregates (data not shown). Analysis of patient details showed that preoperative prostate-specific antigen levels were slightly higher in the nine P. acnes-positive prostate cancer patients (median, 7.8 ng/ml; range, 4.3 to 40 ng/ml) than in the five P. acnes-negative patients (median, 5.5 ng/ml; range, 3.9 to 22 ng/ml) (P = 0.68; unpaired t test). The median Gleason scores were almost similar in these two groups (7 and 6.5, respectively).
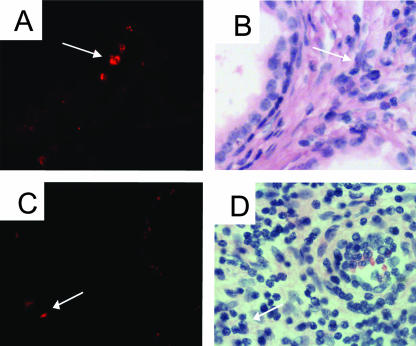
FIG. 4.
Tissue localization of P. acnes in the stroma of samples obtained by prostatectomy. The panels show the results obtained by sequential FISH analysis (A and C) and hematoxylin-eosin staining (B and D) of prostate tissue. The FISH signal coordinates were recorded by using a Mikropositioner glass (Berliner Glas KG), and after probe hybridization the sections were demounted and stained with hematoxylin-eosin. Prominent signals are seen in the vicinity of stromal infiltrating lymphocytes (arrows). Magnifications, ×400.
Detection of P. acnes in sequential prostate samples.
For 10 patients with a prostate sample positive for P. acnes by FISH, a second prostate sample was available for analysis. This second sample was also positive for P. acnes in 3 (30%) of the 10 cases (Table 2). These matched pairs of P. acnes-positive samples included a specimen obtained by needle biopsy 3 months prior to a radical prostatectomy and two specimens obtained by radical prostatectomy removed 4 years and 6 years, respectively, after TURP procedures (Table 2). Both the intracellular and stromal bacterial aggregates of P. acnes were seen in the tissues obtained by TURP, while the sample obtained by biopsy showed only the stromal intracellular form. These results are evidence of persistent or recurrent P. acnes infection of the prostate gland in two cases involving BPH patients who progressed to a diagnosis of prostate cancer many years later. P. acnes was detected in only one of the eight needle biopsy series analyzed, and even in that positive case only one of the eight biopsy needle cores contained a positive signal (Table 2), indicating a low detection rate in biopsy tissues, consistent with their small volume. In addition, a total of eight preceding biopsy cores corresponding to four prostatectomy specimens negative for P. acnes by FISH were also analyzed, and all of these were negative (data not shown).
TABLE 2.
Detection of P. acnes by FISH in sequential prostate tissue samples taken from individual patients
| Method by which first specimen was obtaineda,b | FISH results for first specimen | Method by which second specimen was obtaineda | FISH results for second specimen | Time between sampling |
|---|---|---|---|---|
| Needle biopsy | + (1/8)c | Prostatectomyd | + | 3 mo |
| Needle biopsy | − (0/8) | Prostatectomyd | + | 11 mo |
| Needle biopsy | − (0/6) | Prostatectomy | + | 2 yr |
| TURP | + | Prostatectomy | + | 4 yr |
| TURP | + | Prostatectomy | + | 6 yr |
| TURP | + | Needle biopsy | − (0/3) | 7 yr |
| TURP | + | Needle biopsy | − (0/3) | 8 yr |
| TURP | + | Needle biopsy | − (0/2) | 7 yr |
| TURP | + | Needle biopsy | − (0/1) | 10 yr |
| TURP | + | Needle biopsy | − (0/2) | 5 yr |
All specimens obtained by needle biopsy and radical prostatectomy had a diagnosis of prostate cancer.
All specimens obtained by TURP had a diagnosis of BPH only and were PCR positive for the P. acnes 16S rRNA gene (1).
