Abstract
We have developed a real-time quantitative PCR (rt-QPCR) assay to detect and kinetically monitor BK virus viruria and viremia in renal transplant recipients (RTRs). A total of 607 urine and 223 plasma samples were collected from 203 individuals including those with BK virus-associated nephropathy (BKVAN) (n = 8), those undergoing routine posttransplant surveillance (SV) (n = 155), those with nontransplant chronic kidney disease (NT-CKD) (n = 20), and healthy living kidney donors (LD) (n = 20). The rt-QPCR assay was found to be highly sensitive and specific, with a wide dynamic range (2.4 to 11 log10 copies/ml) and very good precision (coefficient of variation, ∼5.9%). There was a significant difference in the prevalences of viruria and viremia between the BKVAN (100% and 100%) and SV (23% and 3.9%) groups (P < 0.001). No viruria or viremia was detected in LD or in NT-CKD patients. The median (range) peak levels of BK virus viruria and viremia, in log10 copies/ml, were 10.26 (9.04 to 10.83) and 4.83 (3.65 to 5.86) for the BKVAN group versus 0 (0 to 10.83) and 0 (0 to 5.65) for the SV group, respectively (P < 0.001). When the BK virus load in the urine was <7.0 log10 copies/ml, no BK virus viremia was detected. When the BK virus load in the urine reached 7.0, 8.0, 9.0, and ≥10.0 log10 copies/ml, the corresponding detection of BK virus viremia increased to 20, 33, 50, and 100%, respectively. We propose monitoring of BK virus viruria in RTRs, with plasma BK virus load testing reserved for those with viruria levels of ≥7.0 log10 copies/ml.
BK virus (BKV) is a polyomavirus with a circular DNA genome of approximately 5,300 bp. BKV causes ubiquitous infection in early childhood, with seroprevalence in adults ranging from 60 to 100% (31). Following primary infection, the virus remains latent in the urogenital tract (30). BKV reactivation with urinary shedding of infected urothelial cells occurs in 10 to 60% of renal transplant recipients (RTR) (19, 25). Although early studies found that only sporadic cases of graft dysfunction were associated with viral activation (1, 14, 15), more-recent studies demonstrate BKV-associated nephropathy (BKVAN) in as many as 8% of renal allograft recipients, with as many as 50% of patients experiencing graft loss over the next 2 to 3 years of follow-up (8, 9, 11, 20-24, 27, 28, 29). Accumulated data suggest that prospective monitoring of patients at risk for BKVAN may identify those with active infection before renal function deteriorates (3, 8, 13, 18, 22). Early identification provides the opportunity for intervention with reduction of the immunosuppression in an effort to control BKV replication and prevent BKVAN (4, 16).
Progression to BKVAN occurs without clinical signs or symptoms except for increasing serum creatinine concentrations. The “gold standard” for diagnosis of BKVAN continues to be renal biopsy with demonstration of viral cytopathic tubulointerstitial changes (7, 23). Sometimes, however, the biopsy result can be falsely negative due to the focal nature of the disease (10). The clinical utility of noninvasive methods, such as detection of the presence of decoy cells in the urine and detection of BKV in the urine by electron microscopy (EM) or conventional PCR, is limited because of the ubiquitous nature of the virus and its consequently low positive predictive value for tissue-invasive disease (2, 6, 13, 21, 22). A few studies have reported an association between quantitative BKV viruria and viremia and the cytopathic changes of BKVAN (13, 18, 22). More-recent studies have argued that quantitative BKV viremia showed a significant correlation with BKVAN but that BKV viruria had no consistent correlation with BKVAN (13). The relationship between BKV viruria and viremia, and the cutoffs and predictive values of BKV viruria and viremia for the occurrence of BKVAN, are still largely unknown.
