Abstract
Aggregatibacter actinomycetemcomitans is frequently associated with localized aggressive periodontitis (LAP); however, longitudinal cohort studies relating A. actinomycetemcomitans to initiation of LAP have not been reported. A periodontal assessment was performed on 1,075 primarily African-American and Hispanic schoolchildren, ages 11 to 17 years. Samples were taken from each child for A. actinomycetemcomitans. A cohort of 96 students was established that included a test group of 38 A. actinomycetemcomitans-positive students (36 periodontally healthy and 2 with periodontal pockets) and 58 healthy A. actinomycetemcomitans-negative controls. All clinical and microbiological procedures were repeated at 6-month intervals. Bitewing radiographs were taken annually for definitive diagnosis of LAP. At the initial examination, clinical probing attachment measurements indicated that 1.2% of students had LAP, while 13.7% carried A. actinomycetemcomitans, including 16.7% of African-American and 11% of Hispanic students (P = 0.001, chi-square test). A. actinomycetemcomitans serotypes a, b, and c were equally distributed among African-Americans; Hispanic students harbored predominantly serotype c (P = 0.05, chi-square test). In the longitudinal phase, survival analysis was performed to determine whether A. actinomycetemcomitans-positive as compared to A. actinomycetemcomitans-negative students remained healthy (“survived”) or progressed to disease with attachment loss of >2 mm or bone loss (failed to “survive”). Students without A. actinomycetemcomitans at baseline had a significantly greater chance to remain healthy (survive) compared to the A. actinomycetemcomitans-positive test group (P = 0.0001). Eight of 38 A. actinomycetemcomitans-positive and none of 58 A. actinomycetemcomitans-negative students showed bone loss (P = 0.01). A. actinomycetemcomitans serotype did not appear to influence survival. These findings suggest that detection of A. actinomycetemcomitans in periodontally healthy children can serve as a risk marker for initiation of LAP.
Aggregatibacter actinomycetemcomitans was first identified as a possible periodontal pathogen in 1975 in studies of localized juvenile periodontitis, now known as localized aggressive periodontitis (LAP) (36, 43). Since then, most cross-sectional studies have shown A. actinomycetemcomitans to be highly associated with periodontal disease in adolescents (44, 51). However, longitudinal cohort studies relating A. actinomycetemcomitans to the initiation and progression of periodontal disease in adolescents are lacking (7). The discovery that A. actinomycetemcomitans produced a toxin that killed polymorphonuclear leukocytes and monocytes coupled with the identification of the leukotoxin (ltx) gene operon, and the establishment of the relationship of this toxin to other RTX toxins were instrumental in establishing a biological basis for A. actinomycetemcomitans virulence (4, 29, 30). The more recent discovery of an A. actinomycetemcomitans cytolethal distending toxin, a toxin found in many other human “pathogens” such as Shigella spp., Campylobacter jejeuni, and Haemophilus ducreyi, has provided added evidence of the virulent nature of A. actinomycetemcomitans (37, 46). Further confirmation of the role of A. actinomycetemcomitans in disease was shown in a rat model that followed Koch's postulates and demonstrated that A. actinomycetemcomitans isolated from an LAP subject could initiate bone loss when added to a reduced oral flora in a healthy animal (41).
The impetus for the current study resulted from the dearth of longitudinal data related to the role of A. actinomycetemcomitans in the initiation LAP. LAP is the most prevalent periodontal disease found in children and occurs in 20.5 per 1,000 African-American children, 10 per 1,000 Hispanic children, and 1.4 per 1,000 children of other ethnic groups (32). If untreated, teeth can become mobile and severely compromised due to progressive bone and attachment loss and ultimately may have to be removed (2, 8). Since a high percentage of school-age children from Newark, NJ, are African-American and Hispanic and thus more likely to be susceptible to LAP, the Newark school system is an ideal place to conduct a longitudinal study. To test our hypothesis, we designed a study in two phases. In the first, cross-sectional phase, our goal was to perform a thorough oral examination for Newark schoolchildren, ages 11 to 17 years, in order to identify the periodontal health and A. actinomycetemcomitans status of each child. The second phase of the study involved a longitudinal design in which a group of periodontally healthy A. actinomycetemcomitans-positive students (test group) and a group of A. actinomycetemcomitans-negative periodontally healthy students (control group), ages 11 to 16 years, were identified and followed every 6 months for 2 to 3 years. The longitudinal study was planned as a cohort study and as such was designed to follow a group of healthy A. actinomycetemcomitans-positive and A. actinomycetemcomitans-negative children to determine whether those harboring A. actinomycetemcomitans were more likely to develop bone loss over time than their A. actinomycetemcomitans-negative controls.
We hypothesized that periodontally healthy children who were susceptible to LAP and who harbored A. actinomycetemcomitans would be at greater risk for developing LAP than healthy children who did not harbor this organism. Currently, LAP can only be definitively diagnosed after clinically observable soft tissue changes and irreversible bone loss have occurred (1). Thus, if this hypothesis were proven to be correct, a screening method to identify A. actinomycetemcomitans could be used to detect healthy individuals likely to develop LAP.
In this paper, we present a report focusing on cross-sectional clinical and microbiological data from the 1,075 students examined and on longitudinal clinical and microbiological data for 38 A. actinomycetemcomitans-positive students, 36 of whom were classified as periodontally healthy (test group) and 58 A. actinomycetemcomitans-negative healthy students (control group) that were followed for at least 1 year after their initial examination. The issues addressed in this study were (i) the prevalence of LAP in this population, (ii) the prevalence of A. actinomycetemcomitans carriage in this population, (iii) the relationship of A. actinomycetemcomitans carriage to disease initiation, (iv) the most appropriate site for A. actinomycetemcomitans recovery in the oral cavity of healthy students and the relationship of the site of recovery to disease initiation, and (v) the relationship of the A. actinomycetemcomitans strain or clonal type to disease initiation. We chose bone loss as the primary end point for the definitive diagnosis of LAP. We also conducted survival analyses to assess the impact of A. actinomycetemcomitans carriage on progression from health to disease (failure to survive) by comparing the A. actinomycetemcomitans-positive test group and the A. actinomycetemcomitans-negative control group. In this study, survival was defined as those students who were periodontally healthy at baseline and who remained healthy throughout the recall period. In comparison, criteria for failure to survive were subdivided into four end points as follows: (i) those students who developed two 5-mm pockets, (ii) those who developed three 5-mm pockets, (iii) those who subsequently developed one or more pockets of 6 mm or greater with attachment loss of greater than 2 mm, or (iv) those who developed radiographic evidence of bone loss. The analysis of host factors related to disease susceptibility in these two groups will be the subject of a subsequent article.
MATERIALS AND METHODS
This study was reviewed and approved by the Institutional Review Board of the University of Medicine and Dentistry of New Jersey. Signed consent was received from the student's parent or guardian, as was assent, which was obtained from the student. Both consent and assent were received prior to student participation in the study. Medically healthy subjects were examined for dental caries and periodontal disease, sampled for A. actinomycetemcomitans, and then categorized as either A. actinomycetemcomitans positive or A. actinomycetemcomitans negative. The methods described in the section below will be divided into a clinical section and a microbiological section. The study consisted of two phases: a screening (cross-sectional) phase and a recall (longitudinal) phase with an exit treatment strategy for those students who have developed disease.
Clinical methods. (i) Calibration of examiners.
In preparation for the study, we conducted several extensive calibration exercises for measurement of periodontal disease. In each case, 10 subjects were chosen for the calibration exercise. Subjects were obtained from a patient pool of 11- to 17-year-old adolescents who reported to the New Jersey Dental School (NJDS) orthodontic clinic for treatment. The parents or guardians of each student volunteer were required to sign a consent form prior to initiation of the calibration procedure. Three experienced examiners were included in the exercise, one of whom served as the “gold standard” clinician. Interexaminer agreement and intraexaminer repeatability were determined, with a target agreement level of 80% or greater as the goal. For pocket depth measurements, examiners were considered to be in agreement if 80% of sites had identical readings and the remaining 20% of sites were within ±1 mm. For attachment level measurements, examiners were considered to be in agreement if sites were within ±1 mm. This level is well below the range typically used to identify differences in statistical analyses (usually ≥3-mm change) (6). Results of the calibration exercise demonstrated 80% interexaminer agreement and 90% intraexaminer repeatability.
