Abstract
This investigation describes drug resistance patterns and genotyping data on a total of 145 Mycobacterium tuberculosis strains isolated between 2000 and 2004 in Malatya, Turkey. Drug susceptibility results indicated a total of 20% resistant and 4.8% of multidrug-resistant isolates. Spoligotyping resulted in 25 unique patterns and 120 strains in 19 clusters (2 to 33 strains per cluster). When the results were compared to an international spoligotyping database, 19 of 25 unique patterns matched existing shared spoligotype international types (SITs). This led to the description of 38 SITs with 139 strains and 6 orphan patterns (not previously reported). Five of the SITs (SIT759, SIT1936, SIT1937, SIT1938, and SIT2285) were newly created. The most prevalent spoligotype was SIT41 (LAM7-TUR) with 33 (23.9%) isolates. The repartition of strains according to major M. tuberculosis clades (in decreasing order) was as follows: ill-defined T clade (45.7%) > Latin American and Mediterranean (LAM; 29%) > Haarlem (15.9%). Strains belonging to Central Asian (CAS), East-African Indian (EAI), Beijing, and Africanum clades were absent in this setting. IS6110-restriction fragment length polymorphism (RFLP) resulted in 19 clusters (52 strains), with a final clustering rate of 35.9% and a recent transmission rate of 22.8%. Typing based on mycobacterial interspersed repetitive units (MIRUs) permitted us to identify 65 patterns (23 orphan patterns and 42 patterns that matched existing MIRU international types in an updated database). The combination of the three typing methods allowed us to calculate a final clustering rate of 22% and a significantly lower transmission rate of 13.1%. The discrimination achieved by IS6110-RFLP/MIRUs was not significantly improved by adding spoligotyping results (1.4%). We conclude that our patient population is infected by diverse M. tuberculosis populations; however, the majority of the ongoing transmission is due to “evolutionary recent” tuberculosis lineages belonging to principal genetic group 2 (PGG2; Haarlem and LAM) and PGG3 (ill-defined T clade), and most of it is attributable to the LAM7-TUR sublineage with an enhanced phylogeographical specificity for Turkey. An absence of lineages belonging to PGG1 clones (EAI, CAS, and Beijing, essentially found in Central, South, and Southeast Asia), is noteworthy.
Although tuberculosis (TB) incidence has declined from 172 cases per 100,000 people in 1945 to 26/100,000 people in 2005, Turkey is still among the countries with moderate TB incidence. According to the World Health Organization's records, TB incidence in Turkey in 2004 was 28/100,000 with a mortality rate of 5 persons per 100,000 inhabitants. Annually, 18,000 to 20,000 TB patients have been reported throughout the country; 69% of the patients have pulmonary TB, >60% of the cases are male, and for both sexes the highest prevalence is observed in 15- to 24-year age group (Tuberculosis Control Department, Turkish Ministry of Health). A directly observed therapy short-course strategy was started as a pilot study in 2003 but has not yet been implemented throughout the country. Since the beginning of the 1950s, BCG vaccination has been routinely applied to all children at 2 months of age. TB control is performed by 248 dispensaries around the country and 167 laboratories of the national tuberculosis control department. However, only a few medical centers are able to perform accurate and rapid culture and susceptibility testing of the Mycobacterium tuberculosis isolates; this may be indirectly responsible for the high prevalence of drug-resistant TB in Turkey.
Malatya, a city in the east Anatolia region, has a TB incidence of about 32/100,000 inhabitants, which is slightly higher than the country's overall incidence, and 270 to 300 new TB cases with about 80 to 90 positive cultures are reported annually. Two TB dispensaries, two governmental hospitals, and a university hospital provide health services for about 850,000 persons who reside in Malatya. TB patients have been mainly diagnosed by microscopy, by culture, and in some cases by PCR. Drug susceptibility testing for the first isolate of each patient is performed in the university hospital, and patients are treated free of charge. Contact investigations were performed in and around patients' families. An epidemiological analysis showed that most of the TB patients (95%) in Malatya had very low household incomes (less than 150 dollars per month), about one-third of the patients had at least one TB patient in their family either in the past or at the present time, and only 36% of the TB patients had been BCG vaccinated (8).
Molecular tools have enhanced our understanding on the epidemiology of TB by providing new insight on the transmission dynamics, source, and spread of M. tuberculosis (9, 16). By helping to differentiate an ongoing exogenous infection from reactivation, these tools are also helpful for evaluating TB control and treatment programs in a given population (1). At the phylogenetic level, the distribution of specific M. tuberculosis clones (e.g., Beijing/W, LAM7-TUR, etc.) or multidrug-resistant (MDR) TB strains can be easily monitored by molecular typing methods regionally, by country, and even at a global level (4, 16, 22). Characterization of prevailing M. tuberculosis lineages and clones may be focused on different geographical levels such as continents, countries, regions, or cities in order to locate the origin, evolution, and spreading dynamics of a particular M. tuberculosis clone, which are often difficult to identify by traditional epidemiological investigations alone. Following a preliminary report that described the LAM7-TUR clade of M. tuberculosis with a phylogeographical specificity for Turkey (37), the present investigation targeted a detailed population-based study of M. tuberculosis and drug resistance patterns in the city of Malatya, Turkey. We used three different typing methods (spoligotyping, IS6110-restriction fragment length polymorphism [RFLP], and 12-locus mycobacterial interspersed repetitive units [MIRUs]) to determine the rate of recently transmitted TB, as well as to have a precise understanding of the circulating M. tuberculosis clades. The findings are discussed with regard to the drug resistance, origin, evolution, and transmission dynamics of predominant M. tuberculosis clones in Turkey.