Recently, several studies have demonstrated that real-time quantitative PCR (rt-QPCR) is an accurate and cost-effective method for the determination of BKV DNA loads, with high sensitivity and specificity (5, 12, 17, 18, 25, 26, 32). Most of those studies were carried out retrospectively using stored clinical samples and locally developed rt-QPCR assays. Different outcomes, due to differences in targeting sequences, design of primer pairs, DNA extraction methods, and conditions and duration of sample storage, have led to different conclusions regarding the cutoffs and predictive values of BKV viruria and viremia for BKVAN. We have developed an rt-QPCR assay for detection and monitoring of the BKV DNA load based on the LightCycler probing system in order to study the relationship between BKV viruria and viremia and to examine the value of routine monitoring. Here we describe the performance characteristics of this assay for RTR as well as patients with stage V chronic kidney disease (CKD) and healthy living kidney donors (LD).
MATERIALS AND METHODS
Patients and clinical specimens.
All RTR at the University of Alberta Hospital were prospectively enrolled for BKV monitoring from November 2004 to November 2005. Urine samples were collected posttransplantation, monthly from months 2 to 6 and then bimonthly to month 12. Monitoring of BKV viremia was initiated only if BKV viruria was documented. A total of 533 urine and 106 plasma samples were collected from 155 RTR (surveillance group [SV]). The median frequency of urine sampling was 3 times (range, 1 to 12). Three control groups, consisting of 20 LD, 20 nontransplant patients with CKD (NT-CKD patients), and 8 historical patients with biopsy-proven BKVAN, were studied. For the last group, urine and plasma samples for BKV load determination were collected at various time points following the diagnosis of BKVAN.
Urine was collected as midstream samples in a sterile container without concentration. Plasma samples were collected in EDTA-blood tubes. Samples collected from outpatients in remote clinics were shipped to the laboratory using ice packs within 48 h. Both urine and plasma samples were stored at −80°C and assayed within 7 days of collection.
Primers and probes.
The specific primers and probes were designed from the region of the VP1 gene of BKV by utilizing LightCycler probe design software (Roche Diagnostics). An on-line search in GenBank (NCBI) with tentative sequences of primers and probes was subsequently conducted for further confirmation of their specificity as well as their homology with all submitted sequences of different BKV strains. The primer pair was expected to yield a 309-bp amplicon by PCR amplification. All primers and probes were synthesized by TIB Molbiol LLC (Adelphia, NJ). The sequences and locations of primers and probes are given in Table 1.
TABLE 1.
Sequences and locations of primers and probes for the BKV VP1 genea
| Primer or probe | Sequence (5′ to 3′) | Location |
|---|---|---|
| BKpangF | ATGTGACCA ACACAGC | 2270-2285 |
| BKpangR | CTG TGCCATCAAACACC | 2578-2562 |
| BKpangP1 | AGGAGAACCCAGAGAGTGGA-fluorescein | 2497-2516 |
| BKpangP2 | LC-Red 640-GGCAGCCTATGTATGGTATGGAA-phosphate | 2519-2541 |
GenBank accession number V01108.
Establishment of a standard DNA for rt-QPCR.
A 584-bp DNA fragment was amplified using primers designed from the same BKV VP1 region covering the full length of the target sequences (forward, 5′-GTACTATAACCCCTAAAAACC-3′; reverse, 5′-ATGTACAATAAAAGCACCTG-3′; GenBank accession number V01108). The DNA fragment was purified from the PCR product and inserted into plasmid DNA using a QIAGEN PCR clone kit according to the manufacturer's protocol (QIAGEN Inc., Ontario, Canada). Plasmid DNA was extracted using the Mini Prep kit (QIAGEN Inc.), and the quantity of plasmid DNA was measured with a spectrophotometer (Ultrospec 3000; Pharmacia Biotech, Cambridge, England). A series of log dilutions (from 9.3 to 0.3 genome copies) was later prepared to establish an external standard curve for the rt-QPCR.
LightCycler PCR.
DNA was extracted from 200 μl of urine and/or plasma using a QIAGEN DNA minikit according to the manufacturer's protocol (QIAGEN Inc., Ontario, Canada) and was eluted from the column with 200 μl of elution buffer for urine and 50 μl for plasma. Twenty microliters of the PCR mixture, containing 10 μl DNA solution, 4 mM MgCl2, 0.5 μM each primer, 0.2 μM each probe, and 2 μl of the reagent from a LightCycler-FastStart DNA Master hybridization probe kit (Roche Diagnostics), was added to the LightCycler capillaries (Roche Diagnostics). The thermal cycles were as follows: an initial 10 min at 95°C, followed by 45 cycles of 5 s of denaturation at 95°C, 10 s of annealing at 50°C, and 15 s of extension at 72°C. The specificity of the fluorescence signal was checked by a melting curve analysis after each reaction. The melting temperature of the specific probes was 62 ± 0.5°C.
