Abstract
Purpose
This randomized clinical trial tests the hypothesis that a psychological intervention can reduce emotional distress, improve health behaviors and dose-intensity, and enhance immune responses.
Patients and Methods
We studied 227 women who were surgically treated for regional breast cancer. Before adjuvant therapy, women completed interviews and questionnaires assessing emotional distress, social adjustment, and health behaviors. A 60-mL blood sample was drawn for immune assays. Patients were randomly assigned to either the intervention group or assessment only group. The intervention was conducted in small patient groups, with one session per week for 4 months. The sessions included strategies to reduce stress, improve mood, alter health behaviors, and maintain adherence to cancer treatment and care. Reassessment occurred after completion of the intervention.
Results
As predicted, patients receiving the intervention showed significant lowering of anxiety, improvements in perceived social support, improved dietary habits, and reduction in smoking (all P < .05). Analyses of adjuvant chemotherapy dose-intensity revealed significantly more variability (ie, more dispersion in the dose-intensity values) for the assessment arm (P < .05). Immune responses for the intervention patients paralleled their psychological and behavioral improvements. T-cell proliferation in response to phytohemagglutinin and concanavalin A remained stable or increased for the Intervention patients, whereas both responses declined for Assessment patients; this effect was replicated across three concentrations for each assay (all P < .01).
Conclusion
These data show a convergence of significant psychological, health behavior, and biologic effects after a psychological intervention for cancer patients.
INTRODUCTION
There is ongoing debate about the impact of psychological interventions on cancer survival. Significant reductions in cancer patients’ emotional distress can be achieved with interventions,1,2 particularly for those patients with high levels of distress.3,4 Whether or not these changes in distress are related to improved survival rates is unknown. Of the eight prior randomized studies, four reported a survival benefit,5–8 and four did not. 4,9–11 Most of the studies were designed as psychotherapy studies to reduce stress or enhance coping and not designed to test for survival effects.
If psychological interventions improve survival, the mechanism is unknown. Three have been proposed. One hypothesized mechanism is social support.5 Indeed, social isolation and low levels of perceived social support may confer increased risk for morbidity or death in coronary heart disease.12–14 Second, stress reduction from a psychosocial intervention might also lead to changed health behaviors (eg, healthier diet and increased physical activity6) or improved adherence to medical treatments,7 thus affecting survival. With two exceptions,6,7 previous intervention studies have neither manipulated nor included intervention components to change health behaviors or adherence.1,2 Finally, it is hypothesized that stress reduction interacts with neuroendocrine and/or immune responses15,16 to yield improved illness or disease outcomes. Designing trials to include multiple behavioral and biologic outcomes should be the thrust for future behavioral intervention trials for many illnesses and conditions,17 including cancer.2
A clinical trial is underway that tests the hypothesis that a multicomponent biobehavioral intervention would impact the incidence of and time to recurrence for women with regional breast cancer. As in previous trials,1 the intervention was designed to reduce stress, lower emotional distress, and improve quality of life. Proposed cofactors (social support, health behaviors, and adherence) were also included as specific targets of the intervention. Another possible mechanism, immune function, was assessed. Positive changes in the latter variables along with reductions in distress and improvements in quality of life and health behaviors represent plausible hypotheses for the direct or indirect effects of a psychological intervention on disease end points. Data on the efficacy of the intervention on emotional distress, health behaviors, chemotherapy dose-intensity, and immune responses are reported here.
PATIENTS AND METHODS
Patient Eligibility
Women who were diagnosed with stage II or III breast cancer, surgically treated, and awaiting adjuvant therapy were eligible. Exclusion criteria included prior cancer diagnosis; refusal of cancer treatment; age less than 20 years or more than 85 years; residence more than 90 miles from the research site; and diagnoses of mental retardation, severe or untreated psychopathology (eg, schizophrenia), neurologic disorders, dementia, or any immunologic condition or disease.
Study Arms
Assessment only
The baseline assessment occurred after breast surgery and before randomization and the start of adjuvant therapy. The initial assessment gathered psychological, behavioral, and medical and treatment information and data. Research assistants conducted individual, structured interviews that included questionnaire completion. A 60-mL blood sample was also drawn. Assessments were scheduled in the morning to maintain routine and minimize diurnal variability. Patients were paid $25.00 per assessment. At 4 months (corresponding to the end of the intervention), patients were similarly reassessed.
Intervention
An identical baseline assessment protocol was used. The intervention was provided in small cohorts (n = 13), ranging from eight to 12 patients. Each session was conducted by two clinical psychologists. Cohorts met weekly for 1.5 hours for 18 sessions (27 therapy hours during 4 months). For absences, a therapist telephoned the patient to provide support and to discuss the session’s topic. The topics and techniques used are consistent with psychosocial interventions1,2 but also included diet, exercise, smoking, and adherence components (Table 1).
Table 1.
Intervention Targets, Sessions, Components, and Measured Variables
| Intervention Target | Session No. | Intervention Component | Measured Variables |
|---|---|---|---|
| Stress | 1–18 | Understanding stress responses, progressive muscle relaxation training | Impact of Events Scale |
| Quality of life | |||
| Emotional distress | 10–15 | Relaxation training, positive coping, problem solving | Profile of Mood States |
| Social adjustment | 4–9 | Identify social network, identify support needed and specific social contact, communication skills training | Social Network Index, family and friends |
| Health behaviors | 13–16 | ||
| Diet | Food intake diary, low-fat/high-fiber information, food substitution, intake and energy balance information | Food Frequency Questionnaire | |
| Exercise | Stretching and walking protocol (20 min/d three times a week) | Seven-Day Exercise Recall | |
| Smoking | Referral information, group support for cessation | Tobacco intake (cigarettes per day) | |
| Adherence | 2–3, 10 | Disease/treatment information, assertive communication skills, monitoring of treatment/follow-up appointments, goal setting | Dose-intensity, Chemotherapy refusal/dropout, Loss to follow-up |
| Immunity | NA | NA | Cellular immune assays |
| Disease end points | NA | NA | Event/time to recurrence |
Abbreviation: NA, not applicable.
Measures
Individual differences in stress
Evidence suggests that interventions may be differentially effective depending on characteristics of the patient.3,4,18 A measure of the most relevant type of stress, cancer-specific stress, is tested as an individual difference variable that may covary with the effectiveness of the intervention for reducing emotional distress (Profile of Mood States [POMS], see Emotional distress). The Impact of Events Scale (IES)19 examines stress-related intrusive thoughts, denial of thoughts, and avoidant behaviors relevant to cancer diagnosis and treatment. For the present sample, coefficient alpha reliability is 0.87, and 4-month test-retest reliability is 0.78. Reliability data were calculated similarly for the measures listed below.
Emotional distress
The POMS20 assesses negative mood. A Total Mood Disturbance score is the sum of five scales (Anxiety, Depression, Anger, Fatigue, and Confusion) minus the score of a Vigor scale. Cronbach’s alpha coefficient reliability is 0.92, and test-retest reliability is 0.78.
Social adjustment
This construct is examined as a multivariate linear composite of four measures. The first measure is social network. The Social Network Index21,22 documents an individual’s direct contact with family, friends, and the community. Test-retest reliability is 0.71. The second and third measures involve social support. The Perceived Social Support Scales (PSS) for Friends and Family23 assesses the need for and perception of receiving support from friends or family members. Alpha reliability is 0.82 and 0.88 and test-retest reliability is 0.79 and 0.80 for the PSS-Friends and PSS-Family, respectively. The fourth measure is dyadic satisfaction. The global satisfaction item from the Dyadic Adjustment Scale24 assesses relationship satisfaction among married or cohabiting couples. Test-retest reliability is 0.64.