For radiographic analysis, calibration was performed with 20 bitewing radiographs obtained from age appropriate NJDS clinic patients. These horizontal bitewing radiographs were chosen because they demonstrated bone loss ranging from no loss to modest loss (less than 2 mm of crestal resorption) and thus represented the level of disease expected in our study population. The criterion used for calibration was percent agreement on the presence or absence of interproximal bone loss at each interproximal site on each radiograph. Two examiners repeated the analysis three times to determine the accuracy of their readings. In this case, a 95% inter- and intraexaminer agreement was required.
(ii) Screening (cross-sectional) phase.
The purpose of the screening phase of the study was threefold: (i) to determine those children who were A. actinomycetemcomitans positive and those who were A. actinomycetemcomitans negative, (ii) to record pocket depths and probing attachment levels of all children to determine their entering periodontal status, and (iii) to determine the association of A. actinomycetemcomitans with the periodontal status of the entering student. All clinical examinations and sampling were performed in a mobile dental van (graciously donated by the Colgate-Palmolive Company) after the medical history was thoroughly reviewed. Students requiring antibiotic prophylaxis prior to invasive dental procedures or with documented bleeding disorders were excluded from the study. The screening visit included an oral soft tissue examination, full mouth caries and periodontal examination, and sample collection. The following samples were collected from each student: unstimulated saliva, buccal epithelial cells (BECs), epithelial cells from the dorsum of the tongue (for a subset of 425 students), and subgingival plaque from pockets 5 mm or greater. BECs, tongue cells, and subgingival plaque were collected to characterize students as either A. actinomycetemcomitans positive or A. actinomycetemcomitans negative. A prophylaxis and oral hygiene instructions were provided to each student participant.
(iii) Periodontal examination.
All examinations were performed using a Michigan 0 probe with Williams markings, front-faced dental mirrors, and overhead dental operatory lighting. All permanent teeth were probed at six sites per tooth. Any pocket of 5 mm or greater was reexamined to determine whether there was attachment loss of >2 mm at that site. Gingivitis was recorded as mild, moderate, or severe per arch per student. Because of the rapidly progressive nature of LAP and our desire to detect disease as early as possible, we used Loe and Brown's definition of incipient disease in the cross-sectional phase of the study for comparison to the NIDCR epidemiological database (32). As such, for the Loe-Brown criteria, we referred any students with attachment loss of >2 mm at a molar plus an incisor and/or one other tooth for radiographs to assess alveolar bone levels in order to confirm or reject a diagnosis of LAP (39).
In addition, the following classifications were used throughout the study. Students were considered to be “healthy” if they had one or fewer pockets 5 mm or less. Students were considered to be “borderline” if they had two or more 5-mm pockets and attachment loss, if any, of less than 2 mm. Students were considered to have “potential disease” if they had pocket depths of ≥6 mm and attachment loss of >2 mm in one or more teeth. This classification was particularly important for the screening visit because the initial diagnosis reported at this visit was used to determine disease development or progression in the longitudinal study. Although all teeth were assessed, we were particularly concerned with molars and incisors based on the fact that LAP diagnosis is primarily based on those teeth (32). Bitewing radiographic evidence of bone loss was required to establish the diagnosis of LAP and to place a student in the “disease” category. The diagnostic criterion was based on the fact that variability in probing measurement of attachment loss as well as pocket depths have been reported in children with a mixed dentition (10). Therefore, subjects with potential disease, including those with the Loe-Brown criteria for LAP, were rescheduled for reprobing and attachment level measurements as well as radiographic evaluation to establish a definitive LAP diagnosis. Radiographs were scheduled for students in the potential LAP category within 3 months of screening. If radiographic evidence confirmed the initial provisional diagnosis, the student was exited from the study and referred for treatment. These students were given the option of receiving treatment at no cost in the NJDS private practice facility.
(iv) Sample collection.
Salivary samples were collected by having subjects expectorate up to 5 ml of saliva into a 50-ml wide-mouth polystyrene tube that was held over ice. Saliva was then subjected to centrifugation at 10,000 × g for 30 min. The supernatant was decanted to obtain clarified saliva that was stored at −80°C for future testing(16).
BEC samples were obtained from each student by gently scraping the surface of the buccal mucosa with a wooden tongue depressor to obtain approximately 5 × 104 BECs that were suspended in 2 ml of phosphate-buffered saline (PBS). A hemocytometer was used to determine the number of epithelial cells per milliliter of PBS. Samples of BECs were vigorously vortexed, and a 100-μl aliquot containing the suspended cells was removed for plating on A. actinomycetemcomitans growth medium (AAGM) agar for detection of A. actinomycetemcomitans. Tongue epithelial cell samples were collected with a tongue depressor from a subset of 425 students and treated in a similar manner to BECs (15). All epithelial cells were stored at −80°C.
Subgingival pocket samples were collected from all pockets ≥5 mm using two sterile absorbent endodontic paper points placed in the pocket for 10 s. Samples from all sites in a given subject were pooled in 2 ml of PBS. After vigorous vortex agitation, a 100-μl aliquot was removed for plating on AAGM agar. In addition, subgingival plaque collected with a Morse 00 detachable sterile scaler from each pocket and a contralateral healthy site was placed into site-specific tubes containing 100 μl of hybridization buffer (10 mM Tris, 1 mM EDTA, pH 7.6), to which 100 μl of 0.1 N NaOH was added, and stored for future analysis by DNA/DNA checkerboard hybridization (45).
(v) Recall/longitudinal phase.
For the longitudinal phase of the study, periodontally healthy students with A. actinomycetemcomitans comprised the test group and approximately twice the number of periodontally healthy control students who did not have A. actinomycetemcomitans were placed in the control group. All A. actinomycetemcomitans-positive students between the ages of 11 and 16 years at screening were entered in the longitudinal phase of the study. All individuals in each group were scheduled for recall appointments at 6-month intervals. In the recall/longitudinal phase of the study, the students received the same clinical assessments and sampling that were performed at the screening visit, with the addition of a yearly radiographic analysis consisting of two to four horizontal bitewing radiographs. The purpose of the recall phase was to detect students who develop increasing pocket depth, attachment loss, and bone loss and to relate this to the prior or current presence or absence of A. actinomycetemcomitans. Since yearly radiographs are used to establish the definitive LAP diagnosis, it is possible that some diagnoses could be delayed if the onset of disease were to occur in the interim period between yearly radiographic analyses. To minimize the possible delay in LAP diagnosis based on radiograph, a student classified as having “potential disease” at any visit was scheduled for radiographs within the following 3 months. If radiographs do not show bone loss, these students were returned to the 6-month recall schedule. A student was considered to have LAP if radiographs indicated loss of lamina dura and interproximal alveolar bone resorption in the contiguous crestal region of one or more molars or incisors.
To confirm that the control subjects were, in fact, A. actinomycetemcomitans negative, the stored aliquots of their BECs or tongue or pocket samples were screened for A. actinomycetemcomitans by PCR as described below to confirm the results of the negative culture.
(vi) Exit treatment strategy.
All students with disease (bone loss) were exited from the study and offered treatment at NJDS at no cost. In the case of periodontal disease, one bone lesion was considered sufficient to exit the child from the study.
Microbiological methods. (i) Identification of A. actinomycetemcomitans-positive subjects.
AAGM plates were labeled with the student number, and 100-μl aliquots from BECs, tongue, and pocket were plated. After streaking, the plates were placed in a GasPak jar charged with CO2 for transport from the dental van to NJDS. At NJDS, cells were grown for 3 to 4 days in an incubator at 37°C in 10% CO2. After incubation and growth, colonies that were catalase positive and had the classical morphological characteristics of A. actinomycetemcomitans were selected for subculture. Definitive identification of A. actinomycetemcomitans was made by biochemical testing and by PCR using procedures developed in our laboratory (18). Strains confirmed as A. actinomycetemcomitans positive were coded and restreaked on selective media for preservation at −80°C in AAGM plus 10% dimethyl sulfoxide for subsequent analysis.
(ii) Confirmation of A. actinomycetemcomitans-positive cultures: DNA extraction from isolated strains of A. actinomycetemcomitans for PCR.