Precision was analyzed based on (i) the noise band crossing point values of 60 replicated plasmid DNAs (3.3 and 6.3 log10 copies) for the external standard curves during routine PCR performance and (ii) log10 copies per milliliter for 6 replicates of two clinical urine and plasma samples. Plasmid DNA containing a whole JC virus genomic fragment and clinical samples known to be positive for simian virus 40 (SV40), Epstein-Barr virus, or cytomegalovirus were cross-analyzed using selected primers for BKV to determine the specificity of the assay.
Statistical methods.
The correlation of log BKV loads in urine and plasma was analyzed with a linear regression model. The BKV detection rates for the study and control groups were analyzed by the Fisher exact test. Differences between the peak BKV loads for different groups were calculated using the nonparametric Kruskal-Wallis analysis of variance test (STATISTICA, Tulsa, OK). The precision of the rt-QPCR for detecting BKV was expressed by a coefficient of variation (CV). The confidence interval was set at 95% with the significant level at a P value of <0.05.
RESULTS
Evaluation of the rt-QPCR assay.
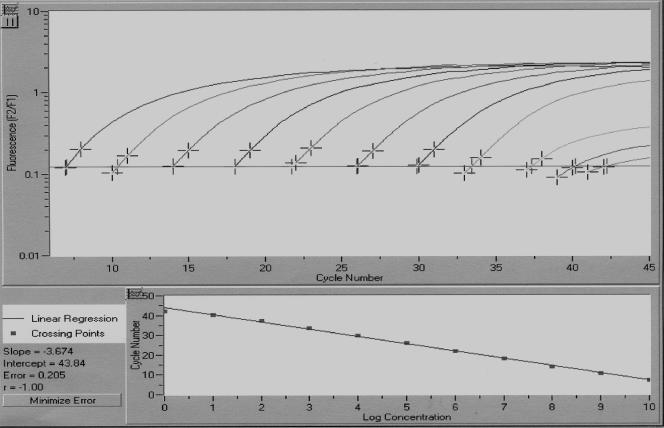
A minimum of 2 copies of target genomic BKV DNA in a reaction mixture could be detected in 1 out of 5 repeat rt-QPCR runs (20%), and the range of 1.0 to 10 log10 copies was detected in all of 5 rt-QPCR runs (100%) against known standard plasmid DNA (data not shown). Since DNAs from 40 μl of plasma and 10 μl of urine were used for starting the PCR, the low thresholds for BKV DNA detection were 2.4 log10 copies/ml of plasma and 3.0 log10 copies/ml of urine. The correlation between the noise band crossing points and log DNA copy numbers revealed a good negative linear relationship (r = −1; P < 0.0001) (Fig. 1). The other selected viruses, JC virus, SV40, Epstein-Barr virus, and cytomegalovirus, did not cross-react with BKV in the rt-QPCR. The intra-assay CVs were 5.7% and 5.9% for 60 replicates of 6.3 and 3.3 log10 copies of plasmid DNA versus 4.1% and 5.0% for 6 replicates of urine and plasma samples, respectively.
FIG. 1.
Standard curve for BKV rt-QPCR. BKV plasmid DNA, in serial dilutions ranging from 0 to 10.0 log10 copies, was amplified by rt-QPCR. Fluorescence intensity was plotted against cycle number. Slope, −3.67; intercept, 43.04; error, 0.21; r = −1.
Detection and quantitation of BKV viruria and viremia.
Overall, BKV viruria only or both viruria and viremia were detected for 100% (8 out of 8) of RTR in the BKVAN group, 23% (36 out of 155) of RTR in the SV group, and none of the LD (0 of 20) or NT-CKD patients (0 of 20). There was a significant difference in BKV viruria and viremia rates between samples from the BKVAN and SV groups (P < 0.001). In samples obtained at various time points after the diagnosis of BKVAN, 34 out of 34 urine samples (100%) and 46 out of 77 plasma samples (60%) were positive. In the SV group, 119 of 533 urine samples (22%) were positive. Of 106 plasma samples obtained from viruric patients in this group, 25 (24%) were positive. Rates of BKV detection in urine and plasma samples by group are summarized in Table 2.
