Abstract
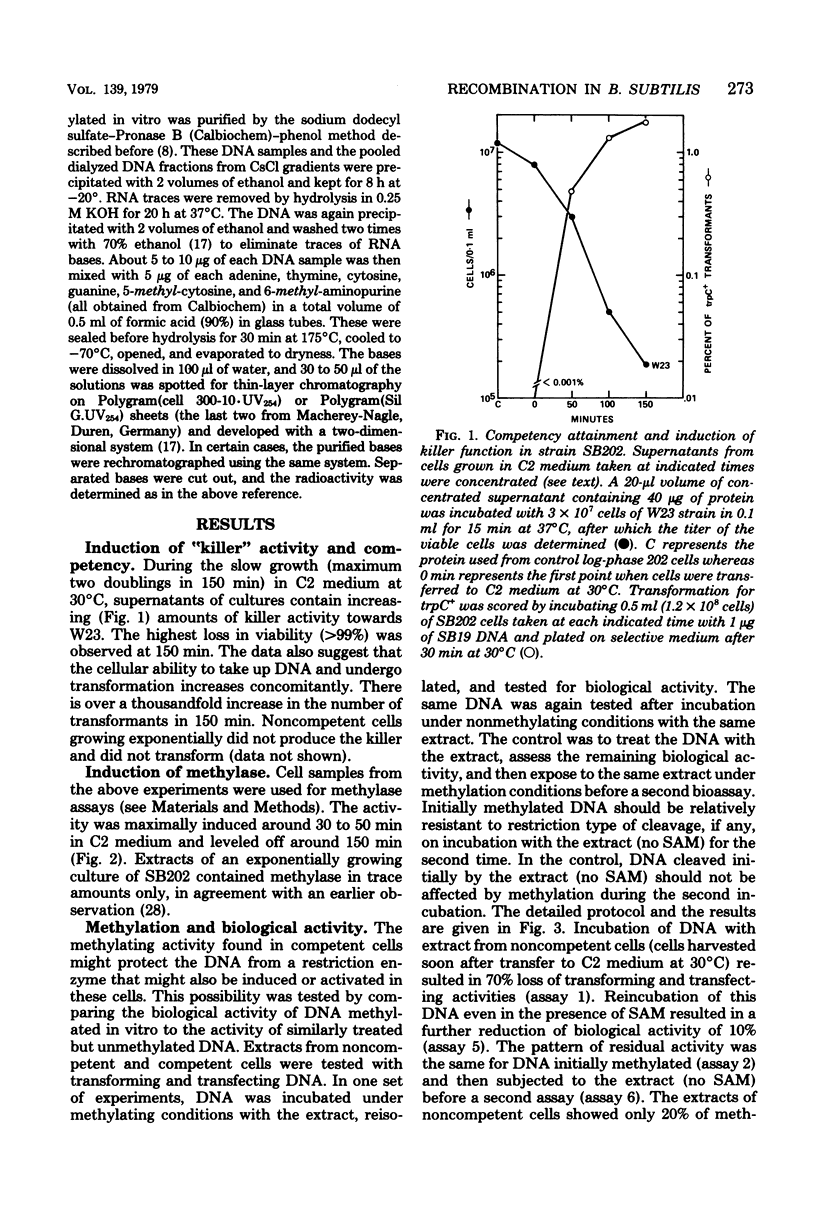
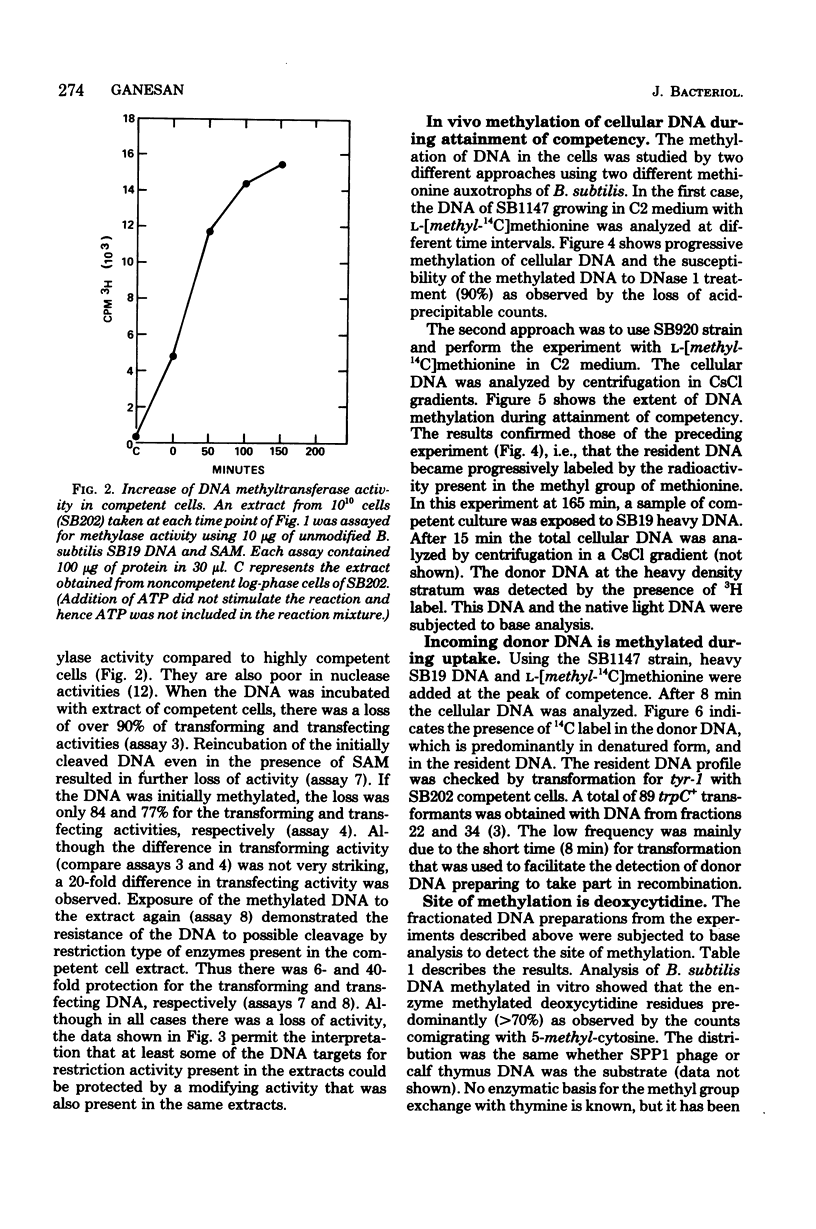
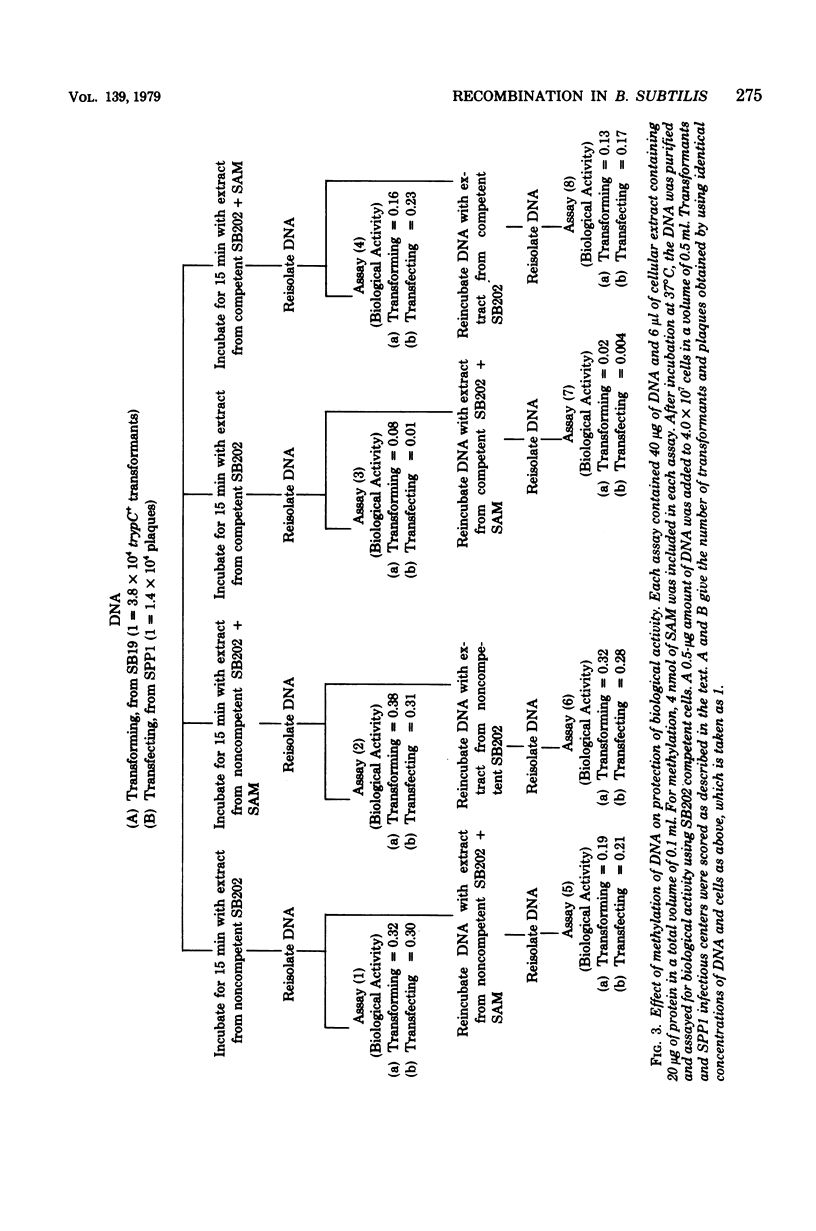
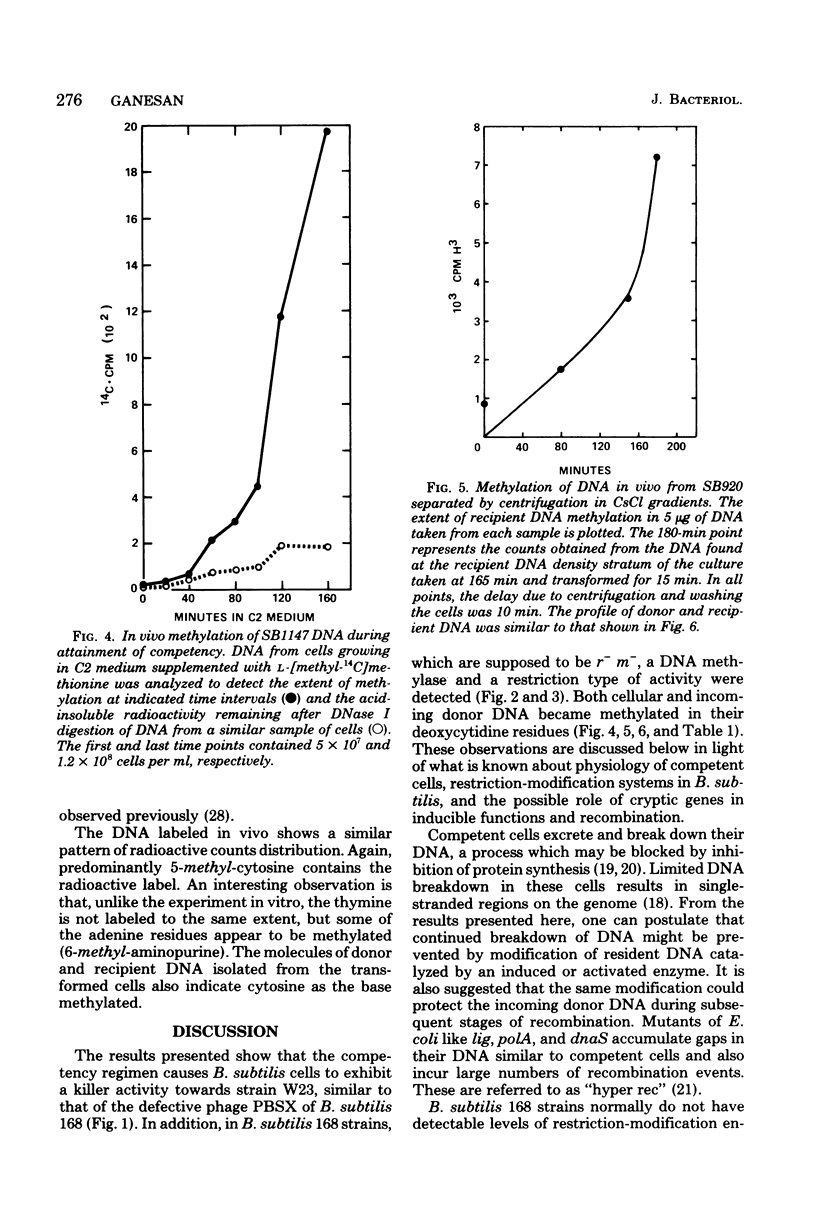
In Bacillus subtilis the ability to take up deoxyribonucleic acid (DNA) and undergo genetic transformation may coincide with the induction of defective phage(s) and the expression of possibly related cryptic genes. A restriction-modification enzyme system appears to be expressed. Targets of the restriction activity on the DNA can be blocked my methylation catalyzed by the methyl transferase. It is shown that cellular DNA becomes progressively methylated and reaches the maxium level during the peak of competency. Deoxycytidine residues of both incoming donor and resident DNA are methylated. The possible participation of these enzymes in recombination and the general role of cryptic genes in inducible functions are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. J., Ganesan A. T. Temperature-sensitive deoxyribonucleic acid replication in a dnaC mutant of Bacillus subtilis. J Bacteriol. 1975 Jan;121(1):173–183. doi: 10.1128/jb.121.1.173-183.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert F., Rutberg L. Restriction and modification in Bacillus subtilis. Induction of a modifying activity in Bacillus subtilis 168. Mol Gen Genet. 1974;133(2):175–177. doi: 10.1007/BF00264838. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron S., Murray K. Restriction and modification in B. subtilis. Nucleotide sequence recognised by restriction endonuclease R. Bsu R from strain R. Mol Gen Genet. 1975 Dec 30;143(1):25–33. doi: 10.1007/BF00269417. [DOI] [PubMed] [Google Scholar]
