Abstract
Varicella-zoster virus (VZV) is a member of the Herpesviridae family, primary infection with which causes varicella, more commonly known as chicken pox. Characteristic of members of the alphaherpesvirus subfamily, VZV is neurotropic and establishes latency in sensory neurons. Reactivation of VZV causes herpes zoster, also known as shingles. The most frequent complication following zoster is chronic and often debilitating pain called postherpetic neuralgia (PHN), which can last for months after the disappearance of a rash. During episodes of acute zoster, VZV viremia occurs in some, but not all, patients; however, the effect of the viral load on the disease outcome is not known. Here we describe the development of a highly specific, sensitive, and reproducible real-time PCR assay to investigate the factors that may contribute to the presence and levels of baseline viremia in patients with zoster and to determine the relationship between viremia and the development and persistence of PHN. VZV DNA was detected in the peripheral blood mononuclear cells (PBMCs) of 78% of patients with acute zoster and in 9% of healthy asymptomatic blood donors. The presence of VZV in the PBMCs of patients with acute zoster was independently associated with age and being on antivirals but not with gender, immune status, extent of rash, the age of the rash at the time of blood sampling, having a history of prodromal pain, or the extent of acute pain. Prodromal pain was significantly associated with higher baseline viral loads. Viral load levels were not associated with the development or persistence of PHN at 6, 12, or 26 weeks.
Varicella-zoster virus (VZV) is ubiquitous in most populations worldwide. Primary infection causes varicella (chicken pox). The virus is neurotropic and establishes latency in sensory neurons. Reactivation of latent VZV causes herpes zoster (shingles), during which the virus replicates and spreads within the ganglion before migration via sensory nerves to the epidermis. The most common complication associated with zoster in healthy individuals is postherpetic neuralgia (PHN). PHN is variably defined as pain that persists for more than 4 weeks or 3 months after the development of the rash (11). The condition can be extremely distressing and may persist for several years (11).
The pathogenesis of PHN appears to be related to the destruction of cells and tissue scarring of affected sensory ganglia, which damages afferent nerve fibers, particularly type C nociceptors (4, 10). The mechanism for this may be direct viral destruction of ganglionic cells, or it may result from immunopathological events occurring in response to viral infection. Subsequently, aberrant neuronal regrowth occurs, producing nerve sprouts prone to unprovoked discharge (1). This leads to altered sensory perception and pain in the area supplied by the affected nerves (1). The onset and severity of PHN are associated with older age, female gender, the presence of prodromal pain, the extent and location of the rash, and the severity of pain at zoster onset (3, 5, 13, 21). Antiviral agents prescribed within 72 h of rash onset have been shown to reduce the duration of PHN in patients over age 50 years, and treatment with antiviral agents is currently recommended for the management of these patients (12).
Studies of the nucleoside inhibitor acyclovir and its prodrug, valacyclovir, which produces 10 times higher blood levels of active drug, have shown that the risk of PHN is inversely correlated with the total amount of active drug present in the blood over the first 72 h (2). This supports the notion that an early reduction in the viral load is likely to be key to preventing ganglionic tissue damage and PHN. Several groups have detected VZV DNA in peripheral blood mononuclear cells (PBMCs) and whole blood during acute chicken pox and shingles (7, 14, 15), but there has been little work to correlate clinical features with viral load. De Jong et al. (7) reported that the viral load was increased with multidermatomal involvement. We previously reported on an analysis of 119 patients in which an association between viremia and the duration of pain was noted. However, not all patients who were viremic during acute zoster developed PHN (17). Indeed, the predictive value of known risk factors is poor; for example, 81% of those over age 50 years recovered within 3 months (5). The better identification of those patients at the greatest risk would allow the targeting of treatment and the more effective evaluation of newer management regimens. The need for better prognostic markers to enable a more targeted approach to management has been highlighted. To investigate whether the baseline VZV viral load is related to clinical outcome, we have evaluated a real-time PCR assay with samples from 130 patients with acute zoster who were monitored for 6 to 12 months.
MATERIALS AND METHODS
Patient recruitment.
Blood samples for viral load analysis were obtained from a prospective study of 130 patients with active zoster presenting to family doctors in south and east London between 1997 and 2001. The diagnosis was confirmed clinically and by PCR of vesicle fluid. The patients were asked questions related to their age, gender, immune status (underlying illness and whether they were on any treatment, including inhaled steroids), and antiviral therapy; the age of the rash (how long they had had their zoster-associated rash before they went to see their doctor); the occurrence of prodromal pain, and the severity of their acute pain (achieved by using the visual analogue score [VAS]). The patients were asked to mark a cross on a 10-cm horizontal line according to the extent of their zoster-associated acute pain. A cross on the left hand terminus of the line was indicative of no pain. A cross on the right-hand terminus of the line was indicative of very severe/intolerable pain).
