Abstract
Forty-six strains of Malassezia spp. with atypical biochemical features were isolated from 366 fresh clinical isolates from human subjects and dogs. Isolates obtained in this study included 2 (4.7%) lipid-dependent M. pachydermatis isolates; 1 (2.4%) precipitate-producing and 6 (14.6%) non-polyethoxylated castor oil (Cremophor EL)-assimilating M. furfur isolates; and 37 (34.3%) M. slooffiae isolates that were esculin hydrolyzing, 17 (15.7%) that were non-tolerant of growth at 40°C, and 2 (1.9%) that assimilated polyethoxylated castor oil. Although their colony morphologies and sizes were characteristic on CHROMagar Malassezia medium (CHROM), all strains of M. furfur developed large pale pink and wrinkled colonies, and all strains of M. slooffiae developed small (<1 mm) pale pink colonies on CHROM. These atypical strains were distinguishable by the appearance of their colonies grown on CHROM. Three clinically important Malassezia species, M. globosa, M. restricta, and M. furfur, were correctly identified by their biochemical characteristics and colony morphologies. The results presented here indicate that our proposed identification system will be useful as a routine tool for the identification of clinically important Malassezia species in clinical laboratories.
Members of the genus Malassezia are among the microbiological flora of the skin of homoiothermic animals. Most species of this genus are lipid-dependent yeasts which colonize the seborrheic part of the skin, and they have been reported to be associated with pityriasis versicolor, seborrheic dermatitis, Malassezia folliculitis, and atopic dermatitis (1, 6, 19, 20, 24, 29). Although M. furfur was previously thought to be the causative agent or trigger factor in all of these skin disorders, Guého et al. (9) reclassified this genus into five species in 1996. Malassezia has since been reclassified into seven species based on molecular biological analysis of nuclear ribosomal DNA/RNA (9, 10), and the results agreed with those of mitochondrial ribosomal DNA analyses (30). As members of the genus Malassezia share similar morphological and biochemical characteristics, it was thought that differentiating between them based on phenotypic features would be difficult. While molecular biological techniques are the most reliable for the identification of Malassezia, they are not available in most clinical laboratories. Therefore, culture methods for the identification of Malassezia species are required. Some of these identification or differentiation methods have been reported previously. Guillot et al. reported a method of identification based on lipid usage pattern, catalase reaction, growth temperature, and cell shape (11). Hammer and Riley reported the production of a precipitate by some Malassezia strains on Dixon's agar (12); for example, M. furfur, M. obtusa, and M. slooffiae were precipitate-negative strains, while M. sympodialis and M. globosa were precipitate-positive strains. Mayser et al. reported that some Malassezia species hydrolyzed esculin and assimilated polyethoxylated castor oil (Cremophor EL; Sigma, St. Louis, MO) (18), and these properties could be used to differentiate among species (8). However, it is necessary to develop an updated phenotype-based identification method applicable to new Malassezia species (25, 26). We reported previously that CHROMagar Candida medium with vital growth factors for Malassezia, CHROMagar Malassezia medium (CHROM), could be used for isolating and differentiating between Malassezia and Candida spp. simultaneously (14) and that a biological feature-based identification method was developed for nine species of Malassezia (15). However, we found that some strains showed atypical features in fresh clinical isolates. Here, we (i) report the incidence of atypical biochemical features in Malassezia species and (ii) propose a culture-based identification system for three clinically important Malassezia species, M. furfur, M. globosa, and M. restricta.
MATERIALS AND METHODS
Organisms.
Three hundred sixty-six fresh clinical isolates of Malassezia (42 M. pachydermatis, 83 M. sympodialis, 13 M. globosa, 40 M. furfur, 107 M. slooffiae, 3 M. obtusa, 70 M. restricta, 3 M. dermatis, and 5 M. yamatoensis isolates) obtained from human subjects or dogs, as described below, as well as type and standard strains of Malassezia (Table 1), were used in this study. All strains were identified by molecular biological analysis (16, 17).
TABLE 1.