TABLE 2.
Detection and quantitation of BKV loads in urine and plasma specimens from study and control groups
| Group | No. of patients | No. of specimens
|
BKV PCR result and viral load
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Urine | Plasma | Urine
|
Plasma
|
||||||
| No. (%) of positive patients | No. (%) of positive samples | Median log10 copies/ ml (range) | No. (%) of positive patients | No. (%) of positive samples | Median log10 copies/ml (range) | ||||
| RTR | |||||||||
| SV | 155 | 533 | 106 | 36 (23.2) | 119 (22.3) | 0 (0-10.83) | 6 (3.9) | 25 (23.6) | 0 (0-5.65) |
| BKVAN | 8 | 34 | 77 | 7 (100) | 34 (100) | 9.51 (6.49-10.83) | 8 (100) | 46 (60) | 2.84 (0-5.86) |
| Control groups | |||||||||
| LD | 20 | 20 | 20 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| NT-CKD | 20 | 20 | 20 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
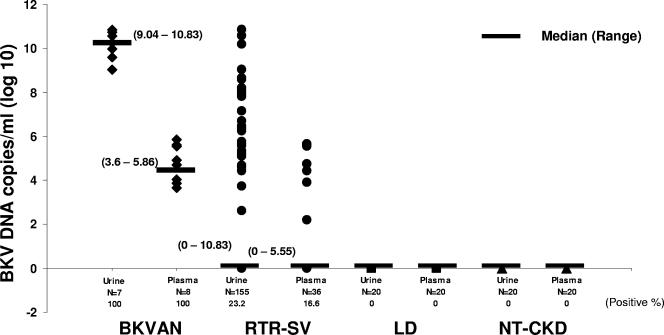
Quantitation of BKV viruria showed that the median BKV loads for the BKVAN and SV groups were 9.51 (range, 6.49 to 10.83) and 0 (range, 0 to 10.83) log10 copies/ml, respectively. The median BKV loads in plasma for the BKVAN and SV groups were 2.84 (range, 0 to 5.86) and 0 (range, 0 to 5.63) log10 copies/ml, respectively. To examine the relationships between viruria and viremia in the BKVAN and SV groups, we took the sample with the highest viral load obtained from each patient and designated it the peak BKV load for the individual. The median (range) peak BKV loads for viruria versus viremia were 10.26 (9.04 to 10.83) versus 4.83 (3.65 to 5.86) log10 copies/ml for the BKVAN group and 0 (0 to 10.83) versus 0 (0 to 5.63) log10 copies/ml for the SV group, respectively (P < 0.001) (Fig. 2).
FIG. 2.
Distribution of peaks of BKV loads (expressed as log10 BKV DNA copies per milliliter) in urine and plasma from four groups: BKVAN, SV, LD, and NT-CKD. The highest viral load was detected for the BKVAN group, followed by the SV group. Short lines indicate medians, and ranges are given in parentheses.
Correlation of BKV loads in urine and plasma.
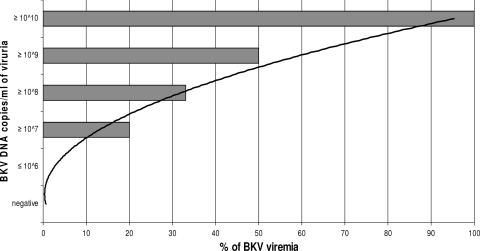
There were 135 occasions on which both plasma and urine samples were available for BKV PCR analysis at the same time point for individuals in the BKVAN and SV groups. Of these paired urine and plasma samples, 64% (86 out of 135) of urine samples and 19% (26 out of 135) of plasma samples were BKV positive. The median BKV loads in urine and plasma samples were 4.95 (range, 0 to 10.83) and 0 (range, 0 to 5.63) log10 copies/ml. BKV viremia was never detected when viruria levels were <6.0 log10 copies/ml. Conversely, BKV viremia was detected in 20%, 33%, 50%, and 100% of patients with urine BKV loads of 7.0, 8.0, 9.0, and ≥10.0 log10 copies/ml, respectively (Fig. 3).