Separation of PBMCs and preparation of DNA.
PBMCs were isolated from 50 ml of heparinized blood (1:200 volume of 1% preservative-free heparin) by layering blood samples onto 12.5 ml (15% total volume) of a Ficoll-Hypaque gradient (Amersham Pharmacia Biotech AB, Sweden). Following centrifugation (20 min, 1,000 × g), the PBMCs were removed and decanted into 45 ml of phosphate-buffered saline, mixed, and centrifuged (12 min, 1,000 × g). This step was repeated, after which the PBMCs were resuspended in 3 ml of phosphate-buffered saline and 1 ml of PBMCs was decanted into three sterile 1.5 ml Eppendorf tubes. DNA was extracted from the PBMCs with a QIAamp DNA blood mini kit (Qiagen Ltd., United Kingdom), according to the manufacturer's instructions. The eluted DNA was stored at −20°C. For each set of DNA extractions, a negative control was used by replacing sample material with 200 μl of DNase- and RNase-free water (Sigma, United Kingdom).
PCR standards for real-time PCR.
Two primers (forward primer, 5′-CACGTATTTTCAGTCCTCTTCAAGTG-3′; reverse primer, 5′-TTAGACGTGGAGTTGACATCGTTT-3′) and a probe (5′-TACCGCCCGTGGAGCGCG-3′) were designed by using Primer Express software (version 3.0; Applied Biosystems, United Kingdom) to target a 77-bp region of open reading frame (ORF) 29 in the VZV genome. The probe was synthesized with the 6-carboxyfluorescein fluorophore at its 5′ terminus and a 6-carboxytetramethylrhodamine quencher at its 3′ terminus (Crunched Ltd., United Kingdom). The primers and the probe were titrated, and the optimum working dilutions were determined to be 25 pmol for the primers and 5 pmol for the probe (data not shown). The PCR fragment amplified from ORF 29 was cloned into the EcoRI site of a pUC-based pCR2.1 vector by using a TA cloning kit, in accordance with the manufacturer's instructions (Invitrogen, United Kingdom), and transformed into One Shot competent TOP10 Escherichia coli cells (Invitrogen). Clones containing the vector plus the insert were isolated, and the DNA was purified with a QIAprep spin miniprep kit (Qiagen), according to the manufacturer's instructions. The DNA concentration was measured on a Gene Spec I spectrophotometer (Nalca Instruments Ltd., United Kingdom), and the plasmid copy number was calculated. Purified plasmid DNA was subsequently serially diluted and used to construct a standard curve. The specificity of the assay was confirmed by comparison of the sequences of the PCR products to all available sequences in the GenBank database by using the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/). No PCR products were amplified from 1 μg of herpes simplex virus, cytomegalovirus, or Epstein-Barr virus DNA.
Real-time PCR.
One microliter of each plasmid standard (8, 8 × 101, 8 × 102, 8 × 103, 8 × 104, 8 × 105, 8 × 106, or 8 × 107 copies per μl) or 9 μl of DNA extracted from lymphocytes was amplified with 12.5 μl of 2× TaqMan universal PCR master mix (which contains AmpliTaq Gold DNA polymerase, AmpErase uracil-N-glycosylase, deoxynucleoside triphosphates [with dUTP], passive reference 1 [see below], and optimized buffer components [proprietary formula]), 25 pmol of the forward and the reverse primers, and 5 pmol of the TaqMan probe. Reaction mixtures containing patient PBMC DNA also included 1.25 μl of the β-actin-predeveloped TaqMan assay reagents internal control kit (Applied Biosystems). The β-actin-predeveloped TaqMan assay reagents control kit contained primers and a probe designed to detect human β-actin and was used to ensure that any VZV-negative samples did not contain PCR inhibitors and that the DNA extractions were successful. All reaction volumes were corrected to 25 μl with DNase- and RNase-free water (Sigma). All PCRs were performed in an ABI 7700 real-time PCR system (Applied Biosystems), in triplicate, in a 96-well reaction plate. Following an initial step of 50°C for 2 min and 95°C for 10 min, the reaction mixtures were subjected to 45 cycles of amplification (55°C for 55 s and 60°C for 1 min). All reaction mixtures contained dUTP. This allows the use of uracil-N-glycosylase pretreatment of the PCR mixtures, which prevents false-positive results due to the carryover of amplimers. All reactions also contained the fluorescent dye ROX (6-carboxy-X-rhodamine; passive reference 1). This acted as a passive reference to normalize the fluorescent reporter dye signal and to eliminate the fluorescence variation caused by differences in reaction volumes. Analysis was performed with SDS (version 2.0) software (Applied Biosystems). The authenticity of all cycle threshold (CT) values was confirmed by manually visualizing the amplification plots generated to eliminate false-positive results due to aberrant light emission.