Health behaviors
Three measures assess three behaviors. The first measure is dietary patterns. The Food Habits Questionnaire25 assesses dietary choices and eating patterns with five scales (avoiding fat, food substitution with lower-fat alternatives, modification of food preparation, replacing high-fat with low-fat foods, and fruit and vegetable intake). Alpha reliability is 0.79, and test-retest reliability is 0.77. The second measure is exercise. A 7-day report of moderate and vigorous physical activity, based on the Seven-Day Exercise Recall of the Stanford Heart Disease Prevention Program,26 provides a summary index of energy expenditure. Normative data are available.26 The third measure is smoking. Patients were queried as to their smoking status, and if smoking, they were asked their daily intake (participants used cigarettes only; one cigarette = one tobacco unit). Test-retest reliability is 0.93.
Adherence to chemotherapy
Relative dose-intensity for each drug was calculated.27,28 Because multiple chemotherapy agents often comprise a regimen, the value for each patient was the sum of the relative dose-intensities divided by the number of agents. The incidence of refusal, premature termination of chemotherapy, and the case lost to medical follow-up was also recorded.
Functional status
A research nurse, blind to randomization status, provided Karnofsky performance status29–31 ratings based on a clinical interview and medical charts and records. Interrater reliability ranges from 0.70 to 0.97.30,31
Immune Assays
Blood separation procedures
Peripheral blood leukocytes (PBLs) were isolated from 60 mL of venous blood by using Ficoll density gradient centrifugation (Pharmacia Biotech Inc, Piscataway, NJ). The isolated leukocytes were then washed in calcium-and magnesium-free Dulbecco’s phosphate-buffered saline (PBS) and counted on a Coulter counter (Beckman Coulter Inc, Fullerton, CA). Aliquots of 6 × 106 isolated PBLs were suspended again in 0.6 mL of RPMI-1640 medium supplemented with 10% fetal bovine serum (HyClone, Logan, UT); 2-mercaptoethanol (BME; Sigma, St Louis, MO); and 100× antibiotic-antimycotic stock, HEPES, sodium bicarbonate, and L-glutamine (all from Gibco BRL, Grand Island, NY).
Quantification of T lymphocytes, T-cell subsets, and natural killer (NK) cells
PBLs were labeled with florescent-conjugated monoclonal antibodies (MAbs) specific for the following cell surface markers: total T cells (CD3, fluorescein isothiocyanate), T4 subset (CD4, rhodamine), T8 subset (CD8, fluorescein isothiocyanate), and NK cells (CD56, rhodamine). MAbs were purchased from Coulter Corp. Briefly, an aliquot of PBLs was treated with erythrocyte lysis buffer, resuspended in Dulbecco’s PBS, and centrifuged for 5 minutes at 3,300 rpm. Cells (0.5 × 106) were incubated with the appropriate MAb for 15 minutes in the dark on ice. After the incubation, the cells were washed, and the labeled blood cells were fixed with Dulbecco’s PBS containing 2% formaldehyde. Dual-labeled immunoglobulin was used to determine nonspecific immunofluorescence binding. Samples were analyzed with a Coulter EPICS XL-MCL flow cytometer (Beckman Coulter Inc).
Blastogenic response to phytohemagglutinin (PHA) and concanavalin A (Con A)
The serial dilutions used for PHA and Con A were 2.5, 5.0, and 10.0 μg/mL. For the assays, isolated PBLs, resuspended in supplemented RPMI without phenol red, were seeded in triplicate at 0.5 × 105 per well and incubated for 68 hours at 37°C, with 5% CO2, in sterile 96-well flat-bottomed plates. Wells were pulsed for the final 4 hours with 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (Promega Corp, Madison, WI), and phenazine methosulfate, an electron-coupling reagent, to measure proliferative response. Briefly, the 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay is a nonradioactive calorimetric procedure that labels metabolically active cells via reduction of a colored substrate. The amount of proliferation was determined via optical density readings of the suspension in the well compared with cells and media alone using an HTS7000 Bioassay microplate reader (Perkin Elmer, Wellesley, MA) at a determination wavelength of 492 nm and a reference wavelength of 690 nm, as previously noted.28,32 No standard curves were used in these assays. The average coefficient of variation (ACV) among replicate wells was 9.6% for the unstimulated cell control condition. For the PHA-stimulated condition, the ACV was 4.6% for the 2.5 μg/mL dilution, 4.7% for the 5 μg/mL dilution, and 6% for the 10 μg/mL dilution. For the Con A–stimulated condition, the ACV was 4% for the 2.5 μg/mL dilution, 4.7% for the 5 μg/mL dilution, and 5% for the 10 μg/mL dilution.
NK cell cytotoxicity
Briefly, PBLs were resuspended in complete medium at a density of 2.5 × 106 cells/mL and seeded into 96-well V-bottom microtiter plates in a volume sufficient to provide an effector to target cell ratio of 100:1, 50:1, 25:1, 12.5:1, 6.25:1, and 3.13:1 (triplicate wells). Complete medium was added to each well to give a total volume of 200 μL. The NK-sensitive human myeloid K562 cell line was used as the target in this assay.27 K562 cells were harvested from culture, labeled with chromium-51 (51Cr), and washed. 51Cr-labeled K562 target cells (5 × 103) were added to each well in a volume of 100 μL. Plates were centrifuged at 300 × g for 5 minutes and then incubated for 5 hours in 5% CO2 at 37°C. After this incubation, the plates were again centrifuged at 300 × g for 5 minutes, and 100 μL of supernatant was harvested and counted using a Beckman 5500 gamma counter (Beckman Coulter Inc). Minimum and maximum 51Cr release was determined using target cells that had been incubated in complete medium or 5% sodium dodecyl sulfate detergent solution, respectively. Cytotoxicity was calculated using the following equation: experimental 51Cr release – minimum release/maximum release – minimum release.
Statistics
Accrual and randomization
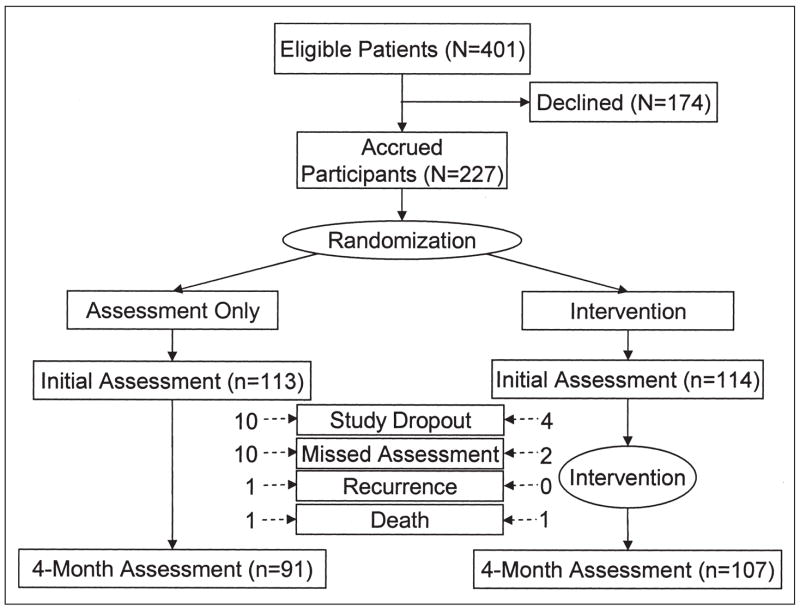
Power analyses suggested a total number of 200 patients, and 227 patients were accrued. The following two sources were used: consecutive patients at a university-affiliated National Cancer Institute– designated Comprehensive Cancer Center (n = 189) and self- and physician-referred patients from the community (n = 38). After the initial assessment, White and Freedman’s minimization method33 was used for randomization. Prognostic and psychosocial strata were as follows: (1) extent of disease and treatment (nodal status, tumor size, and anticipated bone marrow transplantation [BMT] treatment; the four levels included: negative nodes but tumor more than 2 cm, one to three positive nodes, more than four positive nodes with BMT, and more than four positive nodes without BMT); (2) hormone receptor status (positive v negative); (3) menopausal status (pre- or perimenopausal v postmenopausal); and (4) partner status (spouse or partner v none). See Figure 1 for trial flow diagram.