MATERIALS AND METHODS
M. tuberculosis strains.
The present study was performed on 145 M. tuberculosis strains isolated from 145 pulmonary TB patients over a period of 5 years (from 2000 to 2004). Our sample was 34% representative overall of the culture-positive TB patients in Malatya in the study period. Preliminary identification of the isolates as M. tuberculosis complex was performed by using the BACTEC NAP test (Becton Dickinson, Sparks, MD). Conventional biochemical tests and growth characteristics were used to identify M. tuberculosis as reported previously (7). Susceptibility testing to isoniazid (0.1 μg/ml), rifampin (2 μg/ml), streptomycin (2 μg/ml), and ethambutol (2.5 μg/ml) was performed by the modified 1% proportion method in the BACTEC 460 radiometric system (Becton Dickinson). Multidrug resistance was defined as resistance to at least isoniazid and rifampin (15).
Patients.
The study population included about one-third of the culture-positive TB patients reporting to TB dispensaries, two governmental hospitals, and a university hospital in the Malatya region. Epidemiological and demographic data such as age, sex, city of birth, address at the time of diagnosis, place of residence, schooling, work and social activities, and clinical characteristics of the illness were prospectively collected.
Spoligotyping and database comparison.
Standard spoligotyping was performed with the Dra and Drb primers, with Dra biotinylated in 5′, as described previously (12). H37Rv and M. bovis BCG strains were used as positive controls, and distilled water was used as a negative control. The biotin-labeled PCR product was hybridized on probe derived from spacer sequences covalently bound to a membrane. Spoligotypes in binary format were entered in an Excel spreadsheet and compared to the updated international spoligotyping database of the Pasteur Institute of Guadeloupe. The SpolDB4 database (4; an online version is available [http://www.pasteur-guadeloupe.fr:8081/SITVITDemo]) initially contained 39,295 patterns distributed into 1,939 shared types (a pattern shared by two or more patient isolates) and 3,370 orphans (patterns reported for a single isolate). An updated version at the time of this comparison contained about 60,000 patterns distributed into 2,300 spoligotype international types (SITs). Major phylogenetic clades were assigned according to signatures provided in SpolDB4 (4), which defines 62 genetic lineages and sublineages. Designation of clades as “ancestral,” “modern,” or “evolutionary-recent” lineages (based on the presence or absence of a specific deletion TbD1 [3]), and the determination of the principal genetic groups (PGG) based on katG-gyrA polymorphism (27) was performed as summarized recently (4, 22). Briefly, M. tuberculosis can be divided into “ancestral” TbD1-positive and “modern” TbD1-negative strains. The TbD1-positive strains are invariably classified as PGG1 upon katG-gyrA polymorphism, as opposed to “modern” TbD1-negative strains that may belong to either of the three PGG subgroups (27). The PGG2 and PGG3 subgroups are also termed as “evolutionary-recent” (LAM, Haarlem, X, and T) as opposed to the PGG1 subgroup (Beijing, East-African Indian [EAI], Central Asian [CAS], and Africanum); however, only EAI and M. africanum are classified as “ancestral” sensu stricto (3, 22).
IS6110-RFLP.
IS6110-RFLP was performed by using standardized methodology (33). Briefly, 2 μg of genomic DNA was digested with PvuII. DNA fragments were separated by electrophoresis on agarose gels, denatured, and blotted onto nylon membrane by the alkaline transfer procedure. Hybridization was performed on PvuII-restricted genomic DNA with a chemiluminescence-labeled 521-bp IS6110 fragment. The results were analyzed by using H37Rv as an international standard, and comparison was done using Bionumerics (Applied Maths, Sint-Martens-Latem, Belgium). The results were analyzed with Taxotron; a pairwise distance matrix was built by using the Dice Index.
MIRU typing and database comparison.
Genomic DNA of the M. tuberculosis isolates were amplified from 12 MIRU loci using four different multiplex PCRs as described previously (2). DNA fragments were separated by capillary electrophoresis using an ABI Prism 3100 Avant genetic analyzer (Applied Biosystems, Belgium). Sizing of the PCR fragments and assignment of the various MIRU alleles were done by using the GeneScan and customized Genotyper software packages (Applied Biosystems, Lennik, Belgium). MIRU typing was performed at the Pasteur Institute in Brussels, Belgium, and the exact MIRU copy number corresponding to 12 loci was sent to Pasteur Institute of Guadeloupe for database comparison. The MIRU data were entered into an “in-house” database, which at the time of this comparison contained 12 locus MIRU patterns on about 6,000 isolates, and 800 shared types, referred as MITs (for MIRU international type). The discriminatory power of individual loci was calculated by using the Hunter and Gaston Discriminatory Index (HGDI) (11) as applied to MIRUs previously (26, 29).
Statistical analysis.
Taxotron (P. Grimont, Institut Pasteur) and Bionumerics (v 3.1; Applied Maths) were used to analyze the molecular typing results. IS6110-RFLP patterns were analyzed as fingerprint types, whereas MIRU patterns were analyzed as character types. The pairwise distance between patterns was computed by using UPGMA (unweighted pair-group method using arithmetic averages) and the Jaccard index (23). This methodology has proven useful for defining major phylogeographical clades within the M. tuberculosis complex (26). We carried out two comparisons of genotyping technique: spoligotyping versus MIRUs and spoligotyping versus IS6110-RFLP. Two different software programs were used to make comparative analysis: TAXOTRON for spoligotyping versus IS6110-RFLP and PAUP for spoligotyping versus MIRUs. The clustering rate defined the percentage of isolates sharing an identical pattern after a typing method (or a combination of methods), whereas the transmission rate was determined by using the “N-1 method” as described by Small et al. (25), assuming that each cluster includes one index case.
RESULTS
Patients and drug resistance.