Origin and distribution of Malassezia strains
| Species | No. of total strains | No. of stock strains | No. of fresh isolates | Origin | Comments |
|---|---|---|---|---|---|
| M. pachydermatis | 43 | 1 | CBS 1879 | ||
| 30 | Dog, external ear | External otitis | |||
| 12 | Dog, external ear | Healthy dogs | |||
| M. sympodialis | 84 | 1 | CBS 7222 | ||
| 80 | Human | Healthy adults | |||
| 1 | Human | Psoriasis vulgaris | |||
| 1 | Human | Atopic dermatitis | |||
| 1 | Human, external ear | External otitis | |||
| M. globosa | 14 | 1 | CBS 7966 | ||
| 10 | Human | Pityriasis versicolor | |||
| 3 | Human | Seborrheic dermatitis | |||
| M. dermatis | 5 | 2 | JCM11348 and JCM11470 | ||
| 2 | Human | Healthy adults | |||
| 1 | Human | Seborrheic dermatitis | |||
| M. furfur | 41 | 1 | CBS 1878 | ||
| 38 | Human | Healthy adults | |||
| 1 | Human | Psoriasis vulgaris | |||
| 1 | Human, external ear | External otitis | |||
| M. slooffiae | 108 | 1 | CBS 7956 | ||
| 95 | Human, external ear | Healthy adults | |||
| 8 | Human, external ear | External otitis | |||
| 3 | Human | Seborrheic dermatitis | |||
| 1 | Human | Psoriasis vulgaris | |||
| M. obtusa | 4 | 1 | CBS 7876 | ||
| 3 | Human | Healthy adults | |||
| M. restricta | 71 | 1 | CBS 7877 | ||
| 31 | Human, external ear | Healthy adults | |||
| 35 | Human | Seborrheic dermatitis | |||
| 2 | Human | Psoriasis vulgaris | |||
| 2 | Human | Pityriasis versicolor | |||
| M. japonica | 2 | 2 | 0 | M9966, M9967 | |
| M. yamatoensis | 5 | 0 | 2 | Human | Healthy adults |
| 2 | Human | Psoriasis vulgaris | |||
| 1 | Human | Seborrheic dermatitis | |||
| Total | 377 | 11 | 366 |
Culture media.
Strains of Malassezia were maintained on modified Leeming and Notman agar composed of (per liter) 10 g of peptone (Oxoid, Basingstoke, United Kingdom), 10 g of glucose, 2 g of yeast extract (Oxoid), 8 g of ox bile (Oxoid), 10 ml of glycerol, 0.5 g of glycerol monostearate, 5 ml of Tween 60, 20 ml of olive oil, and 15 g of agar (Oxoid) and sterilized by autoclaving.
The following specific media were used in this study. CHROMagar Malassezia medium (CHROM) was composed (per liter) of 56.3 g of CHROMagar Malassezia basal medium (CHROMagar, Paris, France) and 10 ml of Tween 40 (15). Sabouraud's dextrose agar (SDA) was composed (per liter) of 10 g of mycological peptone, 40 g of glucose, and 15 g of agar. Cremophor EL (Sigma, St. Louis, MO) agar (EL slant) was composed (per liter) of 65 g of SDA and 10 ml of Cremophor EL (15). Tween 60-esculin agar (TE slant) was composed (per liter) of 10 g of peptone, 10 g of glucose, 2 g of yeast extract, 5 ml of Tween 60, 0.5 g of ferric ammonium citrate, 1 g of esculin, and 15 g agar (15).
Clinical specimens.
Two hundred eighteen clinical specimens from the body surface of patients with atopic dermatitis, seborrheic dermatitis, psoriasis vulgaris, and external otitis and from healthy adults were obtained from Teikyo University Hospital (Tokyo, Japan), Kitasato University Hospital (Kanagawa, Japan), and Takinomiya General Hospital (Kagawa, Japan) and from 65 dogs with and without external otitis from Nihon University Veterinary Hospital (Kanagawa, Japan) and veterinary clinics in the Kanto area. Samples from the body surface were taken using adhesive tape (10 mm by 10 mm) as reported by Padilha-Goncalves (22), which was then placed on CHROM. External ear samples were taken with swabs and then streaked on CHROM. All isolates observed for CHROM were checked by colony morphology and size after incubation in air at 32°C for 4 to 7 days.
Phenotypic feature testing.