Fig 1.
Experimental design and flow diagram.
Intervention integrity and attendance
To achieve reliability, therapists followed a session-by-session manual, and patients received a companion manual. Equivalence of content was evaluated with session videotapes and patient ratings. Analyses of variance (ANOVAs) found no significant differences (all P > .40) between the 13 cohorts in the frequency of use of the intervention components (eg, relaxation), rated helpfulness of each component, and rated importance of 20 group experiences (eg, getting support).
Compliance with the intervention was excellent, with 81% of the women (92 of 114 women) participating. Of the 22 nonparticipants, one died, four dropped from the clinical trial, and 17 (15%) were intervention dropouts but continued in the trial. There was no difference between cohorts in attendance (P > .20). Participants completed 94% of the intervention sessions, either in person (mean, 13 of 18 sessions; standard deviation, 2.96 sessions) or combined with the telephone follow-ups (mean, 17 of 18 sessions; standard deviation, 1.88 sessions). Absences were caused by employment obligations or treatment toxicities requiring home stay.
Data availability
Both study arms had excellent retention (Fig 1). Excluding the three cases of recurrence or death (three of 227 patients), there was 94% (210 of 224 patients) retention at 4 months. Notably, six (43%) of the 14 study dropouts also failed to return for their medical follow-up, suggesting nonparticipation was not specific to the trial. Only 12 (5%) of 224 patients missed the 4-month assessment.
Data is analyzed according to intention to treat. Thus, findings include data from 15% of the intervention patients who did not participate but remained in the trial. The overall proportion of patients with valid data for the 4-month assessment is as follows: stress (IES), 87%; emotional distress (POMS), 88%; social adjustment, 88%; health behaviors including food habits, 87%, smoking 85%, and exercise, 96%; and compliance, 100%. Numbers are lower for the 4-month immune assays because 85% of the sample was undergoing chemotherapy; there were difficulties with poor venous access and low cell counts. When samples were insufficient, assays were prioritized. At 4 months, data were available for 83% of the NK cell assays and 75% of the Con A and PHA assays. Finally, data availability was equivalent between study arms for all measures.
Analysis plan
The study arms are compared with respect to entry characteristics and outcome measures using χ2 or ANOVA models as appropriate. A repeated measures ANOVA model was used for the social, health behavior, and dose-intensity variables. This was most appropriate because of the strong within-group pretreatment/post-treatment correlations for the variables. The effect of primary interest was the two-way interaction, with group (intervention or assessment) as the between-subjects factor and time (initial or 4-month) as the within-subjects factor. For emotional distress only, a mixed, three-factor repeated measures ANOVA was used, with group and initial cancer stress (low or high, defined by an IES median split) as the between-subjects factors and time as the within-subjects factor. The three-way interaction tested whether the effectiveness of the intervention in reducing emotional distress was greater among patients with initially higher cancer stress. The likelihood of type I errors was reduced by using a contingent, two-step analytic procedure. For measures containing subscales (eg, POMS), the total score was analyzed first, and only if the score was significant did the analysis for the subscales follow. Four measures were used to assess the construct of social adjustment. For this, a multivariate ANOVA (MANOVA) model was used to examine the effect of the intervention on the four measures simultaneously; if significant, ANOVA followed for each measure.
In contrast to the psychosocial variables, the immune outcomes exhibited low within-group pretreatment/post-treatment correlations, and the ANOVA model was not optimal. In this circumstance, an analysis of covariance (ANCOVA) model was best suited. Use of the initial (baseline) score as covariate reduces the error term and provides greater power.34 Again, steps were taken to reduce the likelihood of type I errors. As a conservative strategy, a multivariate analysis of covariance (MANCOVA), with initial values as covariates, simultaneously tested all the dilutions and ratios within an assay (eg, dilutions 2.5, 5.0, and 10.0 μg/mL for Con A). Only if the results were significant did follow-up ANCOVAs test for a group effect at each dilution and ratio.
RESULTS
Description of the Sample
Accrual rate for the cancer center was 52%, which was higher than similar trials.4,9,18 Accrual from the community was essentially 100% because all nonparticipants fell into excluded categories, such as diagnosis of stage I disease. In combination, the overall accrual was 57%. Contrasts between cancer center and community accruals on demographics, disease and prognostic characteristics, or cancer treatment variables were not significant (all P > .09). Analyses contrasting participants versus nonparticipants also found no significant differences between study arms (all P > .10). Reasons for refusal were “too far” (25%, > 60 miles), “insufficient time” (20%), “not interested” (17%), “too stressed” (10%), and miscellaneous or not specified (28%).
Table 2 lists descriptive data. The data are similar to those of the Ohio Cancer Incidence Surveillance System35 and the Surveillance, Epidemiology, and End Results36 database. Analyses of sociodemographic variables revealed no significant differences between study arms (all P > .27). Furthermore, there were no significant differences in disease or prognostic factors or treatments received or planned (all P > .23). Groups were equivalent on most of the outcome variables, and the few differences were small in magnitude (Table 3).
Table 2.
Initial Equivalence of Study Arms on Sociodemographic, Prognostic, Treatment, and Performance Status Variables
| Total (N = 227)
|
Assessment (n = 113)
|
Intervention (n = 114)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | % | SD | Mean | % | SD | Mean | % | SD |
| Sociodemographic | |||||||||
| Age, years | 50.82 | 10.76 | 51.07 | 10.89 | 50.56 | 10.68 | |||
| Race, % white | 90 | 90 | 90 | ||||||
| Education, years | 14.75 | 2.74 | 14.34 | 2.57 | 15.16 | 2.85 | |||
| Family income, thousand $/year | 67.98 | 71.41 | 66.30 | 84.68 | 69.64 | 55.59 | |||
| Marital status, % married | 67 | 67 | 67 | ||||||
| Significant other, % yes | 74 | 72 | 75 | ||||||
| Prognostic | |||||||||
| Stage, II v III, %II | 90 | 92 | 89 | ||||||
| Nodes, No. positive | 3.05 | 5.45 | 3.06 | 5.27 | 3.04 | 5.64 | |||
| Tumor size, cm | 3.02 | 1.77 | 2.91 | 1.75 | 3.12 | 1.78 | |||
| ER/PR, % positive | 68 | 68 | 68 | ||||||
| Menopausal status, % premenopausal | 54 | 52 | 55 | ||||||
| Treatment received/recommended | |||||||||
| Surgery, % segmental mastectomy | 43 | 43 | 43 | ||||||
| Radiation therapy, % yes | 54 | 51 | 56 | ||||||
| Hormonal therapy, % yes | 75 | 80 | 71 | ||||||
| Chemotherapy, % yes | 84 | 85 | 83 | ||||||
| Doxorubicin | 74 | 73 | 75 | ||||||
| Cyclophosphamide | 82 | 86 | 79 | ||||||
| Methotrexate | 12 | 14 | 10 | ||||||
| Fluorouracil | 18 | 19 | 17 | ||||||
| Paclitaxel | 21 | 20 | 21 | ||||||
| Performance status | |||||||||
| Karnofsky performance status* | 85.11 | 7.95 | 86.55 | 6.91 | 83.68 | 8.65 | |||
Abbreviations: SD, standard deviation; ER, estrogen receptor; PR, progesterone receptor.