VZV viremia in patients with acute zoster and asymptomatic blood donors.
DNA from the PBMCs of 130 patients with zoster and from 53 asymptomatic blood donors was amplified by real-time PCR. Samples were considered positive for VZV if at least two CT values of each triplicate were within one CT of each other when they were amplified with the VZV-specific primers and probe. Samples were considered negative for VZV if all three CT values in a triplicate were 45 when they were amplified with the viral target primers and probe and if at least two CT values of that triplicate were below 45 and within one CT of each other with regard to the CT values for the β-actin primers and probe. The optical density of the DNA extracted from 200 μl of PBMCs from each patient was measured by using a Gene Spec I spectrophotometer (Gene Teach, Hong Kong). The optical density was converted into the DNA concentration (μg/μl), which was multiplied by 9 (to account for the 9 μl of DNA extracted from PBMCs used in the real-time assay for each patient) and divided into the viral copy number in 9 μl of PBMCs. This allowed the VZV copy number per μg of human genomic DNA (105 PBMCs) to be compared between patients.
Statistical methods. (i) Intra- and interassay variations.
The intra-assay variability of the TaqMan assay was assessed by comparing the CT values from triplicate amplifications of the plasmid standards and by comparing the VZV copy number obtained from the amplification of six replicates of five patient PBMC samples (see Tables 1 and 2, respectively). The interassay reproducibility of the TaqMan assay was assessed by comparing the VZV copy number obtained from five samples (samples A to E) amplified in replicates of six in two consecutive assays (see Table 2).
TABLE 1.
Triplicate CT values from amplification of plasmid standardsa
| VZV copy no. |
CT value
|
CV (%) | ||||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | Avg | SD | ||
| 8.00E + 07 | 15.04 | 15.50 | 15.14 | 15.23 | 0.24 | 1.96 |
| 8.00E + 06 | 19.11 | 19.23 | 19.12 | 19.15 | 0.07 | 0.36 |
| 8.00E + 05 | 22.01 | 22.43 | 22.11 | 22.18 | 0.22 | 0.99 |
| 8.00E + 04 | 25.40 | 25.21 | 24.93 | 25.18 | 0.24 | 0.95 |
| 8.00E + 03 | 29.29 | 29.10 | 29.42 | 29.27 | 0.16 | 0.54 |
| 8.00E + 02 | 34.10 | 33.92 | 34.11 | 34.04 | 0.11 | 0.32 |
| 8.00E + 01 | 36.09 | 37.10 | 35.00 | 36.06 | 1.05 | 2.91 |
| 8.00E + 00 | 40.00 | 40.11 | 41.00 | 40.37 | 0.55 | 1.36 |
R1 to R3, replicates 1 to 3, respectively; SD, standard deviation.
TABLE 2.
Intra- and interassay reproducibilitiesa
| Patient sample | Assay 1
|
Assay 2
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VZV copies (log10) for 103 copies/105 PBMCs
|
CV (%) | VZV copies (103)/105 PBMCs
|
CV (%) | |||||||||||||||||
| R1 | R2 | R3 | R4 | R5 | R6 | Median | Mean | SD | R1 | R2 | R3 | R4 | R5 | R6 | Median | Mean | SD | |||
| A | 1.03 | 0.99 | 1.09 | 1.21 | 1.11 | 0.94 | 1.06 | 1.06 | 0.1 | 9.43 | 0.97 | 1.03 | 1.01 | 1 | 1.01 | 1.12 | 1.01 | 1.02 | 0.05 | 4.9 |
| B | 3 | 3.12 | 3.03 | 3.1 | 3.2 | 3.08 | 3.09 | 3.09 | 0.07 | 2.26 | 2.99 | 3.09 | 3.01 | 3.11 | 3.09 | 2.93 | 3.05 | 3.04 | 0.07 | 2.3 |
| C | 5 | 5.05 | 4.9 | 5.1 | 4.99 | 5.01 | 5.01 | 5.01 | 0.07 | 1.39 | 5.08 | 5.19 | 5 | 5.11 | 5.15 | 4.99 | 5.1 | 5.09 | 0.08 | 1.57 |
| D | 7 | 7.13 | 7.02 | 6.94 | 6.99 | 7.03 | 7.01 | 7.02 | 0.06 | 0.85 | 7.12 | 7.02 | 6.98 | 7.01 | 6.94 | 7.02 | 7.02 | 7.02 | 0.06 | 0.85 |
| E | 10 | 10.02 | 10.04 | 10.01 | 9.97 | 10.02 | 10.02 | 10.01 | 0.02 | 0.19 | 10.06 | 10.02 | 9.95 | 10.13 | 10 | 10.02 | 10.02 | 10.03 | 0.06 | 0.59 |
R1 to R6, replicates 1 to 6, respectively; SD, standard deviation.
