Abstract
The prevalence of urogenital Chlamydia trachomatis infection was determined with a PCR-based test of women from low- and high-risk populations in Iloilo City, Philippines, between August 2002 and March 2006. Two rapid tests for C. trachomatis, Clearview Chlamydia MF and the Chlamydia Rapid Test (CRT), were also evaluated in these resource-limited settings. Specimens were obtained from female sex workers (FSWs; n = 1,484) attending a social hygiene clinic (SHC) and from women (n = 838) attending an obstetrics-gynecology (OB-GYN) clinic. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the rapid tests were determined, with PCR as the gold standard. The PCR positivity rate for SHC participants (72% asymptomatic) ranged from 17.9 to 32.0% during the study period. Compared with those of PCR, the sensitivities and specificities of the Clearview test were 53.5 and 99.1%, respectively, with endocervical swab specimens (CS; n = 822) from the FSWs and 31.1 and 95.2%, respectively, with vaginal swab specimens (VS; n = 333) from these women. The sensitivity, specificity, PPV, and NPV of the CRT with VS from the FSWs were 71.0, 99.0, 97.1, and 87.9%, respectively. At the OB-GYN site, the PCR positivity rate with VS was 6.3%. The sensitivity, specificity, PPV, and NPV of the CRT with these specimens were 86.8, 99.6, 93.9, and 99.1%, respectively. The performance of the Clearview test at the SHC was thus markedly lower with VS than with CS, whereas the CRT performed well with VS from both populations.
Chlamydia trachomatis is responsible for the most common sexually transmitted bacterial infection and the leading cause of pelvic inflammatory disease in women (34, 38). In 1999, the World Health Organization (WHO) estimated that 92 million new chlamydial infections occur worldwide annually, with the largest proportion (43 million) of these infections being contracted in south and Southeast Asia (38), a region that is mostly resource limited with respect to infectious disease diagnosis and management.
Although up to 94% of women with urogenital chlamydial infections do not manifest obvious symptoms (20, 28), the consequences of such untreated infections, including pelvic inflammatory disease, ectopic pregnancy, and infertility (27), generate a global financial burden of billions of dollars annually. Because most infected individuals are either asymptomatic or have mild, nonspecific symptoms, C. trachomatis infection poses a problem for health control programs. National screening and prevention programs for C. trachomatis infection have been implemented, usually among young adults, in industrialized countries (11, 14, 16). With the exception of sporadic testing of commercial sex workers, Chlamydia screening is rare in developing countries, which have the highest incidence of new chlamydial infections (7, 9, 17, 25). The major barriers to screening programs in the developing world include the high cost of testing and the lack of appropriate staff and diagnostic tools. Such countries have thus not benefited from the advent of highly sensitive nucleic acid amplification tests (NAATs). Indeed, rather than relying on microbiological testing, most clinics in resource-limited settings continue to implement treatment based on syndromic management algorithms for sexually transmitted infections (STIs), an approach that lacks specificity for C. trachomatis infection in women (6, 7). The availability of point-of-care (POC) rapid tests that are robust, sensitive, specific, and cost-effective would allow expanded diagnosis and screening for C. trachomatis infection in these settings. With this goal in mind, the WHO STD Diagnostic Initiative established the ASSURED criteria for POC tests, which outline the essential requirements for such assays: affordable, sensitive, specific, user friendly, rapid and robust, equipment free, and deliverable to end users (29).
Previous estimates of the prevalence of chlamydial infection among female sex workers (FSWs) in the Philippines, where such infection is a notifiable disease, have ranged from 27 to 36% (8, 35). Although these estimates were based on the results of diagnostic tests with differing performance characteristics, they suggest that chlamydial infection is common in this population (8). Despite its high prevalence, testing for C. trachomatis infection is not performed at the social hygiene clinics (SHCs), which are present in 140 cities in the Philippines. The SHCs constitute a network with the aim of promoting sexual health among commercial sex workers and preventing STI transmission (39).
The present study was initiated in 2002 to determine the prevalence of C. trachomatis infection among FSWs attending an SHC in Iloilo City, Philippines. The study was later expanded to include attendees of an obstetrics-gynecology (OB-GYN) clinic of the Western Visayas Medical Center (WVMC), also in Iloilo City. C. trachomatis was detected with a NAAT (Amplicor CT/NG PCR; Roche Diagnostic Systems, Branchburg, NJ) performed with vaginal swab (VS) or endocervical swab (CS) specimens or both. The performance of a rapid test for Chlamydia (Clearview Chlamydia MF; Inverness Medical, Bedford, United Kingdom) was evaluated relative to these results. In 2005, when a new rapid test for Chlamydia (CRT), developed at the University of Cambridge, became available, its performance was also evaluated at both the SHC and OB-GYN clinic in Iloilo City.
MATERIALS AND METHODS
Participants.