Significant group difference, P < .05.
Table 3.
Initial Equivalence of Study Arms on Individual Differences in Stress and Outcome Variables
| Total (N = 227)
|
Assessment (n = 113)
|
Intervention (n = 114)
|
||||
|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Mean | SD |
| Individual difference: stress | ||||||
| Impact of Events Scale | 26.27 | 14.41 | 26.28 | 14.46 | 26.25 | 14.42 |
| Emotional distress | ||||||
| Profile of Mood States* | 36.32 | 34.26 | 31.38 | 32.11 | 41.42 | 35.67 |
| Anxiety* | 13.10 | 7.39 | 12.02 | 6.91 | 14.17 | 7.72 |
| Depression | 11.76 | 10.37 | 10.83 | 9.32 | 12.68 | 11.28 |
| Anger | 7.85 | 6.44 | 7.49 | 6.70 | 8.22 | 6.19 |
| Confusion | 8.97 | 5.49 | 8.19 | 5.37 | 9.75 | 5.53 |
| Fatigue | 9.58 | 6.29 | 8.65 | 5.97 | 10.49 | 6.49 |
| Vigor | 14.94 | 6.36 | 16.00 | 6.43 | 13.89 | 6.13 |
| Social adjustment | ||||||
| Social Network Index | 6.05 | 2.91 | 5.98 | 2.85 | 6.12 | 2.98 |
| Perceived Social Support | ||||||
| Family | 16.39 | 4.29 | 16.54 | 4.25 | 16.24 | 4.34 |
| Friends* | 16.83 | 3.49 | 16.23 | 3.79 | 17.42 | 3.07 |
| Dyadic Satisfaction Scale* | 3.69 | 1.38 | 3.94 | 1.28 | 3.46 | 1.43 |
| Health behaviors | ||||||
| Food Habits Questionnaire | 43.13 | 9.14 | 42.91 | 9.20 | 43.35 | 9.12 |
| Avoiding fat | 7.30 | 2.82 | 7.29 | 2.76 | 7.30 | 2.90 |
| Food substitution | 12.17 | 3.54 | 12.15 | 3.70 | 12.19 | 3.39 |
| Meat modification | 11.60 | 3.33 | 11.30 | 3.37 | 11.89 | 3.27 |
| Food replacement | 5.72 | 2.12 | 5.85 | 2.20 | 5.58 | 2.03 |
| Fruit/vegetable | 6.34 | 2.00 | 6.32 | 1.81 | 6.37 | 2.19 |
| Exercise, 7-day recall | 17.50 | 27.55 | 19.15 | 29.88 | 15.64 | 24.76 |
| Smoking, cigarettes/d/person† | 10.36 | 9.52 | 9.00 | 8.56 | 12.78 | 11.13 |
| Chemotherapy: recommended, mg/m2/wk‡ | ||||||
| Doxorubicin, n = 168 | 37.49 | 8.39 | 36.27 | 7.27 | 38.67 | 9.22 |
| Cyclophosphamide, n = 187 | 516.28 | 427.24 | 483.02 | 357.08 | 552.14 | 491.36 |
| Methotrexate, n = 28 | 40.14 | 25.73 | 39.61 | 29.94 | 40.96 | 18.76 |
| Fluorouracil, n = 41 | 444.64 | 118.31 | 441.35 | 118.38 | 448.45 | 121.36 |
| Paclitaxel, n = 48 | 121.38 | 29.18 | 123.03 | 30.18 | 119.86 | 28.77 |
| Immune measures | ||||||
| T-lymphocyte counts, K/μL | ||||||
| T3 | 1.28 | 0.59 | 1.25 | 0.51 | 1.32 | 0.66 |
| T4 | 0.90 | 0.43 | 0.86 | 0.39 | 0.93 | 0.47 |
| T8 | 0.43 | 0.22 | 0.42 | 0.19 | 0.43 | 0.25 |
| Blastogenenic response, optical density readings | ||||||
| Unstimulated cells, control | 0.21 | 0.14 | 0.23 | 0.15 | 0.18 | 0.13 |
| Con A, μg/mL, with control values substracted | ||||||
| 10.0 | 0.12 | 0.10 | 0.11 | 0.09 | 0.12 | 0.10 |
| 5.0 | 0.19 | 0.13 | 0.18 | 0.12 | 0.20 | 0.14 |
| 2.5 | 0.19 | 0.14 | 0.18 | 0.12 | 0.21 | 0.16 |
| PHA, μg/mL, with control values subtracted | ||||||
| 10.0 | 0.31 | 0.15 | 0.30 | 0.16 | 0.32 | 0.15 |
| 5.0 | 0.30 | 0.15 | 0.28 | 0.15 | 0.31 | 0.15 |
| 2.5 | 0.28 | 0.15 | 0.27 | 0.15 | 0.29 | 0.15 |
| Natural killer cell count, K/μL | 216.7 | 137.2 | 219.9 | 130.7 | 213.5 | 143.8 |
| Lysis at effector to target ratios, % | ||||||
| 100:1 | 56.38 | 19.33 | 57.12 | 19.45 | 55.63 | 19.27 |
| 50:1 | 47.95 | 18.98 | 48.06 | 18.99 | 47.84 | 19.06 |
| 25:1 | 34.76 | 15.39 | 34.57 | 15.31 | 34.95 | 15.55 |
| 12.5:1 | 23.48 | 12.20 | 23.34 | 12.56 | 23.61 | 11.90 |
| 6.25:1 | 14.32 | 8.37 | 14.41 | 8.74 | 14.23 | 8.04 |
| 3.125:1 | 7.29 | 4.85 | 7.23 | 4.82 | 7.35 | 4.89 |
Abbreviations: SD, standard deviation; Con A, concanavalin A; PHA, phytohemagglutinin.
Significant group difference, P < .05.
Means based on the 25 smokers in the sample.
Means based on the number of patients receiving the treatment.
Emotional Distress and Individual Differences
The two-way interaction for Total Mood Disturbance was not significant. A significant three-way interaction was found (F1,192 = 4.55, P = .03), such that Total Mood Disturbance decreased more in the intervention arm than the assessment arm (F1,93 = 4.13, P = .04) for subjects with high initial cancer stress. Follow-up analysis of the POMS scales was conducted for general clarification of the intervention effect. Of particular relevance is the Anxiety scale because the intervention focused on reducing stress and anxiety. The two-way interaction was significant (F1,193 = 4.15, P = .04), such that there was a greater reduction of anxious moods in the intervention arm than in the assessment arm. The three-way interaction was not significant; the intervention was equally effective in reducing anxiety for patients with low or high cancer stress. For the Fatigue scale, the two-way interaction was not significant, but a significant three-way interaction was found (F1,193 = 5.14, P = .02). The intervention lowered fatigue for patients with high cancer stress but not for patients with low cancer stress. Interaction effects for the remaining scales approached significance (.05 < P < .08), and changes were in the hypothesized direction (ie, greater reductions in distress for the intervention arm).
Social Adjustment, Health Behaviors, and Adherence
As noted, analyses focused exclusively on the two-way interaction. A significant two-way interaction for social adjustment (F4,140 = 2.41, P = .048) was found. Follow-up univariate analyses revealed a significant two-way interaction for PSS-Family (F1,143 = 5.36, P = .02). Perceived support from the family significantly increased in the intervention arm but decreased in the assessment arm.