- Bron S., Murray K., Trautner T. A. Restriction and modification in B. subtilis. Purification and general properties of a restriction endonuclease from strain R. Mol Gen Genet. 1975 Dec 30;143(1):13–23. doi: 10.1007/BF00269416. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. In vivo site-specific genetic recombination promoted by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4811–4815. doi: 10.1073/pnas.74.11.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S. D. Replication and expression of plasmids from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1680–1682. doi: 10.1073/pnas.74.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J., Marmur J. Defective bacteriophages. J Cell Physiol. 1970 Dec;76(3):253–263. doi: 10.1002/jcp.1040760305. [DOI] [PubMed] [Google Scholar]
- Gunthert U., Schweiger M., Stupp M., Doerfler W. DNA methylation in adenovirus, adenovirus-transformed cells, and host cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3923–3927. doi: 10.1073/pnas.73.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günthert U., Pawlek B., Stutz J., Trautner T. A. Restriction and modification in Bacillus subtilis: inducibility of a DNA methylating activity in nonmodifying cells. J Virol. 1976 Oct;20(1):188–195. doi: 10.1128/jvi.20.1.188-195.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. J., Barr G. C. Mechanism of transformation in B. subtilis. Mol Gen Genet. 1971;113(4):331–344. doi: 10.1007/BF00272333. [DOI] [PubMed] [Google Scholar]
