Abstract
In this study, we present a novel genotyping scheme to classify German wild-type varicella-zoster virus (VZV) strains and to differentiate them from the Oka vaccine strain (genotype B). This approach is based on analysis of four loci in open reading frames (ORFs) 51 to 58, encompassing a total length of 1,990 bp. The new genotyping scheme produced identical clusters in phylogenetic analyses compared to full-genome sequences from well-characterized VZV strains. Based on genotype A, D, B, and C reference strains, a dichotomous identification key (DIK) was developed and applied for VZV strains obtained from vesicle fluid and liquor samples originating from 42 patients suffering from varicella or zoster between 2003 and 2006. Sequencing of regions in ORFs 51, 52, 53, 56, 57, and 58 identified 18 single-nucleotide polymorphisms (SNPs), including two novel ones, SNP 89727 and SNP 92792 in ORF51 and ORF52, respectively. The DIK as well as phylogenetic analysis by Bayesian inference showed that 14 VZV strains belonged to genotype A, and 28 VZV strains were classified as genotype D. Neither Japanese (vaccine)-like B strains nor recombinant-like C strains were found within the samples from Germany. The novel genotyping scheme and the DIK were demonstrated to be practical and simple and allow the highly efficient replication of phylogenetic patterns in VZV initially derived from full-genome DNA sequence analyses. Therefore, this approach may allow us to draw a more comprehensive picture of wild-type VZV strains circulating in Germany and Central Europe by high-throughput procedures in the future.
Varicella-zoster virus (VZV), genus Varicellovirus, subfamily Alphaherpesvirinae of the Herpesviridae, is the causative agent of chicken pox (varicella) in children. After primary infection, a lifelong latent infection is established in the dorsal root and trigeminal ganglia. Reactivation of VZV in elderly or immunocompromised persons can cause shingles (zoster). The VZV genome is highly conserved and consists of 125 kb of linear, double-stranded DNA and is predicted to code for 71 proteins. Within the VZV genome, one long region and one short unique region, each flanked by inverted repeats and five internal repeat regions, have been identified (4).
With the introduction of routine varicella vaccination in Germany in 2004, surveillance of VZV strains in cases of chickenpox and zoster became necessary. Genetic diversity among Central European VZV strains was initially described using either restriction enzyme mapping (12) or variations in the copy number at the tandem repeat regions (13). However, these techniques have substantial limitations to VZV strain genotyping, principally due to low fidelity and complexity (14). Therefore, individual VZV strain genotyping based on partial or total DNA genome sequences is essential for the continued monitoring of vaccine impact in Central Europe. Phylogenetic analysis of DNA sequences can also provide insights into VZV molecular evolution, such as recombination events between major circulating clades (8, 10). Previous studies based on DNA sequence analysis suggested that VZV strain distribution is associated with climate factors (6) or migration patterns (18).
Several VZV genotyping schemes based on partial VZV genome sequences were proposed for the classification of different genotypes (2, 6, 18). Based on phylogenetic analysis of a short region (447 bp) in open reading frame 22 (ORF22), three genotypes, genotypes E (European), J (Japanese), and M (mosaic), were classified (6). Another approach based on phylogenetic analysis of six genes (“glycoprotein/IE62 scheme”) encompassing 13 kbp resulted in the definition of four genotypes, genotypes A (European/North American), B (Japanese/Asian), C (recombinant), and D (European/North American) (18). Similarly, a third genotyping scheme (“scattered scheme”) (2) based on phylogenetic analysis of ORFs 1, 21, 50, and 54, encompassing 2,021 bp, classified four genotypes, but these genotypes differ from those defined by the “glycoprotein/IE62 scheme”: A (Africa, Asia, and Far East), B and C (European/North American), and J (Japanese). In addition to the discrepancies of the definition of genotypes by the three schemes described above, they also differ in their resolution, ease of use, and cost.
Alternatively, full-genome sequence analysis allows the identification of a large number of single-nucleotide polymorphisms (SNPs) that can be used for the identification of stable VZV subclades, especially within the European clades (8, 16). It was further shown to be the only method for identifying recombination between major circulating VZV clades (8, 10). However, full-genome sequence analysis is costly and labor-intensive. In this study, we present a novel VZV genotyping scheme based on the DNA sequence analysis of four loci in ORF51 to ORF58 that allows the efficient distinction among German wild-type VZV strains and differentiation from the Oka vaccine strain and other strains.