All FSWs attending the SHC in Iloilo City for routine STI screening for gonorrhea, syphilis, and nongonococcal urethritis (NGU) during six 2- to 3-week periods between August 2002 and March 2006 were invited to join the study. The FSWs worked in nightclubs and massage parlors, and some were also recruited to the study by SHC nurses and doctors in an outreach program. Consecutive patients attending the OB-GYN clinic at WVMC for antenatal care (pregnant women), family planning, or assessment of genitourinary symptoms between October 2005 and March 2006 were also recruited to the study. The SHC and OB-GYN components of the study were both approved by the Cambridge Research Ethics Committee and the Ethics Committee of WVMC. Patients from both clinics were eligible for the study if they were female, at least 18 years of age, willing to participate, literate, and not currently menstruating and had not taken antibiotics within the previous month. Written informed consent was obtained from all participants prior to specimen collection. Participants who withdrew from the study were not recorded.
Specimen collection.
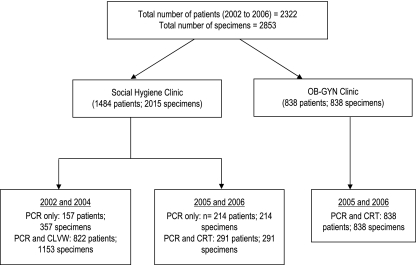
For SHC attendees, CS and/or VS specimens were collected from each participant by the attending clinician (Fig. 1). In an outreach program in 2002, the participants collected their own VS specimens. In 2004, subsets of specimens were collected as follows: paired CS specimens only in February 2004 and matched CS (n = 2) and VS (n = 2) specimens in June 2004 for PCR and Clearview Chlamydia MF testing. The VS and CS specimens were tested randomly with respect to order of collection with both PCR and Clearview Chlamydia MF assays. The SHC participants recruited in outreach settings were provided with written and verbal instructions on how to obtain two paired self-collected VS specimens. Between October 2005 and March 2006, only two VS specimens were collected by attending clinicians from each SHC and OB-GYN participant for PCR and/or CRT testing. All specimens were coded to ensure patient anonymity, and test results were conveyed to participants with utmost confidentiality. Specimens for PCR testing were frozen at −20°C at the time of sampling before they were transported to Cambridge and stored at −20°C until analysis. The VS or CS specimens were tested on-site with the rapid tests on the day of swab collection. Personnel performing the PCR and rapid tests were blinded to the order of collection of the two swabs, to comparator test results, and to clinical findings.
FIG. 1.
Specimen collection and testing algorithm followed in the study. CLVW, Clearview Chlamydia MF (Inverness Medical, Bedford, United Kingdom).
Testing by PCR.
CSs and VSs (collected either by the participant [SCVSs] or a clinician [CCVSs]) were collected with the BD Culturette EZ (Becton Dickinson Biosciences, San Jose, CA) and screened for the presence of C. trachomatis DNA with the Amplicor CT/NG PCR-based assay (Roche Molecular Systems, Branchburg, NJ), which targets a conserved region of the cryptic plasmid. All swabs were stored at 4°C within 1 h at the collection site and were transported to the testing site on cold packs. At the testing site, swabs were then placed in 3.0 ml of predispensed M4RT medium tubes of a specimen collection kit (Roche). Specimens were vigorously vortexed and stored overnight at 4°C to enhance the release of the adsorbed cells and tested with the Amplicor assay according to the instructions of the manufacturer. In brief, 100 μl of M4RT medium was added to 100 μl of CT/NG lysis buffer, provided by the manufacturer (specimen preparation kit; Roche) in a 1.7-ml SlickSeal microcentrifuge tube (Bioquote, York, United Kingdom) and mixed well by vortexing for 10 min at room temperature. With a new aerosol barrier tip for each sample, 200 μl of CT/NG specimen diluent was added to each tube, vortexed, and incubated for 10 min at room temperature and kept for less than 2 h before aliquots were transferred to reaction tubes containing the CT/NG reaction mixture (Roche). The reaction volume was 100 μl (50 μl of processed specimens and 50 μl of CT/NG reaction mixture). Samples that were inhibitory to PCR (as shown by failed amplification of the internal control) were diluted 1:1 with sample dilution buffer, heated at 95 to 100°C for 10 min, and then amplified. Amplification was performed with an 9700 thermal cycler (Applied Biosystems, Foster City, CA) according to the following protocol: 1 cycle of 50°C for 2 min and 95°C for 5 min, followed by 36 cycles of 93°C for 20 s, 61°C for 1 min, and 71°C for 40 s and then 1 cycle of 72°C for 5 min, after which samples were held at 72°C (>24 h) until detection.
Testing by Clearview Chlamydia MF.
In August 2002 and June 2004, paired CS and VS specimens were tested with the Clearview enzyme immunoassay performed on-site by study personnel according to the instructions of the manufacturer. These results were compared to the PCR results from paired CS specimens.
Testing by CRT.
VS specimens from the OB-GYN clinic participants and the FSWs were tested with the CRT by clinic personnel. All testers had passed the proficiency testing requirements according to Clinical and Laboratory Standards Institute guidelines (24). CRT testing was performed as described by Michel et al. (22), with some modifications. Briefly, each swab was subjected to extraction by sequential addition of 400 μl of reagent 1, 300 μl of reagent 2, and 100 μl of reagent 3 to the swab in a tapered sample preparation tube, with gentle mixing between additions. The sample preparation reagents were supplied in unit doses, thereby eliminating the need for precise pipetting. The extraction tube was then capped and used as a dropper to deliver 5 drops (∼100 μl) of the extracted sample to a tube containing the lyophilized amplification and detection reagents. The resulting mixture was agitated gently until a clear pink solution was obtained, after which the test strip, coated with a monoclonal antibody to chlamydial lipopolysaccharide and including a procedural control, was added to the solution and allowed to stand for 25 min before the result was read. Each swab was subjected to extraction twice in order to ensure that chlamydial antigen was extracted optimally from the viscous VS sample. Both CRT test strips were used in the interpretation of the result; a clearly visible test line on one or both test strips indicated a positive result, provided that the control lines were also visible on each test strip.
