Abstract
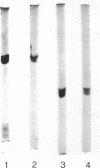
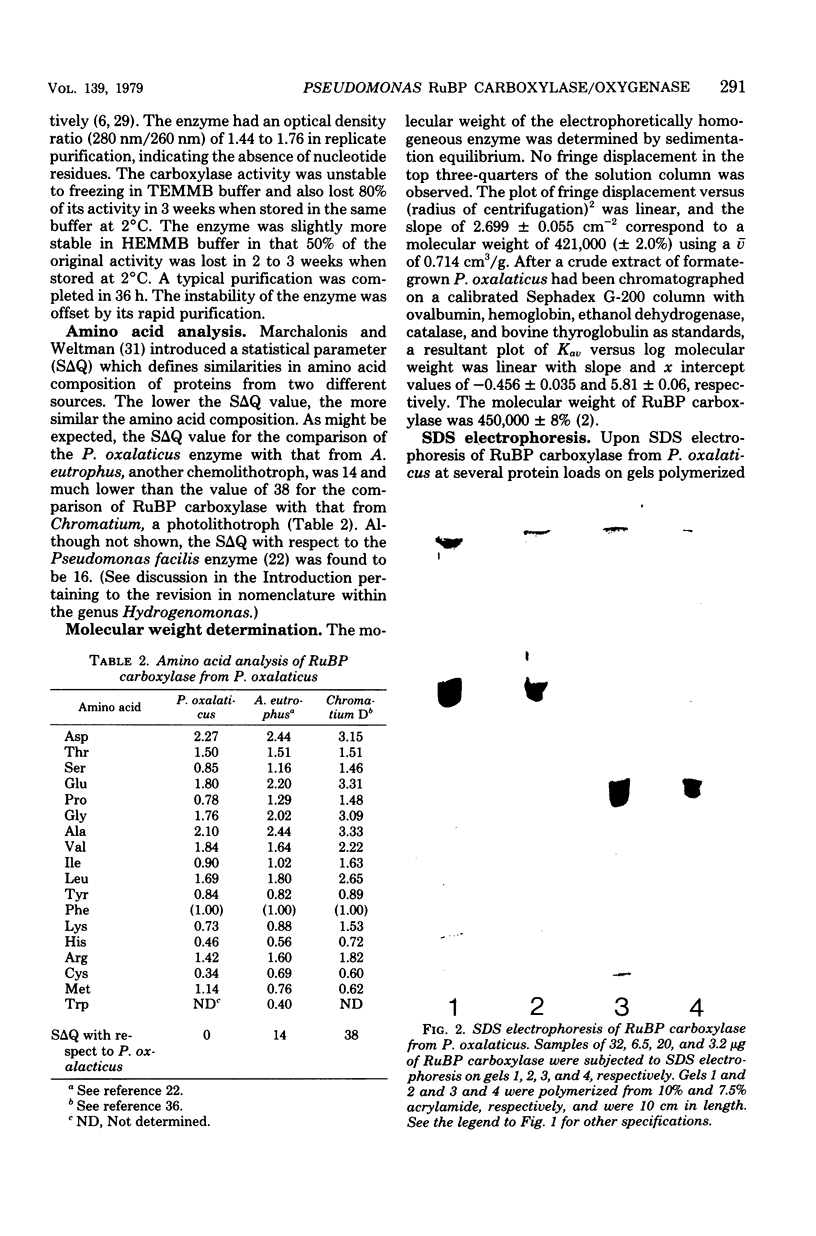
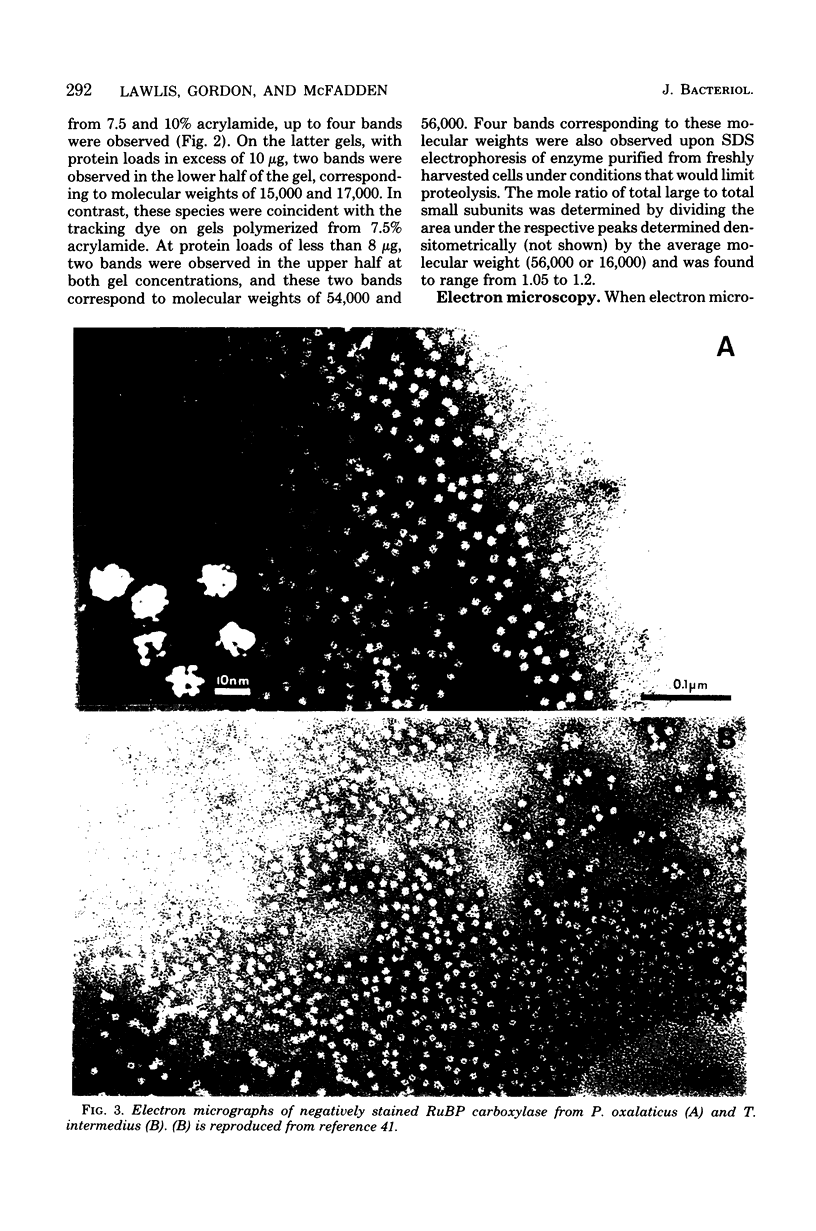
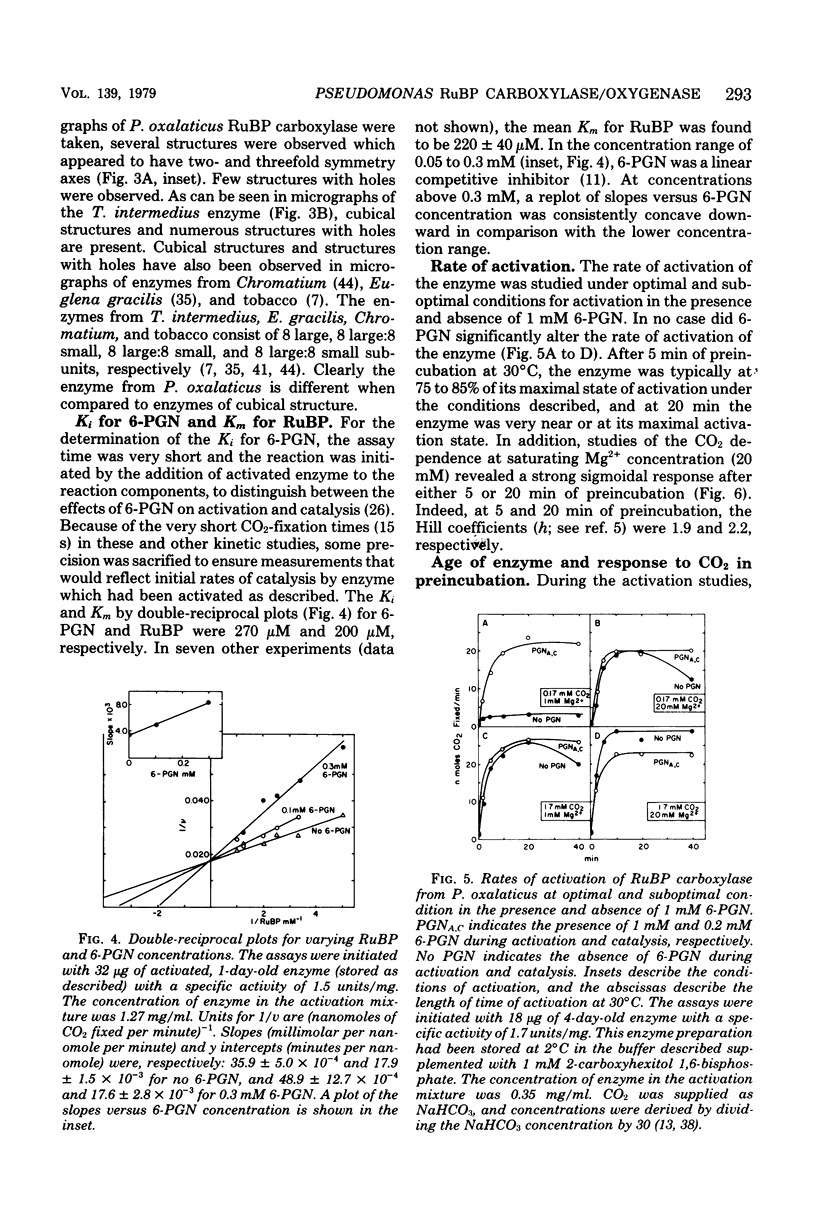
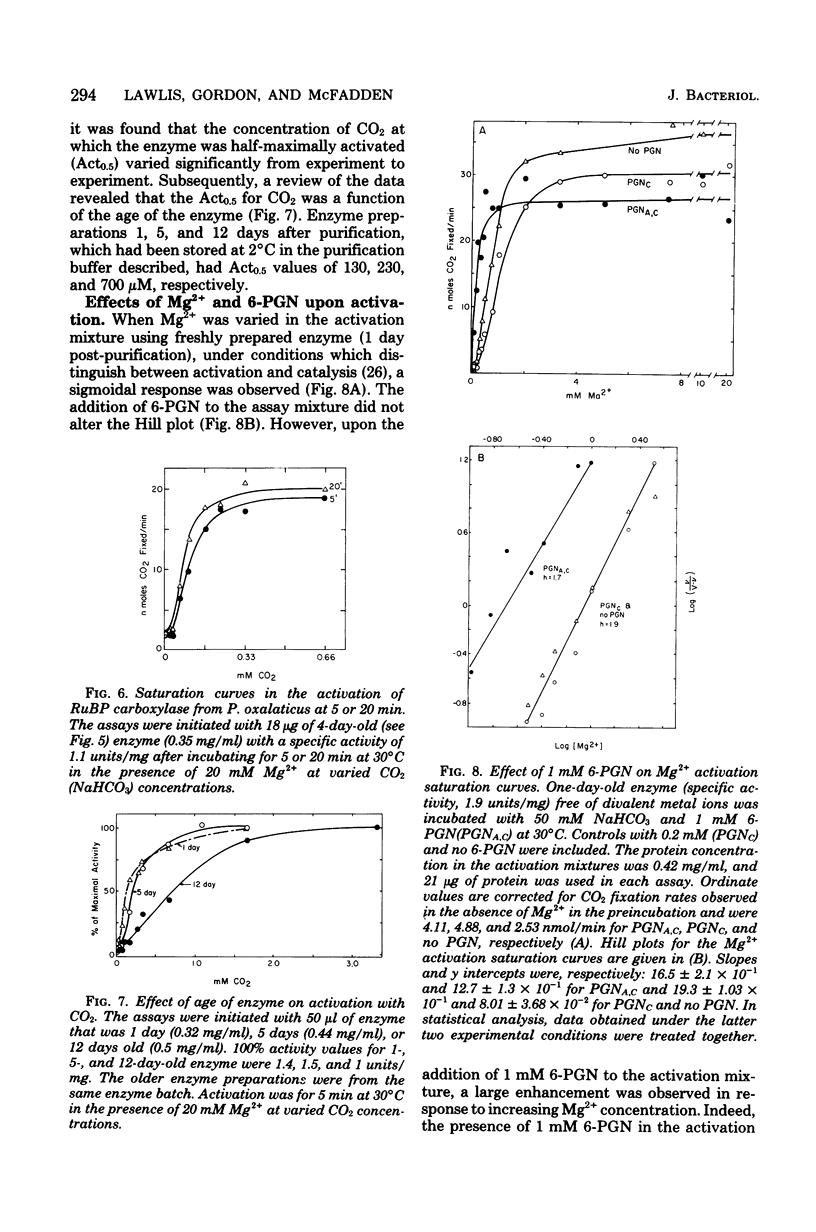
Ribulose 1,5-bisphosphate carboxylase/oxygenase was purified by a rapid, facile procedure from formate-grown Pseudomonas oxalaticus. The electrophoretically homogeneous enzyme had specific activities of 1.9 mumol of CO2 fixed per min per mg of protein and 0.15 