Plus signs, positive results; minus signs, negative results. The values in parentheses are the number of needle core biopsy specimens positive/number of needle core biopsy specimens tested.
Culture positive for P. acnes (6).
DISCUSSION
Several factors hamper efforts to detect P. acnes in human tissues. P. acnes is difficult to detect in vivo by Gram stain (10) and is extremely slowly growing, requiring extended culture under anaerobic conditions (6). Furthermore, detection of P. acnes in prostate tissue by culture or PCR cannot distinguish between tissue-invasive infection, colonization of prostatic ducts, and contamination with skin bacteria during surgical procedures or tissue processing. In this study we report on the development of a multicolor FISH assay for the detection and localization of P. acnes in prostate tissue. A cocktail of five P. acnes-specific probes was used to visualize the bacterial genome in the tissue. To increase the specificity of the assay we used large overlapping probes covering approximately 14 kb of the P. acnes genome. A similar approach based on large probes (2 kb) was recently successful in detecting a novel gamma retrovirus in human prostate cancer tissues (37).
In our study, 5 (50%) of 10 randomly selected patients with prostate cancer were shown to harbor P. acnes in their prostatic stromal tissue. The rate of detection by FISH was higher than the 35% previously reported for P. acnes by culture (6), which may be attributed to a higher sensitivity of FISH and also the larger proportion of tissue examined by this method. The demonstration of P. acnes in BPH tissues by FISH is in accord with the results of previous reports on the isolation of P. acnes from prostate tissue of patients with idiopathic prostatic inflammation as well as patients with BPH (4, 23, 27, 34, 36). However, it is beyond the scope of the present study to draw conclusions on causal associations between infection with P. acnes and the development of BPH or prostate cancer, due to the small number of patient samples analyzed and our inability to obtain control prostate tissue from healthy men without prostate disease for comparison. This work was designed as a small, semiquantitative pilot study to evaluate our assay for its ability to detect P. acnes in prostate tissue and to search for evidence of genuine infection; in this respect, our results do confirm tissue-invasive infection of the prostate gland rather than ductal colonization in the positive cases analyzed.
In addition, two separate lines of evidence point to the possibility that P. acnes can establish persistent infection in the prostate gland. First, we have detected P. acnes in sequential prostate samples from individual patients taken up to 6 years apart. Second, the major form of P. acnes infection in the prostate gland was stromal intracellular bacteria, which is more consistent with persistent rather than acute infection. The localization of P. acnes in macrophages has been described in both experimentally infected animals and patients with sarcoidosis (15, 22, 39). P. acnes displays low sensitivity to the bactericidal and degradative functions of human monocytes (38) and may therefore persist intracellularly.
Sessile bacterial growth, as exemplified by a biofilm, represents another microbial strategy used to establish chronic infection (7). Biofilms adherent to epithelial or artificial surfaces represent the most common form, but intracellular (3) and stromal (8, 12) localizations have also been reported. Biofilms attached to ductal walls have been demonstrated in patients with prostatitis (26), but to the best of our knowledge, a stromal biofilm has never been demonstrated in prostate tissue. Given the organized nature of the bacterial aggregates observed in this study and their envelopment in diffusely staining material suggestive of a secreted matrix, it is tempting to speculate that P. acnes may establish biofilm forms in prostate tissue. The ability of P. acnes (prostate isolate, type 1B) to form biofilm-like aggregates on cultured prostate epithelial cells further supports the possibility of biofilm formation in the pathogenesis of P. acnes infection in the prostate gland. It is noteworthy that scant inflammatory cells were seen in the vicinity of stromal P. acnes aggregates in prostatic tissue, which is similar to the relative lack of an inflammatory response reported against biofilms, where the extracellular matrix is thought to mask bacterial antigens and protect the sessile bacterial communities (7). Whether P. acnes may indeed form tissue biofilms requires further study, for example, to show the expression of genes linked to the production of extracellular matrix components.