- Harris W. J., Barr G. C. Some properties of DNA in competent Bacillus subtilis. J Mol Biol. 1969 Jan;39(2):245–255. doi: 10.1016/0022-2836(69)90314-3. [DOI] [PubMed] [Google Scholar]
- Harris W. J., Barr G. C. Structural features of DNA in competent Bacillus subtilis. Mol Gen Genet. 1971;113(4):316–330. doi: 10.1007/BF00272332. [DOI] [PubMed] [Google Scholar]
- Konrad E. B. Method for the isolation of Escherichia coli mutants with enhanced recombination between chromosomal duplications. J Bacteriol. 1977 Apr;130(1):167–172. doi: 10.1128/jb.130.1.167-172.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller C. A., Cohen S. N. Phenotypically cryptic EcoRI endonuclease activity specified by the ColE1 plasmid. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1265–1269. doi: 10.1073/pnas.75.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W., STOCKER B. A. BIOSYNTHETIC LATENCY IN EARLY STAGES OF DEOXYRIBONUCLEIC ACIDTRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1963 Oct;86:785–796. doi: 10.1128/jb.86.4.785-796.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K. Mechanism of bacterial transformation and transfection. Prog Nucleic Acid Res Mol Biol. 1974;14(0):39–100. doi: 10.1016/s0079-6603(08)60205-6. [DOI] [PubMed] [Google Scholar]