mumol of O2 consumed per min per mg of protein. The amino acid composition was similar to that of other bacterial sources of the enzyme. The molecular weights determined by sedimentation equilibrium and by gel filtration were 421,000 and 450,000, respectively. Upon sodium dodecyl sulfate electrophoresis of enzyme purified under conditions which would limit proteolysis, two types of large (L) subunits and two types of small (S) subunits were observed with apparent molecular weights of 57,000, 55,000, 17,000 and 15,000. By densitometric scans at two different protein concentrations the stoichiometry of the total large to total small subunits was 1:1, implying an L6S6 structure. Electron micrographs of the enzyme revealed an unusual structure that was inconsistent with a cubical structure. The enzyme had an unusually high Km for ribulose 1,5-bisphosphate (220 microM) and was strongly inhibited by 6-phosphogluconate in the ribulose 1,5-bisphosphate carboxylase assay (Ki = 270 microM). One, 5, and 12 days after purification the enzyme was half-maximally activated at 0.13 microM, 0.23 mM, and 0.70 mM CO2, respectively, at saturating Mg2+. At saturating CO2, enzyme 1 day afer purification responded sigmoidally to Mg2+ and was half-maximally activated by 0.85 mM Mg2+ in the absence of 6-phosphogluconate (Hill coefficient, h = 2.0) and by 0.19 mM Mg2+ in the presence of mM 6-phosphogluconate (h = 1.7).
