Abstract
Two temporally and geographically clustered cases of meningitis caused by Streptococcus agalactiae expressing the infrequent Ib serotype are reported. Characterization by pulsed-field gel electrophoresis and multilocus sequence typing revealed that the isolates were identical and represented the widely distributed ST10/ST8 lineage associated with serotype Ib.
CASE REPORTS
Case 1.
A 69-year-old woman with no known risk factors for group B streptococcal (GBS) infection was admitted on 7 January 2007 to a tertiary-care hospital in Lisbon with headache, nausea and vomiting, disturbances of consciousness, and blurred vision with an onset 24 h prior to admission. On presentation, she was apyretic and confused and had no signs of meningeal irritation. Hematological investigations revealed a white blood cell (WBC) count of 4,630 × 106/liter (with 85% granulocytes) and a C-reactive protein level of 370 mg/liter. A lumbar puncture was performed, and blood samples were taken for culture. The cerebrospinal fluid (CSF) exhibited a WBC (granulocyte) count of 113/mm3, a glucose concentration of 1 mg/dl, and a protein concentration of 533 mg/dl, and Gram staining showed gram-positive cocci. A head computed axial tomography scan was unremarkable.
On the basis of the initial results, empirical therapy consisting of ceftriaxone (4 g daily, given intravenously) and ampicillin (12 g daily, given intravenously) was started.
Clinical evolution was rapid with loss of consciousness and multiple organ failure, and the patient died 12 h after admission.
Both CSF and blood cultures were positive 24 h later for Streptococcus agalactiae susceptible to penicillin and ceftriaxone.
Case 2.
Within a few hours of the first case, a second case was encountered at the same hospital. A 58-year-old woman was admitted with headache, prostration, fever, polyarthralgia, and diarrhea with an onset 48 h prior to admission. On presentation, she was comatose and febrile and had positive signs of meningeal irritation. The WBC count was 17,900 × 106/liter (with 88% granulocytes), and the C-reactive protein level was 292 mg/liter. A lumbar puncture was performed, and blood samples were taken for culture. The CSF revealed >500 WBC (granulocytes)/mm3, a glucose concentration of <1 mg/dl, a protein concentration of 736 mg/dl, and the presence of gram-positive cocci. A head computed axial tomography scan was unremarkable.
On the basis of the initial results, empirical therapy consisting of ceftriaxone (4 g daily, given intravenously) and ampicillin (12 g daily, given intravenously) was started.
Both CSF and blood cultures were positive 24 h later for S. agalactiae susceptible to penicillin and ceftriaxone. On the basis of the microbiological results, the antimicrobial therapy was changed to penicillin G (24 × 106 U daily, given intravenously).
Urine culture, a vaginorectal swab culture for detection of GBS, and another set of blood cultures performed 24 h after admission were all negative. A review of the patient's clinical history revealed a mastectomy due to breast cancer 10 years prior to the current episode as the only possible risk factor for GBS infection. On discharge, the patient revealed no motor deficits but was diagnosed with neurosensory deafness and diplopia.
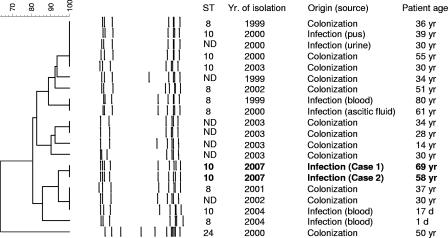
The patients lived on the same street, but interviews of the members of their households were not able to establish a prior social acquaintance. Isolates from both cases were serotyped by using specific sera (Denka Seiken, Japan) (5) and a genotyping method (9) as type Ib. Further characterization by pulsed-field gel electrophoresis (PFGE) (11) revealed identical profiles (Fig. 1). To evaluate if the PFGE profiles were unusual among isolates expressing serotype Ib, 18 isolates from an ongoing nationwide survey of GBS infections focusing primarily on invasive infections (5, 11) were also characterized by both PFGE and multilocus sequence typing (8) and the results are reported in Fig. 1.