Treatment of PCR-positive participants.
Participants who tested positive for C. trachomatis by PCR were recalled to the clinic and offered antibiotic treatment according to the guidelines of the Philippine Department of Health: 200 mg of ofloxacin and 100 mg of doxycycline twice daily for 14 days from 2002 through 2003 or a single dose (1 g) of azithromycin (from 2004 through 2006). Pregnant women positive for C. trachomatis by PCR were administered 500 mg amoxicillin three times a day for 7 days.
Statistical analysis.
Data analysis was performed with SAS version 9.1 software. The 95% confidence intervals (CI) for sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated as an exact binomial. The prevalence of C. trachomatis infection at each site was calculated on the basis of PCR positivity, with the 95% CI being calculated according to the Wald method, and differences were statistically compared by chi-square test. Differences in the proportion of positive test results in a subset of data from paired specimens in June 2004 were assessed with McNemar's test. The Mantel-Henszel trend test was used to analyze the NGU, gonorrhea, and syphilis prevalence rates recorded by the SHC.
RESULTS
SHC.
Chlamydia testing by PCR was performed on a total of 2,015 (1,036 VS and 979 CS) specimens from FSWs (Table 1). Matched VS and CS specimens were obtained from participants attending the clinic in 2002 and in June 2004 as well as from FSWs in 18 different outreach settings in Iloilo City in November 2002. FSWs visit the SHC regularly for routine STI testing to comply with a government requirement for their profession. From 2000 to 2004, the SHC recorded annual prevalence rates of 10.5 to 26.0% for NGU, 1.5 to 3.7% for gonorrhea, and 2.1 to 5.4% for syphilis, with syphilis and gonorrhea showing significantly decreasing rates over time (P = 0.0079 and P < 0.0001, respectively). In contrast, NGU showed a significant increase in prevalence (P < 0.0001) over the same time period.
TABLE 1.
Prevalence of CT infection between 2002 and 2006 as determined by PCR at the SHC in Iloilo City (n = 1,484 patients)
| Date of sampling | Site of sample collection | Specimen type | No. of specimens | No. of positives | C. trachomatis positivity ratea (%) by PCR (95% CI) |
|---|---|---|---|---|---|
| August 2002 | Clinic | VS | 194 | 36 | 18.6 (13.1-24.0) |
| August 2002 | Clinic | CS | 196 | 35 | 17.9 (12.5-23.2) |
| November 2002 | On-siteb | VS | 200 | 36 | 18.0 (12.7-23.3) |
| November 2002 | Clinic | CS | 201 | 38 | 18.9 (13.5-24.3) |
| February 2004 | Clinic | CS | 300 | 62 | 20.7 (16.1-25.2) |
| June 2004 | Clinic | VS | 137 | 25 | 18.2 (11.8-24.7) |
| June 2004 | Clinic | CS | 282 | 57 | 20.2 (15.5-24.9) |
| October 2005 | Clinic | VS | 291 | 93 | 32.0 (26.6-37.3) |
| March 2006 | Clinic | VS | 214 | 47 | 22.0 (16.4-27.5) |
No significant difference (P = 0.87, χ2 test) in the C. trachomatis positivity rate (by PCR) over the study period was apparent with CS specimens. However, the C. trachomatis positivity rates (by PCR) with VS specimens (P = 0.0020) and with both CS and VS specimens (P = 0.0002) did show significant changes.
Specimens were collected by the participants in an outreach program.
The study was primarily aimed at determining the prevalence of C. trachomatis infection by PCR in these two sites. On certain testing occasions, when either Clearview or the CRT was available, the performance and utility of these two rapid tests were also determined.
The combined data from all patients (n = 1,484) attending the SHC using both specimen types indicated that the prevalence of C. trachomatis infection ranged from 17.9% (35/196) to 20.7% (62/300) in 2002 and 2004, increased to 32.0% (93/291) in 2005, and then decreased to 22.0% (47/214) in 2006 (P = 0.0002 for 2002 to 2004 versus 2006; Table 1). Analysis of C. trachomatis positivity rate by sample type revealed no significant difference over the study period for CS specimens (P = 0.87), but a statistically significant increase for VS specimens was apparent in 2005 (P = 0.002). In a subset of specimens consisting of paired CCVSs and CSs collected in 2004, C. trachomatis positivity determined from CCVS specimens from FSWs did not differ significantly (P = 0.2482) from that for CS specimens (Table 2).
TABLE 2.