Analyses of the overall dietary habits measure indicated a significant two-way interaction (F1,194 = 5.01, P = .03), in which only the intervention arm significantly increased healthy food habits by 4 months. Follow-up ANOVAs revealed significant interactions for avoiding fat (F1,194 = 3.92, P = .049) and food substitution scales (F1,193 = 4.41, P = .04). Analyses of the exercise recall measure approached significance for the interaction (P = .08), with a greater increase in physical activity in the intervention arm than in the assessment arm.
Analyses for smoking also showed a significant interaction (F1,195 = 4.52, P = .03), indicating that the number of cigarettes smoked daily decreased for the intervention participants but increased for the assessment participants. When restricted to only active smokers, the interaction was again significant (F1,15 = 4.94, P = .04). Descriptively, there were changes in smoking status from 12 months before diagnosis to the 4-month assessment. Ninety percent (90%) of the assessment smokers either resumed or continued smoking in contrast to 30% of the intervention smokers.
Regarding dose-intensity of chemotherapy, the distribution of values was too skewed for parametric statistics because 67% of the patients received more than 90% of their recommended regimen dose. Dose-intensity between the intervention and assessment arms did not differ significantly for either means (90.2% v 87.7%, respectively) or medians (95.7% v 94.0%, respectively). However, the variance of dose-intensity in the assessment arm was significantly greater (P = .03; Conover’s squared rank test37), indicating more individual variability in dose-intensity values for the assessment patients compared with those for the intervention patients.
Regarding adherence from the initial to the 4-month assessment, seven of 227 women discontinued chemotherapy against medical advice; five of these women were from the assessment arm. Six of 227 patients were lost to medical follow-up; five of these women were from the assessment arm. These descriptive data likely underestimate the incidence of noncompliant behaviors because both the clinical trial and the treating institutions were aggressive in maintaining follow-up (eg, providing transportation funds to the clinic, if needed).
Immune Analyses
T-lymphocyte counts
ANCOVAs revealed no significant group effect on CD3, CD4, or CD8 counts.
T-cell blastogenesis
The MANCOVA revealed a significant effect for Con A–induced T-cell blastogenesis (F3,154 = 6.37, P = .0004). Follow-up ANCOVAs were significant for all Con A dilutions (all P < .01). For each, there was an identical pattern of increased proliferation for the intervention arm and decreased proliferation for the assessment arm from the initial to the 4-month assessments. For illustration, data for the 5.0 μg/mL dilution (F1,158 = 14.80, P = .0002) are provided (Fig 2A). For interested readers, when this data was analyzed using a repeated measures MANOVA model, results were similar (F3,157 = 8.67, P = .00002).
Fig 2.
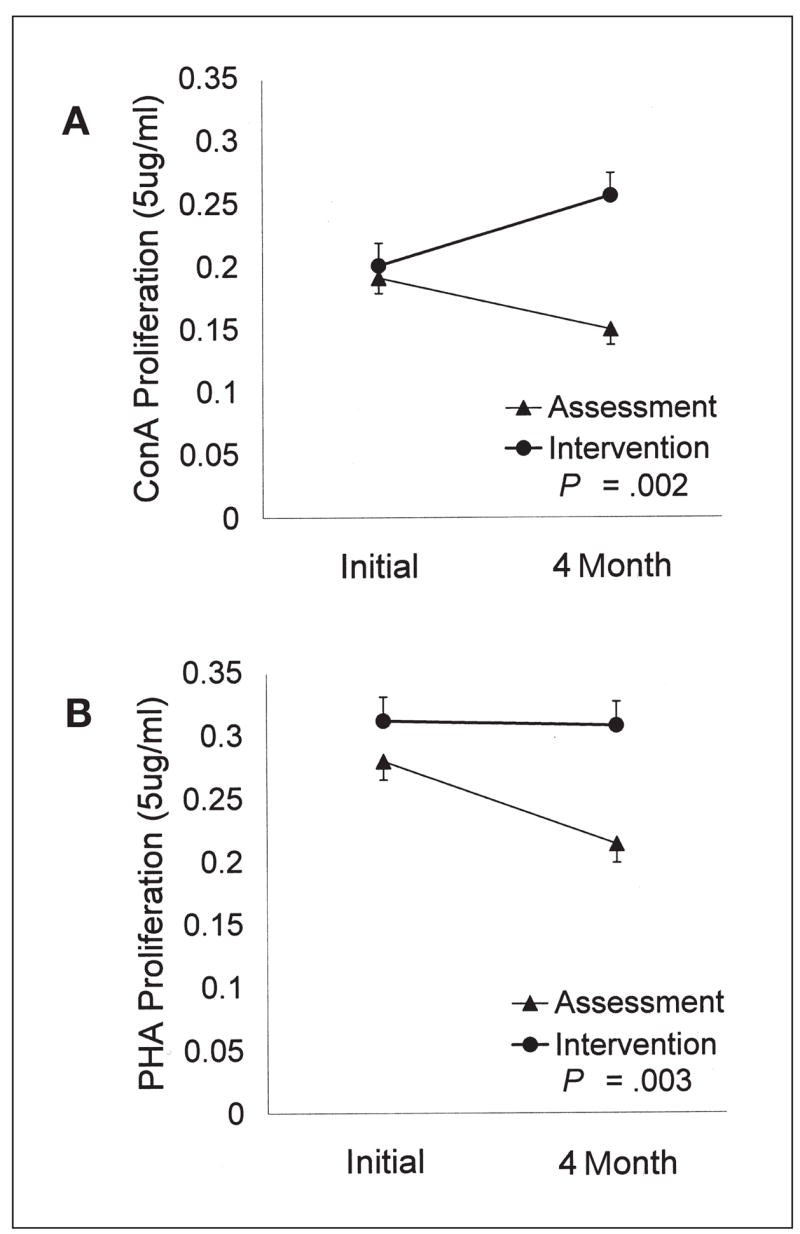
(A) Example of significant effects for Con A–induced proliferation. Optical density readings for 5.0 μg/mL dilution are displayed, with error bars representing one SE. (B) Example of significant effects for PHA-induced proliferation. Optical density readings for 5.0 μg/mL dilution are displayed, with error bars representing one SE.
There were similar, significant findings for PHA. The MANCOVA (F3,154 = 3.85, P = .01) and follow-up ANCOVAs for each dilution were significant (all P < .05). Across dilutions, the pattern was the same; proliferation remained constant for the intervention arm but significantly declined for the assessment arm. Data for the 5.0 μg/mL dilution, (F1,158 = 8.64, P = .004) are provided (Fig 2B). Again, for interested readers, when this data was analyzed using a repeated measures MANOVA model, results were similar (F3,157 = 2.35, P = .075).
NK cell count and cell lysis
The findings for cell count were not significant. Findings for NK lysis, using either the MANCOVA or repeated measures MANOVA model, were not significant.