FIG. 3.
Clustered bar graph showing the relationship between the detectable BKV load in the urine and the corresponding proportion of RTR whose plasma samples were positive for BKV. Among 86 BKV viruria samples, 31 had ≤6.0, 13 had ≥7.0, 18 had ≥8.0, 15 had ≥9.0, and 9 had ≥10.0 log10 DNA copies/ml. The trend line indicates a possible prediction of the chance of detectable BKV in plasma based on the level of BKV viruria.
DISCUSSION
Measurement of BKV loads in the urine and plasma of RTR is a powerful tool for identifying patients at risk of developing BKVAN and for monitoring the virologic response to therapy by such patients (13, 19, 26). In the present study, we described a novel rt-QPCR using the LightCycler probing system for detection and quantitation of BKV viruria and viremia. The rt-QPCR assay demonstrated superior sensitivity in the detection of BKV viruria compared to either EM or urine cytology performed on the same samples (unpublished data). A further benefit of monitoring with rt-QPCR is that urine and plasma samples are stable during transport and preparation for rt-QPCR. In contrast, specimens for EM or urine cytology must be processed within a relatively short time frame, a particular problem in monitoring patients who live far away from the transplant center. Thus, routine monitoring for viruria by rt-QPCR appears to be a very useful strategy for prospective surveillance of RTR.
The rt-QPCR showed very good precision in BKV viral quantitation in this study. The intra-assay CV was below 5.9% for plasmid DNA and 5.0% for clinical samples in precision testing. Although the acceptable limits for CVs for the evaluation of the precision of a quantitative PCR assay have not been well defined, the values in the current study were better than those observed in other, similar studies (12, 28). We believe that our rt-QPCR assay, developed in-house, provided a reliable diagnostic tool for monitoring BKV levels sequentially in both urine and plasma samples over time for transplant recipients. Since BKV shares high sequence homology with JC virus and SV40 (as much as 75%), the sequence region for designing specific primers is restricted. Selected primers yielding a longer amplicon in real-time PCR may lead to low sensitivity. We demonstrated that specific primers designed for the 309-bp amplicon were able to differentiate BKV from JC virus and SV40 specifically and that their sensitivity was not compromised in the rt-PCR assay in this study.
A wide range of viral DNA loads, from 2.4 to 11 log10 DNA copies per ml, could be detected in both urine and plasma samples with this assay. A wide dynamic range allows identification of patients with very high viral loads in the urine who may be at particular risk of developing BKVAN while still proving useful for prospective monitoring of patients with low-level BKV reactivation. In addition, the wide range offers the advantage of being able to quantify both the large amount of BKV in the urine and the typically low BKV load in plasma samples in the same PCR run, providing a reliable method for studying the relationship between BKV viruria and viremia.
The role of quantitation of viruria levels in routine surveillance is still uncertain. Few studies have directly examined the association between BKV viremia and viruria (5, 12, 17, 19, 26). High levels of viruria are probably predictive of a greater risk of BKV viremia and BKVAN. Randhawa et al. and Herman et al. reported that BKV viremia was associated with significantly higher urinary viral shedding (12, 26). However, there was no clear threshold level of urinary viral load that could predict viremia, although a linear correlation between viruria and viremia was observed. In the study reported here, BKV viremia was never detected when the urine viral load was less than 7.0 log10 copies/ml. However, detectable viremia increased to 20, 33, 50, and 100% when the quantitation of viruria reached 7.0, 8.0, 9.0, and ≥10.0 log10 copies/ml, correspondingly. Therefore, 7.0 log10 copies/ml of BKV DNA in the urine may be a cutoff that predicts the risk of viremia. This finding supports a BKV monitoring strategy based primarily on urinary surveillance by rt-QPCR, with monitoring of plasma only if urine viral loads rise above this threshold. We have not seen a single case in which viremia was identified without detectable viruria (data not shown). Urine samples are easily collected and more stable than blood during transportation, and implementation of the rt-QPCR as the first-line test for BK monitoring in RTR would be more cost-effective than plasma monitoring.