The “typical phenotypic features” of Malassezia species were defined as shown in Table 2. All isolates of Malassezia were inoculated onto CHROM and specific media (SDA, EL slant, and TE slant) and incubated at 32°C for 4 days before observation. SDA was used to determine the isolates' lipid dependence, EL slants for the isolates' abilities to utilize polyethoxylated castor oil, and TE slants for the isolates' abilities to hydrolyze esculin and utilize Tween 60. Fresh cultures grown on CHROM were subjected to catalase testing with 3% hydrogen peroxide. Colony size on CHROM was determined by measuring well-isolated single colonies and assessed as small (<1 mm), medium (1 to 2 mm), or large (2 to 5 mm). Additional phenotypic characterization of the ability to grow at 40°C was performed if the isolate was thought to be a strain of M. slooffiae. All test strains of Malassezia species were identified by molecular analysis.
TABLE 2.
Typical phenotypic features in nine species of Malasseziaa
| Species | Growth on SDA | Growth on mDixon at
|
Precipitate production on CHROM | Utilization of
|
Esculin | Polyethoxylated castor oil | Catalase reaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 32°C | 37°C | 40°C | 10% Tw 20 | 0.5% Tw 40 | 0.5% Tw 60 | 0.1% Tw 80 | ||||||
| M. pachydermatisb | + | + | + | + | +f | + | + | + | + | ±e | ±e | + |
| M. sympodialisb | − | + | + | + | +f | − | + | + | + | +e | −e | + |
| M. globosab | − | + | ± or − | − | +f | − | − | − | − | −e | −e | + |
| M. dermatisc | − | + | + | + | +f | + | + | + | + | −f | −f | + |
| M. furfurb | − | + | + | + | −f | + | + | + | + | −e | +e | + |
| M. slooffiaeb | − | + | + | + | −f | ± or + | + | + | − | −e | −e | + |
| M. obtusab | − | + | ± or + | − | −f | − | − | − | − | +e | −e | + |
| M. restrictab | − | + | + | − | −f | − | − | − | − | −e | −e | − |
| M. japonicad | − | + | + | − | −f | − | ± | + | − | +f | −f | + |
Molecular analysis.
DNA was extracted by the procedure of Makimura et al. (16). The internal transcribed spacer 1 (ITS1) region was sequenced directly from PCR products by using the primer pair 18SF1 and 58SR1 (17). The PCR products were sequenced on an ABI PRISM 310 genetic analyzer according to the manufacturer's instructions (Applied Biosystems, Foster City, CA).
RESULTS
Phenotypic features of Malassezia and incidence of atypical phenotypic features found in Malassezia species.
The phenotypic features of nine Malassezia species are shown in Table 3.
TABLE 3.
Biological features and incidence of a typical phenotype of nine species of Malassezia
| Species identified by molecular biological analysis | No. of strains | Colony characteristic on CHROM (% of incidence)a
|
Growth characteristic (% of incidence)b
|
Catalase reaction | |||||
|---|---|---|---|---|---|---|---|---|---|
| Size | Color/morphology | Precipitate | SDA | TE slant | EL slant | 40°C | |||
| M. pachydermatis | 43 | Large (100) | Pale pink/smooth (100) | + (100) | Growth (95.3)d | Growth and produced a black zone (95.3)d | Growth (95.3)d | NT | + (100) |
| M. sympodialis | 84 | Large (100) | Pale pink/smooth (100) | + (100) | No growth (100) | Growth and produced a black zone (100) | No growth (100) | NT | + (100) |
| M. globosa | 14 | Small (100) | Purple/smooth (100) | + (100) | No growth (100) | No growth and no change (100) | No growth (100) | NT | + (100) |
| M. dermatis | 5 | Large (100) | Pale pink to purple/smooth (100) | + (100) | No growth (100) | Growth and no change (100) | No growth (100) | NT | + (100) |
| M. furfur | 41 | Large (100) | Pale pink/wrinkled (100) | − (97.6)c | No growth (100) | Growth and produced a black zone (100) | Growth (85.4) | NT | + (100) |
| M. slooffiae | 108 | Small (100) | Pale pink/smooth (100) | − (100) | No growth (100) | Growth and no change (65.7)e | No growth (98.1) | Growth (84.3)e | + (100) |
| M. obtusa | 4 | Medium (100) | Pink/rough (100) | − (100) | No growth (100) | No growth but produced a black zone (100) | No growth (100) | NT | + (100) |
| M. restricta | 71 | Small (100) | Pink/smooth (100) | − (100) | No growth (100) | No growth and no change (100) | No growth (100) | NT | − (100) |
| M. japonica | 2 | Large (100) | Pink/smooth (100) | − (100) | No growth (100) | Growth and produced a black zone (100) | No growth (100) | No growth (100) | + (100) |
Incubated at 32°C for 4 to 7 days.