(ii) Univariate and multivariate analyses.
The presence and the absence of viremia at the time of diagnosis of zoster was analyzed in relation to age, gender, immune status, the age of the rash at the time of blood sampling, a history of prodromal pain, the use of antivirals, the pain VAS, and the extent of the rash (one dermatome versus more than one dermatome affected) by univariate analysis by the use of chi-squared tests (see Table 3). Analysis of the viral load excluded those patients in whom no virus was detectable (see Table 4). In this case, the log (ln) viral load was analyzed univariately in relation to age, gender, a history of prodromal pain, the use of antivirals, and the extent of the rash by use of the two-sample t test and in relation to immune status, the age of the rash at the time of blood sampling, and the pain VAS by one-way analysis of variance. Logistic regression by a stepwise procedure with a 10% significance level was performed by using SAS/STAT software (version 8; SAS Institute Inc., Cary, NC) to identify which combination of factors was the most strongly associated with the presence of viremia and with the probability of being in pain at 6, 12, and 26 weeks.
TABLE 3.
Determinants of VZV viremia in patients with acute zoster
| Variable | Category | Median viral load | Sample size | No. (row %) of patients
|
P value | |
|---|---|---|---|---|---|---|
| Viremia positive | Viremia negative | |||||
| Age (yr) | ≤50 | 690 | 55 | 36 (65) | 19 (35) | 0.004a |
| >50 | 1,000 | 75 | 65 (87) | 10 (13) | ||
| Gender | Female | 784 | 64 | 52 (81) | 12 (19) | 0.337 |
| Male | 1,000 | 66 | 49 (74) | 17 (26) | ||
| Immune status | Competent | 720 | 89 | 64 (72) | 25 (28) | 0.066b |
| Compromised | 1,000 | 31 | 28 (90) | 3 (10) | ||
| Inhaled steroids | 1,515 | 10 | 9 (90) | 1 (10) | ||
| Rash age (days) | 0 to 2 | 1,000 | 34 | 29 (85) | 5 (15) | 0.412 |
| 3 to 4 | 640 | 33 | 22 (67) | 11 (33) | ||
| 5 to 6 | 1,030 | 33 | 27 (82) | 6 (18) | ||
| 7 to 8 | 670 | 19 | 14 (74) | 5 (26) | ||
| >8 | 1,665 | 10 | 8 (80) | 2 (20) | ||
| Prodromal pain | Yes | 1,020 | 80 | 64 (80) | 16 (20) | 0.424 |
| No | 680 | 50 | 37 (74) | 13 (16) | ||
| On antivirals | Yes | 1,000 | 99 | 84 (85) | 15 (15) | 0.0005a |
| No | 400 | 31 | 17 (55) | 14 (45) | ||
| VAS | 0 | 2,185 | 8 | 6 (75) | 2 (25) | 0.881 |
| 1 to 2 | 930 | 26 | 22 (85) | 4 (15) | ||
| 3 to 4 | 510 | 23 | 17 (74) | 6 (26) | ||
| 5 to 6 | 690 | 25 | 20 (80) | 5 (20) | ||
| 7 to 8 | 1,140 | 32 | 25 (78) | 7 (22) | ||
| 9 to 10 | 635 | 16 | 11 (69) | 5 (31) | ||
| No. of dermatomes | 0 to 1 | 1,775 | 46 | 36 (78) | 10 (22) | 0.881 |
| >1 | 2,110 | 83 | 64 (77) | 19 (23) | ||
Significant at the 1% level.
Significant at the 10% level.
TABLE 4.