MATERIALS AND METHODS
Patients and samples.
Samples were obtained between 2003 and 2006 from a total of 42 patients (21 males and 21 females) who were referred to the hospital of the Johann Wolfgang Goethe University, Frankfurt am Main, Germany, or to the Clinic of Dermatology and Venereology, University of Rostock, Rostock, Germany. There was no epidemiological relationship, in either time or place, between the patients. All 38 vesicle fluid samples were obtained as swabs in viral transport medium originating from 32 patients suffering from zoster and 6 patients suffering from varicella as diagnosed by clinical presentation. Liquor was obtained from two patients suffering from varicella and zoster encephalitis, respectively, by lumbar puncture. The vitreous body was obtained from one patient with zoster ophthalmicus, and a biopsy was obtained by gastroscopy from one patient suffering from stomach cancer. Samples were sent to the Institute of Medical Virology, Frankfurt am Main, Germany, to confirm VZV infection by real-time PCR according to methods described previously (17).
DNA isolation and PCR.
DNA was isolated from the samples using the QIAamp DNA Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. DNA amplification reactions were carried out in 50-μl volumes with 10 μl of extract or control DNA, 0.5 μl proofreading Pfu polymerase (Fermentas, St. Leon-Rot, Germany) corresponding to 1.25 U, 5 μl 10× Pfu buffer containing 25 mM MgSO4, 1 μl of each forward primer (primers GT1-F [5′-GCAATATTGGGGGCGTGT-3′], GT2-F [5′-TCTTTCAGGACTCCTATGTA-3′], GT5-F [5′-GGGGGTACTATCCGTAA-3′], and GT6-F [5′-TGAGCGGGTGTAATATCG-3′]) and the corresponding reverse primer (primers GT1-R [5′-TTCGGCATGCGGGGCCA-3′], GT2-R [5′-CACCGAAAAATGTTCAGT-3′], GT5-R [5′-CGTTGGTACCAGTGTTT-3′], and GT6-R [5′-TGTTCGATCCCAAATTTTAA-3′]) corresponding to 1 μM, 1 μl deoxynucleotide triphosphate mix at a final concentration of 200 μM of each deoxynucleotide triphosphate, and 31.5 μl water. Thermal cycling conditions were comprised of an initial hot start at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at primer pair-specific temperatures for 30 s, and polymerization at 72°C for 1 min. Finally, an extension step at 72°C for 10 min was carried out. The PCR products were visualized on a UV transilluminator following separation on 2.5% agarose gels containing ethidium bromide.
DNA sequencing and phylogenetic analysis.
PCR products were purified using YM-100 microconcentrators (Celera Diagnostics, Alameda, CA) according to the manufacturer's protocol. Purified PCR products were sequenced on an ABI 3100 Avant DNA sequencer by using the ABI BigDye Terminator version 1.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol.
Sequences were aligned using CLUSTAL X (15) and manually checked in the BIOEDIT sequence editing program (5). Phylogenetic analysis was performed using the MrBayes 3.0 program (11) with a general time-reversible substitution model. The consensus trees were drawn with the TreeView program (9).
RESULTS
All 42 patient samples were found to be positive for VZV DNA when analyzed by real-time PCR, with threshold cycle values varying from 17 to 34, and by conventional PCR amplification of four loci in ORF51 to ORF58 according to the new protocol (data not shown). This concordance of real-time PCR and the novel genotyping PCR protocol demonstrated the sensitivity of the new approach.