Performance of Clearview Chlamydia MF (relative to PCR) with CS and VS specimens at the SHC in Iloilo Citya
| Yr | Total no. tested | Sample type | Sensitivity (%), 95% CI | Specificity (%), 95% CI | PPV (%), 95% CI | NPV (%), 95% CI |
|---|---|---|---|---|---|---|
| 2002 | 397 | CS | 56.2 (41/73), 44.8-67.6 | 99.4 (322/324), 98.5-100 | 95.4 (41/43), 89.1-100 | 91.2 (322/353), 88.3-94.2 |
| 2004 | 425 | CS | 51.2 (44/86), 40.6-61.7 | 98.8 (335/339), 97.7-100 | 91.7 (44/48), 83.9-99.5 | 88.9 (335/377), 85.7-92.0 |
| Total | 822 | CS | 53.5 (85/159), 45.7-61.2 | 99.1 (657/663), 98.4-99.8 | 93.4 (85/91), 88.3-98.5 | 90.0 (657/730), 87.8-92.2 |
| 2002 | 196 | SCVS | 25.0 (9/36), 10.9-39.2 | 93.8 (150/160), 90.0-97.5 | 47.4 (9/19), 24.9-69.8 | 84.7 (150/177), 79.4-90.0 |
| 2004 | 137 | CCVS | 40.0 (10/25), 20.8-59.2 | 97.3 (109/112), 94.3-100 | 76.9 (10/13), 54.0-99.8 | 87.9 (109/124), 82.2-93.6 |
| Total | 333 | SCVS and CCVS | 31.1 (19/61), 19.5-42.8 | 95.2 (259/272), 92.7-97.8 | 59.4 (19/32), 42.4-76.4 | 86.0 (259/301), 82.1-90.0 |
No significant difference in test performance between subsets of paired CS and VS specimens was apparent in 2004 (P = 0.2482; McNemar's test).
With PCR as the reference test, the sensitivity and specificity of the Clearview rapid test were 53.8% (85/158,) and 99.1% (657/663) for CS specimens, respectively, and 31.1% (19/61) and 95.2% (259/272) for VS specimens, respectively (Table 2). The PPV and NPV of the Clearview rapid test were 93.4 and 90.0%, respectively, for CS specimens and 59.4 and 86.0%, respectively, for VS specimens (Table 2). In comparison, CRT testing of the paired VS specimens showed a sensitivity of 71.0% (66/93) and a specificity of 99.0% (196/198), with a PPV and NPV of 97.1 and 87.9%, respectively (Table 3).
TABLE 3.
Performance of a new CRT on VSs using PCR as reference test in women attending the SHC and an OB-GYN clinic in Iloilo City
| Clinic (no. of patients) | Sensitivity (%), 95% CI | Specificity (%), 95% CI | PPV, 95% CI | NPV, 95% CI |
|---|---|---|---|---|
| OB-GYN (838) | 86.8 (46/53), 77.7-95.9 | 99.6 (782/785), 99.2-100.0 | 93.9 (46/49), 87.2-100.0 | 99.1 (782/789), 98.5-99.8 |
| SHC (291) | 71.0 (66/93), 60.5-79.7 | 99.0 (196/198), 96.0-99.8 | 97.1 (66/68), 88.8-99.5 | 87.9 (196/223), 82.7-91.7 |
The SHC attendees during the study period were derived from at least 18 registered nightclubs and massage parlors that employed approximately 310 FSWs. Demographic analysis of a subset of these women (n = 782, from June 2004 to March 2006) revealed an age range of 18 to 56 years (median, 25.8 years). Most of the study participants were asymptomatic at the time of specimen collection, with only 28% reporting genitourinary symptoms. Self-reported methods of contraception included condom use by partner (53%); oral contraception (12%); other means such as tubal ligation, intrauterine device, or depot medroxyprogesterone acetate (1%); and none (34%).
A subset of participants who were positive for C. trachomatis infection by PCR in October 2005 and who had been treated were followed up and retested in March 2006. Of the 88 women who had originally tested positive, 16 were retested and 4 (25%) were again found to be positive for C. trachomatis by PCR.
OB-GYN clinic.
Most of the participants at the OB-GYN clinic were pregnant women attending for routine antenatal care. The prevalence of C. trachomatis infection in this “low-risk” population was 6.3% (53/838) on the basis of PCR testing of VS specimens. Taking the PCR results as reference, the CRT showed a sensitivity and specificity of 86.8% (46/53) and 99.6% (782/785), respectively, with a PPV and NPV of 93.9 and 99.1%, respectively (Table 3).
The rates of PCR inhibition for the SHC and the OB-GYN samples were 2.4% (7/291) and 5% (42/838), respectively, during the 2005 and 2006 testing. Further processing by heating (at 95°C for 10 min) of inhibitory samples resulted in the resolution of 6 negatives and 1 positive for SHC and 41 negatives and 1 positive for OB-GYN. In the earlier testing dates at the SHC, when further processing by heating was not performed, about 4% of the inhibitory samples were unresolved and excluded from the study.
DISCUSSION
C. trachomatis infection is a reportable disease in the Philippines, yet routine screening is not performed, even among high-risk populations such as FSWs attending SHCs, because of financial and technical constraints. We have determined the prevalence of C. trachomatis infection among FSWs attending an SHC and among low-risk attendees of an OB-GYN clinic in Iloilo City. Both clinics test for gonorrhea and NGU by microscopic analysis of Gram-stained vaginal and urethral smears and for syphilis by the Venereal Disease Research Laboratory syphilis test but are not equipped to perform NAATs or enzyme immunoassays and do not have access to a stable power supply.