Given the high rate of isolation of P. acnes from the male urinary tract (33), an ascending mechanism for prostate invasion seems logical. Bladder catheterization, prostate biopsy, and TURP procedures may also introduce P. acnes into the prostate gland. Our findings of stromal intracellular P. acnes in the initial biopsy material from one patient, along with both intracellular and biofilm-like stromal forms in the initial specimens obtained by TURP, suggests that the infection was already well established in the prostate tissue when these procedures were performed and supports a biological rather than iatrogenic route of infection, at least in some patients.
P. acnes is recognized for its capacity to activate and induce proinflammatory cytokine production by cells of the mononuclear phagocyte system (17). It likely plays a contributory role in the high level of prostatic inflammation associated with positive cultures for P. acnes in prostate cancer patients (6), and we suggest that it may also be involved in the inflammation associated with the development and progression of BPH. For example, P. acnes is known to induce Toll-like receptor 4 (18) and interleukin 15 (28), both of which are up-regulated in BPH (14, 19).
In conclusion, our results provide direct confirmation of P. acnes infection in the prostate glands of patients with BPH and prostate cancer. Our findings of P. acnes in both intracellular locations and biofilm-like aggregates within the stroma, along with its detection in sequential samples taken up to 6 years apart, suggest infection of a persistent nature. Further study is needed to clarify the link between this bacterium and prostatic inflammation, which may lead to a better understanding and the better management of common prostate diseases.
Acknowledgments
We thank the Kempe Foundation, the Lion's Cancer Research Foundation, the Capio Research Foundation, the Magnus Bergvall Foundation, and the Medical Faculty, Umeå University, for financial support. Satu Koskiniemi at Laboratory Medicine/Clinical Bacteriology, University Hospital of Northern Sweden, is greatly acknowledged for help with the bacterial strains.
Footnotes
Published ahead of print on 19 September 2007.
REFERENCES
- 1.Alexeyev, O., J. Bergh, I. Marklund, C. Thellenberg-Karlsson, F. Wiklund, H. Gronberg, A. Bergh, and F. Elgh. 2006. Association between the presence of bacterial 16S RNA in prostate specimens taken during transurethral resection of prostate and subsequent risk of prostate cancer (Sweden). Cancer Causes Control 17:1127-1133. [DOI] [PubMed] [Google Scholar]
- 2.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 4.Berger, R. E., J. N. Krieger, I. Rothman, C. H. Muller, and S. L. Hillier. 1997. Bacteria in the prostate tissue of men with idiopathic prostatic inflammation. J. Urol. 157:863-865. [PubMed] [Google Scholar]
- 5.Bruggemann, H., A. Henne, F. Hoster, H. Liesegang, A. Wiezer, A. Strittmatter, S. Hujer, P. Durre, and G. Gottschalk. 2004. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science 305:671-673. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, R. J., B. A. Shannon, J. E. McNeal, T. Shannon, and K. L. Garrett. 2005. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J. Urol. 173:1969-1974. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Costerton, W., R. Veeh, M. Shirtliff, M. Pasmore, C. Post, and G. Ehrlich. 2003. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 112:1466-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Marzo, A. M., V. L. Marchi, J. I. Epstein, and W. G. Nelson. 