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- Akazawa T., Kondo H., Shimazue T., Nishimura M., Sugiyama T. Further studies on ribulose 1,5-diphosphate carboxylase from Chromatium strain D. Biochemistry. 1972 Mar 28;11(7):1298–1303. doi: 10.1021/bi00757a028. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews T. J., Lorimer G. H., Tolbert N. E. Ribulose diphosphate oxygenase. I. Synthesis of phosphoglycolate by fraction-1 protein of leaves. Biochemistry. 1973 Jan 2;12(1):11–18. doi: 10.1021/bi00725a003. [DOI] [PubMed] [Google Scholar]
- Badger M. R., Lorimer G. H. Activation of ribulose-1, 5-bisphosphate oxygenase, The role of Mg2+, CO2, and pH. Arch Biochem Biophys. 1976 Aug;175(2):723–729. doi: 10.1016/0003-9861(76)90565-8. [DOI] [PubMed] [Google Scholar]
- Blackmore M. A., Quayle J. R., Walker I. O. Choice between autotrophy and heterotrophy in Pseudomonas oxalaticus. Utilization of oxalate by cells after adaptation from growth on formate to growth on oxalate. Biochem J. 1968 May;107(5):699–704. doi: 10.1042/bj1070699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A. A kinetic study of ribulose bisphosphate carboxylase from the photosynthetic bacterium Rhodospirillum rubrum. Biochem J. 1978 Aug 1;173(2):467–473. doi: 10.1042/bj1730467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Filmer D. The active species of "CO2" utilized by ribulose diphosphate carboxylase. J Biol Chem. 1969 Feb 10;244(3):1081–1083. [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Different molecular forms of D-ribulose-1,5-bisphosphate carboxylase from Rhodopseudomonas sphaeroides. J Biol Chem. 1977 Feb 10;252(3):943–949. [PubMed] [Google Scholar]
- Goldthwaite J. J., Bogorad L. A one-step method for the isolation and determination of leaf ribulose-1,5-diphosphate carboxylase. Anal Biochem. 1971 May;41(1):57–66. doi: 10.1016/0003-2697(71)90191-6. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- JAYASURIYA G. C. The isolation and characteristics of an oxalate-decomposing organism. J Gen Microbiol. 1955 Jun;12(3):419–428. doi: 10.1099/00221287-12-3-419. [DOI] [PubMed] [Google Scholar]
- Kuehn G. D., McFadden B. A. Ribulose 1,5-diphosphate carboxylase from Hydrogenomonas eutropha and Hydrogenomonas facilis. I. Purification, metallic ion requirements, inhibition, and kinetic constants. Biochemistry. 1969 Jun;8(6):2394–2402. doi: 10.1021/bi00834a021. [DOI] [PubMed] [Google Scholar]
- Kuehn G. D., McFadden B. A. Ribulose 1,5-diphosphate carboxylase from Hydrogenomonas eutropha and Hydrogenomonas facilis. II. Molecular weight, subunits, composition, and sulfhydryl groups. Biochemistry. 1969 Jun;8(6):2403–2408. doi: 10.1021/bi00834a022. [DOI] [PubMed] [Google Scholar]
- Kung S. D., Gray J. C., Wildman S. G., Carlson P. S. Polypeptide composition of fraction 1 protein from parasexual hybrid plants in the genus Nicotiana. Science. 1975 Jan 31;187(4174):353–355. doi: 10.1126/science.187.4174.353. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T. A model for the kinetics of activation and catalysis of ribulose 1,5-bisphosphate carboxylase. Biochem J. 1976 Dec 1;159(3):563–570. doi: 10.1042/bj1590563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlis V. B., Gordon G. L., McFadden B. A. Regulation of activation of ribulose bisphosphate carboxylase from Pseudomonas oxalaticus. Biochem Biophys Res Commun. 1978 Oct 16;84(3):699–705. doi: 10.1016/0006-291x(78)90761-1. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Brown R. H. Purification and Some Properties of Chlorella fusca Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1975 Feb;55(2):360–364. doi: 10.1104/pp.55.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Andrews T. J., Tolbert N. E. Ribulose diphosphate oxygenase. II. Further proof of reaction products and mechanism of action. Biochemistry. 1973 Jan 2;12(1):18–23. doi: 10.1021/bi00725a004. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- McFadden B. A. Autotrophic CO2 assimilation and the evolution of ribulose diphosphate carboxylase. Bacteriol Rev. 1973 Sep;37(3):289–319. doi: 10.1128/br.37.3.289-319.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A., Lord J. M., Rowe A., Dilks S. Composition, quaternary structure, and catalytic properties of D-ribulose-1, 5-bisphosphate carboxylase from Euglena gracilis. Eur J Biochem. 1975 May;54(1):195–206. doi: 10.1111/j.1432-1033.1975.tb04129.x. [DOI] [PubMed] [Google Scholar]