A. actinomycetemcomitans strains isolated from students were stored frozen and then regrown for confirmation of A. actinomycetemcomitans and for A. actinomycetemcomitans serotype analysis. DNA from A. actinomycetemcomitans strains was obtained for PCR analysis using the protocol developed by the DNeasy tissue kit (QIAGEN, Inc., Valencia, CA) for gram-negative bacteria(27). Briefly, a 3-day culture of a given A. actinomycetemcomitans strain was grown on AAGM agar in 10% CO2 at 37°C. Colonial morphology was confirmed using a dissecting microscope at ×25 magnification using transmitted light. DNA was processed from pure cultures and extracted as described by QIAGEN, Inc. (26). The quality of the DNA was checked by 0.8% agarose gel electrophoresis to ensure that no undue shearing had occurred. All DNA solutions were adjusted to a concentration of 1 μg/ml before PCR analysis and used as template DNA for a given isolated strain of A. actinomycetemcomitans. PCR was performed in Micro Amp tubes (PE Biosystems, Norwalk, CT) with a PE480 DNA thermal cycler.
(iii) Determination of A. actinomycetemcomitans serotype and leukotoxin promoter type.
The DNA sequences of the gene clusters responsible for the synthesis of A. actinomycetemcomitans a, b, c, d, e, and f serotype-specific O antigens that have been reported were used for analysis(26). PCR primers that amplify unique regions in each of the six A. actinomycetemcomitans serotype-specific gene clusters were used to determine the frequency of each serotype in our strain collection (Table 1). The PCR product sizes for each pair of serotype-specific primers were 293 bp (serotype a), 333 bp (serotype b), 268 bp (serotype c), 411 bp (serotype d), 311 bp (serotype e), and 232 bp (serotype f) (Fig. 1). In addition, ltx primer pairs allow for identification of the type of ltx promoter using A. actinomycetemcomitans template DNA. A. actinomycetemcomitans strains with the JP2-type promoter region yielded 492-bp size fragments, while those with the 652-type promoter yielded 1,022-bp fragments (26, 27). Template DNA from an isolated strain of A. actinomycetemcomitans was reacted in separate PCR microcentrifuge tubes with the appropriate primers to determine if the strain was serotype a, b, or c and whether the leukotoxin promoter present was either JP2 or the 652 type. If there was no result found with the serotype testing, then the template was tested with the d, e, or f primer pairs. Primers are described in Table 1. In this manner, we were able to distinguish one serotype from the next and one ltx promoter type from the other in a highly sensitive and reproducible manner starting with DNA isolated from A. actinomycetemcomitans grown on an agar plate.
TABLE 1.
A. actinomycetemcomitans serotype-specific PCR assay
| Assay | Primer | Sequencea | Serotype | PCR product size (bp) | Region amplifiedb | GenBank accession no. |
|---|---|---|---|---|---|---|
| 1 | P11 | TCTCCACCATTTTTGAGTGG (f) | b | 333 | 12780-13112 | AB002668 |
| P12 | GAAACCACTTCTATTTCTCC (r) | c | 268 | 12808-13075 | AB010415 | |
| P13 | CCTTTATCAATCCAGACAGC (f) | 232 | 4813-5044 | AF310164 | ||
| P14 | ARAAYTTYTCWTCGGGAATG (r) | |||||
| 2 | P15 | TGGGTCATGGGAGGTACTCC (f) | a | 293 | 8021-8313 | AB046360 |
| P16 | GCTAGGAACAAAGCAGCATC (r) | |||||
| 3 | P17 | TGGAACGGGTATGGGAACGG (f) | d | 411 | 11462-12034 | AB041226 |
| P18 | GGATGCTCATCTAGCCATGC (r) | |||||
| 4 | P19 | ATTCCAGCCTTTTGGTTCTC (f) | e | 311 | 17118-17427 | AB030032 |
| P20 | TGGTCTGCGTTGTAGGTTGG (r) |
f, forward; r, reverse. R = A or G, Y = C or T, and W = A or T.
GenBank nucleotide sequence coordinates.
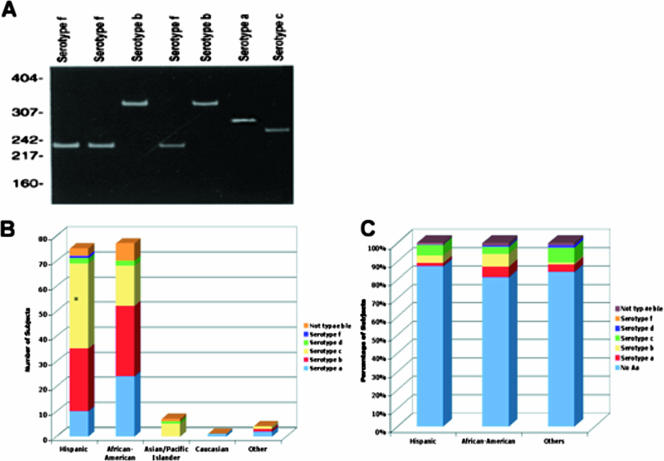
FIG. 1.
Aggregatibacter actinomycetemcomitans serotypes: relationship to site of recovery and to ethnicity. (A) Example of PCR assay for serotype identification. Lane labels indicate serotype. The sizes (in base pairs) of the molecular weight standards run are shown on the left. Fragments were run on a 5% polyacrylamide gel. (B) Histogram of A. actinomycetemcomitans serotypes in relation to ethnicity of A. actinomycetemcomitans carrier. Serotype c is significantly greater in the Hispanic children who carry A. actinomycetemcomitans (P = 0.05). No differences were seen in serotype distribution in African-American children who carry A. actinomycetemcomitans. (C) Histogram of percentage of subjects who had A. actinomycetemcomitans and the specific serotypes of A. actinomycetemcomitans found in relation to the ethnicity of the A. actinomycetemcomitans carrier. Note that the overwhelming majority of students did not carry A. actinomycetemcomitans (No Aa).
(iv) Confirmation of A. actinomycetemcomitans culture-negative students: extraction of DNA from A. actinomycetemcomitans adhering to BECs and paper points.
Approximately 1 ml of a solution of 1 × 104 BECs in PBS and/or 1 ml from paper point samples was pelleted in a microcentrifuge tube by centrifugation at 11,000 × g, and the supernatant was discarded. DNA was extracted using the QIAGEN DNeasy kit (catalog no. 69504) gram-negative bacterial protocol as described above.
(v) PCR confirmation of culture negatives.
BECs were harvested from each student at each collection period. In addition, pocket samples were collected at each visit from pockets 5 mm or greater. BECs and paper point samples were stored frozen for future analysis by PCR to identify the presence of A. actinomycetemcomitans. To confirm that students who were initially categorized as A. actinomycetemcomitans negative were, in fact, A. actinomycetemcomitans negative, the following analysis was performed. In brief, DNA template was prepared from stored BECs and pocket samples collected at each visit with a QIAGEN DNeasy kit as described above. Template DNA was then amplified using a Genomeplex whole-genome amplification kit (Sigma Chemical, Inc.; catalog no. WGA1), which amplifies all template DNA 100 to 1,000 times, increasing the sensitivity to A. actinomycetemcomitans template DNA. The presence of A. actinomycetemcomitans DNA was determined by screening the amplified template DNA for the ltx promoter gene that is unique to A. actinomycetemcomitans. A positive ltx PCR response indicates the presence of A. actinomycetemcomitans in the sample. A minimum of two separate PCR evaluations was performed on stored BECs and/or pocket samples to confirm that a student was A. actinomycetemcomitans negative. Thus far, we have evaluated BECs and pocket samples from 90 A. actinomycetemcomitans culture-negative students by using PCR to check for A. actinomycetemcomitans negativity. None of these students was A. actinomycetemcomitans positive, whereas BECs and pockets from all 10 randomly chosen A. actinomycetemcomitans-positive students tested for the presence of A. actinomycetemcomitans by PCR were shown to be A. actinomycetemcomitans positive.
(vi) Data analysis.
The main hypothesis was that the presence of A. actinomycetemcomitans could be used to predict an increased risk for LAP in initially periodontally healthy individuals as determined by radiographic criteria. Subjects from the cross-sectional portion of the study who were found to harbor A. actinomycetemcomitans were compared over time to a cohort of individuals who did not initially or at any time in the study harbor the bacterium (age-, gender-, and race-matched controls). To ensure that the two groups were balanced, potential differences in age were evaluated with an unpaired t test and a Pearson chi-square test of independence was used to evaluate differences in ethnic and gender distributions of the two groups. A P value of ≤0.05 was considered to be statistically significant in all analyses. Analysis of the distribution of the presence of A. actinomycetemcomitans by ethnic group was performed by chi-square as was A. actinomycetemcomitans serotype distribution within each ethnic group.