1999. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am. J. Pathol. 155:1985-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteban, J., G. Garcia-Calvo, P. Jimenez-Castillo, and F. Soriano. 1996. Failure of Gram stain to detect Propionibacterium acnes in specimens from clinically significant infections. J. Clin. Microbiol. 34:2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezaki, T., and S. Suzuki. 1982. Achromopeptidase for lysis of anaerobic gram-positive cocci. J. Clin. Microbiol. 16:844-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulcher, T. P., J. K. Dart, L. McLaughlin-Borlace, R. Howes, M. Matheson, and I. Cree. 2001. Demonstration of biofilm in infectious crystalline keratopathy using ruthenium red and electron microscopy. Ophthalmology 108:1088-1092. [DOI] [PubMed] [Google Scholar]
- 13.Gronberg, H. 2003. Prostate cancer epidemiology. Lancet 361:859-864. [DOI] [PubMed] [Google Scholar]
- 14.Handisurya, A., G. E. Steiner, U. Stix, R. C. Ecker, S. Pfaffeneder-Mantai, D. Langer, G. Kramer, N. Memaran-Dadgar, and M. Marberger. 2001. Differential expression of interleukin-15, a pro-inflammatory cytokine and T-cell growth factor, and its receptor in human prostate. Prostate 49:251-262. [DOI] [PubMed] [Google Scholar]
- 15.Hiramatsu, J., M. Kataoka, Y. Nakata, K. Okazaki, S. Tada, M. Tanimoto, and Y. Eishi. 2003. Propionibacterium acnes DNA detected in bronchoalveolar lavage cells from patients with sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 20:197-203. [PubMed] [Google Scholar]
- 16.Hochreiter, W. W., J. L. Duncan, and A. J. Schaeffer. 2000. Evaluation of the bacterial flora of the prostate using a 16S rRNA gene based polymerase chain reaction. J. Urol. 163:127-130. [PubMed] [Google Scholar]
- 17.Ingham, E. 1999. The immunology of Propionibacterium acnes and acne. Curr. Opin. Infect. Dis. 12:191-197. [DOI] [PubMed] [Google Scholar]
- 18.Jugeau, S., I. Tenaud, A. C. Knol, V. Jarrousse, G. Quereux, A. Khammari, and B. Dreno. 2005. Induction of Toll-like receptors by Propionibacterium acnes. Br. J. Dermatol. 153:1105-1113. [DOI] [PubMed] [Google Scholar]
- 19.Konig, J. E., T. Senge, E. P. Allhoff, and W. Konig. 2004. Analysis of the inflammatory network in benign prostate hyperplasia and prostate cancer. Prostate 58:121-129. [DOI] [PubMed] [Google Scholar]
- 20.Kramer, G., and M. Marberger. 2006. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr. Opin. Urol. 16:25-29. [PubMed] [Google Scholar]
- 21.Krieger, J. N., D. E. Riley, R. L. Vesella, D. C. Miner, S. O. Ross, and P. H. Lange. 2000. Bacterial DNA sequences in prostate tissue from patients with prostate cancer and chronic prostatitis. J. Urol. 164:1221-1228. [PubMed] [Google Scholar]
- 22.Kuwata, K., H. Watanabe, S. Y. Jiang, T. Yamamoto, C. Tomiyama-Miyaji, T. Abo, T. Miyazaki, and M. Naito. 2003. AIM inhibits apoptosis of T cells and NKT cells in Corynebacterium-induced granuloma formation in mice. Am. J. Pathol. 162:837-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, J. C., C. H. Muller, I. Rothman, K. J. Agnew, D. Eschenbach, M. A. Ciol, J. A. Turner, and R. E. Berger. 2003. Prostate biopsy culture findings of men with chronic pelvic pain syndrome do not differ from those of healthy controls. J. Urol. 169:584-587. [DOI] [PubMed] [Google Scholar]
- 24.MacLennan, G. T., R. Eisenberg, R. L. Fleshman, J. M. Taylor, P. Fu, M. I. Resnick, and S. Gupta. 2006. The influence of chronic inflammation in prostatic carcinogenesis: a 5-year followup study. J. Urol. 176:1012-1016. [DOI] [PubMed] [Google Scholar]