Incubated at 32°C for 4 days. NT, not tested; +, positive; −, negative.
Only one strain of fresh clinical isolate produced a precipitate.
Only two strains of fresh clinical isolates did not grow on SDA and EL and did not produce a black zone on TE.
Thirty-seven strains of fresh clinical isolates produced a black zone on TE, and 17 strains did not grow on modified Leeming and Notman agar at 40°C for 4 days.
(i) Precipitate production and colony morphology on CHROM.
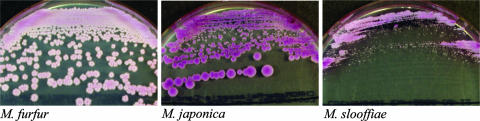
All of the type and reference strains and fresh clinical isolates of M. pachydermatis (43/43), M. sympodialis (84/84), M. globosa (14/14), and M. dermatis (5/5) produced precipitates after incubation at 32°C for 4 days on CHROM. Other strains of the species M. furfur (40/41), M. slooffiae (108/108), M. obtusa (4/4), M. restricta (71/71), and M. japonica (2/2) did not produce such precipitates, with the exception of only one (2.4%) strain of a fresh clinical isolate of M. furfur. Malassezia furfur colonies, including this one atypical strain, were easily distinguishable from those of other Malassezia species on CHROM due to their characteristically large pale pink and wrinkled colonies (Fig. 1). Colony size on CHROM was measured from well-isolated single colonies, and isolates were divided into three groups: small (M. globosa, M. slooffiae, and M. restricta), medium (M. obtusa), and large (M. pachydermatis, M. sympodialis, M. dermatis, M. furfur, and M. japonica).
FIG. 1.
Colony characteristics of M. furfur, M. japonica, and M. slooffiae on CHROM were observed after incubation at 32°C for 4 days. Colonies of M. furfur were large, pale pink, and wrinkled and did not produce precipitates. Colonies of M. japonica were larger (2 to 5 mm) than those of M. slooffiae (<1 mm). Their sizes were measured in well-isolated single colonies.
(ii) Lipid dependence.
Only M. pachydermatis (41/43) grew on the lipid-free culture medium (SDA), and other Malassezia species did not grow. This biological feature of M. pachydermatis was specific, but two (4.7%) atypical strains of lipid-dependent M. pachydermatis were obtained.
(iii) Utilization of polyethoxylated castor oil.
EL slants were used to determine the ability to utilize polyethoxylated castor oil, and we obtained six (14.6%) atypical strains of M. furfur and two (4.7%) of M. pachydermatis that did not grow on EL slants. Two (1.9%) M. slooffiae strains grew on EL slants. This biological feature was not acceptable as a key identifying feature of M. furfur.
(iv) Catalase reaction.
Malassezia restricta was the only catalase-negative species. This biological feature was acceptable as a key identifying feature of M. restricta.
(v) Hydrolysis of esculin and utilization of Tween 60.
Forty-one (95.3%) isolates of M. pachydermatis, 84 (100%) of M. sympodialis, 41 (100%) of M. furfur, and 2 (100%) of M. japonica showed production of a black zone around the colonies due to esculin hydrolysis products and ferrous iron in TE slants. On the other hand, 5 (100%) M. dermatis and 71 (65.7%) M. slooffiae isolates did not produce such a zone. None of the strains of M. globosa or M. restricta utilized Tween 60 or grew on TE slants. None of the strains of M. obtusa utilized Tween 60, but they hydrolyzed esculin and produced a black zone on TE slants.
(vi) Tolerance of 40°C.
None of the strains of M. japonica and 17 (15.7%) M. slooffiae strains did not grow on modified Leeming and Notman agar at 40°C. This biological feature was not acceptable for distinguishing between M. slooffiae and M. japonica species. Other Malassezia species were not tested.
Proposal for an identification system for Malassezia species.