Survival analysis was used to evaluate the initiation of periodontal disease over time. In our study, survival was defined as a student who at the start of the study was healthy and who remained healthy at recall. Conversely, a student who started as healthy with no pockets or with one 4- to 5-mm pocket and progressed to two or more pockets or to attachment loss and/or bone loss failed to survive. In all cases, students in the A. actinomycetemcomitans-positive group were compared to students in the A. actinomycetemcomitans-negative group. The survival data are presented by Kaplan-Meier plots (25). To test the hypothesis that differences in initiation of LAP can be seen when the A. actinomycetemcomitans-negative control group and the A. actinomycetemcomitans-positive test group were compared, both the log-rank test and the Wilcoxon test were used. The log-rank test places more weight on longer survival times and is most useful when the ratio of hazard functions in the groups being compared is approximately constant. The Wilcoxon test places more weight on early survival times and is the optimum rank test if the error distribution is logistic. Both tests were required to be significant before any difference between groups was considered statistically significant (11). The survival analysis of primary interest was the comparison of the levels of development of bone loss in initially healthy students in the test and control groups. Further analysis was performed in the A. actinomycetemcomitans-positive group by comparing the initial site of A. actinomycetemcomitans recovery (buccal versus pocket) to increasing pocket depth and attachment loss over time. Finally, survival analysis was also used to determine the significance of harboring specific A. actinomycetemcomitans serotypes (a, b, and c) at baseline in relation to disease initiation using the criteria of two or three pockets 5 mm or greater.
RESULTS
The results reported herein represent data obtained from the first 1,075 students that were seen at the initial examination (cross-sectional data) and the 96 students who were recalled for a minimum of 1 year following that screening visit (longitudinal data).
Cross-sectional data.
Table 2 shows the demographic distribution of students seen at the initial screening examination visit. The student composition was as follows: 654 were female, 421 were male, 410 were African-American, 598 were Hispanic, 25 were Asian, 23 were Caucasian non-Hispanic, and 28 were considered “other” (i.e., ethnicity not specified). The students’ ages ranged from 11 to 17 years, with a mean age (± standard deviation) of 15 ± 2.2 years.
TABLE 2.
Demographic distribution of students at screening by ethnicity, gender, and age
| Ethnicity (n) | No. of students by age (yr)
|
|||||
|---|---|---|---|---|---|---|
| Female
|
Male
|
|||||
| 11-14 | 15-16 | ≥17 | 11-14 | 15-16 | ≥17 | |
| Hispanic (589) | 227 | 104 | 20 | 144 | 83 | 11 |
| African-American (410) | 147 | 95 | 14 | 108 | 37 | 9 |
| Asian (25) | 4 | 7 | 3 | 4 | 4 | 3 |
| Caucasian (27) | 7 | 7 | 6 | 3 | 2 | 2 |
| Other (24) | 5 | 5 | 3 | 5 | 5 | 1 |
| Total | 390 | 218 | 46 | 264 | 131 | 26 |
One of the major goals of this study was to determine the relationship of A. actinomycetemcomitans to disease initiation in the longitudinal phase of the study; however, to accomplish this goal it was necessary to establish the presence of A. actinomycetemcomitans in each student at screening and at each recall visit thereafter. Table 3 shows the distribution of A. actinomycetemcomitans in the population at screening. Overall, 13.7% of students carried A. actinomycetemcomitans. Eighty-six of the A. actinomycetemcomitans-positive students were periodontally healthy, 33 were in the borderline health category (these typically had two to three 5-mm pockets), and 28 were in the potential disease category. The distributions of A. actinomycetemcomitans-positive students among the student subgroups were 16.6% of the African-American students, 11.5% of the Hispanic students, 20% of the Asian or Pacific Islanders, 4% of Caucasians, and 17.6% of the “others.” There were too few Asian or Pacific islanders, Caucasians, or “others” to determine whether carriage of A. actinomycetemcomitans by enrolled students in one of these ethnic groups was higher than that seen in any of the other groups. However, there was a sufficient number of subjects to make this comparison for African-American and Hispanic students, and African-Americans had a significantly greater prevalence of A. actinomycetemcomitans than Hispanics (P = 0.0147).
TABLE 3.
Recovery of A. actinomycetemcomitans from students at screeninga
| Ethnicity (n) | No. of females/males | Mean age (yr) of females/males | No. of A. actinomycetemcomitans- positive females/males |
|---|---|---|---|
| Hispanic (589) | 349/240 | 14.2/14.2 | 35/33 |
| African-American (410) | 251/159 | 14.2/14.2 | 40/28 |
| Asian (25) | 15/10 | 16.2/16.3 | 4.0/1.0 |
| Caucasian (23) | 16/7 | 16.2/15.2 | 1.0/0.0 |
| Other (28) | 17/11 | 15.2/14.2 | 3.0/2.0 |
| Total | 648/427 | 83/64 |
Shown is the demographic distribution of A. actinomycetemcomitans-positive students by ethnicity, gender, and age.
Table 4 addresses the clinical status of the students examined at screening. Of the 1,075 students examined to date, 105 were borderline, while 42 were categorized as having potential disease (pockets 6 mm or greater and associated attachment loss of >2 mm). As per the data seen in Table 4, almost 4% of the population screened met the criterion for potential disease: i.e., involvement of one or more sites ≥6 mm with attachment loss of >2 mm represented. Of these, 66.7% showed the presence of A. actinomycetemcomitans. Thirteen of these 42 (1.2%) also met the Loe-Brown criterion for incipient LAP (32) at screening: i.e., involvement of at least two sites ≥6 mm with associated attachment loss (Table 4). As noted in Table 4, 2% of the African-American students and 0.7% of the Hispanic students had a level of disease consistent with the Loe-Brown criterion. Ten of these 13, or 67.9% of students in this group, were A. actinomycetemcomitans positive. Only 2 of the 13 had radiographic evidence of bone loss: one whose attachment loss at screening required immediate confirmation of disease by radiographic evaluation and another student who subsequently developed radiographic evidence of bone loss 1 year after screening. Both of these students were A. actinomycetemcomitans positive.
TABLE 4.
Demographics of students with potential disease at screening by ethnicity, gender, and age
| Ethnicity and periodontal status (n) | No. of females/males | Mean age (yr) of females/males |
|---|---|---|
| ≥1 pocket >6 mm with >2 mm of attachment loss (42) | ||
| Hispanic (23) | 10/13 | 15.2/14.9 |
| African-American (19) | 11/8 | 15.8/14.7 |
| Total | 21/21 | |
| ≥2 pockets >6 mm with >2 mm of attachment loss (13) | ||
| Hispanic (4) | 1/3 | 14/14.3 |
| African-American (8) | 5/3 | 16.4/14.0 |
| Asian (1) | 0/1 | 0/18 |
| Total | 6/7 |
Given our hypothesis that the presence of A. actinomycetemcomitans can serve as a risk marker for disease, one of our goals was to determine the most efficient method of sampling that could be used to detect A. actinomycetemcomitans in periodontally healthy individuals. As a result we surveyed several oral sites that have been reported to harbor A. actinomycetemcomitans. While the obvious target site for A. actinomycetemcomitans is the pocket, pocket colonization could represent a later stage of infection that follows oral colonization of a periodontally healthy individual. We, therefore, looked for the presence of A. actinomycetemcomitans on BECs, in periodontal pockets, and in some cases on the dorsum of the tongue. As seen in Table 5, A. actinomycetemcomitans was recovered from BECs alone in 59.3% of healthy students (maximum of one 4- to 5-mm pocket), which represents 34.7% of the total number of individuals in which A. actinomycetemcomitans was found (51 of 147). In students with two or more 5-mm pockets, A. actinomycetemcomitans was recovered from pockets alone in 22.4% of cases, while A. actinomycetemcomitans was recovered from either BECs alone or from both BECs and pockets 76.9% of the time. We sampled the tongues of 425 students and recovered A. actinomycetemcomitans from only 5 students’ tongue scrapings (1.4%). Four of the five students who harbored A. actinomycetemcomitans on their tongues also had A. actinomycetemcomitans on their BECs but not in their pockets (data not shown). Thus, the buccal mucosa appears to provide the most efficient oral site for sampling A. actinomycetemcomitans in healthy individuals. Furthermore, sampling for A. actinomycetemcomitans from BECs and pooled pockets sites 5 mm or greater allows for recovery of A. actinomycetemcomitans from almost 99% of the students who were A. actinomycetemcomitans positive when recovery from BEC alone, a pocket alone, or a combination of the two sampling sites was considered (Table 5).