- McFadden B. A., Tabita F. R. D-ribulose-1, 5-diphosphate carboxylase and the evolution of autotrophy. Biosystems. 1974 Oct;6(2):93–112. doi: 10.1016/0303-2647(74)90002-1. [DOI] [PubMed] [Google Scholar]
- Murai T., Akazawa T. Homotropic effect of CO 2 in ribulose-1,5-diphosphate carboxylase reaction. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2121–2126. doi: 10.1016/0006-291x(72)90768-1. [DOI] [PubMed] [Google Scholar]
- Purohit K., McFadden B. A., Cohen A. L. Purification, quaternary structure, composition, and properties of D-ribulose-1,5-bisphosphate carboxylase from Thiobacillus intermedius. J Bacteriol. 1976 Jul;127(1):505–515. doi: 10.1128/jb.127.1.505-515.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit K., McFadden B. A. Heterogeneity of large subunits of ribulose-1,5-bisphosphate carbosylase from Hydrogenomonas eutropha. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1220–1227. doi: 10.1016/0006-291x(76)90784-1. [DOI] [PubMed] [Google Scholar]
- Purohit K., McFadden B. A. Quaternary structure and oxygenase activity of D-ribulose-1,5-bisphosphate carboxylase from Hydrogenomonas eutropha. J Bacteriol. 1977 Jan;129(1):415–421. doi: 10.1128/jb.129.1.415-421.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAYLE J. R., KEECH D. B. Carbon assimilation by Pseudomonas oxalaticus (OX1). 343. Oxalate utilization during growth on oxalate. Biochem J. 1960 Jun;75:515–523. doi: 10.1042/bj0750515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M., Saluja A., McFadden B. A. Ribulose bisphosphate carboxylase from methanol-grown Paracoccus denitrificans. J Bacteriol. 1978 Jun;134(3):1123–1132. doi: 10.1128/jb.134.3.1123-1132.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Ito T., Akazawa T. Subunit structure of ribulose 1,5-diphosphate carboxylase from Chlorella ellipsoidea. Biochemistry. 1971 Aug 31;10(18):3406–3411. doi: 10.1021/bi00794a014. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. II. Quaternary structure, composition, catalytic, and immunological properties. J Biol Chem. 1974 Jun 10;249(11):3459–3464. [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. Molecular and catalytic properties of ribulose 1,5-bisphosphate carboxylase from the photosynthetic extreme halophile Ectothiorhodospira halophila. J Bacteriol. 1976 Jun;126(3):1271–1277. doi: 10.1128/jb.126.3.1271-1277.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. One-step isolation of microbial ribulose-1,5-diphosphate carboxylase. Arch Microbiol. 1974;99(3):231–240. doi: 10.1007/BF00696237. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A., Pfennig N. D-ribulose-1,5-bisphosphate carboxylase in Chlorobium thiosulfatophilum Tassajara. Biochim Biophys Acta. 1974 Mar 21;341(1):187–194. doi: 10.1016/0005-2744(74)90079-5. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., Stevens S. E., Jr, Gibson J. L. Carbon dioxide assimilation in blue-green algae: initial studies on the structure of ribulose 1,5-bisphosphate carboxylase. J Bacteriol. 1976 Feb;125(2):531–539. doi: 10.1128/jb.125.2.531-539.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita R. F., Stevens S. E., Jr, Quijano R. D-ribulose 1, 5-diphosphate carboxylase from blue-green algae. Biochem Biophys Res Commun. 1974 Nov 6;61(1):45–52. doi: 10.1016/0006-291x(74)90531-2. [DOI] [PubMed] [Google Scholar]
- Urey H. C. On the Early Chemical History of the Earth and the Origin of Life. Proc Natl Acad Sci U S A. 1952 Apr;38(4):351–363. doi: 10.1073/pnas.38.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]