All of the 145 patients were characterized with pulmonary TB and originated from Malatya (there were no foreign-born cases). The mean age was 32.5 years, and the male-to-female ratio was 1.8. The percentages of isolates according to the age of the patients were as follows: 0 to 10 years, 6.2%; 11 to 20 years, 22.8%; 21 to 30 years, 26.2%; 31 to 40 years, 16.5%; 41 to 50 years, 13.1%; 51 to 60 years, 6.9%; 61 to 70 years, 3.4%; and older than 70 years, 4.8%. Thus, nearly two of three cases occurred among adults compared to only 8.2% among the elderly (older than 60 years). The age and gender composition of patients in our sample reflects the predominance of TB among the young male population in Malatya. Drug susceptibility testing showed that 29 (20%) of the 145 M. tuberculosis isolates were resistant to at least one of the tested anti-TB drugs. Seven strains (4.8%) were MDR. The resistance rates to streptomycin and isoniazid were equal (13.5% for each), and there was a 5.3% resistance rate to rifampin and ethambutol. As shown below, despite the same geographic origin, traditional epidemiological investigations did not reveal a direct relationship among the majority of the patients. Furthermore, resistance rates did not change significantly among unique and clustered isolates.
Analysis of spoligotyping data.
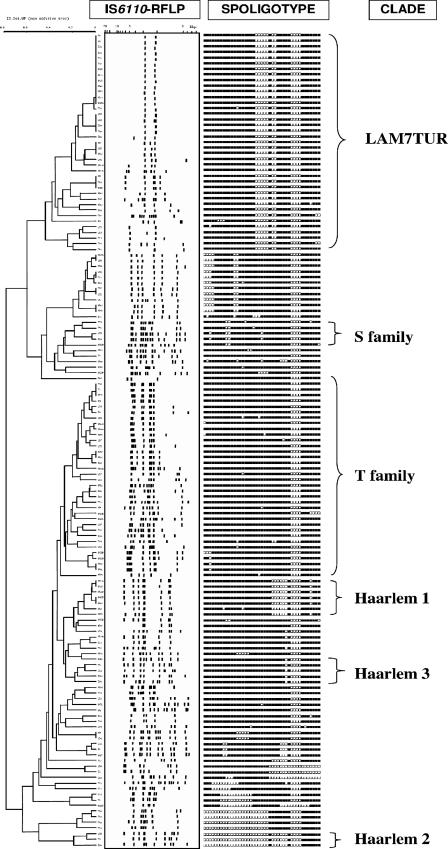
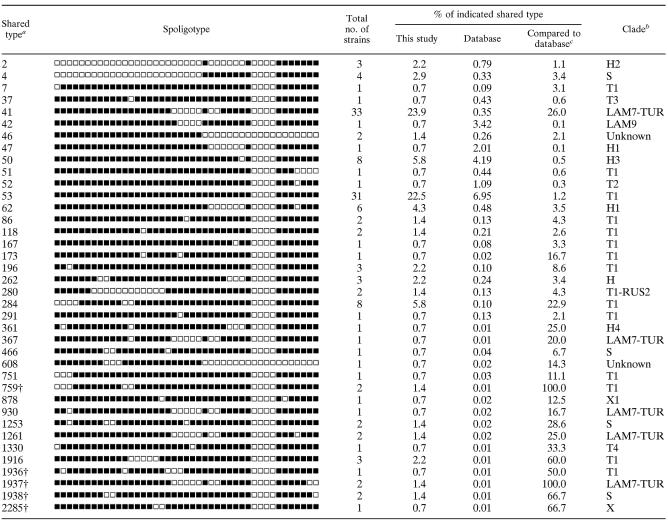
Results obtained on a total of 145 strains (summarized in Fig. 1) showed 44 different patterns. A total of 120 clinical isolates (82.8%) were found to be distributed into 19 clusters containing from 2 to 33 isolates, and the remaining 25 isolates (17.2%) were unique, 6 of which were considered orphans (i.e., not found in SpolDB4). Five newly created SITs were defined between similar spoligotype patterns (n = 8) from Turkey and other countries: four isolates from Turkey matched together in two new SITs (SIT759 and SIT1937), two isolates matched an orphan strain from The Netherlands (SIT1938), one matched with a strain from Sweden (SIT1936), and another one matched with a strain from South Africa (SIT2285). The distribution of SITs from Malatya is detailed in Table 1. SIT41 (LAM7-TUR family) was the predominant family (23.9%), followed by SIT53 (22.5%), SIT50 (5.8%), SIT284 (5.8%), and SIT62 (4.3%). SIT41 and SIT53 were much more prevalent in Turkey than in the rest of the world (23.9% versus 0.35% and 22.5% versus 6.95% in SpolDB4, respectively). SIT284 was significantly more prevalent in the study population (5.8%) than in the database (0.1%), whereas SIT50 was distributed equally worldwide and in our study (Table 1). The major clades found in the present study were as follows: ill-defined T clade (45.7%), LAM (29%), Haarlem (15.9%), S (3.6%), and X (0.7%).
FIG. 1.
UPGMA tree made by numerical analysis of spoligotyping data. Major clades are shown on the right side of the picture. IS6110-RFLP patterns and spoligotypes of the 145 studied isolates are presented.
TABLE 1.
Distribution of the 139 isolates in 38 shared types in this study and the corresponding clade designations (six orphans are not shown here)
a†, Newly created shared types in SpolDB4. ST759 and ST1937 included only two Turkish isolates each, ST1936 matched with an orphan from Sweden, two strains of ST1938 matched with the orphan from The Netherlands, and ST2285 matched with orphan from South Africa.
bDetermination of clades was done as described previously (4).
cPercentage of the total number of strains of the shared type compared to the global database.
IS6110-RFLP typing.
Of the 145 strains fingerprinted, 42 (29%) had five IS6110 bands or fewer (low-copy-number strains); the remaining 103 isolates (71%) were high-copy-number strains (6 to 18 copies of IS6110). A total of 52 of 145 isolates (clustering rate, 35.9%) were split into 19 clusters containing from 2 to 13 isolates, and 93 isolates (64.1%) were unique. The recent transmission rate was calculated to be 22.8% (52 − 19/145) by supposing that there was one index case in each cluster identified.