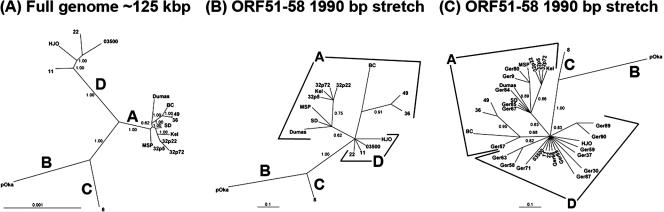
Samples from 42 patients, 39 originating from patients with German nationality and 3 originating from patients with Ethiopian and Iranian nationalities, were investigated with the new genotyping scheme. Initially, the phylogenetic resolution of the new genotyping scheme was tested using sequence data from well-characterized VZV reference strains of genotypes A, D, B, and C. The phylogenetic analysis of sequence data from the four sites in ORF51 to ORF58 produced phylogenetic clusters that were identical to those obtained by full-genome sequence analysis of these strains (Fig. 1A and B).
FIG. 1.
Phylogenetic trees of VZV strains from previous studies based on full-genome sequences (A) and based on the 1,990-bp stretch only that is used in the new genotyping scheme. Selected wild-type strains detected with the new method in this study are included in C. All strains from Germany cluster with either clade A or D. Posterior probabilities are shown on each branch.
The 1,990-bp-long ORF51 to ORF58 VZV sequences of the 42 patients were compared to the corresponding sequences of genotype A, D, B, and C reference strains. Analysis by Bayesian inference of the VZV sequences from the 42 patients resulted in the identification of 14 genotype A and 28 genotype D strains (Fig. 1C). Sequence comparison of 10 genotype A, 4 genotype D, 1 genotype B, and 1 genotype C reference strains (Table 1) allowed us to identify SNPs and to generate a simple dichotomous identification key (DIK) (Fig. 2). The validity of the DIK was verified by analysis of the 42 patient samples. In line with the phylogenetic analysis, the application of the DIK resulted in the identification of exclusively genotype A (14/39) and genotype D (28/39) sequences. Three specimens originating from patients with Ethopian and Iranian nationalities were classified as belonging to genotypes D and A, respectively (Table 1). No significant differences in the genotype distribution within the samples were observed when analyzed by age, sex, or immunocompromising factors.
TABLE 1.
SNPs in a 1,990-bp stretch including ORF51 to ORF58 of reference genotype A, B, C, and D strains and German VZV strainsa
| Strain | Type | SNP at position:
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORF51
|
ORF52
|
ORF53
|
ORF56
|
ORF57
|
ORF58
|
|||||||||||||||||||||||||||
| 89724 | 89727 | 89886 | 89905 | 90088 | 90103 | 90202 | 90217 | 92375 | 92432 | 92523 | 92535 | 92670 | 92792 | 92861 | 99186 | 99189 | 99227 | 99228 | 99229 | 99421 | 99442 | 99452 | 99570 | 99709 | 99789 | 99981 | 100088 | 100114 | 100123 | 100151 | ||
| Dumas | A | A | T | T | T | T | C | G | T | A | T | A | A | C | A | T | T | A | T | T | C | T | C | C | C | A | T | C | A | T | T | T |
| BC | A | T | C | G | C | G | ||||||||||||||||||||||||||
| 36 | A | C | T | C | G | |||||||||||||||||||||||||||
| 49 | A | C | T | C | G | |||||||||||||||||||||||||||
| MSP | A | T | C | |||||||||||||||||||||||||||||
| 32p5 | A | T | G | |||||||||||||||||||||||||||||
| 32p22 | A | T | G | |||||||||||||||||||||||||||||
| 32p72 | A | T | G | |||||||||||||||||||||||||||||
| Kel | A | T | G | |||||||||||||||||||||||||||||
| SD | A | T | ||||||||||||||||||||||||||||||
| 11 | D | T | G | |||||||||||||||||||||||||||||
| 22 | D | T | G | |||||||||||||||||||||||||||||
| 03-500 | D | T | G | |||||||||||||||||||||||||||||
| HJO | D | T | T | G | ||||||||||||||||||||||||||||
| 8 | C | C | T | C | G | G | A | |||||||||||||||||||||||||