Using our rt-QPCR, we were able to detect BKV viruria and viremia in 100% of the BKVAN group and in 23% and 3.9% of the SV group of RTR, respectively. Importantly, no sample obtained from patients in the negative-control groups (LD and NT-CKD patients) was positive by rt-QPCR. The prevalences of viruria and viremia in this study are consistent with those in previous studies (12, 13, 22, 25). One of the major challenges in establishing a reliable diagnostic assay is to be able to differentiate between asymptomatic viruria/viremia and disease, namely, BKVAN. Viremia is a better predictor of BKVAN than viruria, with 100% sensitivity and a positive predictive value of 85% in one study (23). Randhawa et al. suggested predictive values for active BKVAN of 7.0 and 3.7 log10 copies/ml of BKV in viruria and viremia, respectively (26). In the study reported here, no patient in the SV group developed biopsy-proven BKVAN during the study period. Thus, the degree of viruria or viremia predictive of clinical disease using our rt-QPCR assay cannot yet be determined. The value of prospective monitoring of BKV infection in RTR remains controversial. Two published prospective studies on BKV monitoring of RTR have reached different conclusions. Hirsch et al. observed BKV replication indicated by decoy cell shedding in urine, BKV viremia detected by PCR, and BKVAN detected by histopathology in 30%, 13%, and 8% of RTR, respectively, and noted that BKV viremia appeared several weeks to months prior to the histopathological changes of BKVAN. They concluded that monitoring of viruria and viremia by PCR was useful in identifying patients at risk for BKVAN, since immunosuppressive therapy for such patients could be tailored for those with viremia (13). However, Bressollette-Bodin et al. followed all the RTR in their region during the first year after transplantation and found that BKV viruria and viremia were not useful in predicting BKVAN (5).
A detailed analysis of the correlation of BKV viruria and viremia with the clinical course, immunosuppressive regimen, and occurrence of BKVAN during follow-up is under way with a larger cohort of patients in our center. However, we believe that the rt-QPCR assay developed in our laboratory, with its sensitive, specific, and accurate quantification of BKV loads in urine and plasma, will be a useful tool for identifying patients at risk for BKVAN, allowing for preemptive management through modification of immunosuppression.
Acknowledgments
We acknowledge Jennifer Yang and Kimberly Martin for technical support in the study.
This study was funded by Astellas Canada Inc.
Footnotes
Published ahead of print on 12 September 2007.
REFERENCES
- 1.Andrews, C. A., K. V. Shah, R. W. Daniel, M. S. Hirsch, and R. H. Rubin. 1988. A serological investigation of BK virus and JC virus infections in recipients of renal allografts. J. Infect. Dis. 158:176-181. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, R. R., S. Dagostin, and K. V. Shah. 1989. Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J. Clin. Microbiol. 27:1174-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binet, I., V. Nickeleit, H. H. Hirsch, O. Prince, P. Dalquen, F. Gudat, M. J. Mihatsch, and G. Thiel. 1999. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation 67:918-922. [DOI] [PubMed] [Google Scholar]
- 4.Brennan, D. C., I. Agha, D. L. Bohl, M. A. Schnitzler, K. L. Hardinger, M. Lockwood, S. Torrence, R. Schuessler, T. Roby, M. Gaudreault-Keener, and G. A. Storch. 2005. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am. J. Transplant. 5:582-594. [DOI] [PubMed] [Google Scholar]