To determine the prevalence of C. trachomatis infection in these two populations, all specimens were tested by PCR. Due to logistical constraints, only a subset of these specimens were tested by Clearview or CRT. In the earlier studies, only CS specimens were tested using Clearview following the manufacturer's claim for direct qualitative detection of C. trachomatis in CSs and not in VSs. With subsequent testing, the sensitivity of Clearview on both CSs and VSs was determined to evaluate its performance on noninvasive specimens from sex workers.
The Roche Amplicor CT/NG PCR test was used as the reference because of its high sensitivity and specificity (2). Although this test is not intended for use with VS specimens, other studies have found that the performance of NAATs for C. trachomatis CSs specimens is similar to that for VS specimens (10, 15, 30, 33). This finding was supported by the results of the present study, as demonstrated by the similar PCR positivity rates for the matched CS and VS specimens (Table 1). For women infected with C. trachomatis, CS specimens contain the highest organism load, followed in decreasing order by VS specimens, urethral swabs, and first-void urine (23). However, despite their lower organism load than CS specimens, VS specimens are gaining acceptance as the specimen of choice for screening and diagnostic purposes because their collection is noninvasive, easier, and less expensive and their chlamydial load is sufficient for most tests to maintain sensitivity (33).
The prevalence of C. trachomatis infection among the FSWs remained high despite antibiotic treatment of C. trachomatis-infected women between each study interval. This finding is most likely due to chlamydial reinfection, given that only 53% of FSWs reported regular condom use. It is also possible that the increased prevalence observed in 2005 was due to new FSWs attending the clinic from outlying areas, given that geographically mobile sex workers are common in the Philippines. It was difficult to monitor the incidence of new FSWs attending the SHC, as some attendees would reregister at the clinic under different names. Furthermore, a 2004 update by WHO/Joint United Nations Programme on HIV/AIDS reported that Filipino FSWs averaged two to four clients per week and that these clients had two or more other sex partners, suggesting that reinfection might occur readily within this population, especially with a low rate of condom use (35). Repeat infection with C. trachomatis has been reported in 13 to 29% of previously infected women within 6 months of initial diagnosis and treatment (18), similar to the rate seen in the present study.
The SHC and OB-GYN clinic of the present study are resource-limited settings that do not perform routine C. trachomatis testing and were therefore selected for evaluation of the utility of rapid tests (Clearview and CRT) as potential screening and diagnostic tools for C. trachomatis infection. For the SHC population, the CRT had a sensitivity of 71.0% with VS specimens whereas Clearview Chlamydia MF had a sensitivity of 53.8% with CS specimens and 31.1% with VS specimens. The Clearview results are below the recommended sensitivity of 60% required for POC tests to be able to assist STI treatment interventions (36). Although testing by Clearview and the CRT was not performed side-by-side on the same specimens, the targeted populations were shown to be similar based on PCR positivity for C. trachomatis infection. Similar sensitivities for VS (32.8%) and CS (49.7%) specimens were described in a recent WHO study from China comparing Clearview Chlamydia MF with PCR for 1,497 women attending STI clinics or reeducation centers (40). Earlier studies comparing Clearview with other tests showed variable performance depending on the comparator test. For example, with cervical swabs, sensitivity and specificity against cell culture were 62.3% and 99.7%, respectively (19). With the PACE 2 assay and cell culture as comparator tests, Clearview showed a sensitivity of 72.9% and specificity of 98.9% (1). In another study, where patients were considered positive for C. trachomatis if three or more assays (Clearview, PACE 2 assay, AMP CT by Gen-Probe, and LCx [Abbott] assays; swab and/or urine) were positive, Clearview showed a sensitivity of 50% in 787 women attending an OB-GYN clinic (21).
The performance of the CRT was compared with PCR for both the FSWs and women attending the OB-GYN clinic. Although the analytical sensitivity of Amplicor PCR for VSs tends to be low (3, 4) because of inhibitors (3), this study included an internal control to monitor the potential effect of inhibition on the PCR results. Additional processing of inhibitory specimens resolved most inhibitory samples, thereby increasing the accuracy of PCR results. The CRT showed a higher sensitivity with the OB-GYN population (86.8%) than with the SHC population (71.0%). This finding was expected given that most FSWs used creams and other feminine hygiene products vaginally, which can interfere with the CRT (data not shown). Furthermore, the observed practice of repeated vaginal douching prior to STI screening to reduce the possibility of microscopic STI diagnosis may have lowered the C. trachomatis organism load in the VS specimens of the FSWs. Despite the lower sensitivity of the CRT with FSWs than with OB-GYN clinic attendees, its superior sensitivity and specificity, noninvasive sample type, instrument independence, and shorter total assay time offer clear advantages over the Clearview assay for use in the SHC setting.
The CRT uses a generic signal amplification system (SAS) that enhances the visual signal on a dipstick and a sample preparation procedure that overcomes signal inhibition and allows more efficient extraction of the target antigen. The SAS principle is based on an increase in the valency (and thus the size) of the colored immune complex, making it more visible. In the case of the conventional dipstick assay, the analyte-specific antibody (primary antibody) is conjugated to a colored particle such as gold or latex. In the SAS format, the detection signal is amplified by chemically coupling multiple copies of a hapten to the primary detection antibodies (amplification conjugate). To visualize the immune complex, an antihapten monoclonal antibody is conjugated to colored particles such as gold or colored latex. The labeling of the primary antibody with multiple haptens increases the valency of the immune complex, and the resulting lattice formed between the analyte, multiple-hapten-labeled primary antibody, and the antihapten color conjugate yields a larger complex, thus producing a stronger visual signal. The combined effect of the SAS and improved sample preparation procedure may have enabled the CRT to overcome the inherent limitations of other immunobased POC assays.