| pOka | B | G | C | T | C | G | —d | — | — | G | G | T | A | G | ||||||||||||||||||
| Ger1 | A | T | ||||||||||||||||||||||||||||||
| Ger4 | A | T | ||||||||||||||||||||||||||||||
| Ger46c | A | T | ||||||||||||||||||||||||||||||
| Ger53 | A | T | ||||||||||||||||||||||||||||||
| Ger54 | A | T | ||||||||||||||||||||||||||||||
| Ger67 | A | T | ||||||||||||||||||||||||||||||
| Ger70 | A | T | ||||||||||||||||||||||||||||||
| Ger81 | A | T | ||||||||||||||||||||||||||||||
| Ger84 | A | T | G | — | — | — | ||||||||||||||||||||||||||
| Ger85 | A | T | ||||||||||||||||||||||||||||||
| Ger8 | A | T | C | |||||||||||||||||||||||||||||
| Ger9 | A | T | C | |||||||||||||||||||||||||||||
| Ger80c | A | T | C | |||||||||||||||||||||||||||||
| Ger57 | A | T | C | G | ||||||||||||||||||||||||||||
| Ger6 | D | T | G | |||||||||||||||||||||||||||||
| Ger25 | D | T | G | |||||||||||||||||||||||||||||
| Ger29 | D | T | G | |||||||||||||||||||||||||||||
| Ger31 | D | T | G | |||||||||||||||||||||||||||||
| Ger33 | D | T | G | |||||||||||||||||||||||||||||
| Ger43 | D | T | G | |||||||||||||||||||||||||||||
| Ger45 | D | T | G | |||||||||||||||||||||||||||||
| Ger47 | D | T | G | |||||||||||||||||||||||||||||
| Ger51 | D | T | G | |||||||||||||||||||||||||||||
| Ger55 | D | T | G | |||||||||||||||||||||||||||||
| Ger60 | D | T | G | |||||||||||||||||||||||||||||
| Ger62 | D | T | G | |||||||||||||||||||||||||||||
| Ger66 | D | T | G | |||||||||||||||||||||||||||||
| Ger69 | D | T | G | |||||||||||||||||||||||||||||
| Ger78 | D | T | G | |||||||||||||||||||||||||||||
| Ger79 | D | T | G | |||||||||||||||||||||||||||||
| Ger82 | D | T | G | |||||||||||||||||||||||||||||
| Ger83 | D | T | G | |||||||||||||||||||||||||||||
| Ger88 | D | T | G | |||||||||||||||||||||||||||||
| Ger89 | D | T | G | G | ||||||||||||||||||||||||||||
| Ger90 | D | T | G | G | ||||||||||||||||||||||||||||
| Ger58 | D | C | T | G | ||||||||||||||||||||||||||||
| Ger63 | D | C | T | G | ||||||||||||||||||||||||||||
| Ger30 | D | C | T | A | G | |||||||||||||||||||||||||||
| Ger37b | D | T | T | G | ||||||||||||||||||||||||||||
| Ger59 | D | T | C | G | ||||||||||||||||||||||||||||
| Ger71 | D | C | T | T | G | |||||||||||||||||||||||||||
| Ger87 | D | T | T | G | C | |||||||||||||||||||||||||||
Nucleotide positions refer to the European strain Dumas. Reference strains of previous studies are included: Dumas (GenBank accession number NC001348), The Netherlands; BC (accession number AY548171), British Columbia, Canada; 36 (accession number DQ479958), New Brunswick, Canada; 49 (accession number DQ479959), New Brunswick, Canada; MSP (accession number AY548170), Minnesota; 32p5 (accession number DQ479961), Texas; 32p22 (accession number DQ479962), Texas; 32p72 (accession number DY479963), Texas; Kel (accession number DQ479954), Iowa; SD (accession number DQ479953), South Dakota; 11 (accession number DQ47995), New Brunswick, Canada; 22 (accession number DQ479956) New Brunswick, Canada; 03-500 (accession number DQ479957), Alberta, Canada; HJO (accession number AJ871403), Germany; 8 (accession number DQ479960) New Brunswick, Canada; pOka (accession number AB097933), Japan.
Originating from a patient from Ethiopia.
Originating from patients from Iran.
—, nucleotide deletion compared to Dumas reference strain data.
FIG. 2.
DIK for genotyping of German wild-type VZV strains using sequence information from ORF51 to ORF58 and SNPs therein. nt, nucleotide.