Factors influencing viral load in patients with VZV viremia
| Variable | Category | Mean viral load log (ln) no. | Sample size (no. of patients) | P value |
|---|---|---|---|---|
| Age (yr) | ≤50 | 7.341 | 36 | 0.352 |
| >50 | 7.135 | 65 | ||
| Gender | Female | 7.062 | 52 | 0.170 |
| Male | 7.363 | 49 | ||
| Immune status | Competent | 7.217 | 64 | 0.993 |
| Compromised | 7.186 | 28 | ||
| Inhaled steroids | 7.212 | 9 | ||
| Rash age (days) | 0 to 2 | 7.117 | 29 | 0.867 |
| 3 to 4 | 7.077 | 22 | ||
| 5 to 6 | 7.314 | 27 | ||
| 7 to 8 | 7.234 | 14 | ||
| >8 | 7.570 | 8 | ||
| Prodromal pain | Yes | 7.376 | 64 | 0.043a |
| No | 6.918 | 37 | ||
| On antivirals | Yes | 7.138 | 84 | 0.233 |
| No | 7.553 | 17 | ||
| VAS | 0 | 8.033 | 6 | 0.256 |
| 1 to 2 | 7.210 | 22 | ||
| 3 to 4 | 7.171 | 17 | ||
| 5 to 6 | 6.949 | 20 | ||
| 7 to 8 | 7.395 | 25 | ||
| 9 to 10 | 6.857 | 11 | ||
| No. of dermatomes | 0 to 1 | 7.235 | 46 | 0.863 |
| >1 | 7.197 | 83 | ||
| Pain at 6 wk | Yes | 7.144 | 47 | 0.674 |
| No | 7.239 | 50 | ||
| Pain at 3 mo | Yes | 7.037 | 29 | 0.380 |
| No | 7.259 | 68 | ||
| Pain at 6 mo | Yes | 7.191 | 20 | 0.993 |
| No | 7.188 | 76 |
Significant at the 5% level.
RESULTS
Sensitivity and reproducibility.
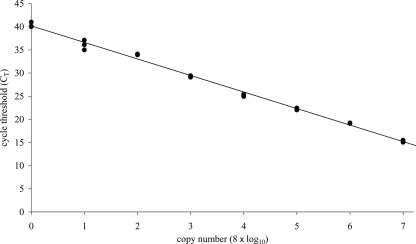
The real-time PCR assay was sensitive down to less than 10 VZV copies, which was detected at an average CT of 40.02 (Fig. 1). The relationship of CT with the log copy number was strong, with a correlation coefficient of 99.7%. The least dilute standard, which contained 8 × 107 copies, was detected at an average CT of 15.23 (Fig. 1). The intra-assay reproducibility was good, with the minimum and maximum coefficient of variation (CV) for the plasmid triplicates being 0.32% and 2.91%, respectively (Table 1). The intra-assay CV for actual patient samples increased as the copy number decreased and ranged from 0.19% for approximately 10 × 103 copies per 105 PBMCs to 9.43% for approximately 1 × 103 copies per 105 PBMCs (Table 2). The maximum interassay CV difference was 4.53% (assay 2, sample A, CV of 4.90% subtracted from assay 1, sample A, CV of 9.43%) (Table 2). The minimum interassay CV difference was 0% (assay 2, sample D, CV of 0.85% subtracted from assay 1, sample D, CV of 0.85%) (Table 2).
FIG. 1.
Standard curve generated by real-time PCR. Serial dilutions of each viral plasmid standard (•), ranging from 8 to 8 × 107 copies per reaction mixture, were used to generate the standard curve. The CT values that corresponded to the PCR cycle number were plotted against the copy number of each viral standard. The slope of the line was −3.57, the y intercept was 40.2, and the correlation coefficient was 99.7%.
VZV viremia in asymptomatic blood donors.
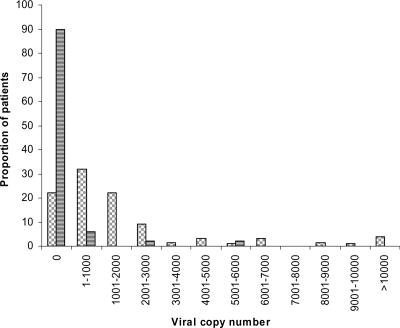
Of 53 asymptomatic blood donors, 5 (9%) had measurable PBMC viremia, with VZV copy numbers of 600, 902, 783, 2,006, and 5,009 per 105 PBMCs, respectively (Fig. 2). The remaining 48 patients were negative.
FIG. 2.
Distribution of VZV viral loads among 130 patients with zoster and 53 asymptomatic blood donors. Bars with horizontal stripes, asymptomatic blood donors; bars with gray blocks, represent zoster patients.
VZV viremia in patients with acute zoster.