Whereas the CRT has a lower sensitivity than NAATs, it can be a useful tool for screening and diagnosis of C. trachomatis infection in resource-limited settings, where such testing is not currently available. Given that test results are available within 30 min, the CRT also enables the clinician to treat infected individuals immediately and to instigate contact tracing, potentially reducing onward transmission of infection, preventing disease progression, and overcoming the failure to treat individuals who are tested but lost to follow-up. This scenario exemplifies the “rapid test paradox” (12), in which a less sensitive rapid test combined with immediate on-site treatment ensures that more infected patients receive treatment than if screening is performed with a NAAT whose results are not available immediately. In the present study, it took 3 to 4 weeks for C. trachomatis-infected women to receive treatment for their infections. Treatment based on the CRT results would enable most infected women to be treated promptly, reducing adverse sequelae and onward transmission of infection. The CRT targets chlamydial lipopolysaccharide, so it is also capable of detecting C. trachomatis variants that might be missed by current plasmid-based NAATs (31).
C. trachomatis infections with low organism loads are most likely to elude detection by the CRT. However, these infections in women are less likely to be associated with multiple symptoms or clinical signs (23). It is those infections with high organism load that are more likely to lead to cervicitis or pelvic inflammatory disease.
In view of the consistently high prevalence of C. trachomatis infection among FSWs in the present study, as well as the potential synergistic role of STIs in human immunodeficiency virus (HIV) transmission (26, 37), it would be prudent to implement C. trachomatis screening within this population. A recent study of street- and brothel-based FSWs in Angeles City in the Philippines showed that the prevalence of gonorrhea and C. trachomatis infection declined after implementation of an intensive outreach and treatment program (39). Although the prevalence of C. trachomatis infection of 6.3% for the OB-GYN clinic was substantially lower than that for the SHC, it is still relatively high for a general population, especially given that most attendees are pregnant women, at risk of C. trachomatis-related pregnancy complications (5, 32) and neonatal ocular infection (13). A test such as the CRT would also enable effective intervention in these women as well as help to achieve long-term goals of STI and HIV control through sustained access to effective preventive and treatment services. Future research is warranted to determine the clinical significance and transmission dynamics of such infections in both men and women. Further studies are also required to evaluate the cost-effectiveness of the CRT versus NAATs and to determine its utility in other settings.
Acknowledgments
This work was supported by a grant (049366/Z/96/Z) from The Wellcome Trust, United Kingdom.
We thank the study participants of the SHC and OB-GYN clinic in Iloilo City; the nursing, clinical, and laboratory staff of the clinics for their assistance during the study; J. P. Allain and J. White for comments on the manuscript; and M. Cheang of the University of Manitoba for performing statistical analysis.
The authors from the University of Cambridge are equity holders of a spin-off company, Diagnostics for the Real World Ltd. (DRW), based on the rapid test technologies developed at the university. The University of Cambridge is also an equity holder of DRW. The remaining authors declare no potential conflicts of interest.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Blanding, J., L. Hirsch, N. Stranton, T. Wright, S. Aarnaes, L. M. de la Maza, and E. M. Peterson. 1993. Comparison of the Clearview Chlamydia, the PACE 2 assay, and culture for detection of Chlamydia trachomatis from cervical specimens in a low-prevalence population. J. Clin. Microbiol. 31:1622-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalker, V. J., H. Vaughan, P. Patel, A. Rossouw, H. Seyedzadeh, K. Gerrard, and V. L. A. James. 2005. External quality assessment for detection of Chlamydia trachomatis. J. Clin. Microbiol. 43:1341-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chernesky, M., D. Jang, K. Luinstra, S. Chong, M. Smieja, W. Cai, B. Hayhoe, E. Portillo, C. MacRitchie, C. Main, and R. Ewert. 2006. High analytical sensitivity and low rates of inhibition may contribute to detection of Chlamydia trachomatis in significantly more women by the Aptima Combo 2 assay. J. Clin. Microbiol. 44:400-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chernesky, M., S. Castriciano, D. Jang, and M. Smieja. 2006. Use of flocked swabs and a universal transport medium to enhance molecular detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J. Clin. Microbiol. 44:1084-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, I., J. C. Veille, and B. M. Calkins. 1990. Improved pregnancy outcome following successful treatment of chlamydial infection. JAMA 263:3160-3163. [PubMed] [Google Scholar]
- 6.Dallabetta, G. A., A. C. Gerbase, and K. K. Holmes. 1998. Problems, solutions, and challenges in syndromic management of sexually transmitted diseases. Sex. Transm. Infect. 74(Suppl. 1):S1-S11. [PubMed] [Google Scholar]
- 7.Davies, S. C., B. Otto, S. Partohudoyo, V. A. M. A. Chrisnadarmani, G. A. Neilsen, L. Ciaffi, J. Patten, T. Samson, and I. N. Sutama. 2003. Sexually transmitted infections among female sex workers in Kupang, Indonesia. Sex. Transm. Dis. 30:671-679. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health, Philippines, and WHO Regional Office of the Western Pacific. 2000. Consensus report on S. T. I., HIV and AIDS epidemiology: Philippines, p. 1-26. http://www.wpro.who.int/NR/rdonlyres/B1D6D9DB-B27D-4BC4-A382-AF16A42E4E16/0/Consensus_Report_PHL_2000.pdf.