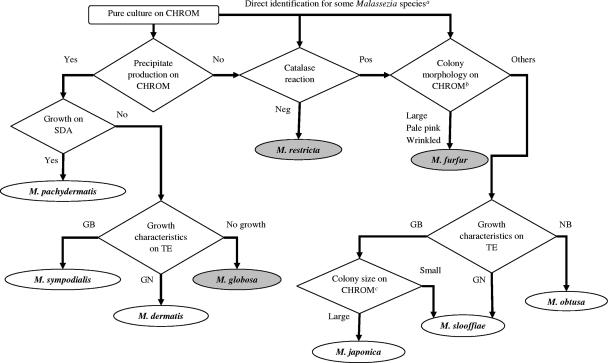
Our proposed identification system for nine species of Malassezia using 366 fresh isolates and 11 type and reference strains identified by molecular analysis is shown in Fig. 2. First, some Malassezia species could be identified directly. M. furfur developed characteristically large pale pink and wrinkled colonies on CHROM and could be differentiated from other Malassezia species. M. restricta was the only catalase-negative species. Second, only M. pachydermatis grew on SDA, but 2 (4.7%) atypical strains were lipid dependent and were incorrectly identified as M. dermatis. Third, with the exception of M. yamatoensis, other Malassezia species were correctly identified by this system. This system was not applicable for the identification of M. yamatoensis. Table 4 shows the numbers of correct and incorrect results and the sensitivity and specificity of our identification system. Three clinically important Malassezia species, M. furfur (100% [41 of 41]), M. globosa (100% [14 of 14]), and M. restricta (100% [71 of 71]), were correctly identified by this system, and the rate of concordance of this system with molecular analysis was 98.1% (370/377).
FIG. 2.
Proposed identification workflow for nine species of Malassezia. Pos, positive; Neg, negative; GB, growth and black zone; GN, growth and no change; NB, no growth and black zone. a, Direct identification by catalase reaction and features of colonies on CHROM for M. restricta and M. furfur. b, Colony morphology on CHROM as shown in Fig. 1. c, Colony size of M. japonica and M. slooffiae on CHROM as shown in Fig. 1.
TABLE 4.
Identification results and sensitivity and specificity of the proposed identification system
| Species identified by molecular biological analysis | No. of test strains | No. of correct results | No. of incorrect results | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| M. pachydermatis | 43 | 41 | 0 | 95.4 | 100 |
| M. sympodialis | 84 | 84 | 2a | 100 | 97.7 |
| M. globosa | 14 | 14 | 0 | 100 | 100 |
| M. dermatis | 5 | 5 | 2b | 100 | 71.4 |
| M. furfur | 41 | 41 | 0 | 100 | 100 |
| M. slooffiae | 108 | 108 | 3c | 100 | 97.3 |
| M. obtusa | 4 | 4 | 0 | 100 | 100 |
| M. restricta | 71 | 71 | 0 | 100 | 100 |
| M. japonica | 2 | 2 | 0 | 100 | 100 |
| M. yamatoensis | 5 | 0 | 0 | NAd | NA |
| Total | 377 | 370 | 7 |
Two strains of fresh clinical isolates of M. yamatoensis were incorrectly identified as M. sympodialis by this modified identification method.
Two strains of fresh clinical isolates of M. pachydermatis were incorrectly identified as M. dermatis by this modified identification method.
Three strains of fresh clinical isolates of M. yamatoensis were incorrectly identified as M. dermatis by this modified identification method.
NA, this method was not applicable.
Cost effectiveness of the proposed identification system.
Cost comparison results were based on the commercial price of each system (Table 5), but the labor costs associated with testing were not included. The cost of identifying Malassezia was reduced by about $9,100 in this study.
TABLE 5.
Cost comparison in U.S. dollars of the molecular biological analysis system and that of the proposed system
| Item | Molecular biological analysis
|
Proposed biological identification system
|
||
|---|---|---|---|---|
| Cost per test | Total costa | Cost per test | Total costa | |
| CHROM | 2.00 | 566 | 2.00 | 566 |
| Molecular biological analysis | 25.00 | 9,425 | 0 | 0 |
| 3% hydrogen peroxideb | 0 | 0 | 0.417 | 0.0264 |
| SDA slant | 0 | 0 | 0.417 | 157.2 |
| TE slant | 0 | 0 | 0.417 | 157.2 |
| Total | 27.0 | 9,991 | 3.251 | 880 |
During the present study.