VZV DNA was detected in the PBMCs of 101 (78%) of the 130 patients with acute zoster studied, which was significantly greater than the rate of detection from asymptomatic blood donors (P < 0.0001 from a test of two proportions). The distribution of viral loads is shown in Fig. 2 and ranged from 40 to 29 × 103 copies/105 PBMCs, with a median value of 925 copies/105 PBMCs. Although the numbers of patients in each group were small, there was no clear difference in the viral loads between asymptomatic and symptomatic patients. Even though the patients were bled between 0 and 29 days after the onset of their rash, no relationship between either the presence of virus (Table 3) or the levels of the viral load and the duration of the rash (Table 4) at the time of sampling was found.
The detection of VZV in PBMCs at the baseline was weakly associated with the patient's immune status (P = 0.066) and was significantly associated with the patient's age (P = 0.004) and taking antiviral agents (P = 0.0005) (Table 3). There was no association with gender, immune status, the age of the rash, the severity of pain, having a history of prodromal pain, or the extent of the rash. When this was further analyzed by logistic regression, only age (P = 0.01) remained significant.
By fitting a logistic regression model to predict a high or a low viral load by using the median viral load of 925 copies/105 PBMCs as the cutoff (the high viral load was greater than 925 copies/105 PBMCs), no significant association with age, gender, immune status, the age of the rash at the time of blood sampling, a history of prodromal pain, the use of antivirals, or the pain VAS was identified.
Exclusion of the 29 patients without a measurable viral load allowed us to log transform the viral load data from the remaining 101 viremic patients. Univariate analysis showed that a higher viral load was significantly associated only with prodromal pain (P = 0.04) (Table 4). Again, there was no association with gender, immune status, the age of the rash at the time of blood sampling, the severity of pain, or the extent of the rash.
By using data on pain and disability collected from these patients at 6, 12, and 26 weeks following the rash, we were able to examine the putative relationship between the baseline viral load and the development and persistence of PHN. Logistic regression was used to obtain a model of the baseline factors that predicted pain at each of these time points. On multivariate analysis, age over 50 years (P = 0.001), female gender (P = 0.054), and immunosuppression (P = 0.02) but not viral load were independently predictive of pain at 6 weeks. Age and gender remained independently predictive of higher pain scores at 12 weeks (P = 0.005 and 0.097, respectively) and 26 weeks (P = 0.036 and 0.023, respectively).
DISCUSSION
The data obtained in our study demonstrate that the real-time PCR assay for VZV viral load determination is sensitive, with a lower detection limit of eight copies of the VZV genome per microliter. This is comparable to the findings presented in previously published reports of about 10 copies per assay (equivalent to 12.5 copies for our assay) (19), 250 copies/ml (equivalent to approximately 6 copies for our assay) (18), and 50 to 80 copies/μg genomic DNA (equivalent to 40 copies for our assay) (15). The assay developed by De Jong et al. (7) was even more sensitive, detecting single genomes, equivalent to 20 copies/ml from serum and 80 copies/ml from whole blood (equivalent to 16 copies for our assay). The specificity of our assay for VZV was confirmed by searches with the BLAST program and the failure to amplify DNA from other herpesviruses (herpes simplex virus, cytomegalovirus, and Epstein-Barr virus), which all share some degree of sequence homology (6). The intra-assay and interassay reproducibilities were high, with maximum CVs of 9.43% and 4.53%, respectively.
The PBMCs of 9% (5/53) of randomly sampled patients undergoing phlebotomy were positive for VZV. None of these patients had clinical signs of varicella or zoster, none had had VZV-related disease within the previous 12 months, and their age and immune status were unknown. The significance of this finding is not known. Schunemann and colleagues (16) reported the detection of VZV in PBMCs from 2% (5/233) of patients seropositive for VZV but with no clinical signs of VZV illness. Mainka et al. (15) detected VZV in the PBMCs from 3% of immunocompetent individuals and 4% of immunocompromised individuals who were seropositive for VZV but who had no VZV-induced symptoms. Devlin and colleagues (8) detected VZV in 21% of PBMCs from VZV-seropositive patients without a history of zoster. In contrast, De Jong et al. (7) did not detect VZV in any of 30 asymptomatic controls using a highly sensitive assay which detected 1 copy per reaction mixture (equivalent to 20 to 80 copies per ml). The asymptomatic reactivation of latent virus may be important for boosting host immunity to the virus. Wilson et al. (20) found that asymptomatic and symptomatic reactivation of VZV in 19% of patients following allogeneic bone marrow transplantation was associated with improved measures of cell-mediated immunity compared with the measures in those who did not reactivate virus.