MIRU typing.
Interpretable results on all of the 12 loci were obtained for 142 strains, which permitted us to identify 65 MIRU profiles corresponding to a total of 98 clustered isolates (21 clusters) and 44 unique isolates. When the patterns were compared to an updated database, our study sample contained a total of 42 MITs and 23 orphan patterns. The MIT310 was the most prevalent MIRU type, including 23 strains (16.2%), followed by MIT43 (12 strains, 8.4%), MIT194 (8 strains, 5.6%), and MIT8 (7 strains, 4.9%). The 98 clinical isolates were found to be distributed into 21 clusters (the clustering rate was 98 of 142 [69%]), and 44 isolates (31%) were unique (including 23 orphans).
Determination of clustering rates.
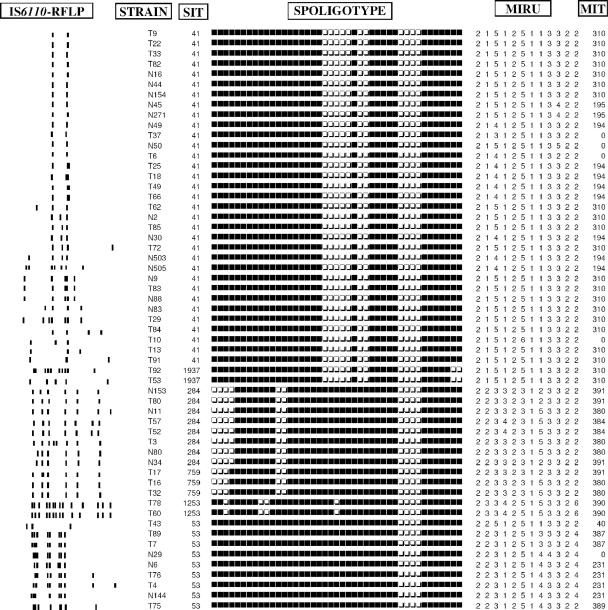
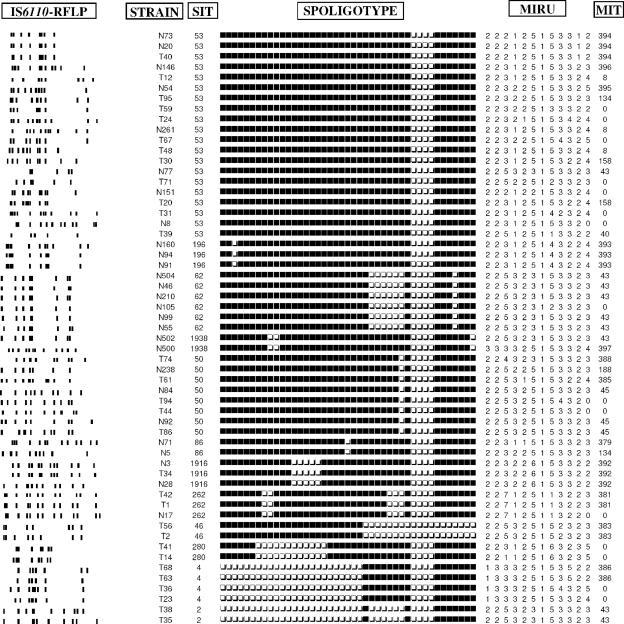
The combination of the typing methods decreased the clustering rate and led to an increase in the number of clusters. Spoligotype clusters were divided into several new clusters either by using the combined analysis of spoligotyping and IS6110-RFLP or of spoligotyping and MIRUs. For example, SIT41 which formed a block of 33 strains, was split into four subgroups (each including 3 to 13 strains) and 10 unique IS6110 patterns. Likewise, with the combination of spoligotyping and MIRUs, these 33 strains were divided into three subgroups (each including 2 to 19 strains) and four unique MIRU patterns. The subdivision of five predominant SITs by IS6110-RFLP and MIRUs is shown in Table 2. Analysis by combining the three techniques of spoligotyping, MIRUs, and IS6110-RFLP showed that the large IS6110 cluster (n = 13) with two copies IS6110 was divided into two subclusters including two and seven strains, respectively, and four unique profiles. Commonly, strains with low IS6110 copy patterns were easily subdivided by MIRUs, whereas strains with identical or close high IS6110 copy patterns were generally grouped by MIRU typing (Fig. 2). The clustering rates of various combinations of the typing schemes were as follows: spoligotyping and MIRUs, 48.9%; IS6110-RFLP and spoligotyping, 29.7%; IS6110-RFLP and MIRUs, 23.4%; and IS6110-RFLP, spoligotyping, and MIRUs, 22% (the lower the clustering rate, the higher the discrimination). The discriminatory powers of different MIRU-VNTR (for variable number of tandem DNA repeats) loci using HGDI calculations summarized in Table 3 showed that seven loci with the highest diversity (loci 4, 10, 16, 23, 26, 31, and 40) reached >86% of the discriminatory power provided by all 12 loci. When the three typing methods were combined, the final clustering and transmission rates were estimated to be 22 and 13.1%, respectively.
TABLE 2.
Subdivision of the major spoligotyping defined STs by IS6110-RFLP and MIRU-VNTRs
| Major shared spoligotype (n)a | Spoligotyping + IS6110-RFLP | Spoligotyping + MIRU-VNTR |
|---|---|---|
| ST41 (33) | Four subclusters (3 to 13 strains/each); 10 unique strains | Three subclusters (2 to 19 strains/each); 4 unique strains |
| ST53 (29) | Two subclusters (3 to 4 strains/each); 22 unique strains | Four subclusters (2 to 4 strains/each); 20 unique strains |
| ST284 (8) | Three subclusters (2 strains/each); 2 unique strains | Four subclusters (2 strains/each) |
| ST50 (8) | All unique | One cluster with 3 strains; 5 unique strains |
| ST62 (6) | Two subclusters (2 to 4 strains/each) | One cluster with 5 strains; 1 unique strain |
n, Number of strains.