TABLE 5.
Sites of recovery of A. actinomycetemcomitans at screening visita
| Periodontal status | No. of students with A. actinomycetemcomitans recovery at site(s):
|
|||
|---|---|---|---|---|
| Buccal | Buccal and pocket | Total | ||
| Healthy or one 4- or 5-mm pocket | 51 | 18 | 17 | 86 |
| At least two 5-mm pockets | 5 | 14 | 14 | 33 |
| At least one 6-mm pocket with ≥2 mm of attachment loss | 0 | 1 | 26 | 27 |
| Total | 56 | 33 | 57 | 146 |
One student had A. actinomycetemcomitans recovered from his tongue and had a pocket of 7 mm.
Figure 1 illustrates the distribution of A. actinomycetemcomitans in students. Figure 1A shows the serotype specific DNA profile that we used to establish the A. actinomycetemcomitans serotype of each sample tested. As seen in Fig. 1B, the distribution of serotypes is significantly skewed toward serotype c in the Hispanic children (P ≤ 0.0017), while in the African-American children there appears to be a more equal distribution of serotypes with elevations of serotypes b and a. Of seven students with the JP2 strain, six were African-American and one was Hispanic. Taken together, serotypes a, b, and c accounted for 95.8% of the typeable serotypes found (Fig. 1B and C). Serotype d was found in five students, and serotype f was found in only one student, while serotype e was not found and 9.5% of the serotypes evaluated were nontypeable (data not shown). (To date none of the students with these strains has developed disease.)
Longitudinal data.
For the longitudinal study, we selected a group of healthy or borderline A. actinomycetemcomitans-positive students identified at screening. Since carriage of A. actinomycetemcomitans is not widespread, we first selected healthy students who were A. actinomycetemcomitans positive and then matched them with A. actinomycetemcomitans-negative students by age, sex, and race. At this point we have 38 A. actinomycetemcomitans-positive and 58 A. actinomycetemcomitans-negative students who have been followed for 1 year or more from the time of screening. Of those 38 A. actinomycetemcomitans-positive students, 36 were in the healthy or borderline category, while 2 were in the potential disease category.
Table 6 presents the baseline characteristics for students who had been in the longitudinal phase of the study for at least 1 year. Of the A. actinomycetemcomitans-positive students (n = 38), 18 were Hispanic and 18 were African-American, with a mean age of approximately 15.5 years, approximately half of whom were female. Of the A. actinomycetemcomitans-negative students (n = 58), 33 were Hispanic and 25 were African-American, with a mean age of 14.4 years.
TABLE 6.
Baseline demographics of students in the longitudinal phase of the study
| Characteristic | No. (%) of studentsa
|
|
|---|---|---|
| A. actinomycetemcomitans positive (n= 38) | A. actinomycetemcomitans negative (n = 58) | |
| Ethnicity | ||
| African-American | 18 (47.7) | 25 (43.1) |
| Hispanic | 18 (47.7) | 33 (56.9) |
| Asian | 1 (2.6) | 0 (0.0) |
| Other | 1 (2.6) | 0 (0.0) |
| Gender | ||
| Male | 20 (52.6) | 28 (48.2) |
| Female | 18 (47.4) | 30 (51.7) |
The mean ± standard deviation ages of the A. actinomycetemcomitans-positive and -negative groups are 15.7 ± 0.3 and 14.4 ± 0.2 years, respectively.
Of the eight students with bone loss at recall, all had A. actinomycetemcomitans at screening. Four had been classified as healthy, two were borderline, and two had been classified as having potential disease. Five of the eight students were African-American (age, 15.75 ± 1.0 years), and three were Hispanic (age, 15.0 ± 1.0 years). Among these students, four had serotype c, one had serotype a, and three had serotype b. Of those with the b serotype, two had the JP2 phenotype, while the third with serotype b at the visit at which bone loss was detected had serotype c at screening. Moreover, seven students of the 147 who harbored A. actinomycetemcomitans had the JP2 phenotype. Of these seven with the JP2-type promoter, two of the African-American students developed bone loss. Most importantly, none of the 58 A. actinomycetemcomitans-negative students showed any evidence of bone loss.
Nine of the 38 A. actinomycetemcomitans-positive students recalled showed more than one serotype when A. actinomycetemcomitans from one visit was compared to A. actinomycetemcomitans isolated at another visit. This confirms reports that more than one serotype of A. actinomycetemcomitans can exist in a patient either simultaneously or sequentially (48, 49).
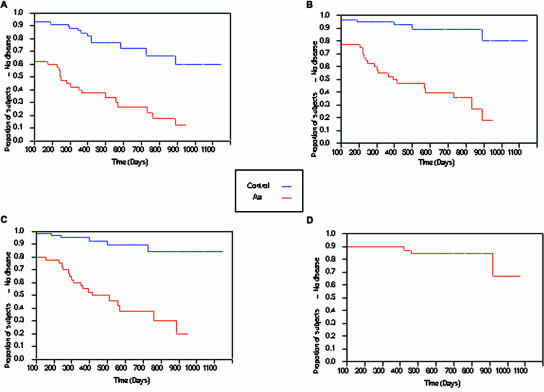
Survival analysis was used to determine the relationship of the presence or absence of A. actinomycetemcomitans at baseline to the finding of initiation or progression of disease at subsequent recall visits. In addition, survival analysis was used to determine whether or not the initial site of A. actinomycetemcomitans recovery or the initial A. actinomycetemcomitans serotype found was related to movement from health at baseline to two 5-mm pockets or to the potential disease or disease categories at recall. Throughout Fig. 2, we defined survival as those students who remained healthy during the recall examination. In Fig. 2A, we defined failure to survive as a student who progressed from health at baseline to two sites with pockets of 5 mm or more at recall. As seen in Fig. 2A, the A. actinomycetemcomitans-negative control group had a survival rate of about 70% and the A. actinomycetemcomitans-positive test group had a statistically significant lower survival rate of about 10% (P < 0.0001). If the end point for failure to survive is changed to three 5-mm pockets, as seen in Fig. 2B, the A. actinomycetemcomitans-negative control group had a survival rate of 85% and the A. actinomycetemcomitans-positive test group had a significantly lower survival rate of 20% (P < 0.0001) and thus the separation between the two groups was found to be greater. When the end point for failure to survive was placed at pockets of 6 mm or greater with attachment loss of >2 mm (Fig. 2C), survival was once again significantly lower in the A. actinomycetemcomitans-positive group than the A. actinomycetemcomitans-negative control group (P < 0.0001). Potential covariates (age, race, and gender) were modeled in the survival analysis for all three criteria (two 5-mm pockets, three 5-mm pockets, and attachment loss). In no case were these findings affected by these covariates.
FIG. 2.
Survival plots of healthy A. actinomycetemcomitans-positive (test group, n = 38) compared to healthy A. actinomycetemcomitans-negative (control group, n = 58) students at initial screening visit and how their periodontal status progressed over the recall period. The x axis shows the time in days after the screening visit, and the y axis shows the proportion of students who remained healthy and had no disease over the recall period. (A) Students who survived and thus did not have two 5-mm pockets at recall. The A. actinomycetemcomitans-negative group (control group) is seen in the top curve, while the A. actinomycetemcomitans-negative group (test group) is seen in the lower curve. Of those students who never developed two 5-mm pockets over the recall period, 65% had no A. actinomycetemcomitans, while only 10% of those who had A. actinomycetemcomitans failed to develop two pockets. Therefore, 90% of those who had A. actinomycetemcomitans did develop two pockets (P = 0.0001). (B) Students who survived and thus did not have three 5-mm pockets at recall. The control group is seen in the top curve, and the test group is seen in the lower curve. The number of students in the A. actinomycetemcomitans-negative group who developed three 5-mm pockets was only 10%, while over 80% of those in the A. actinomycetemcomitans-positive group developed three pockets (P = 0.0001). (C) Students who survived and thus did not have pockets greater than 6 mm with attachment loss 2 mm or greater at recall. The control group is seen in the top curve, and the test group is seen in the lower curve. Of those who never developed one 6-mm pocket, over 85% had no A. actinomycetemcomitans. Of those who did develop one or more 6-mm pockets with attachment loss, over 85% had A. actinomycetemcomitans (P = 0.0001). (D) Students who survived and thus did not have bone loss at recall. The control group as seen in the top curve cannot be discerned because there was no bone loss throughout the recall period. The test group is seen in the lower curve. Only those students in the test group had bone loss (P = 0.01).