Seventy-eight percent (101/130) of the patients with acute zoster in our study had VZV DNA detected in their PBMCs at the time of presentation. This is higher than the rate in other reported studies of PBMCs. Mainka and colleagues (15) found that 16% of 71 patients with acute herpes zoster were positive for VZV DNA in their PBMCs, and a second study found a comparable percentage (20%) to be positive (14). The differences may reflect differences in the methods of cell separation, DNA extraction, and quantification. Although in our study the median level of viremia in healthy controls (902 copies per 105 PBMCs) was lower than that for symptomatic patients (1,160 copies per 105 PBMCs), they were not significantly different. From our study, therefore, we cannot identify a VZV load in PBMCs that correlates with the development of clinically evident zoster.
Of the previous studies, only De Jong et al. (7) correlated the viral load with demographic data and clinical symptoms. They found that the viral load was significantly higher in patients with multidermatomal zoster than in those with unidermatomal zoster and that in a single patient, the viral load increased as the extent of the rash increased. We did not find any significant association between the presence or levels of viremia in relation to a unidermatomal rash versus a multidermatomal rash. The extent of rash has been shown to be an independent risk factor for PHN when data from clinical trials have been analyzed (9). However, those data were based on the extent of the rash at a common time point (day 4) (9). Since in clinical practice most patients are seen at different stages during the natural history of their rash, this might not be a useful prognostic indicator.
We have previously shown by univariate analysis of 204 patients with zoster that the detection of viral DNA in PBMCs by a gel-based PCR is associated with age over 50 years and the persistence of pain (17). This finding is supported by univariate and multivariate analyses of the results from the current study and by the data from Devlin and colleagues (8). That group also found that the presence of VZV DNA in the PBMCs of patients with zoster correlated significantly with older age, postulating that underlying their findings was the failure of the immune system in older patients to control the viral load as effectively as the immune system in younger patients. Our observation that VZV viremia is more prevalent in immunocompromised patients than in immunocompetent patients provides a further rationale for this hypothesis. In our study we also found antiviral therapy to be associated with the presence of viremia, although this association disappeared on multivariate analysis. We have previously reported that being on antiviral therapy is an independent “risk factor” for the development of PHN, presumably because those patients who are prescribed antivirals have been assessed as being at the highest risk (17). In our previous study (17), the level of viremia at the baseline was also associated with continued pain or abnormal sensations at 3 and 6 months. However, in the current study no relationship between viral load and PHN was identified.
To examine these findings further, we analyzed only those patients with a measurable viral load at presentation. When the whole data set was considered, there were no associations with having a viral load greater or less than the median (925 copies per 105 PBMCs). Excluding the 29 (22%) patients with undetectable viral loads, only prodromal pain was significantly associated with viral load (Table 4). The findings may be confounded by the low numbers and by the heterogeneity of the remaining patients, who differed widely in age and by the day of sampling in relation to the age of the rash at the time of blood sampling. The exact mechanism behind prodromal pain is not known but probably relates to the earlier or the more extensive initial replication of VZV in sensory neurons prior to the appearance of the rash (12).
In summary, using a real-time PCR, we have confirmed earlier findings that VZV viremia is significantly more common in patients with shingles than in healthy controls. Our data suggest that age is an independent risk factor for viremia in patients with shingles. Age is also the strongest risk factor for the development of prolonged zoster-associated pain; however, the sample sizes used in the current study were too small to determine whether viremia is an independent risk factor for PHN in older people. Further studies are still needed to identify prognostic markers for patients who progress to PHN to aid in the targeting of more effective treatment and management regimens. Our data do not show any associations of viral load with risk factors for PHN, other than prodromal pain. These results may be confounded by the small and heterogeneous group analyzed. The assay developed here could be used for larger clinical trials to determine the association of viral load with PHN or for the quantification of viral DNA during therapy.
Acknowledgments
This work was supported by the research advisory board of Barts and the London special trustees.
Footnotes
Published ahead of print on 12 September 2007.
REFERENCES
- 1.Bennett, G. J. 1994. Neuropathic pain, p. 201-224. In P. D. Wall and R. Melzack (ed.), Textbook of pain. Churchill Livingstone, Edinburgh, United Kingdom.