Two new SNPs were detected in the VZV sequences of Ger58/Ger63 and Ger89/Ger90. The T>C nucleotide exchange in ORF51 (SNP 89727) of Ger58/Ger63 is synonymous, whereas the nonsynonymous A>G mutation in ORF52 (SNP 92792) of Ger89/Ger90 results in an amino acid exchange (asparagine to serine). Interestingly, a deletion of three nucleotides in ORF56 resulting in a deletion of serine was detected in both Ger85 and pOka (Table 1). Bayesian inference nevertheless clusters Ger85 within the genotype A clade since the sequence of pOka is otherwise very distinct (Fig. 1C).
Overall, the new VZV genotyping scheme allows a clear distinction between the Japanese (vaccine)-like B and recombinant-like C genotypes from the European/North American A and D genotypes.
DISCUSSION
Genotyping schemes that are intended to be a useful tool must be simple to perform and able to yield reliable results compared to full-genome sequence analysis. The genotyping scheme described herein, encompassing a total of 1,990 bp, is sensitive and simple to perform and allows the typing of a large number of specimens. Our data are in line with data from previous studies showing that the VZV genome is extremely stable and that small numbers of SNPs from across the genome can be used to distinguish at least four main genotypes: genotypes A, B, C, and D (10, 18). Full-genome sequence analysis of wild-type VZV strains originating from North America (n = 13) and The Netherlands (n = 1) reveals 19 clade-specific SNPs for genotype A, 54 clade-specific SNPs for genotype B, 47 clade-specific SNPs for genotype C, and 50 clade-specific SNPs for genotype D (10).
The present study is the first one to determine genotypes from wild-type VZV strains originating from Germany and to define a simple method for genotyping of VZV strains in Germany and perhaps other central European countries. All investigated wild-type VZV strains were demonstrated to belong to genotype A or D. Neither Japanese (vaccine)-like B strains nor recombinant-like C strains were found. This is in contrast to previous studies that found recombinant-like C strains as well as Japanese (vaccine)-like B strains in France and Spain (7), Ireland (3), and the United Kingdom (1, 2). Interestingly, differentiation of German wild-type VZV strains from the Oka vaccine strain by restriction enzyme mapping resulted in the detection of one Oka vaccine type within 116 investigated samples (13). Nevertheless, restriction enzyme mapping has substantial limitations to VZV strain genotyping, such as no differentiation between A and D clades and subclades, respectively. Our investigation demonstrated that strain Ger85, with a deletion of three neighboring nucleotide residues, which resembles that observed in the Oka vaccine strain, however, due to a large level of divergence in the investigated region of ORF51 to ORF58, was clearly grouped into the genotype A clade.
It has been suggested that the heterogeneity of VZV strains in the United Kingdom is related to the pattern of immigration to those countries (1, 2). Immigration patterns in Germany are not comparable to those in the United Kingdom, and therefore, a more homogeneous distribution of VZV genotypes should be expected. Therefore, the genotyping scheme discriminating between the European A and D genotypes described herein should be used to determine VZV genotypes in German specimens.
The newly identified SNP 89727 and SNP 92792 distinguish Ger89, Ger90, Ger58, and Ger63 from the other clade D strains. More DNA sequence data for these strains are required to assess the subclade status. The classification of new subclades should be limited to full-genome sequence analysis, at least for the glycoprotein/IE62 scheme. Assignment of new subclades based on the phylogenetic analysis of only a limited number of SNPs across the genome can be misleading because such classifications are sometimes based on one particular SNP that is shared or that differs between strains and may not be indicative of the true divergence of the strains (10).
In summary, the novel genotyping scheme presented here is a simple and useful tool for the typing of wild-type VZV strains originating from Germany and perhaps other countries in Central Europe. It will be a reliable method to monitor trends in molecular evolution such as an increase in Japanese (vaccine)-like B strains or recombinant-like C strains due to the introduction of routine varicella vaccination in Germany.
Acknowledgments
We acknowledge Karoline Bleymehl, Regina Allwinn, Annemarie Berger, and Holger Felix Rabenau for helpful discussions and Marhild Kortenbusch and Margarethe Podporski for technical assistance.
The work was supported by the Hospital of the Johann Wolfgang Goethe University.
Footnotes
Published ahead of print on 12 September 2007.