FIG. 1.
Characteristics of the GBS isolates responsible for the two cases of meningitis reported here and of other serotype Ib isolates recovered in Portugal. The dendrogram was derived by the unweighted-pair group method using average linkages by using the Dice coefficient of the SmaI PFGE profiles of the isolates generated by using the Bionumerics software (Applied Maths, Sint-Martens-Latem, Belgium). The horizontal scale at the top represents the percentage of relatedness between isolates. STs were determined by multilocus sequence typing. ND, not determined. Patient ages are indicated in years (yr) or days (d). The two isolates responsible for the cases reported are in bold.
Although the incidence of invasive GBS infections in nonpregnant adults has increased over the last decades, meningitis remains an uncommon manifestation (3). The incidence of GBS meningitis in adults in the United States is estimated to be 0.15 case per 100,000 adults (2), but it results in a high case fatality rate (27 to 34%) (3). The incidence in Portugal is not known, but a prior study at a large hospital in the Lisbon area did not identify any cases (5) and our ongoing nationwide survey started in 2003 identified only three cases, apart from the two reported here, among 916 GBS isolates responsible for infections in adults (136 of those were recovered from normally sterile sites).
The majority of GBS meningitis cases are closely linked to the presence of underlying conditions (2, 4) or to the perinatal period (6), but in some cases no patient risk factors could be identified (1). In some of these cases, evidence of an external source of infection was presented (7). In the two cases reported here, only the patient in the second case had a known risk factor for invasive GBS infection—a mastectomy following a breast cancer diagnosis. Farley et al. have suggested that a mastectomy may continue to enhance the risk for invasive GBS infection many years after it was performed, but the presentation of the patients is cellulitis of the arm or chest wall on the side that underwent the mastectomy (4). This did not occur in our case, and in fact, even though sometimes a distant focus of infection can be established (2), this was not possible in either of the cases presented here. Moreover, in the second case, vaginorectal colonization could not be established, suggesting an exogenous source.
The temporal and geographical clustering of these two cases prompted further characterization of the isolates. The same serotype (Ib) was identified in both isolates. Serotype Ib is not among the most frequent serotypes associated with invasive disease in adults in the United States (3), and our previous study identified a single case of adult bacteremia due to this serotype (n = 1/21) (5). Among neonatal infections and colonization of pregnant women in Portugal, serotype Ib was equally unremarkable in prevalence (11). Furthermore, none of the three adult GBS meningitis cases detected by our ongoing survey was associated with this serotype nor were any of the GBS infections in adults identified since January 2007 in the hospital where the two cases were detected (these isolates were serotyped as part of an enhanced surveillance triggered by these meningitis cases).
Characterization by PFGE revealed identical profiles (Fig. 1), indicating that the isolates responsible for these two cases belong to the same bacterial clone. The characterization by PFGE of the isolates in our collection as serotype Ib identified a major cluster, and although no other isolate revealed the same PFGE profile as the isolates responsible for the two cases described here, these were included in the same cluster as the majority of the Ib isolates. This cluster included isolates associated with colonization and responsible for different clinical presentations in diverse age groups (Fig. 1). Multilocus sequence typing analysis confirmed the distinction of the isolates in two clusters, in agreement with the PFGE analysis, since two sequence types (STs), ST8 and ST10, are single-locus variants and therefore closely related, whereas ST24 differs at five out of seven loci in both of them. Again, the isolates responsible for the two cases presented here had the same ST—ST10. This was not an unusual ST among Ib isolates and was found in four additional isolates associated with both colonization and infection, including invasive disease (Fig. 1). ST8 and ST10 are also the most frequent STs found among serotype Ib isolates from various sources and geographic origins (8, 10). A previous study could not establish an association between a particular genetic lineage and invasive disease in adults, but a significant fraction of infections caused by clonal complex CC9, where ST8 and ST10 are included, was noted (10).