- 5.Bressollette-Bodin, C., M. Coste-Burel, M. Hourmant, V. Sebille, E. Andre-Garnier, and B. M. Imbert-Marcille. 2005. A prospective longitudinal study of BK virus infection in 104 renal transplant recipients. Am. J. Transplant. 5:1926-1933. [DOI] [PubMed] [Google Scholar]
- 6.Chan, P. K., K. W. Ip, S. Y. Shiu, E. K. Chiu, M. P. Wong, and K. Y. Yuen. 1994. Association between polyomaviruria and microscopic haematuria in bone marrow transplant recipients. J. Infect. 29:139-146. [DOI] [PubMed] [Google Scholar]
- 7.Colvin, R. B., and S. Mauiyyedi. 2001. Differential diagnosis between infection and rejection in renal allografts. Transplant Proc. 33:1778-1779. [DOI] [PubMed] [Google Scholar]
- 8.Drachenberg, C. B., C. O. Beskow, C. B. Cangro, P. M. Bourquin, A. Simsir, J. Fink, M. R. Weir, D. K. Klassen, S. T. Bartlett, and J. C. Papadimitriou. 1999. Human polyoma virus in renal allograft biopsies: morphological findings and correlation with urine cytology. Hum. Pathol. 30:970-977. [DOI] [PubMed] [Google Scholar]
- 9.Drachenberg, C. B., J. C. Papadimitriou, R. Wali, J. Nogueira, S. Mendley, H. H. Hirsch, C. B. Cangro, D. K. Klassen, M. R. Weir, S. T. Bartlett, and E. Ramos. 2004. Improved outcome of polyoma virus allograft nephropathy with early biopsy. Transplant Proc. 36:758-759. [DOI] [PubMed] [Google Scholar]
- 10.Drachenberg, C. B., J. C. Papadimitriou, R. Wali, C. L. Cubitt, and E. Ramos. 2003. BK polyoma virus allograft nephropathy: ultrastructural features from viral cell entry to lysis. Am. J. Transplant. 3:1383-1392. [DOI] [PubMed] [Google Scholar]
- 11.Drachenberg, R. C., C. B. Drachenberg, J. C. Papadimitriou, E. Ramos, J. C. Fink, R. Wali, M. R. Weir, C. B. Cangro, D. K. Klassen, A. Khaled, R. Cunningham, and S. T. Bartlett. 2001. Morphological spectrum of polyoma virus disease in renal allografts: diagnostic accuracy of urine cytology. Am. J. Transplant. 1:373-381. [PubMed] [Google Scholar]
- 12.Herman, J., M. Van Ranst, R. Snoeck, K. Beuselinck, E. Lerut, and R. Van Damme-Lombaerts. 2004. Polyomavirus infection in pediatric renal transplant recipients: evaluation using a quantitative real-time PCR technique. Pediatr. Transplant. 8:485-492. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch, H. H., W. Knowles, M. Dickenmann, J. Passweg, T. Klimkait, M. J. Mihatsch, and J. Steiger. 2002. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N. Engl. J. Med. 347:488-496. [DOI] [PubMed] [Google Scholar]
- 14.Hogan, T. F., B. L. Padgett, D. L. Walker, E. C. Borden, and J. A. McBain. 1980. Rapid detection and identification of JC virus and BK virus in human urine by using immunofluorescence microscopy. J. Clin. Microbiol. 11:178-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan, T. F., E. C. Borden, J. A. McBain, B. L. Padgett, and D. L. Walker. 1980. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann. Intern. Med. 92:373-378. [DOI] [PubMed] [Google Scholar]
- 16.Kadambi, P. V., M. A. Josephson, J. Williams, L. Corey, K. R. Jerome, S. M. Meehan, and A. P. Limaye. 2003. Treatment of refractory BK virus-associated nephropathy with cidofovir. Am. J. Transplant. 3:186-191. [DOI] [PubMed] [Google Scholar]
- 17.Leung, A. Y., M. Chan, S. C. Tang, R. Liang, and Y. L. Kwong. 2002. Real-time quantitative analysis of polyoma BK viremia and viruria in renal allograft recipients. J. Virol. Methods 103:51-56. [DOI] [PubMed] [Google Scholar]
- 18.Limaye, A. P., K. R. Jerome, C. S. Kuhr, J. Ferrenberg, M. L. Huang, C. L. Davis, L. Corey, and C. L. Marsh. 2001. Quantitation of BK virus load in serum for the diagnosis of BK virus-associated nephropathy in renal transplant recipients. J. Infect. Dis. 183:1669-1672. [DOI] [PubMed] [Google Scholar]