DISCUSSION
The present trial is part of the ongoing effort to test for changes in biobehavioral outcomes and disease end points after a psychological intervention. Lowering stress is the main goal for interventions,1,2 and the most successful interventions reduce patients’ anxiety.38,39 When untreated, anxieties are common and arise from many sources,40 including diagnosis,41 diagnostic tests,42 surgery,43 radiation,44 chemotherapy,45 BMT,46 and cancer symptoms.47,48 Here, anxiety was significantly reduced in the intervention arm. Analyses also contrasted patients who differed in the magnitude of cancer-specific stress. For women with high stress, the intervention provided a 36% reduction in total mood disturbance compared with the 12% reduction found for the assessment arm. Parallel effects emerged for feelings of fatigue. This may be important because fatigue can affect every aspect of a patient’s life,49 yet it is often not recognized. Oncologists attest that pain, for example, is routinely treated (95%), whereas fatigue receives little to no attention (5%).50
Many, if not all, interventions attempt to provide social support to patients, but it is rare that specific therapeutic techniques are used to improve a patient’s social adjustment in the real world to enable or facilitate social support from specific others.1,2 We are aware of only four intervention studies that have noted strategies to help patients to use social support,18 enhance interpersonal relationships,51 or address relationship difficulties.52,53 In two other studies, patients were encouraged to return to prior social activities and maintain relationships.54,55 Even so, studies have not included social adjustment or social support from others as an intervention outcome1,2 as was done here.
The patients’ initial assessment occurred shortly after illness onset and early treatment because it was regarded as providing a more realistic reflection of how a patient’s family and friends actually respond in a crisis.56 The group format may have provided, in general, social support, but more importantly, the intervention components helped patients to make their established network of relationships function more effectively for them. Maintaining existing relationships is important because data indicate that quality of life is poorer for breast cancer patients who are socially isolated.57 We do not know if the intervention patients actually received or only perceived more support from their family. When compared, it is perceived support that may actually be more important for adjustment58–60 and health effects.12
Some interventions with cancer patients focus only on dietary,61–64 exercise,65,66 or smoking cessation change.67,68 The intervention model for these studies has been to have a professional (eg, dietician) meet with a patient for one to several sessions. To our knowledge, psychological interventions for cancer patients have not previously included health behaviors as intervention targets or outcomes. The single exception offered skin protection information to melanoma patients receiving a psychoeducational intervention.69
Despite the brevity of the health behavior component (four of 18 sessions), significant changes in both positive and negative behaviors occurred. The dietary sessions provided information, sampling of low-fat snacks, food intake diaries, and other components. Reductions in fat intake were readily achieved, which was consistent with lengthier dietary interventions.25,61,62 There were no differential changes in activity or exercise, likely because of the minimal time spent. However, even large randomized trials offering lengthy exercise interventions (eg, 26 weeks) have reported significant change on some measures but not on others.66 Finally, the smoking reductions in the intervention arm were significant, despite the low number of smokers in the trial. Recent research indicates that individuals who continue to smoke after a cancer diagnosis endorse reasons such as “I am more relaxed when I smoke” and “smoking relieves tension”.70 Our intervention was focused on stress reduction, and the specific recommendations for smoking cessation were similar to those in the National Cancer Institute’s 4 A’s program used by physicians (ie, Ask, Advise to quit, Assist the patient to stop, and Arrange follow-up monitoring),71 which has been effective in increasing cessation rates.72,73
Poor adherence to treatment is a behavioral problem and one that has received minimal investigation despite its importance.74 Within the assessment group, there was a greater dispersion of chemotherapy dosages, more premature terminations of treatment, and more lost to follow-up cases. The data are consistent and in the predicted direction. However, in future research, a larger sample size and disease sites yielding more variability in dose-intensity values and/or higher rates of nonadherence are needed.
Finally, the often-proposed mechanism for survival effects is improved emotions and/or lowered stress modulating the immune system, which, in turn, alters disease progression. Several intervention studies have tested for changes in NK cell lysis, and, with one exception,75 null effects have been reported.76–80 We too observed no change in NK cell lysis, but we found significant, reliable effects with blastogenesis; a pattern that has been reported by others.81
Blastogenesis remained stable or increased across time for intervention patients, whereas responses declined for assessment patients. These experimental data extend our correlational report showing a negative relationship between high stress and low immune responses as women entered the trial.32 It is notable that the intervention effects on blastogenesis were reliable within and across the Con A and PHA assays. Even so, some might suggest that these intervention effects could be explained by other factors. We do not believe this is the case, which is a viewpoint bolstered by the successful randomization equating the groups, the reliability of blastogenesis findings, and the predicted consistency of the blastogenesis findings with the psychological and behavioral changes.
We also tested competing explanations for the intervention effect on immunity, testing for effects caused by cell counts, chemotherapy, or radiation. When CD3, CD4, and CD8 cell counts were statistically controlled in MANCOVAs, study arm effects remained (all P < .05). Thus, the changes in blastogenesis were functional and not a result of cell trafficking. Regarding chemotherapy, patients from both study arms were equally likely to have completed chemotherapy at 4 months, and the study arms did not differ significantly in the number of patients receiving chemotherapy (Table 2) or the dosages prescribed (Table 3; all P > .05). Nevertheless, MANCOVAs controlling for the types of chemotherapy received (eg, doxorubicin and paclitaxel) were conducted. They showed the same positive intervention effects on both Con A and PHA (all P < .05). Regarding radiation, the study arms did not differ significantly in the number of patients receiving this treatment. By 4 months, more patients in the intervention arm (n = 25) were still receiving radiation compared with the assessment arm (n = 9; P = .005). When radiation status was controlled, however, the positive effect of the intervention on PHA and Con A blastogenesis remained. Taken together, these analyses rule out competing explanations and provide evidence for a robust effect of the intervention on T-cell blastogenesis.
The immune changes observed in the assessment arm (ie, reduced proliferation in response to T-cell mitogens) may indicate the presence of a functional defect in T-cell immunity, although the precise immune implications of this phenomena cannot be discerned at this point in the study. Indeed, it is possible that the observed alterations in lectin-induced blastogenesis do not represent a distinct immune deficiency. Any reduction in T-cell effector function might theoretically lead to increased rates of breast cancer recurrence in surgically treated stage II and III breast cancer patients because of the role the T cells seem to play in the process of tumor surveillance.82 However, it is important to note that alterations in polyclonal T-cell proliferation in response to ex vivo stimulation of patient peripheral-blood mononuclear cells with Con A or PHA are of uncertain significance with respect to subsequent cancer progression. Our follow-up of the study participants will continue, and we will be better able to determine whether these immune effects or others are related to breast cancer recurrence rates.
If psychological interventions impact cancer survival, the process is likely to be multifactorial, including psychological factors, behavioral factors, biologic responses, and other factors.16 This trial includes tests for the expected reductions in emotional distress plus the additional intervention targets (health behaviors, adherence, and immunity), which may represent plausible routes to improved survival. In previous trials, the intervention effects have been strong,10,52,69,75,83–86 moderate,4 and null.87,88 It is important to note that positive intervention effects are a necessary condition for testing for recurrence or survival effects. In this study, the predicted effects for emotional distress, social support, health behaviors, and immunity were observed, with some effects being stronger than others. In combination, they provide the context for a meaningful test of disease end point hypotheses and a strategy to examine multiple routes by which psychological interventions may affect disease course.
Acknowledgments
We thank the participants in the Stress and Immunity Breast Cancer Project and the professional and research staff.
Supported by American Cancer Society grant No. PBR-89, the Longaberger Company-American Cancer Society Grant for Breast Cancer Research grant No. PBR-89A, the US Army Medical Research Acquisition Activity grant Nos. DAMD17-94-J-4165, DAMD17-96-1-6294, and DAMD17-97-1-7062, National Institute of Mental Health grant No. RO1MH51487, National Cancer Institute grant No. RO1CA92704, P30 CA16058, and General Clinical Research Center grant No. MO1-RR0034.
Footnotes
Authors’ disclosures of potential conflicts of interest are found at the end of this article.