Catalase test.
DISCUSSION
The biological tests described above were designed to identify Malassezia species in clinical laboratories. We investigated the variation of phenotypic features of 377 Malassezia species in this study.
First, CHROMagar was used as the primary culture medium, and we obtained 1 (2.4%) atypical strain of M. furfur that produced precipitates on CHROM. On the other hand, M. pachydermatis, M. sympodialis, M. globosa, and M. dermatis were recognized from their precipitates in the agar, as reported by Kaneko et al. (15). The atypical strain of M. furfur developed characteristically large pale pink and wrinkled colonies on CHROM, and all test strains of M. furfur were characteristically similar without precipitate production. Therefore, M. furfur was identified correctly by colony morphology on CHROM. Although M. globosa and M. obtusa were similar to each other in terms of their phenotypic features (9), lipid usage patterns, catalase reactions, and growth temperatures, their characteristics with regard to precipitation on CHROM were different. Furthermore, M. dermatis and M. japonica are new species reported by Sugita et al. (25, 26), and their biological properties resembled those of M. slooffiae and M. sympodialis, except for their lipid usage patterns, respectively, but their precipitations on CHROM were different (Tables 2 and 3).
Second, SDA was used to determine the lipid dependence; none of the lipid-dependent species grew on SDA. We obtained two (4.7%) atypical M. pachydermatis strains that could not be cultured on lipid-free culture medium (SDA), and the sensitivity and specificity of this culture medium for M. pachydermatis were 95.4% and 100%, respectively. The atypical strains were incorrectly identified as M. dermatis based on their biological features. Duarte et al. (5) reported that one lipid-dependent variant strain of M. pachydermatis was isolated in an investigation of 964 cattle and 6 dogs, and unambiguous identification required sequencing.
Third, EL slants were used to determine the ability to utilize polyethoxylated castor oil for M. furfur. Mayser et al. (18) showed that polyethoxylated castor oil was metabolized only by five strains of M. furfur in the agar diffusion test, but we obtained six atypical strains (14.6%) of M. furfur, two (4.7%) of M. pachydermatis, and two (1.9%) of M. slooffiae. Their biological features were not acceptable as key features of M. furfur, and this culture medium's sensitivity and specificity for M. furfur were 85.4% and 97.3%, respectively. However, all test strains of M. furfur were directly identified by colony morphology on CHROM as described above.
Fourth, TE slants were used to determine the ability to hydrolyze esculin and utilize Tween 60 as key features for differentiation among the precipitate-producing group (M. sympodialis, M. dermatis, and M. globosa) and the nonprecipitate-producing group (M. japonica, M. slooffiae, and M. obtusa). Mayser et al. (18) reported that two tested strains of M. sympodialis were able to split esculin with the black zone, while two tested strains of M. slooffiae remained negative. We confirmed these results previously with three strains each of M. sympodialis and M. slooffiae (15). However, 37 atypical strains (34.3%) of M. slooffiae hydrolyzed esculin and showed a black zone around the colonies on TE slants. In addition, 17 (15.7%) strains of M. slooffiae were not tolerant of growth at 40°C. Therefore, the previously reported biological features (15, 18) were not useful for differentiation between M. slooffiae and M. japonica. On the other hand, all test strains of M. slooffiae developed pale pink colonies that were smaller than those of M. japonica on CHROM and could be differentiated based on colony size.
Fifth, catalase reaction was used as a key feature of M. restricta, and this reaction allowed correct recognition of all test strains except M. restricta.
The results presented here indicated that the clinically important species M. globosa, M. restricta, and M. furfur were identified correctly using our proposed method. Recently, data from several institutions have suggested that M. globosa is associated with pityriasis versicolor (19, 20), that M. restricta is associated with seborrheic dermatitis (24, 29), and that M. furfur infections have been observed in hospitalized neonates with very low birth weight receiving intravenous lipid emulsions (2, 3, 4, 7, 23, 28). In agreement with these reports, we isolated mainly M. globosa from pityriasis versicolor and M. restricta from seborrheic dermatitis in the present study.