REFERENCES
- 1.Barrett-Muir, W. B., R. Nichols, and J. Breuer. 2002. Phylogenetic analysis of varicella-zoster virus: evidence of intercontinental spread of genotypes and recombination. J. Virol. 76:1971-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett-Muir, W., F. T. Scott, P. Aaby, J. John, P. Matondo, Q. L. Chaudhry, M. Siqueira, A. Poulsen, K. Yaminishi, and J. Breuer. 2003. Genetic variation of varicella-zoster virus: evidence for geographical separation of strains. J. Med. Virol. 70(Suppl. 1):S42-S47. [DOI] [PubMed] [Google Scholar]
- 3.Carr, M. J., G. P. McCormack, and B. Crowley. 2004. Genetic variation in clinical varicella-zoster virus isolates collected in Ireland between 2002 and 2003. J. Med. Virol. 73:131-136. [DOI] [PubMed] [Google Scholar]
- 4.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 5.Hall, T. A. 1999. BIOEDIT: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 6.Loparev, V. N., A. Gonzalez, M. Deleon-Carnes, G. Tipples, H. Fickenscher, E. G. Torfason, and D. S. Schmid. 2004. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J. Virol. 78:8349-8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loparev, V., E. Martro, E. Rubtcova, C. Rodrigo, J. C. Piette, E. Caumes, J. P. Vernant, D. S. Schmid, and A. M. Fillet. 2007. Toward universal varicella-zoster virus (VZV) genotyping: diversity of VZV strains from France and Spain. J. Clin. Microbiol. 45:559-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norberg, P., J. A. Liljeqvist, T. Bergstrom, S. Sammons, D. S. Schmid, and V. N. Loparev. 2006. Complete-genome phylogenetic approach to varicella-zoster virus evolution: genetic divergence and evidence for recombination. J. Virol. 80:9569-9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page, R. D. 1996. Treeview: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 10.Peters, G. A., S. D. Tyler, C. Grose, A. Severini, M. J. Gray, C. Upton, and G. A. Tipples. 2006. A full-genome phylogenetic analysis of varicella-zoster virus reveals a novel origin of replication-based genotyping scheme and evidence of recombination between major circulating clades. J. Virol. 80:9850-9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronquist, F., and J. P. Huelsenbeck. 2003. Mrbayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572-1574. [DOI] [PubMed] [Google Scholar]
- 12.Sauerbrei, A., U. Eichhorn, S. Gawellek, R. Egerer, M. Schacke, and P. Wutzler. 2003. Molecular characterisation of varicella-zoster virus strains in Germany and differentiation from the Oka vaccine strain. J. Med. Virol. 71:313-319. [DOI] [PubMed] [Google Scholar]
- 13.Sauerbrei, A., R. Zell, and P. Wutzler. 2007. Analysis of repeat units in the R2 region among different Oka varicella-zoster virus vaccine strains and wild-type strains in Germany. Intervirology 50:40-44. [DOI] [PubMed] [Google Scholar]
- 14.Sergeev, N., E. Rubtcova, V. Chizikov, D. S. Schmid, and V. N. Loparev. 2006. New mosaic subgenotype of varicella-zoster virus in the USA: VZV detection and genotyping by oligonucleotide-microarray. J. Virol. Methods 136:8-16. [DOI] [PubMed] [Google Scholar]
- 15.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyler, S. D., G. A. Peters, C. Grose, A. Severini, M. J. Gray, C. Upton, and G. A. Tipples. 2007. Genomic cartography of varicella-zoster virus: a complete genome-based analysis of strain variability with implications for attenuation and phenotypic differences. Virology 359:447-458. [DOI] [PubMed] [Google Scholar]
- 17.van Doornum, G. J., J. Guldemeester, A. D. Osterhaus, and H. G. Niesters. 2003. Diagnosing herpesvirus infections by real-time amplification and rapid culture. J. Clin. Microbiol. 41:576-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagenaar, T. R., V. T. Chow, C. Buranathai, P. Thawatsupha, and C. Grose. 2003. The out of Africa model of varicella-zoster virus evolution: single nucleotide polymorphisms and private alleles distinguish Asian clades from European/North American clades. Vaccine 7:1072-1081. [DOI] [PubMed] [Google Scholar]