FIG. 2.
Example of clusters obtained by spoligotyping divided into subclusters by IS6110-RFLP and MIRU typing.
TABLE 3.
Discriminatory power of different MIRU-VNTR loci
| MIRU locus | No. of isolates with the specified MIRU copy no.
|
HGDIa | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| 2 | 5 | 136 | 1 | 0.08 | ||||
| 4 | 40 | 93 | 9 | 0.49 | ||||
| 10 | 1 | 6 | 62 | 13 | 57 | 3 | 0.64 | |
| 16 | 81 | 12 | 44 | 5 | 0.57 | |||
| 20 | 5 | 137 | 0.07 | |||||
| 23 | 1 | 25 | 112 | 4 | 0.35 | |||
| 24 | 142 | 0.00 | ||||||
| 26 | 46 | 6 | 3 | 12 | 72 | 3 | 0.63 | |
| 27 | 3 | 134 | 5 | 0.11 | ||||
| 31 | 15 | 118 | 6 | 3 | 0.30 | |||
| 39 | 3 | 136 | 3 | 0.08 | ||||
| 40 | 1 | 64 | 31 | 31 | 9 | 2 | 4 | 0.70 |
The seven loci showing discriminatory power in our setting are indicated in boldface.
Epidemiological analysis.
Detail epidemiological and clinical information for 32 patients harboring isolates clustered by all of the three typing methods was analyzed carefully. All of them shared the same geographic origin; however, no direct links (such as members of the same family, relatives, classmates, or proven social relationship) were established. The possible relationships between the age of the patients, sex, drug resistance, and the genotypic lineages observed were also investigated. Regarding the latter analysis, the isolates were compared for each of the three major clades and the corresponding most prevalent SITs found (LAM7-TUR/SIT41, n = 40/33; Haarlem/SIT50, n = 23/8; and ill-defined T/SIT53, n = 47/29) with regard to each of the variables mentioned. No statistically significant relationship either to sex, age groups, or drug resistance for a given clade or SIT could be definitely established (results not shown). Lastly, we did not find a correlation of clustering with previous TB treatment or drug resistance, and resistance rates did not change significantly among unique and clustered isolates (19.8% versus 20.4%, respectively).
DISCUSSION
Drug resistance.
Drug resistance patterns and epidemiological relationships of circulating M. tuberculosis strains in a given population are useful indicators for the prevention of TB. According to a recent report, the median prevalence of global primary and secondary resistance to at least one anti-TB drug were 10.2 and 18.4%, respectively, compared to 1.1 and 7% for MDR TB (35). Although drug resistance surveillance has not been performed at the national level in Turkey, MDR TB reportedly varied in a range from 1.3 to 4.8% for initial drug resistance and from 4.4 to 16.6% for acquired drug resistance in local studies (5, 8, 31, 32). Globally, any drug resistance and MDR rates ranged from 16 to 24% and from 4.8 to 7.3%, respectively (19, 24, 30). The frequencies of primary and secondary resistance to a single drug varied from 18 to 26.6% and from 28 to 53.4%, respectively (5, 31, 32, 36). The reported resistance rates for the tested drugs varied in the range of 5.6 to 30% for isoniazid, 1.2 to 36.2% for rifampin, 2.4 to 32% for streptomycin, and 1.4 to 10% for ethambutol (31, 32, 36). Thus, the resistance rates to tested drugs in Malatya, which is characterized by a very homogeneous population with limited population movements and no immigrants, are within these ranges. Nonetheless, the high monoresistance (13.5%) to streptomycin and isoniazid and the global resistance to any anti-TB drugs (20%) and MDR (4.8%) rates found in our study indicate that the drug resistance is a widespread problem in Turkey. Similar resistance rates among the unique and clustered isolates in our study sample showed that no specific clones of resistant isolates are actively spreading in Malatya, and the lineages described have no selective advantage over the pansusceptible strains in our setting. Our data also suggest that the bulk of the M. tuberculosis drug resistance in Malatya is developed through selective pressure imposed by poorly constructed or inadequately supervised treatment regimens rather than through the transmission of resistant strains.
Spoligotyping and predominant TB lineages.
The clonal structure of M. tuberculosis has not only important implications for molecular epidemiology but also for phylogeny because clonal species are stable in space and time, a fact that facilitates the utilization of global genotyping databases to track past and present transmission of the tubercle bacilli (4, 22). Spoligotyping analysis showed that nearly 95% of the total strains collected and fingerprinted in the present study belonged to known genotype clades or subclades (Table 1). LAM and Haarlem were the most representative clades. The LAM family was confined to one large subclade, LAM7-TUR (essentially SIT41), whereas Haarlem was split among various subclades underlining a large and variable distribution of this lineage in Malatya. Interestingly, although our study was performed on the strains collected from the Asian part of Turkey, there were no strains belonging to CAS, EAI, and Beijing clades, which are essentially reported from different regions of Asia (4). Interestingly, a recent study from a neighboring country, Iran, which is close to Malatya, reported that as high as 9.4% of the tested M. tuberculosis strains belonged to the Beijing clade (20).