A simple, reliable, and cost-effective identification method is required in most clinical laboratories. CHROM with two specific media (SDA and TE slant) and catalase reactions allowed identification of Malassezia species easily, quickly, and at reasonable cost without requiring any expensive or specialized equipment. For example, automated sequencers are very expensive in Japan (about $84,000). In addition, we estimated that the use of this system will result in cost savings equivalent to about $9,100 per 377 samples in our laboratory. This biological identification system was used to correctly identify three clinically important Malassezia species, and we believe that this system can be adopted easily by most clinical laboratories and thus reduce labor costs and enable rapid reporting of clinically relevant laboratory results. Recently, M. nana, M. yamatoensis, and M. equi were reported as new species of Malassezia (13, 21, 27). Although these species are very rare, we hope that a technique for the easy differentiation of these species will be developed in future. The results presented here indicate that this system will be a useful tool for the routine identification of clinically important Malassezia species.
Acknowledgments
This study was supported in part by Health Science Research Grants for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor, and Welfare of Japan (K.M.).
Footnotes
Published ahead of print on 19 September 2007.
REFERENCES
- 1.Ashbee, H. R., and E. G. Evans. 2002. Immunology of diseases associated with Malassezia species. Clin. Microbiol. Rev. 15:21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boekhout, T., and R. W. Bosboom. 1994. Karyotyping of Malassezia yeasts: taxonomic and epidemiological implications. Syst. Appl. Microbiol. 17:146-153. [Google Scholar]
- 3.Boekhout, T., M. Kamp, and E. Guého. 1998. Molecular typing of Malassezia species with PFGE and RAPD. Med. Mycol. 36:365-372. [DOI] [PubMed] [Google Scholar]
- 4.Danker, W. M., S. A. Spector, J. Fierer, and C. E. Davis. 1987. Malassezia fungemia in neonates and adults complication of hyperalimentation. Rev. Infect. Dis. 9:743-753. [DOI] [PubMed] [Google Scholar]
- 5.Duarte, E. R., M. A. Lachance, and J. S. Hamdan. 2002. Identification of atypical strains of Malassezia spp. from cattle and dog. Can. J. Microbiol. 48:749-752. [DOI] [PubMed] [Google Scholar]
- 6.Faergemann, J. 2002. Atopic dermatitis and fungi. Clin. Microbiol. Rev. 15:545-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gueho, E., R. Simmons, W. Pruitt, S. Meyer, and D. Ahearn. 1987. Association of Malassezia pachydermatis with systemic infections of humans. J. Clin. Microbiol. 25:1789-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guého, E., T. Boekhout, H. R. Ashbee, J. Guillot, A. Van Belkum, and J. Faergemann. 1998. The role of Malassezia species in the ecology of human skin and as pathogens. Med. Mycol. 36:220-229. [PubMed] [Google Scholar]
- 9.Guého, E., G. Midgley, and J. Guillot. 1996. The genus Malassezia with description of four new species. Antonie Leewenhoek 69:337-355. [DOI] [PubMed] [Google Scholar]
- 10.Guillot, J., and E. Guého. 1995. The diversity of Malassezia yeasts confirmed by rRNA sequence and nuclear DNA comparisons. Antonie Leeuwenhoek 67:297-314. [DOI] [PubMed] [Google Scholar]
- 11.Guillot, J., E. Guého, M. Lesourd, G. Midgley, G. Chevrier, and B. Dupont. 1996. Identification of Malassezia species. J. Mycol. Med. 6:103-110. [Google Scholar]
- 12.Hammer, K. A., and T. V. Riley. 2000. Precipitate production by some Malassezia species on Dixon's agar. Med. Mycol. 38:105-107. [DOI] [PubMed] [Google Scholar]