- 25.Nelson, W. G., A. M. De Marzo, T. L. DeWeese, and W. B. Isaacs. 2004. The role of inflammation in the pathogenesis of prostate cancer. J. Urol. 172:S6-S11. [DOI] [PubMed] [Google Scholar]
- 26.Nickel, J. C., and J. W. Costerton. 1993. Bacterial localization in antibiotic-refractory chronic bacterial prostatitis. Prostate 23:107-114. [DOI] [PubMed] [Google Scholar]
- 27.Nickel, J. C., J. Downey, I. Young, and S. Boag. 1999. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 84:976-981. [DOI] [PubMed] [Google Scholar]
- 28.Ohteki, T., H. Tada, K. Ishida, T. Sato, C. Maki, T. Yamada, J. Hamuro, and S. Koyasu. 2006. Essential roles of DC-derived IL-15 as a mediator of inflammatory responses in vivo. J. Exp. Med. 203:2329-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel, D. A., C. H. Bock, K. Schwartz, A. S. Wenzlaff, R. Y. Demers, and R. K. Severson. 2005. Sexually transmitted diseases and other urogenital conditions as risk factors for prostate cancer: a case-control study in Wayne County, Michigan. Cancer Causes Control 16:263-273. [DOI] [PubMed] [Google Scholar]
- 30.Platz, E. A., and A. M. De Marzo. 2004. Epidemiology of inflammation and prostate cancer. J. Urol. 171:S36-S40. [DOI] [PubMed] [Google Scholar]
- 31.Rosenblatt, K. A., K. G. Wicklund, and J. L. Stanford. 2001. Sexual factors and the risk of prostate cancer. Am. J. Epidemiol. 153:1152-1158. [DOI] [PubMed] [Google Scholar]
- 32.Sarma, A. V., J. C. McLaughlin, L. P. Wallner, R. L. Dunn, K. A. Cooney, D. Schottenfeld, J. E. Montie, and J. T. Wei. 2006. Sexual behavior, sexually transmitted diseases and prostatitis: the risk of prostate cancer in black men. J. Urol. 176:1108-1113. [DOI] [PubMed] [Google Scholar]
- 33.Shannon, B. A., R. J. Cohen, and K. L. Garrett. 2006. Polymerase chain reaction-based identification of Propionibacterium acnes types isolated from the male urinary tract: evaluation of adolescents, normal adults and men with prostatic pathology. BJU Int. 98:388-392. [DOI] [PubMed] [Google Scholar]
- 34.Shiina, H., Y. Himeno, and T. Ishibe. 1992. Organisms in the prostate and antibiotics in the treatment of postoperative infections. Urol. Int. 48:187-190. [DOI] [PubMed] [Google Scholar]
- 35.St. Amand, A. L., D. N. Frank, M. A. De Groote, R. J. Basaraba, I. M. Orme, and N. R. Pace. 2005. Use of specific rRNA oligonucleotide probes for microscopic detection of Mycobacterium tuberculosis in culture and tissue specimens. J. Clin. Microbiol. 43:5369-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szoke, I., L. Torok, E. Dosa, E. Nagy, and S. Scultety. 1998. The possible role of anaerobic bacteria in chronic prostatitis. Int. J. Androl. 21:163-168. [PubMed] [Google Scholar]
- 37.Urisman, A., R. J. Molinaro, N. Fischer, S. J. Plummer, G. Casey, E. A. Klein, K. Malathi, C. Magi-Galluzzi, R. R. Tubbs, D. Ganem, R. H. Silverman, and J. L. Derisi. 2006. Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2:e25. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Webster, G. F., J. J. Leyden, R. A. Musson, and S. D. Douglas. 1985. Susceptibility of Propionibacterium acnes to killing and degradation by human neutrophils and monocytes in vitro. Infect. Immun. 49:116-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada, T., Y. Eishi, S. Ikeda, I. Ishige, T. Suzuki, T. Takemura, T. Takizawa, and M. Koike. 2002. In situ localization of Propionibacterium acnes DNA in lymph nodes from sarcoidosis patients by signal amplification with catalysed reporter deposition. J. Pathol. 198:541-547. [DOI] [PubMed] [Google Scholar]