- 2.Beutner, K. R., D. J. Friedman, C. Forszpaniak, P. L. Andersen, and M. J. Wood. 1995. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob. Agents Chemother. 39:1546-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowsher, D. 1999. The lifetime occurrence of herpes zoster and prevalence of post-herpetic neuralgia: a retrospective survey in an elderly population. Eur. J. Pain 3:335-342. [DOI] [PubMed] [Google Scholar]
- 4.Cervero, F., and J. M. Laird. 1996. Mechanisms of allodynia: interactions between sensitive mechanoreceptors and nociceptors. Neuroreport 7:526-528. [PubMed] [Google Scholar]
- 5.Coen, P. G., F. Scott, M. Leedham-Green, T. Nia, A. Jamil, R. W. Johnson, and J. Breuer. 2006. Predicting and preventing post-herpetic neuralgia: are current risk factors useful in clinical practice? Eur. J. Pain 10:695-700. [DOI] [PubMed] [Google Scholar]
- 6.Davison, A. J. 1991. Varicella-zoster virus. The Fourteenth Fleming lecture. J. Gen. Virol. 72:475-486. [DOI] [PubMed] [Google Scholar]
- 7.De Jong, M. D., J. F. Weel, T. Schuurman. P. M. Wertheim-van Dillen, and R. Boom. 2000. Quantitation of varicella-zoster virus DNA in whole blood, plasma, and serum by PCR and electrochemiluminescence. J. Clin. Microbiol. 38:2568-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devlin, M. E., D. H. Gilden, R. Mahalingam, A. N. Dueland, and R. Cohrs. 1992. Peripheral blood mononuclear cells of the elderly contain varicella-zoster virus DNA. J. Infect. Dis. 165:619-622. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin, R. H., R. J. Boon, D. R. Griffin, and D. Phung. 1998. Post-herpetic neuralgia: impact of famciclovir, age, rash severity and acute pain in herpes zoster patients. J. Infect. Dis. 178(Suppl. 1):S76-S80. [DOI] [PubMed] [Google Scholar]
- 10.Fields, H. L., M. Rowbotham, and R. Baron. 1998. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol. Dis. 5:209-227. [DOI] [PubMed] [Google Scholar]
- 11.Gilden, D. H., B. K. Kleinschmidt-DeMasters, J. J. LaGuardia, R. Mahalingam, and R. J. Cohrs. 2000. Neurologic complications of the reactivation of varicella-zoster virus. N. Engl. J. Med. 342:635-645. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, R. W. 2001. Herpes-zoster—predicting and minimizing the impact of post-herpetic neuralgia. J. Antimicrob. Chemother. 47(Suppl. T1):1-8. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, R. W. 2002. Consequences and management of pain in herpes zoster. J. Infect. Dis. 186(Suppl.):S83-S90. [DOI] [PubMed] [Google Scholar]
- 14.Kimura, H., S. Kido, T. Ozaki, N. Tanaka, Y. Ito, R. K. Williams, and T. Morishima. 2000. Comparison of quantitations of viral load in varicella and zoster. J. Clin. Microbiol. 38:2447-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mainka, C., B. Fuss, H. Geiger, H. Hofelmayr, and M. H. Wolff. 1998. Characterization of viraemia at different stages of varicella-zoster virus infection. J. Med. Virol. 56:91-98. [DOI] [PubMed] [Google Scholar]
- 16.Schunemann, S., C. Mainka, and M. H. Wolff. 1999. No acute varicella-zoster virus replication in peripheral blood mononuclear cells during post-herpetic neuralgia. Acta Virol. 43:337-340. [PubMed] [Google Scholar]
- 17.Scott, F. T., M. E. Leedham-Green, W. Y. Barrett-Muir, K. Hawrami, W. J. Gallagher, R. Johnson, and J. Breuer. 2003. A study of shingles and the development of post-herpetic neuralgia in east London. J. Med. Virol. 70(Suppl. 1):S24-S30. [DOI] [PubMed] [Google Scholar]
- 18.Stocher, M., G. Holzl, H. Stekel, and J. Berg. 2004. Automated detection of five human herpes virus DNAs by a set of LightCycler PCRs complemented with a single multiple internal control. J. Clin. Virol. 29:171-178. [DOI] [PubMed] [Google Scholar]
- 19.Weidmann, M., U. Meyer-Konig, and F. T. Hufert. 2003. Rapid detection of herpes simple virus and varicella-zoster virus infections by real-time PCR. J. Clin. Microbiol. 41:1565-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson, A., M. Sharp, C. M. Koropchak, S. F. Ting, and A. M. Arvin. 1992. Subclinical varicella-zoster virus viraemia, herpes zoster, and T lymphocyte immunity to varicella-zoster viral antigens after bone marrow transplantation. J. Infect. Dis. 165:119-126. [DOI] [PubMed] [Google Scholar]
- 21.Wood, M. J., R. Kay, R. H. Dworkin, S. J. Soong, and R. J. Whitley. 1996. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin. Infect. Dis. 22:341-347. [DOI] [PubMed] [Google Scholar]