In Fig. 2D, radiographic bone loss was used as the end point for failure to survive. Eight students in the A. actinomycetemcomitans group had radiographic evidence of bone loss, which was used as the definition of disease. Moreover, none of the initially healthy individuals in the control group developed LAP using bone loss as the diagnostic criterion for disease [P < 0.01].
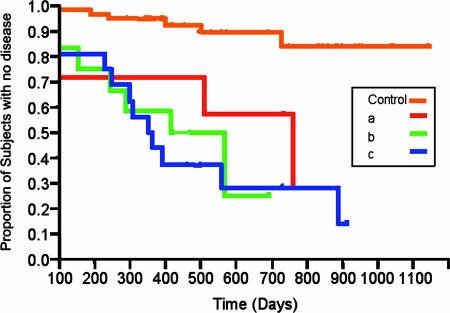
In Fig. 3, the survival rates for individuals with the most frequently occurring serotypes of A. actinomycetemcomitans (a, b, and c) are seen. No differences in survival rates could be found among the three serotypes, suggesting that there is no one A. actinomycetemcomitans serotype that is more highly related to disease initiation than others. Furthermore, no difference in survival was seen when the sites of A. actinomycetemcomitans recovery (buccal versus pocket) were compared. This finding suggests that healthy students who had A. actinomycetemcomitans recovered from buccal cells were just as likely to develop disease as those from whom A. actinomycetemcomitans was recovered from pocket sites.
FIG. 3.
Survival plot comparing students who harbored serotype a, b, or c and how these serotypes related to two or three 5-mm pockets. The top curve shows the plot for the A. actinomycetemcomitans-negative controls. Plots for A. actinomycetemcomitans-positive subjects carrying strains of serotype a, b, or c are shown in the remaining curves. No difference in survival analysis was seen when the strains were compared.
DISCUSSION
LAP (previously known as localized juvenile periodontitis) is a disease of adolescents that can have profound cosmetic, functional, and psychological effects (5). This disease occurs disproportionately in children from ethnic groups that are often economically disadvantaged and have limited access to dental care (31, 40). Children who have LAP are known to harbor A. actinomycetemcomitans, an organism that has been highly associated with LAP; however concrete proof of the relationship of A. actinomycetemcomitans to the initiation of disease is lacking (22, 23). If the presence of A. actinomycetemcomitans is indicative of a greater risk for developing LAP, then early detection of A. actinomycetemcomitans can lead to improved diagnostic and preventive measures. Parenthetically, it is also interesting to note that A. actinomycetemcomitans has been shown to colonize heart tissue and has been implicated as a factor that increases the risk for heart disease (21, 47). Therefore, early detection of A. actinomycetemcomitans in the oral cavity may have an impact on health extending beyond the mouth.
The cross-sectional data presented in this paper are based on the first 1,075 students surveyed. For comparison with previously reported epidemiologic findings, we found that 13 of the 1,075 students fit the Loe-Brown definition of LAP at the initial screening visit as determined by probing attachment level measurements (32). This represented 1.2% of the population evaluated, which is approximately twice the prevalence of 0.54% found in the NHANES II study (32). However, when subgroups of the NHANES data set were considered, the prevalences of LAP in Hispanic and African-American children were 1% and 2%, respectively (32). Thus, our findings are consistent with the NHANES data in that we found that 0.7% of the Hispanic children and 2.0% of the African-American children from Newark had LAP based on clinical probing measurements.
In terms of A. actinomycetemcomitans carriage, our results are consistent with other reports that demonstrate racial and ethnic differences regarding A. actinomycetemcomitans carriage (3, 12, 38, 51). Approximately 95% of the A. actinomycetemcomitans strains isolated were of the a, b, or c serotype. Hispanic children had primarily the c serotype, while there was a more or less equal distribution of serotypes a, b, and c in African-American children (12, 24, 33). It was also noted that nine of the A. actinomycetemcomitans-positive students who had been examined at different recall visits had more than one serotype of A. actinomycetemcomitans; however, these findings cannot be interpreted as more than a casual observation, since a determination of serotype carriage over the course of the study was not a primary objective and consequently no more than two or three colonies were randomly sampled from each plate from which A. actinomycetemcomitans was isolated at each visit.
On another note, the controversy regarding the virulence of the JP2 strain of A. actinomycetemcomitans remains unanswered (28). The literature suggests that the JP2 strain is highly virulent and is often associated with disease (9, 20). In our study, the strain was isolated from only 7 of 147 students who carried A. actinomycetemcomitans. Our group also analyzed data from another study in which 33 strains were collected from patients, 24 of which were obtained from LAP patients in our clinic (27). Of 18 African-American patients with LAP, 7 harbored the JP2 strain while the remaining 11 LAP patients harbored A. actinomycetemcomitans strains other than the JP2 clonal type. Therefore, of the total of 24 patients with disease, 7 harbored the JP2 strain while the majority of LAP patients with A. actinomycetemcomitans harbored other strains. We concluded, therefore, that phylogenetically diverse strains of A. actinomycetemcomitans can carry pathogenic potential (27). In the present study, of the seven students with the JP2 promoter type, two who were initially healthy developed disease (i.e., radiographic evidence of bone loss). However, it is important to note that five A. actinomycetemcomitans-positive students who developed bone loss did not harbor the JP2 promoter type at any time during the study. Based on our data at this point in time, we feel that it is not possible to definitively state whether the JP2 phenotype is more or less virulent than other A. actinomycetemcomitans phenotypes.
With respect to A. actinomycetemcomitans recovery, the buccal mucosal surface was the site most apt to harbor A. actinomycetemcomitans, with approximately 76% of the A. actinomycetemcomitans-positive students having had A. actinomycetemcomitans recovered from this site. Considering that two A. actinomycetemcomitans adhesins, Aae and ApiA, have been shown to impart a high degree of specificity for BECs in vitro, this finding was not totally unexpected (17, 50). What was surprising was the fact that we found so few instances in which A. actinomycetemcomitans could be isolated from the tongue epithelium. This finding is not completely consistent with previous reports (14, 35). However, in the studies that report A. actinomycetemcomitans isolation from the tongue, the subjects were much older and almost exclusively Asian and/or Caucasian (13, 34).
The longitudinal cohort study report represents data obtained from 96 students—38 A. actinomycetemcomitans positive and 58 A. actinomycetemcomitans negative—who were followed for more than 1 year after their initial screening visit. We found that 6 of 36 A. actinomycetemcomitans-positive students who were initially healthy developed bone loss, our primary measure of LAP, while two A. actinomycetemcomitans-positive students in the potential disease category had bone loss. In contrast, none of the 58 A. actinomycetemcomitans-negative initially healthy students developed bone loss (P = 0.01). As a secondary method of analysis, we examined disease initiation as determined by soft tissue measurement of pockets and attachment levels in previously healthy individuals. It is thought that periodontal disease is initiated by extension of supragingival plaque to the subgingival space, forcing the margin of the junctional epithelium to migrate in an apical direction to form a periodontal “pocket” (42). Our rationale for using pocket depth end points as a sign of disease initiation was based on the well-recognized concept that pocket depth can proceed to more advanced attachment loss, which can then lead to bone loss (19, 39). Thus, in students who were healthy at baseline, the assumption was made that finding two or more pockets of 5 mm or greater at a subsequent examination is indicative of the possible initiation of periodontal disease.