Authors’ Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Andersen BL. Psychological interventions for cancer patients to enhance the quality of life. J Consult Clin Psychol. 1992;60:552–568. doi: 10.1037//0022-006x.60.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen BL. Biobehavioral outcomes following psychological interventions for cancer patients. J Consult Clin Psychol. 2002;70:590–610. doi: 10.1037//0022-006X.70.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helgeson VS, Cohen S, Schulz R, et al. Group support interventions for women with breast cancer: Who benefits from what? Health Psychol. 2000;19:107–114. doi: 10.1037//0278-6133.19.2.107. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719–1726. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel D, Bloom JR, Kraemer HC, et al. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2:888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- 6.Fawzy FI, Fawzy NW, Hyun CS, et al. Malignant melanoma: Effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry. 1993;50:681–689. doi: 10.1001/archpsyc.1993.01820210015002. [DOI] [PubMed] [Google Scholar]
- 7.Richardson JL, Zarnegar Z, Bisno B, et al. Psychosocial status at initiation of cancer treatment and survival. J Psychosom Res. 1990;34:189–201. doi: 10.1016/0022-3999(90)90053-7. [DOI] [PubMed] [Google Scholar]
- 8.Kuchler T, Henne-Bruns D, Rappat S, et al. Impact of psychotherapeutic support on gastrointestinal cancer patients undergoing surgery: Survival results of a trial. Hepatogastroenterology. 1999;46:322–335. [PubMed] [Google Scholar]
- 9.Cunningham AJ, Edmonds CV, Jenkins GP, et al. A randomized controlled trial of the effects of group psychological therapy on survival in women with metastatic breast cancer. Psychooncology. 1998;7:508–517. doi: 10.1002/(SICI)1099-1611(199811/12)7:6<508::AID-PON376>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Linn MW, Linn BS, Harris R. Effects of-counseling for late stage cancer patients. Cancer. 1982;49:1048–1055. doi: 10.1002/1097-0142(19820301)49:5<1048::aid-cncr2820490534>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Edelman S, Lemon J, Bell DR, et al. Effects of group CBT on the survival time of patients with metastatic breast cancer. Psychooncology. 1999;8:474–481. doi: 10.1002/(sici)1099-1611(199911/12)8:6<474::aid-pon427>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Berkman LF, Leo-Summers L, Horwitz RI. Emotional support and survival after myocardial infarction: A prospective, population-based study of the elderly. Ann Intern Med. 1992;117:1003–1009. doi: 10.7326/0003-4819-117-12-1003. [DOI] [PubMed] [Google Scholar]
- 13.Hazuda HP. A critical evaluation of U.S., epidemiological evidence and ethnic variation. In: Shumaker SA, editor. Social Support and Cardiovascular Disease. New York, NY: Plenum; 1994. pp. 119–142. [Google Scholar]
- 14.Orth-Gomer K, Rosengren A, Wilhelmsen L. Lack of social support and incidence of coronary heart disease in middle-aged Swedish men. Psychosom Med. 1993;55:37–43. doi: 10.1097/00006842-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kiecolt-Glaser JK, McGuire L, Robles TF, et al. Psychoneuroimmunology: Psychological influences on immune function and health. J Consult Clin Psychol. 2002;70:537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- 16.Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Am Psychol. 1994;49:389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keefe FJ, Buffington ALH, Studts JL, et al. Behavioral medicine: 2002 and beyond. J Consult Clin Psychol. 2002;70:852–856. doi: 10.1037//0022-006x.70.3.852. [DOI] [PubMed] [Google Scholar]
- 18.Antoni MH, Lehman JM, Klibourn KM, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol. 2001;20:20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- 19.Horowitz M, Wilner N, Alvarez W. Impact of Events Scale: A measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 20.McNair DM, Lorr M, Droppleman LF. EITS Manual of the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- 21.Berkman LF, Syme SL. Social networks, host resistance, and mortality: A nine year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 22.Berkman LF. Social networks, host resistance, and mortality: A follow-up study of Alameda County residents. Berkeley, CA: University of California Berkeley; 1977. (dissertation) [Google Scholar]
- 23.Procidano ME, Heller K. Measures of perceived social support from friends and from family: Three validation studies. Am J Community Psychol. 1983;11:1–24. doi: 10.1007/BF00898416. [DOI] [PubMed] [Google Scholar]
- 24.Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. J Marriage Fam. 1976;38:15–28. [Google Scholar]
- 25.Kristal AR, Shattuck AL, Henry HJ, et al. Rapid assessment of dietary intake of fat, fiber, and saturated fat: Validity of an instrument suitable for community intervention research and nutritional surveillance. Am J Health Promot. 1990;4:288–295. doi: 10.4278/0890-1171-4.4.288. [DOI] [PubMed] [Google Scholar]
- 26.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 27.Hryniuk WM. The importance of dose intensity in the outcome of chemotherapy. In: DiVita VT, editor. Important Advances in Oncology. Philadelphia, PA: JB Lippencott; 1988. pp. 121–142. [PubMed] [Google Scholar]
- 28.Hryniuk WM, Levine MN. Analysis of dose intensity of adjuvant chemotherapy trials in stage II breast cancer. J Clin Oncol. 1986;4:1162–1170. doi: 10.1200/JCO.1986.4.8.1162. [DOI] [PubMed] [Google Scholar]
- 29.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- 30.Mor V, Laliberte L, Morris JN, et al. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 31.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky Performance Status. Cancer. 1980;45:2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White SJ, Freedman LS. Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer. 1978;37:849–857. doi: 10.1038/bjc.1978.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girden ER. ANOVA Repeated Measures. Sage University Paper Series on Quantitative Applications in the Social Sciences, 07-084. Newbury Park, CA: Sage; 1992. pp. 1–76. [Google Scholar]
- 35.Ohio Department of Health. Community health assessments and the center for public health data and statistics. Ohio Department of Health; Columbus, OH: 2002. [Google Scholar]
- 36.Surveillance Epidemiology, and End Results Program (SEER) Division of Cancer Control and Population Sciences. Bethesda, MD: National Cancer Institute; 2000. [Google Scholar]
- 37.Conover WJ. Practical Nonparametric Statistics. 2. New York, NY: John Wiley & Sons; 1980. p. 239. [Google Scholar]
- 38.Sheard T, Maguire P. The effect of psychological interventions on anxiety and depression in cancer patients: Results of two meta-analyses. Br J Cancer. 1999;80:1770–1780. doi: 10.1038/sj.bjc.6690596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.v’ant Spijker A, Trijsburg RW, Duivenvoorden HJ. Psychological sequelae of cancer diagnosis: A meta-analytical review of 58 studies after 1980. Psychosom Med. 1997;59:280–293. doi: 10.1097/00006842-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Stark DP, House A. Anxiety in cancer patients. Br J Cancer. 2000;83:1261–1267. doi: 10.1054/bjoc.2000.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weisman AD, Worden JW. The existential plight in cancer: Significance of the first 100 days. Int J Psychiatry Med. 1976;7:1–15. doi: 10.2190/uq2g-ugv1-3ppc-6387. [DOI] [PubMed] [Google Scholar]