- Notani N., Goodgal S. H. On the nature of recombinants formed during transformation in Hemophilus influenzae. J Gen Physiol. 1966 Jul;49(6):197–209. doi: 10.1085/jgp.49.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Marmur J. Purification and properties of deoxyribonucleic acid methylase from Bacillus subtilis. Biochemistry. 1966 Feb;5(2):761–773. doi: 10.1021/bi00866a051. [DOI] [PubMed] [Google Scholar]
- Riva S., Polsinelli M., Falaschi A. A new phage of Bacillus subtilis with infectious DNA having separable strands. J Mol Biol. 1968 Jul 28;35(2):347–356. doi: 10.1016/s0022-2836(68)80029-4. [DOI] [PubMed] [Google Scholar]
- Rottländer E., Trautner T. A. Genetic and transfection studies with B, subtilis phage SP 50. I. Phage mutants with restricted growth on B. subtilis strain 168. Mol Gen Genet. 1970;108(1):47–60. doi: 10.1007/BF00343184. [DOI] [PubMed] [Google Scholar]
- Rudin L., Sjöström J. E., Lindberg M., Philipson L. Factors affecting competence for transformation in Staphylococcus aureus. J Bacteriol. 1974 Apr;118(1):155–164. doi: 10.1128/jb.118.1.155-164.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Allison D. P., Beattie K. L. Relationship between prophage induction and transformation in Haemophilus influenzae. J Bacteriol. 1973 Jul;115(1):153–161. doi: 10.1128/jb.115.1.153-161.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., Ikawa S., Kim C., Ando T. Site-specific deoxyribonucleases in Bacillus subtilis and other Bacillus strains. J Bacteriol. 