In terms of shared spoligotypes, the most predominant SITs in our study were SIT41, SIT50, SIT53, and SIT284, with SIT41 (octal code 777777404760771) being the most prevalent (23.9% of the isolates, Table 1). It is endemic in Turkey and was recently named as a LAM7-TUR sublineage (37). Despite some minor variations shown in Table 1 (SIT367, SIT930, SIT1261, and SIT1937), LAM7-TUR is a specific sublineage of the LAM superfamily, as revealed by the interrogation of the updated database of the Pasteur Institute of Guadeloupe. Consulted on 14 February 2007, the database contained combined MIRU and spoligotyping data for 642 strains belonging to the LAM superfamily, 40 of which were classified as LAM7-TUR. In contrast to all other sublineages of this family that had in common two or more copies of the MIRU-4 locus, all of the LAM7-TUR were unique in having a single copy of MIRU-4. Ongoing studies based on a newly described optimized set of 15-locus MIRU typing (in which only 6 MIRU loci are common to the previously described 12-locus format [28]) suggest that LAM7-TUR could be further divided into at least three subclusters (results not shown). We also looked into the distribution of other predominant shared types in the updated database. SIT284 (octal code 037637777760771) was also predominant in Bulgaria (5.4% of isolates), followed by Turkey (3.3%), Austria (1.9%), and Saudi Arabia (1.4%), suggesting that it could be endemic in Bulgaria, and its geographic proximity with Turkey could partially explain these quite similar percentages. On the other hand, SIT50 and SIT53, belonging to the Haarlem-3 and ill-defined T-1 sublineages, respectively, were widely distributed worldwide in SpolDB4 (Table 1).
Discriminatory power of IS6110-RFLP for high- versus low-copy-number isolates.
IS6110-RFLP is a very sensitive method for strains with a high IS6110 copy number (13, 14, 33), providing more discrimination than 25 MIRU-VNTRs loci alone or in combination with spoligotyping (9). For example, the clustering rate of IS6110-RFLP for the strains collected from patients in two cities in England (16 to 34%) was lower than those of spoligotyping (59 to 62%) and 12 MIRUs (40 to 48%), showing the higher discriminatory power of this approach, particularly for high-copy-number isolates (9). However, high stringency may also lead to an underestimation of the transmission in patients infected with high-copy-number isolates (34). A study from Hamburg showed that an optimized set of 24 MIRU loci including a discriminatory subset of 15 loci gave a comparable or slightly higher predictive value of TB transmission, especially when combined with spoligotyping, than did IS6110-RFLP (18).
In our study, the 35.9% clustering rate of IS6110-RFLP was lower than those of spoligotyping (83%) and 12-locus MIRU (69%) typing. The clustering rate of IS6110-RFLP decreased to 30.1% for the 103 strains with a high copy number of IS6110, and 21 (67.7%) of the 31 clustered strains were also clustered by MIRUs. Unfortunately, some strains from different patients can display identical IS6110 fingerprints for low-copy-number isolates. In the present study we found that 21 (50%) of the 42 IS6110 low-copy-number strains and 13 (93%) of the 14 strains with two copies of IS6110 were clustered by the IS6110-RFLP method. As indicated previously, these low-copy-number strains require retyping by a second method (6, 10) such as MIRU-VNTRs.
Usefulness of MIRU typing.
MIRU typing is adapted for global databases since each typed strain is assigned a 12-digit number matching the number of repeats for each of the 12 loci (17). This coding system makes it easy to carry out interlaboratory analysis, as well as to express the genetic diversity separately for each loci. The allelic diversity detected in each locus was quantified, which allowed us to establish a hierarchy of polymorphism of the 12 loci. As shown in Table 3, a set of seven loci showed discriminatory power in our sample (loci 4, 10, 16, 23, 26, 31, and 40), reaching ca. 86% of the total discrimination provided by all of the 12 loci. The evolution rate of MIRU loci being higher than the rate of IS6110 transposition in IS6110 low-copy-number isolates, MIRUs are ideally more informative for these strains (9, 14). We found that the IS6110 cluster of 13 strains with two copies of IS6110 (among SIT41 isolates) was divided by MIRUs into two clusters of 2 and 7 strains and four unique patterns (Fig. 1 and 2). When we considered the spoligotyping as a secondary typing method for these strains, we found that 12 of these 13 strains were also in a cluster, supporting the idea that spoligotyping used alone is not sufficient for purely epidemiological investigation (21). As shown in Fig. 2, our results also corroborated previous observations that the combined evolution rate of the 12 MIRU loci was slightly lower than that of IS6110-RFLP in strains with high IS6110 copy numbers (17, 29), e.g., SIT284 including eight strains with six to eight copies of IS6110 was split into five subtypes by IS6110-RFLP compared to three subtypes by MIRUs. Similar figures for SIT62 (including six strains with seven to eight copies of IS6110) were three against two subtypes, respectively. However, in the majority of cases and for each SIT given, we found similar splitting by the IS6110-RFLP and MIRU methods (Fig. 1 and 2).
Conclusion.
This investigation described a high rate of drug resistance in Malatya, Turkey, underlining the need for routine drug susceptibility testing for all culture-positive TB cases. Database comparison showed that Turkey has a heterogenic M. tuberculosis population including SITs commonly distributed worldwide (SIT50 and SIT53) and those with high specificity for Turkey and its neighboring countries (SIT41 and SIT284), as well a smaller proportion of strains that did not match any existing isolates in the international database. Interestingly, most of the ongoing transmission in our study happened to be due to “evolutionary-recent” TB lineages belonging to PGG2 (Haarlem and LAM) and PGG3 (ill-defined T clade) clades, one in four TB cases being caused by the LAM7-TUR sublineage. An absence of lineages belonging to PGG1 clones (EAI, CAS, and Beijing, essentially found in Central, South, and Southeast Asia) in our Anatolian setting is amazing considering that its recorded history shows a close link to people of varied ethnic and linguistic traditions, including central Asia (http://en.wikipedia.org/wiki/Anatolia).
Using three typing methods together (spoligotyping, MIRUs, and IS6110-RFLP), a final clustering rate of 22% and a transmission rate of 13.1% were obtained. In the present study, however, we failed to find an association of clustering with gender or age, probably due to the small size of the sample. Last but not least, our study suggests that molecular clocks of the markers currently in use should be studied in relation to the predominant genotypic lineages observed (high versus low IS6110 copy isolates and spoligotyping- and MIRU-defined clades), which may be helpful in allocating different weights to each of the markers in a given setting.