Although a definite common source for the two cases was not established, their temporal clustering and the proximity of the homes of the two patients strongly suggested that such a common origin could exist. The microbiological findings further support this notion. Not only did the isolates present an unusual serotype, but they also displayed identical PFGE profiles, albeit belonging to the major genetic lineage associated with serotype Ib. The virulence of the strain causing these infections is apparent in the fatal outcome of one of the cases and the neurological sequelae of the surviving patient, in spite of appropriate antimicrobial therapy. GBS is an increasingly significant agent of bacterial meningitis in adults, and the high morbidity and mortality associated with these infections (3), even in adults with no risk factors, together with the possibility of community outbreaks, justify continued monitoring to obtain further insights into this important pathogen.
Acknowledgments
This work was partly supported by Fundação para a Ciência e Tecnologia (POCI/SAU-ESP/57646/2004) and by a grant from Fundação Calouste Gulbenkian.
Footnotes
Published ahead of print on 19 September 2008.
REFERENCES
- 1.Barile, A. J., A. J. Kallen, and M. R. Wallace. 1999. Fatal group B streptococcal meningitis in a previously healthy young adult. Clin. Infect. Dis. 28:151. [DOI] [PubMed] [Google Scholar]
- 2.Domingo, P., N. Barquet, M. Alvarez, P. Coll, J. Nava, and J. Garau. 1997. Group B streptococcal meningitis in adults: report of twelve cases and review. Clin. Infect. Dis. 25:1180-1187. [DOI] [PubMed] [Google Scholar]
- 3.Farley, M. M. 2001. Group B streptococcal disease in nonpregnant adults. Clin. Infect. Dis. 33:556-561. [DOI] [PubMed] [Google Scholar]
- 4.Farley, M. M., R. C. Harvey, T. Stull, J. D. Smith, A. Schuchat, J. D. Wenger, and D. S. Stephens. 1993. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. N. Engl. J. Med. 328:1807-1811. [DOI] [PubMed] [Google Scholar]
- 5.Figueira-Coelho, J., M. Ramirez, M. J. Salgado, and J. Melo-Cristino. 2004. Streptococcus agalactiae in a large Portuguese teaching hospital: antimicrobial susceptibility, serotype distribution, and clonal analysis of macrolide-resistant isolates. Microb. Drug Resist. 10:31-36. [DOI] [PubMed] [Google Scholar]
- 6.Guerin, J. M., F. Leibinger, A. Mofredj, and J. M. Ekherian. 1997. Streptococcus B meningitis in post-partum. J. Infect. 34:151-153. [DOI] [PubMed] [Google Scholar]
- 7.Guihot, A., F. Bricaire, and P. Bossi. 2005. Group B streptococcal meningitis in a patient with horizontal transmission: beware of toothbrushing on Sunday mornings. J. Infect. 50:240-241. [DOI] [PubMed] [Google Scholar]
- 8.Jones, N., J. F. Bohnsack, S. Takahashi, K. A. Oliver, M.-S. Chan, F. Kunst, P. Glaser, C. Rusniok, D. W. M. Crook, R. M. Harding, N. Bisharat, and B. G. Spratt. 2003. Multilocus sequence typing system for group B streptococcus. J. Clin. Microbiol. 41:2530-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong, F., S. Gowan, D. Martin, G. James, and G. L. Gilbert. 2002. Serotype identification of group B streptococci by PCR and sequencing. J. Clin. Microbiol. 40:216-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luan, S. L., M. Granlund, M. Sellin, T. Lagergard, B. G. Spratt, and M. Norgren. 2005. Multilocus sequence typing of Swedish invasive group B streptococcus isolates indicates a neonatally associated genetic lineage and capsule switching. J. Clin. Microbiol. 43:3727-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins, E. R., M. A. Pessanha, M. Ramirez, J. Melo-Cristino, and the Portuguese Group for the Study of Streptococcal Infections. 2007. Analysis of group B streptococcal isolates from infants and pregnant women in Portugal reveals two lineages with enhanced invasiveness. J. Clin. Microbiol. 45:3224-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]