- 19.Merlino, C., M. Bergallo, G. Gribaudo, G. Gregori, G. Paolo Segoloni, F. Giacchino, A. N. Ponzi, and R. Cavallo. 2003. Polyomavirus BK DNA quantification assay to evaluate viral load in renal transplant recipients. J. Clin. Virol. 28:265-274. [DOI] [PubMed] [Google Scholar]
- 20.Mylonakis, E., N. Goes, R. H. Rubin, A. B. Cosimi, R. B. Colvin, and J. A. Fishman. 2001. BK virus in solid organ transplant recipients: an emerging syndrome. Transplantation 72:1587-1592. [DOI] [PubMed] [Google Scholar]
- 21.Nickeleit, V., H. H. Hirsch, I. F. Binet, F. Gudat, O. Prince, P. Dalquen, G. Thiel, and M. J. Mihatsch. 1999. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J. Am. Soc. Nephrol. 10:1080-1089. [DOI] [PubMed] [Google Scholar]
- 22.Nickeleit, V., T. Klimkait, I. F. Binet, P. Dalquen, V. Del Zenero, G. Thiel, M. J. Mihatsch, and H. H. Hirsch. 2000. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N. Engl. J. Med. 342:1309-1315. [DOI] [PubMed] [Google Scholar]
- 23.Nickeleit, V., H. H. Hirsch, M. Zeiler, F. Gudat, O. Prince, G. Thiel, and M. J. Mihatsch. 2000. BK-virus nephropathy in renal transplants—tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol. Dial. Transplant. 15:324-332. [DOI] [PubMed] [Google Scholar]
- 24.Ramos, E., C. B. Drachenberg, M. Portocarrero, R. Wali, D. K. Klassen, J. C. Fink, A. Farney, H. Hirsch, J. C. Papadimitriou, C. B. Cangro, M. R. Weir, and S. T. Bartlett. 2002. BK virus nephropathy diagnosis and treatment: experience at the University of Maryland Renal Transplant Program. Clin. Transpl. 2002:143-153. [PubMed] [Google Scholar]
- 25.Randhawa, P., J. Uhrmacher, W. Pasculle, A. Vats, R. Shapiro, B. Eghtsead, and K. Weck. 2005. A comparative study of BK and JC virus infections in organ transplant recipients. J. Med. Virol. 77:238-243. [DOI] [PubMed] [Google Scholar]
- 26.Randhawa, P., A. Ho, R. Shapiro, A. Vats, P. Swalsky, S. Finkelstein, J. Uhrmacher, and K. Weck. 2004. Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J. Clin. Microbiol. 42:1176-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randhawa, P. S., S. Finkelstein, V. Scantlebury, R. Shapiro, C. Vivas, M. Jordan, M. M. Picken, and A. J. Demetris. 1999. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation 67:103-109. [DOI] [PubMed] [Google Scholar]
- 28.Randhawa, P. S., A. Vats, D. Zygmunt, P. Swalsky, V. Scantlebury, R. Shapiro, and S. Finkelstein. 2002. Quantitation of viral DNA in renal allograft tissue from patients with BK virus nephropathy. Transplantation 74:485-488. [DOI] [PubMed] [Google Scholar]
- 29.Randhawa, P. S., M. I. Minervini, M. Lombardero, R. Duquesnoy, J. Fung, R. Shapiro, M. Jordan, C. Vivas, V. Scantlebury, and A. Demetris. 2000. Biopsy of marginal donor kidneys: correlation of histologic findings with graft dysfunction. Transplantation 69:1352-1357. [DOI] [PubMed] [Google Scholar]
- 30.Reploeg, M. D., G. A. Storch, and D. B. Clifford. 2001. BK virus: a clinical review. Clin. Infect. Dis. 33:191-202. [DOI] [PubMed] [Google Scholar]
- 31.Shah, K. V., R. W. Daniel, and R. M. Warszawski. 1973. High prevalence of antibodies to BK virus, an SV40-related papovavirus, in residents of Maryland. J. Infect. Dis. 128:784-787. [DOI] [PubMed] [Google Scholar]
- 32.Whiley, D. M., I. M. Mackay, and T. P. Sloots. 2001. Detection and differentiation of human polyomaviruses JC and BK by LightCycler PCR. J. Clin. Microbiol. 39:4357-4361. [DOI] [PMC free article] [PubMed] [Google Scholar]