- 9.Desai, V. K., J. K. Kosambiya, H. G. Thakor, D. D. Umrigar, B. R. Khandwala, and K. K. Bhuyan. 2003. Prevalence of sexually transmitted infections and performance of STI syndromes against aetiological diagnosis in female sex workers of red light area in Surat, India. Sex. Transm. Infect. 79:111-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domeika, M., and O. Drulyte. 2000. Use of PCR for the detection of genital Chlamydia trachomatis infection on self-obtained mailed vaginal samples. Acta Obstetr. Gynecol. Scand. 79:570-575. [PubMed] [Google Scholar]
- 11.Gaydos, C. A., M. R. Howell, B. Pare, K. L. Clark, D. A. Ellis, R. M. Hendrix, J. C. Gaydos, K. T. McKee, and T. C. Quinn. 1998. Chlamydia trachomatis infections in female military recruits. N. Engl. J. Med. 339:739-744. [DOI] [PubMed] [Google Scholar]
- 12.Gift, T. L., M. S. Pate, E. W. Hook III, and W. J. Kassler. 1999. The rapid test paradox: when fewer cases detected lead to more cases treated: a decision analysis of tests for Chlamydia trachomatis. Sex. Transm. Dis. 26:232-240. [DOI] [PubMed] [Google Scholar]
- 13.Hobson, D. 1983. Chlamydial infection in neonates and older children. Br. Med. Bull. 39:128-132. [DOI] [PubMed] [Google Scholar]
- 14.Holland-Hall, C. M., H. C. Wiesenfeld, and P. J. Murray. 2002. Self-collected vaginal swabs for the detection of multiple sexually transmitted infections in adolescent girls. J. Pediatr. Adolesc. Gynecol. 15:307-313. [DOI] [PubMed] [Google Scholar]
- 15.Hook, E. W., III, K. Smith, C. Mullen, J. Stephens, L. Rhinehardt, M. S. Pate, and H. H. Lee. 1997. Diagnosis of genitourinary Chlamydia trachomatis infections by using the ligase chain reaction on patient-obtained vaginal swabs. J. Clin. Microbiol. 35:2133-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howell, M. R., T. C. Quinn, and C. A. Gaydos. 1998. Screening for Chlamydia trachomatis in asymptomatic women attending family planning clinics: a cost-effective analysis of three strategies. Ann. Internal Med. 128:277-284. [DOI] [PubMed] [Google Scholar]
- 17.Kim, A., L. P. Sun, C. Chhorvann, C. Lindan, F. Van Griensven, P. H. Kilmarx, P. Sirivongrangson, J. K. Louie, H. B. Leng, and K. Page-Shafer. 2005. High prevalence of HIV and sexually transmitted infections among indirect sex workers in Cambodia. Sex. Transm. Dis. 32:745-751. [DOI] [PubMed] [Google Scholar]
- 18.Kjaer, H. O., G. Dimcevski, G. Hoff, F. Olesen, and L. Ostergaard. 2000. Recurrence of urogenital Chlamydia trachomatis infection evaluated by mailed samples obtained at home: 24 weeks' prospective follow up study. Sex. Transm. Infect. 76:169-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kluytmans, J. A. J. W., W. H. F. Goessens, J. W. Mouton, J. H. Van Rijsoort-Vos, H. G. M. Niesters, W. G. V. Quint, L. Habbema, E. Stolz, and J. H. T. Wagenvoort. 1993. Evaluation of Clearview and Magic Lite tests, polymerase chain reaction, and cell culture for detection of Chlamydia trachomatis in urogenital specimens. J. Clin. Microbiol. 31:3204-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korenromp, E. L., M. K. Sudaryo, S. J. de Vlas, R. H. Gray, N. K. Sewankambo, D. Serwadda, M. J. Wawer, and J. D. F. Habbema. 2002. What proportion of episodes of gonorrhoea and Chlamydia becomes symptomatic? Int. J. STD AIDS 13:91-101. [DOI] [PubMed] [Google Scholar]
- 21.Lauderdale, T.-L., L. Landers, I. Thorneycroft, and K. Chapin. 1999. Comparison of the PACE 2 assay, two amplification assays, and Clearview EIA for detection of Chlamydia trachomatis in female endocervical and urine specimens. J. Clin. Microbiol. 37:2223-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel, C.-E. E., A. W. Solomon, J. P. V. Magbanua, P. A. Massae, L. Huang, J. Mosha, S. K. West, E. C. B. Nadala, R. Bailey, C. Wisniewski, D. C. W. Mabey, and H. H. Lee. 2006. Field evaluation of a rapid point-of-care assay for targeting antibiotic treatment for trachoma control: a comparative study. Lancet 367:1585-1590. [DOI] [PubMed] [Google Scholar]
- 23.Michel, C.-E. E., C. Sonnex, C. A. Carne, J. A. White, J. P. V. Magbanua, E. C. B. Nadala, Jr., and H. H. Lee. 2007. Chlamydia trachomatis load at matched anatomic sites: implications for screening strategies. J. Clin. Microbiol. 45:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NCCLS. 2002. User protocol for evaluation of qualitative test performance. Approved guideline EP12-A, vol. 22, no. 14. NCCLS, Wayne, PA.