- 13.Hirai, A., R. Kano, K. Makimura, E. R. Duarte, J. S. Hamdan, M. A. Lachance, H. Yamaguchi, and A. Hasegawa. 2004. Malassezia nana sp. nov., a novel lipid-dependent yeast species isolated from animals. Int. J. Evol. Microbiol. 54:623-627. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko, T., K. Makimura, M. Onozaki, K. Ueda, Y. Yamada, Y. Nishiyama, and H. Yamaguchi. 2005. Vital growth factors of Malassezia species: presumptive identification of Malassezia and Candida species on modified CHROMagar Candida agar. Med. Mycol. 43:699-704. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko, T., K. Makimura, T. Sugita, and H. Yamaguchi. 2006. Tween 40-based precipitate production observed on modified chromogenic agar and development of biological identification kit for Malassezia species. Med. Mycol. 44:227-231. [DOI] [PubMed] [Google Scholar]
- 16.Makimura, K., Y. S. Murayama, and H. Yamaguchi. 1994. Detection of a wide range of medically important fungi by the polymerase chain reaction. J. Med. Microbiol. 40:358-364. [DOI] [PubMed] [Google Scholar]
- 17.Makimura, K., Y. Tamura, M. Kudo, K. Uchida, H. Saito, and H. Yamaguchi. 2000. Species identification and strain typing of Malassezia species stock strains and clinical isolates based on the DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J. Med. Microbiol. 49:29-35. [DOI] [PubMed] [Google Scholar]
- 18.Mayser, P., P. Haze, C. Papavassilis, M. Pickel, K. Gruender, and E. Guého. 1997. Differentiation of Malassezia species: selectivity of cremophor EL, castor oil and ricinoleic acid for M. furfur. Br. J. Dermatol. 137:208-213. [DOI] [PubMed] [Google Scholar]
- 19.Morishita, N., Y. Sei., I. Takiuchi, and T. Sugita. 2005. Examination of the causative agent of pityriasis versicolor. Jpn. J. Med. Mycol. 46:169-170. [DOI] [PubMed] [Google Scholar]
- 20.Nakabayashi, A. 2002. Identification of causative species in Malassezia-associated dermatoses. Jpn. J. Med. Mycol. 43:65-68. [DOI] [PubMed] [Google Scholar]
- 21.Nell, A., S. A. James, C. J. Bond, and M. E. Herrtage. 2002. Identification and distribution of a novel Malassezia yeast species on normal equine skin. Vet. Rec. 150:395-398. [DOI] [PubMed] [Google Scholar]
- 22.Padilha-Goncalves, A. 1996. A single method to stain Malassezia furfur and Corynebacterium minutissimum in scales. Rev. Inst. Med. Trop. Sao Paulo 38:299-302. [DOI] [PubMed] [Google Scholar]
- 23.Richet, H. M., M. M. McNeil, M. C. Edwards, and W. R. Jarvis. 1989. Cluster of Malassezia furfur pulmonary infections in infants in a neonatal intensive-care unit. J. Clin. Microbiol. 27:1197-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sei, Y. 2006. Malassezia related disease. Jpn. J. Med. Mycol. 47:75-80. [DOI] [PubMed] [Google Scholar]
- 25.Sugita, T., M. Takashima, T. Shinoda, H. Suto, T. Unno, R. Tsuboi, H. Ogawa, and A. Nishikawa. 2002. New yeast species, Malassezia dermatis, isolated from patients with atopic dermatitis. J. Clin. Microbiol. 40:1363-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugita, T., M. Takashima, M. Kodama, R. Tsuboi, and A. Nishikawa. 2003. Description of a new yeast species, Malassezia japonica, and its detection in patients with atopic dermatitis and healthy subjects. J. Clin. Microbiol. 41:4695-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugita, T., M. Tajima, M. Takashima, M. Amaya, M. Saito, R. Tsuboi, and A. Nishikawa. 2004. A new yeast, Malassezia yamatoensis, isolated from a patient with seborrheic dermatitis, and its distribution in patients and healthy subjects. Microbiol. Immunol. 48:579-583. [DOI] [PubMed] [Google Scholar]
- 28.Surmont, I., A. Gavilanes, J. Vandepitte, H. Devlieger, and E. Eggermont. 1989. Malassezia furfur fungemia in infants receiving intravenous lipid emulsions: a rarity or just underestimated? Eur. J. Pediatr. 148:435-438. [DOI] [PubMed] [Google Scholar]
- 29.Tajima, M. 2005. Malassezia species in patients with seborrheic dermatitis and atopic dermatitis. Jpn. J. Med. Mycol. 45:137-142. [DOI] [PubMed] [Google Scholar]
- 30.Yamada, Y., K. Makimura, K. Ueda, Y. Nishiyama, K. Uchida, H. Yamaguchi, and M. Osumi. 2003. DNA base alignment and taxonomic study of genus Malassezia based upon partial sequences of mitochondrial large subunit ribosomal RNA gene. Microbiol. Immunol. 47:475-478. [DOI] [PubMed] [Google Scholar]