Acknowledgments
We thank Christophe Sola for helping with the Taxotron analysis and helpful discussions.
R.D. was partially supported by the Molecular and Clinical Microbiology Society, Malatya, Turkey. The study performed at the Pasteur Institute of Guadeloupe was partially funded by the European Regional Development Fund, European Commission (ERDF/FEDER, A34-05). T.Z. received a Ph.D. fellowship awarded by European Union and the Regional Council of Guadeloupe and the International Network of the Pasteur Institutes.
Footnotes
Published ahead of print on 10 October 2007.
REFERENCES
- 1.Achonu, C., F. Jamieson, M. A. Behr, T. Lillebaek, K. Khan, and M. Gardam. 2006. Evidence for local transmission and reactivation of tuberculosis in the Toronto Somali community. Scand. J. Infect. Dis. 38:778-781. [DOI] [PubMed] [Google Scholar]
- 2.Allix, C., P. Supply, and M. Fauville-Dufaux. 2004. Utility of fast mycobacterial interspersed repetitive unit-variable number tandem repeat genotyping in clinical mycobacteriological analysis. CID 39:783-789. [DOI] [PubMed] [Google Scholar]
- 3.Brosch, R., S. V. Gordon, M. Marmiesse, P. Brodin, C. Buchrieser, K. Eiglmeier, T. Garnier, C. Gutierrez, G. Hewinson, K. Kremer, L. M. Parsons, A. S. Pym, S. Samper, and D. van Soolingen, and S. T. Cole. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA 99:3684-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brudey, K., J. R. Driscoll, L. Rigouts, W. M. Prodinger, A. Gori, S. A. Al-Hajoj, C. Allix, L. Aristimuno, J. Arora, V. Baumanis, L. Binder, P. Cafrune, A. Cataldi, S. Cheong, R. Diel, C. Ellermeier, J. T. Evans, M. Fauville-Dufaux, S. Ferdinand, D. Garcia, V. C. Garzelli, L. Gazzola, H. M. Gomes, M. C. Guttierez, P. M. Hawkey, D.P. van Helden, G. V. Kadival, B. N. Kreiswirth, K. Kremer, M. Kubin, S. P. Kulkarni, B. Liens, T. Lillebaek, M. L. Ho, C. Martin, C. Martin, I. Mokrousov, O. Narvskaia, Y. F. Ngeow, L. Naumann, S. Niemann, I. Parwati, Z. Rahim, V. Rasolofo-Razanamparany, T. Rasolonavalona, M. L. Rossetti, S. Rusch-Gerdes, A. Sajduda, S. Samper, I. G. Shemyakin, U. B. Singh, A. Somoskovi, R. A. Skuce, D. van Soolingen, E. M. Streicher, P. N. Suffys, E. Tortoli, T. Tracevska, V. Vincent, T. C. Victor, R. M. Warren, S. F. Yap, K. Zaman, F. Portaels, N. Rastogi, and C. Sola. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics, and epidemiology. BMC Microbiol. 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caglar, A. S., A. M. Cicek, S. Ozkan, and A. Caglar. 2003. Drug resistance among the pulmonary tuberculosis patients in Ankara, p. 90. Congress XXIII. National Tuberculosis and Thorax Diseases, Malatya, Turkey.
- 6.Cowan, L. S., L. Mosher, L. Diem, J. P. Massey, and J. T. Crawford. 2002. Variable-number tandem repeat typing of Mycobacterium tuberculosis isolates with low copy numbers of IS6110 by using mycobacterial interspersed repetitive units. J. Clin. Microbiol. 40:1592-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David, H. L., V. Lévy-Frébault, and M. F. Thorel. 1989. Méthodes de laboratoire pour mycobactériologie clinique, p. 1-87. Commission des Laboratoires de Référence et d'Expertise de l'Institut Pasteur, Institut Pasteur, Paris, France.
- 8.Durmaz, R., I. H. Ozerol, B. Durmaz, S. Gunal, A. Senoglu, and E. Evliyaoglu. 2003. Primary drug resistance and molecular epidemiology of Mycobacterium tuberculosis isolates from patients in a population with high tuberculosis incidence in Turkey. Microbial Drug Resist. 9:361-366. [DOI] [PubMed] [Google Scholar]
- 9.Gopaul, K. K., T. J. Brown, A. L. Gibson, M. D. Yates, and F. A. Drobniewski. 2006. Progression toward an improved DNA amplification-based typing technique in the study of Mycobacterium tuberculosis epidemiology. J. Clin. Microbiol. 44:2492-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez, M. C., N. Ahmed, E. Willery, S. Narayanan, S. E. Hasnain, D. S. Chauhan, V. M. Katoch, V. Vincent, C. Locht, and P. Supply. 2006. Predominance of ancestral lineages of Mycobacterium tuberculosis in India. Emerg. Infect. Dis. 12:1367-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanduma, E., T. D. McHugh, and S. H. Gillespie. 2003. Molecular methods for Mycobacterium tuberculosis strain typing: a user's guide. J. Appl. Microbiol. 94:781-791. [DOI] [PubMed] [Google Scholar]
- 14.Kremer, K., D. van Soolingen, R. Frothingham, W. H. de Haas, P. W. M. Hermans, C. Martin, P. Palittapongarnpim, B. B. Plikaytis, L. W. Riley, M. A. Yakrus, J. M. Musser, and J. A. D. van Embden. 1999. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 37:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long, R. 2000. Drug-resistant tuberculosis. CMAJ 163:425-428. [PMC free article] [PubMed] [Google Scholar]
- 16.Mathema, B., N. E. Kurepina, P. J. Bifani, and B. N. Kreiswrith. 2006. Molecular epidemiology of tuberculosis: current insights. Clin. Microbiol. Rev. 19:658-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazars, E., S. Lesjean, A. L. Banuls, M. Gilbert, V. Vincent, B. Gicquel, M. Tibayrenc, C. Locht, and P. Supply. 2001. High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proc. Natl. Acad. Sci. USA 98:1901-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oelemann, M. C., R. Diel, V. Vatin, W. Haas, S. Rusch-Gerdes, C. Locht, S. Niemann, and P. Supply. 2007. Assessment of an optimized mycobacterial interspersed repetitive unit-variable number of tandem repeat typing system combined with spoligotyping for population-based molecular epidemiology studies of tuberculosis. J. Clin. Microbiol. 45:691-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozturk, C. E., O. A. Balbay, D. Kaya, I. Ceyhan, I. Bulut, and I. Sahin. 2005. The resistance to major antituberculous drugs of Mycobacterium tuberculosis strains isolated from the respiratory system specimens of tuberculosis patients in Duzce, Turkey. Jpn. J. Infect. Dis. 58:47-49. [PubMed] [Google Scholar]
- 20.Ramazanzadeh, R., P. Fania, N. Amirmozafari, F. Ghazi, Z. Ghadertotonchi, J. Kamran, F. Mohammadi, M. Mirsaedi, and M. Masjedi. 2006. Comparison between molecular epidemiology, geographical regions and drug resistance in Mycobacterium tuberculosis strains isolated from Iranian and Afghan patients. Chemotherapy 52:316-320. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi, N. 2006. Comparison of different methods for molecular typing of Mycobacterium tuberculosis, p. 149-150. Sixth National Mycobacteria Symposium, Kizilcahamam, Ankara, Turkey.