1976 Oct;128(1):473–476. doi: 10.1128/jb.128.1.473-476.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Competence for transfection in Staphylococcus aureus. J Bacteriol. 1973 Feb;113(2):576–585. doi: 10.1128/jb.113.2.576-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R. Physical heterogeneity among Bacillus subtilis deoxyribonucleic acid molecules carrying particular genetic markers. J Bacteriol. 1969 Jun;98(3):1239–1247. doi: 10.1128/jb.98.3.1239-1247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner T. A., Pawlek B., Bron S., Anagnostopoulos C. Restriction and modification in B. subtilis. Biological aspects. Mol Gen Genet. 1974;131(3):181–191. doi: 10.1007/BF00267958. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E. DNA repair in Bacillus subtilis. I. The presence of an inducible system. Mol Gen Genet. 1977 Jun 8;153(2):211–218. [PubMed] [Google Scholar]
- Yasbin R. E. DNA repair in Bacillus subtilis. II. Activation of the inducible system in competent bacteria. Mol Gen Genet. 1977 Jun 8;153(2):219–225. [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Effect of lysogeny on transfection and transfection enhancement in Bacillus subtilis. J Bacteriol. 1975 Jan;121(1):305–312. doi: 10.1128/jb.121.1.305-312.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis: evidence for selective induction of prophage in competent cells. J Bacteriol. 1975 Jan;121(1):296–304. doi: 10.1128/jb.121.1.296-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