- 25.Nessa, K., S. A. Waris, A. Alam, M. Huq, S. Nahar, F. A. H. Chawdhary, S. Monira, M. U. Badal, J. Sultana, K. F. Mahmud, J. Das, D. K. Mitra, Z. Sultan, N. Hossain, and M. Rahman. 2005. Sexually transmitted infections among brothel-based sex workers in Bangladesh: high prevalence of asymptomatic infection. Sex. Transm. Dis. 32:13-19. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen, V. T., T. L. Nguyen, D. H. Nguyen, T. T. T. Le, T. T. N. Vo, T. B. V. Cao, and N. O'Farrell. 2005. Sexually transmitted infections in female sex workers in five border provinces of Vietnam. Sex. Transm. Dis. 32:550-556. [DOI] [PubMed] [Google Scholar]
- 27.Paavonen, J., and M. Lehtinen. 1996. Chlamydial pelvic inflammatory disease. Hum. Reprod. Update 2:519-529. [DOI] [PubMed] [Google Scholar]
- 28.Paxton, L. A., N. Sewankambo, R. Gray, D. Serwadda, D. McNairn, C. Li, and M. J. Wawer. 1998. Asymptomatic non-ulcerative genital tract infections in a rural Ugandan population. Sex. Transm. Infect. 74:421-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeling, R. W., K. K. Holmes, D. Mabey, and A. Ronald. 2006. Rapid tests for sexually transmitted infections (STIs): the way forward. Sex. Transm. Infect. 82(Suppl. 5):v1-v6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polaneczky, M., C. Quigley, L. Pollock, D. Dulko, and S. S. Witkin. 1998. Use of self-collected vaginal specimens for detection of Chlamydia trachomatis. Obstetr. Gynecol. 91:375-378. [DOI] [PubMed] [Google Scholar]
- 31.Ripa, T., and P. Nilsson. 2006. A variant of Chlamydia trachomatis with deletion in cryptic plasmid: implications for use of PCR diagnostic tests. Eur. Surveill. 11:E061109 2. [DOI] [PubMed] [Google Scholar]
- 32.Ryan, G. M., T. N. Abdella, S. G. McNeeley, V. S. Baselski, and D. E. Drummond. 1990. Chlamydia trachomatis infection in pregnancy and effect of treatment on outcome. Am. J. Obstetr. Gynecol. 162:34-39. [DOI] [PubMed] [Google Scholar]
- 33.Schachter, J. S., W. M. McCormack, M. A. Chernesky, D. H. Martin, B. Van Der Pol, P. A. Rice, E. W. Hook III, W. E. Stamm, T. C. Quinn, and J. M. Chow. 2003. Vaginal swabs are appropriate specimens for diagnosis of genital tract infection with Chlamydia trachomatis. J. Clin. Microbiol. 41:3784-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scholes, D., A. Stergachis, F. E. Heidrich, H. Andrilla, K. K. Holmes, and W. E. Stamm. 1996. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N. Engl. J. Med. 334:1362-1366. [DOI] [PubMed] [Google Scholar]
- 35.UNAIDS/WHO. 2004. Epidemiological fact sheets on HIV/AIDS and sexually transmitted infections: 2004 update, p. 1-14. http://data.unaids.org/Publications/Fact-Sheets01/philippines_EN.pdf.
- 36.Vickerman, P., C. Watts, M. Alary, D. Mabey, and R. W. Peeling. 2003. Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women. Sex. Transm. Infect. 79:363-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wasserheit, J. N. 1992. Epidemiological synergy: interrelationships between human immunodeficiency virus and other sexually transmitted diseases. Sex. Transm. Dis. 19:61-77. [PubMed] [Google Scholar]
- 38.WHO Department of HIV/AIDS. 1999. Global prevalence and incidence of selected curable sexually transmitted infections. www.who.int/docstore/hiv/GRSTI/000.htm. [PubMed]
- 39.Wi, T., E. R. Ramos, R. Steen, M. C. R. Roces, M. C. Lim-Quizon, G. Neilsen, and G. Dallabetta. 2006. STI declines among sex workers and clients following outreach, onetime presumptive treatment and regular screening of sex workers in the Philippines. Sex. Transm. Infect. 82:386-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin, Y.-P., R. W. Peeling, X.-S. Chen, K. L. Gong, H. Zhou, W.-M. Gu, H.-P. Zheng, Z.-S. Wang, G. Yong, W.-L. Cao, M.-Q. Shi, W.-H. Wei, X.-Q. Dai, X. Gao, Q. Chen, and D. Mabey. 2006. Clinic-based evaluation of Clearview Chlamydia MF for detection of Chlamydia trachomatis in vaginal and cervical specimens from women at high risk in China. Sex. Transm. Infect. 82:33-37. [DOI] [PMC free article] [PubMed] [Google Scholar]