- 22.Rastogi, N., and C. Sola. 2007. Molecular evolution of the Mycobacterium tuberculosis complex, p. 53-91. In J. C. Palomino, S. C. Leão, and V. Ritacco (ed.), Tuberculosis 2007: from basic science to patient care. [Online.] http://www.tuberculosistextbook.com/.
- 23.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 24.Senol, G., B. Komurcuoglu, and A. Komurcuoglu. 2005. Drug resistance of Mycobacterium tuberculosis in Western Turkey: a retrospective study from 1100-bed teaching hospital. J. Infect. 50:306-311. [DOI] [PubMed] [Google Scholar]
- 25.Small, P. M., P. C. Hopewell, S. P. Singh, A. Paz, J. Parsonnet, D. C. Ruston, G. F. Schecter, C. L. Daley, and G. K. Schoolnik. 1994. The epidemiology of tuberculosis in San Francisco: a population-based study using conventional and molecular methods. N. Engl. J. Med. 330:1703-1709. [DOI] [PubMed] [Google Scholar]
- 26.Sola, C., I. Filliol, E. Legrand, S. Lesjean, C. Locht, P. Supply, and N. Rastogi. 2003. Genotyping of the Mycobacterium tuberculosis complex using MIRUs: association with VNTR and spoligotyping for molecular epidemiology and evolutionary genetics. Infect. Genet. Evol. 3:125-133. [DOI] [PubMed] [Google Scholar]
- 27.Sreevatsan, S., X. Pan, K. E. Stockbauer, N. D. Connell, B. N. Kreiswirth, T. S. Whittam, and J. M. Musser. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. USA 94:9869-9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Supply, P., C. Allix, S. Lesjean, M. Cardoso-Oelemann, S. Rusch-Gerdes, E. Willery, E. Savine, P. de Haas, H. van Deutekom, S. Roring, P. Bifani, N. Kurepina, B. Kreiswirth, C. Sola, N. Rastogi, V. Vatin, M. C. Gutierrez, M. Fauville, S. Niemann, R. Skuce, K. Kremer, C. Locht, and D. van Soolingen. 2006. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 44:4498-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Supply, P., S. Lesjean, E. Savine, K. Kremer, D. van Soolingen, and C. Locht. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 39:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Surucuoglu, S., N. Ozkutuk, P. Celik, H. Gazi, G. Dinc, S. Kurutepe, G. Koroglu, Y. Havlucu, and G. Tuncay. 2005. Drug-resistant pulmonary tuberculosis in western Turkey: prevalence, clinical characteristics and treatment outcome. Ann. Saudi Med. 25:313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tahaoglu, K., O. Kizkin, T. Karagoz, M. Tor, M. Partal, and T. Sadoglu. 1994. High initial and acquired drug resistance in pulmonary tuberculosis in Turkey. Tuberc. Lung Dis. 75:324-328. [DOI] [PubMed] [Google Scholar]
- 32.Talay, F., S. Altın, E. Çetinkaya, and S. Kumbetli. 2003. Drug resistant rates in Istanbul Eyüp tuberculosis control dispensary in 1997-2000 years, p. 89. Congress XXIII. National Tuberculosis and Thorax Diseases, Malatya, Turkey.
- 33.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren, R. M., G. D. van der Spuy, M. Richardson, N. Beyers, C. Booysen, M. A. Behr, and P. D. van Helden. 2002. Evolution of the IS6110-based restriction fragment length polymorphism pattern during the transmission of Mycobacterium tuberculosis. J. Clin. Microbiol. 40:1277-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 2004. Anti-tuberculosis drug resistance in the world. Report no. 3: prevalence and trends. World Health Organization, Geneva, Switzerland.
- 36.Yolsal, N., G. Malat, R. Diçsçi, M. Örkün, and Z. Kılıçaslan. 1998. The comparision of 1984-1989 and 1990-1995 years of drug resistant tuberculosis in Turkey: a meta-analysis. Klimik Dergisi. 1:6-9. [Google Scholar]
- 37.Zozio, T., C. Allix, S. Gunal, Z. Saribas, A. Alp, R. Durmaz, M. Fauville-Dufaux, N. Rastogi, and C. Sola. 2005. Genotyping of Mycobacterium tuberculosis clinical isolates in two cities of Turkey: description of a new family of genotypes that is phylogeographically specific for Asia Minor. BMC Microbiol. 5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]