Survival analysis was chosen as a method for comparing differences in the following end points: pocket depth progression, attachment loss, and bone loss in the A. actinomycetemcomitans-positive and A. actinomycetemcomitans-negative groups. In each of the end points analyzed, significantly greater survival (maintenance of health) was seen in the A. actinomycetemcomitans-negative group than in the A. actinomycetemcomitans-positive group (P = 0.0001 to P = 0.01). The two most reliable measures of periodontal disease are loss of attachment level and bone. When attachment loss was used as the basis for diagnosis of disease initiation, the A. actinomycetemcomitans-positive group showed a greater failure to survive than the A. actinomycetemcomitans-negative group (P = 0.0001). Using bone loss as the criterion, we found that 21% of students harboring A. actinomycetemcomitans developed LAP over time. If this relationship between A. actinomycetemcomitans and disease initiation can be further substantiated, it is likely that the presence of A. actinomycetemcomitans in the oral cavity of healthy children can be considered as a useful predictive marker for LAP. If our hypothesis concerning the usefulness of A. actinomycetemcomitans as a marker of risk is correct, then the challenge is to differentiate between the 21% of children with A. actinomycetemcomitans who will develop bone loss compared to the 79% of the children who have remained healthy to this point. We are currently exploring host factors in saliva and gingival crevicular fluid among our longitudinal study subjects to detect susceptibility factors that could distinguish between A. actinomycetemcomitans-positive children who develop LAP and those who remain periodontally healthy.
In conclusion, we have presented cross-sectional and longitudinal data from a large study of aggressive periodontal disease in children from the Newark, NJ, area. To date, we have examined 1,075 children, 11 to 17 years of age, and entered 96 students 11 to 16 years of age in the longitudinal study. The results indicate that (i) A. actinomycetemcomitans is associated with bone loss; (ii) not all subjects who carry A. actinomycetemcomitans will develop LAP; (iii) in addition to the JP2 serotype b phenotype, there are other strains that are equally associated with disease initiation; (iv) A. actinomycetemcomitans serotype presence in African-American students appears to be equally distributed among serotypes a, b, and c, whereas, Hispanic students show a strong association with serotype c; and (v) in healthy adolescents, the buccal mucosa appears to provide the most efficient site for detection of A. actinomycetemcomitans. Most importantly, if A. actinomycetemcomitans continues to be highly associated with disease development, its detection may be used as a marker of risk for disease initiation. Such a marker can be useful in identifying children in underserved populations who are at risk for LAP. Use of such a marker could then allow for the implementation of effective preventive measures designed to address the pressing issue of oral health disparities in this vulnerable population.
Acknowledgments
We extend our thanks and appreciation to the Delta Dental Foundation of New Jersey for providing the financial support for initiation of the cross-sectional portion of the study and acknowledge the generous contribution of the Colgate Palmolive Corporation for allowing our group to use their mobile Dental Van to conduct the study. We further thank the National Institutes of Dental and Craniofacial Research for providing financial support in the form of grant DE-016474 and grant DE-017968 used for the longitudinal phase of the study. In addition, we thank Cecile Feldman for providing us with the ways and means to perform treatment at no cost to student participants from Newark at the NJDS private practice facility. We also thank Marguerite Leuze, Office of Health Services for Newark Public Schools; Marion A. Bolden, State District School Superintendent for Newark Public Schools; and all of the principals and teachers who assisted us in the process.
In addition, Edward Hernandez, La Casa de Don Pedro Youth and Family Services, and Kathleen Duncan, SMART program at UMDNJ, are also thanked for organizing student participation in the program. Jeffrey Linfante, NJDS, is thanked for participation in the early stages of the study, and gratitude is extended to Michael Barnett for thoughtful discussion and comments. Finally we thank all of the teachers, nurses, students, parents, and guardians who have played a vital role in the success of this study.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Albandar, J. M., Y. A. P. Buschi, and M. F. Z. Barbosa. 1991. Destructive forms of periodontal disease in adolescents. A 3-year longitudinal study. J. Periodontol. 62:370-376. [DOI] [PubMed] [Google Scholar]
- 2.Albandar, J. M., and T. E. Rams. 2002. Risk factors for periodontitis in children and young persons. Periodontol. 2000 29:207-222. [DOI] [PubMed] [Google Scholar]
- 3.Asikainen, S., H. Jousimies-Somer, A. Kanervo, and P. Sommanen. 1987. Certain bacterial species and morphotypes in localized juvenile periodontitis and in matched controls. J. Periodontol. 58:224-230. [DOI] [PubMed] [Google Scholar]
- 4.Baehni, P., C. C. Tsai, N. S. Taichman, and W. McArthur. 1978. Interaction of inflammatory cells and oral microorganisms. J. Periodontol. Res. 4:333-348. [DOI] [PubMed] [Google Scholar]
- 5.Baer, P. 1971. The case of periodontosis as a clinical entity. J. Periodontol. 42:516-520. [DOI] [PubMed] [Google Scholar]
- 6.Best, A. M., J. A. Burmeister, J. C. Gunsolley, C. N. Brooks, and H. A. Schenkein. 1990. Reliability of attachment loss measurements in a longitudinal clinical trial. J. Clin. Periodontol. 17:564-569. [PubMed] [Google Scholar]
- 7.Bogert, M., P. Berthold, V. Brightman, R. Craig, J. DiRienzo, C.-H. Lai, E. Lally, J. Oler, T. Rams, B. Shenker, J. Slots, N. Taichman, and R. Tisot. 1989. Longtudinal study of LJP families—two year surveillance. J. Dent. Res. 68(Special issue):312. [Google Scholar]
- 8.Brown, L. J., J. M. Albandar, J. A. Brunelle, and H. Loe. 1996. Early-onset periodontitis: progression of attachment loss during 6 years. J. Periodontol. 67:968-975. [DOI] [PubMed] [Google Scholar]
- 9.Bueno, L. C., M. P. Mayer, and J. M. DiRienzo. 1998. Relationship between conversion of localized juvenile periodontitis-susceptible children from health to disease and Actinobacillus actinomycetemcomitans leukotoxin promoter structure. J. Periodontol. 69:998-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clerehugh, V., H. V. Worthington, M. A. Lennon, and R. Chandler. 1995. Site progression of loss of attachment over 5 years in 14- to 19-year-old adolescents. J. Clin. Periodontol. 22:15-21. [DOI] [PubMed] [Google Scholar]
- 11.Cox, D. R. 1972. Regression models and life-tables. J. Royal Stat. Soc. 34:187-220. [Google Scholar]
- 12.Craig, R., R. Boylan, and J. Yip. 2001. Prevalence and risk indicators for destructive periodontal diseases in 3 urban American minority populations. J. Clin. Periodontol. 28:524-535. [DOI] [PubMed] [Google Scholar]
- 13.Dahlén, G., F. Widar, R. Teanpaisan, P. Papapanou, V. Baelum, and O. Fejerskov. 2002. Actinobacillus actinomycetemcomitans in a rural adult population in southern Thailand. Oral Microbiol. Immunol. 17:137-242. [DOI] [PubMed] [Google Scholar]
- 14.Eger, T., L. Zoller, H. Muller, S. Hoffmann, and D. Lobinsky. 1996. Potential diagnostic value of sampling oral mucosal surfaces for Actinobacillus actinomycetemcomitans in young adults. Eur. J. Oral Sci. 104:112-117. [DOI] [PubMed] [Google Scholar]
- 15.Fine, D. H., and D. Furgang. 2002. Lactoferrin iron levels affect attachment of Actinobacillus actinomycetemcomitans to buccal epithelial cells. J. Periodontol. 73:616-623. [DOI] [PubMed] [Google Scholar]
- 16.Fine, D. H., D. Furgang, and F. Beydouin. 2002. Lactoferrin iron levels are reduced in saliva of patients with localized juvenile periodontitis. J. Periodontol. 73:624-630. [DOI] [PubMed] [Google Scholar]
- 17.Fine, D. H., K. Velliyagounder, D. Furgang, and J. B. Kaplan. 2005. The Actinobacillus actinomycetemcomitans autotransporter adhesin Aae exhibits specificity for buccal epithelial cells from humans and Old World primates. Infect. Immun. 73:1947-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goncharoff, P. D., D. H. Figurski, R. H. Stevens, and D. H. Fine. 1993. Identification of Actinobacillus actinomycetemcomitans: polymerase chain reaction amplification of LktA-specific sequences. Oral Microbiol. Immunol. 8:105-110. [DOI] [PubMed] [Google Scholar]
- 19.Goodson, M. J., A. D. Haffajee, and S. S. Socransky. 1984. The relationship between attachment level loss and alveolar bone loss. J. Clin. Periodontol. 11:348-359. [DOI] [PubMed] [Google Scholar]
- 20.Haraszthy, V. I., G. Hariharan, E. M. Tinoco, J. R. Cortelli, E. T. Lally, E. Davis, and J. J. Zambon. 2000. Evidence for the role of highly leukotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. J. Periodontol. 71:912-922. [DOI] [PubMed] [Google Scholar]
- 21.Haraszthy, V. I., J. J. Zambon, M. Trevisan, M. Zeid, and R. J. Genco. 2000. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 71:1554-1560. [DOI] [PubMed] [Google Scholar]
- 22.Haubek, D., J. M. DiRienzo, E. M. B. Tinoco, J. Westergaard, N. J. López, C.-P. Chung, K. Poulsen, and M. Kilian. 1997. Racial tropism of a highly toxic clone of Actinobacillus actinomycetemcomitans associated with juvenile periodontitis. J. Clin. Microbiol. 35:3037-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haubek, D., O. K. Ennibi, K. Poulsen, N. Benzarti, and V. Baelum. 2004. The highly leukotoxic JP2 clone of Actinobacillus actinomycetemcomitans and progression of periodontal attachment loss. J. Dent. Res. 83:767-770. [DOI] [PubMed] [Google Scholar]
- 24.Holtta, P., S. Alaluusua, M. Saarela, and S. Asikainen. 1994. Isolation frequency and serotype distribution of mutans streptococci and Actinobacillus actinomycetemcomitans, and clinical periodontal status in Finnish and Vietnamese children. J. Dent. Res. 102:113-119. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan, E. L., and P. Meier. 1972. Nonparametric estimation from incomplete observation. J. Am. Stat. Assoc. 53:457-481. [Google Scholar]
- 26.Kaplan, J. B., M. B. Perry, L. L. MacLean, D. Furgang, M. E. Wilson, and D. H. Fine. 2001. Structural and genetic analysis of O polysaccharide from Actinobacillus actinomycetemcomitans serotype f. Infect. Immun. 69:5375-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan, J. B., H. C. Schreiner, D. Furgang, and D. H. Fine. 2002. Population structure and genetic diversity of Actinobacillus actinomycetemcomitans strains isolated from localized juvenile periodontitis patients. J. Clin. Microbiol. 40:1181-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilian, M. 1998. Clonal basis of bacterial virulence. S. Karger, Basel, Switzerland.
- 29.Kolodrubetz, D., T. Dailey, J. L. Ebersole, and E. Kraig. 1989. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect. Immun. 57:1465-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lally, E. T., I. R. Kieba, D. R. Demuth, J. Rosenblum, E. E. Golub, N. S. Taichman, and C. W. Gibson. 1989. Indentification and expression of the Actinobacillus actinomycetemcomitans leukotoxin gene. Biochem. Biophys. Res. Commun. 159:256-262. [DOI] [PubMed] [Google Scholar]
- 31.Locker, D., M. Clarke, and H. Murray. 1998. Oral health status of Canadian-born and immigrant adolescents in North York, Ontario. Commun. Dent. Oral Epidemiol. 26:177-187. [DOI] [PubMed] [Google Scholar]
- 32.Loe, H., and L. J. Brown. 1991. Early onset periodontitis in the United States of America. J. Periodontol. 62:608-616. [DOI] [PubMed] [Google Scholar]
- 33.McNabb, H., A. Mobelli, R. Gmur, S. Mathey-Dinc, and N. P. Lang. 1992. Periodontal pathogens in shallow pockets in immigrants from developing countries. Oral Microbiol. Immunol. 7:267-272. [DOI] [PubMed] [Google Scholar]
- 34.Mombelli, A., R. Gmur, J. Frey, J. Meyer, K. Zee, J. O. Tam, E. C. M. Lo, J. M. DiRienzo, N. P. Lang, and E. F. Corbet. 1998. Actinobacillus actinomycetemcomitans and P. gingivalis in young Chinese adults. Oral Microbiol. Immunol. 13:231-237. [DOI] [PubMed] [Google Scholar]
- 35.Muller, H. P., A. Heinecke, A. Fuhrmann, T. Eger, and L. Zoller. 2001. Intraoral distribution of Actinobacillus actinomycetemcomitans in young adults with minimal periodontal disease. J. Periodontol. Res. 36:114-123. [DOI] [PubMed] [Google Scholar]
- 36.Newman, M. G., S. S. Socransky, E. D. Savitt, D. A. Propas, and A. Crawford. 1976. Studies of the microbiology of periodontosis. J. Periodontol. 47:373-379. [DOI] [PubMed] [Google Scholar]
- 37.Ohara, M., E. Oswald, and M. Sugai. 2004. Cytolethal distending toxin: a bacterial bullet targeted to nucleus. J. Biochem. 136:409-413. [DOI] [PubMed] [Google Scholar]
- 38.Papapanou, P., R. Teanpaisan, N. S. Obiechina, W. Pithpornchaiyakul, S. Pisuithanakan, V. Baelum, O. Fejerskov, and G. Dahlén. 2002. Periodontal microbiota and clinical periodontal status in a rural sample in southern Thailand. Eur. J. Oral Sci. 110:345-352. [DOI] [PubMed] [Google Scholar]
- 39.Rams, T. E., M. A. Listgarten, and J. Slots. 1994. Utility of radiographic crestal lamina dura for predicting periodontitis disease-activity. J. Clin. Periodontol. 21:571-576. [DOI] [PubMed] [Google Scholar]
- 40.Satcher, D. 2000. Oral health in America: a report of the Surgeon General. Department of Health and Human Services, National Insititutes of Dental and Craniofacial Research, National Institutes of Health, Rockville, MD.
- 41.Schreiner, H. C., K. Sinatra, J. B. Kaplan, D. Furgang, S. C. Kachlany, P. J. Planet, B. A. Perez, D. H. Figurski, and D. H. Fine. 2003. Tight-adherence genes of Actinobacillus actinomycetemcomitans are required for virulence in a rat model. Proc. Natl. Acad. Sci. USA 100:7295-7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroeder, H. E., and R. Attstrom. 1980. Pocket formation: an hypothesis, vol. II. Academic Press, London, United Kingdom.
- 43.Slots, J. 1976. The predominant cultivable organisms in juvenile periodontitis. Scand. J. Dent. Res. 84:1-10. [DOI] [PubMed] [Google Scholar]
- 44.Slots, J., H. S. Reynolds, and R. J. Genco. 1980. Actinobacillus actinomyce-temcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect. Immun. 29:1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Socransky, S. S., and A. D. Haffajee. 2005. Periodontal microbial ecology. Periodontol. 2000 38:135-187. [DOI] [PubMed] [Google Scholar]
- 46.Sugai, M., T. Kamamoto, S. Y. Pérès, Y. Ueno, H. Komatsuzawa, T. Fujiwara, H. Kurihara, H. Suginaka, and E. Oswald. 1998. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect. Immun. 66:5008-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor-Robinson, D., J. Aduse-Opoku, P. Sayed, J. M. Slaney, B. J. Thomas, and M. A. Curtis. 2002. Oro-bacteria in various atherosclerotic arteries. Eur. J. Clin. Microbiol. Infect. Dis. 21:755-757. [DOI] [PubMed] [Google Scholar]
- 48.Tinoco, E., M. Sivakumar, and H. Preus. 1998. The distribution and transmission of Actinobacillus actinomycetemcomitans in families with localized juvenile periodontitis. J. Clin. Periodontol. 25:99-105. [DOI] [PubMed] [Google Scholar]
- 49.van Winkelhoff, A., and K. Boutaga. 2005. Transmission of periodontal bacteria and models of infection. J. Clin. Periodontol 32(Suppl. 6):16-27. [DOI] [PubMed] [Google Scholar]
- 50.Yue, G., J. Kaplan, D. Furgang, K. G. Mansfield, and D. Fine. 2007. A second Aggregatibacter actinomycetemcomitans autotransporter adhesin that exhibits specificity for buccal epithelial cells of humans and Old World primates. Infect. Immun. 75:4440-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zambon, J. J., L. A. Christersson, and J. Slots. 1983. Actinobacillus actinomycetemcomitans in human periodontal disease. Prevalence in patient groups and distribution of biotypes and serotypes within families. J. Periodontol. 54:707-711. [DOI] [PubMed] [Google Scholar]