- 42.Melendez JC, McCrank E. Anxiety-related reactions associated with magnetic resonance imaging examinations. JAMA. 1993;270:745–747. doi: 10.1001/jama.1993.03510060091039. [DOI] [PubMed] [Google Scholar]
- 43.Thomas C, Madden F, Jehu D. Psychological effects of stomas—I. Psychosocial morbidity one year after surgery. J Psychosom Res. 1987;31:311–316. doi: 10.1016/0022-3999(87)90050-x. [DOI] [PubMed] [Google Scholar]
- 44.Andersen BL, Karlsson JA, Anderson B, et al. Anxiety and cancer treatment: Response to stressful radiotherapy. Health Psychol. 1984;3:535–551. doi: 10.1037//0278-6133.3.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrow GR, Roscoe JA, Hickok JT, et al. Nausea and emesis: Evidence for a biobehavioral perspective. Support Care Cancer. 2002;10:96–105. doi: 10.1007/s005200100294. [DOI] [PubMed] [Google Scholar]
- 46.Jacobsen PB, Widows MR, Hann DM, et al. Posttraumatic stress disorder symptoms after bone marrow transplantation for breast cancer. Psychosom Med. 1998;60:366–371. doi: 10.1097/00006842-199805000-00026. [DOI] [PubMed] [Google Scholar]
- 47.Rabin C, Ward S, Leventhal H, et al. Explaining retrospective reports of symptoms in patients undergoing chemotherapy: Anxiety, initial symptom experience, and post treatment symptoms. Health Psychol. 2001;20:91–98. [PubMed] [Google Scholar]
- 48.Redd WH, Montgomery GH, DuHamel KN. Behavioral intervention for cancer-treatment side-effects. J Natl Cancer Inst. 2001;93:810–823. doi: 10.1093/jnci/93.11.810. [DOI] [PubMed] [Google Scholar]
- 49.Winningham ML, Nail LM, Burke MB, et al. Fatigue and the cancer experience: The state of the knowledge. Oncol Nurs Forum. 1994;21:23–36. [PubMed] [Google Scholar]
- 50.Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: Results of a tripart assessment survey. The Fatigue Coalition Semin Hematol. 1997;34:4–12. [PubMed] [Google Scholar]
- 51.Capone MA, Good RS, Westie KS, et al. Psychosocial rehabilitation of gynecologic oncology patients. Arch Phys Med Rehabil. 1980;61:128–132. [PubMed] [Google Scholar]
- 52.Spiegel D, Bloom JR, Yalom I. Group support for patients with metastatic cancer: A randomized outcome study. Arch Gen Psychiatry. 1981;38:527–533. doi: 10.1001/archpsyc.1980.01780300039004. [DOI] [PubMed] [Google Scholar]
- 53.Cain EN, Kohorn EI, Quinlan DM, et al. Psychosocial benefits of a cancer support group. Cancer. 1986;57:183–189. doi: 10.1002/1097-0142(19860101)57:1<183::aid-cncr2820570135>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 54.Houts PS, Whitney CW, Mortel R, et al. Former cancer patients as counselors of newly diagnosed cancer patients. J Natl Cancer Inst. 1986;76:793–796. [PubMed] [Google Scholar]
- 55.Maguire P, Brooke M, Tait A, et al. The effect of counseling on physical disability and social recovery after mastectomy. Clin Oncol. 1983;9:319–324. [PubMed] [Google Scholar]
- 56.Reifman A. Social relationships, recovery from illness, and survival: A literature review. Ann Behav Med. 1995;17:124–131. doi: 10.1007/BF02895061. [DOI] [PubMed] [Google Scholar]
- 57.Michael YV, Berkman LF, Colditz GA, et al. Social networks and health-related quality of life in breast cancer survivors: A prospective study. J Psychosom Res. 2002;52:285–293. doi: 10.1016/s0022-3999(01)00270-7. [DOI] [PubMed] [Google Scholar]
- 58.Cohen S, Hoberman HM. Positive events and social supports as buffers of life change stress. J Appl Soc Psychol. 1983;13:99–125. [Google Scholar]
- 59.Wethington E, Kessler RC. Perceived support, received support, and adjustment to stressful life events. J Health Soc Behav. 1986;27:78–89. [PubMed] [Google Scholar]
- 60.Helgeson VS, Cohen S. Social support and adjustment to cancer: Reconciling descriptive, correlational, and intervention research. Health Psychol. 1996;15:135–148. doi: 10.1037//0278-6133.15.2.135. [DOI] [PubMed] [Google Scholar]
- 61.Nordevang E, Callmer E, Marmur A, et al. Dietary intervention in breast cancer patients: Effects on food choice. Eur J Clin Nutr. 1992;46:387–396. [PubMed] [Google Scholar]
- 62.Chlebowski RT, Blackburn GL, Buzzard M, et al. Adherence to a dietary fat intake reduction program in postmenopausal women receiving therapy for early breast cancer. The Women’s Intervention Nutrition Study. J Clin Oncol. 1993;11:2072–2080. doi: 10.1200/JCO.1993.11.11.2072. [DOI] [PubMed] [Google Scholar]
- 63.Pierce JP, Faerber S, Wright FA, et al. Feasibility of a randomized trial of a high-vegetable diet to prevent breast cancer recurrence. Nutr Cancer. 1997;28:282–288. doi: 10.1080/01635589709514589. [DOI] [PubMed] [Google Scholar]
- 64.Kristal AR, Shattuck AL, Bowen DJ, et al. Feasibility of using volunteer research staff to deliver and evaluate a low-fat dietary intervention: The American Cancer Society Breast Cancer Dietary Intervention Project. Cancer Epidemiol Biomarkers Prev. 1997;6:459–467. [PubMed] [Google Scholar]
- 65.MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patient’s functional capacity. Nurs Res. 1989;38:348–351. [PubMed] [Google Scholar]
- 66.Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: Results of randomized controlled trial. J Clin Oncol. 2001;19:657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 67.Gritz ER, Carr CR, Rapkin D, et al. Predictors of long-term smoking cessation in head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 1993;2:261–270. [PubMed] [Google Scholar]
- 68.Wewers ME, Bowen JM, Stanislaw AE, et al. A nurse-delivered smoking cessation intervention among hospitalized postoperative patients—Influence of a smoking-related diagnosis: A pilot study. Heart Lung. 1994;23:151–156. [PubMed] [Google Scholar]
- 69.Fawzy FI, Cousins N, Fawzy NW, et al. A structured psychiatric intervention for cancer patients. I Changes over time in methods of coping and affective disturbance. Arch Gen Psychiatry. 1990;47:720–725. doi: 10.1001/archpsyc.1990.01810200028004. [DOI] [PubMed] [Google Scholar]
- 70.Schnoll RA, James BA, Malstrom M, et al. Longitudinal predictors of continued tobacco use among patients diagnosed with cancer. Ann Behav Med. 2003;25:214–221. doi: 10.1207/S15324796ABM2503_07. [DOI] [PubMed] [Google Scholar]
- 71.Manley M, Epps RP, Husten C, et al. Clinical interventions in tobacco control: A National Cancer Institute training program for physicians. JAMA. 1991;266:3172–3173. [PubMed] [Google Scholar]
- 72.Fiore MC, Bailey WC, Cohen SJ, et al. Smoking cessation. Clinical practice guideline no. 18. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research publication 96-0692; 1996. [Google Scholar]
- 73.Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence: A clinical practice guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Quality Research publication 00-0032; 2000. [Google Scholar]
- 74.Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 75.Fawzy FI, Kemeny ME, Fawzy NW, et al. A structured psychiatric intervention for cancer patients. II Changes over time in immunological measures. Arch Gen Psychiatry. 1990;47:729–735. doi: 10.1001/archpsyc.1990.01810200037005. [DOI] [PubMed] [Google Scholar]
- 76.Elsesser K, van Berkel M, Sartory G, et al. The effects of anxiety management training on psychological variables and immune parameters in cancer patients: A pilot study. Beh Cogn Psychoth. 1994;22:13–23. [Google Scholar]
- 77.Larson MR, Duberstein PR, Talbot NL, et al. A presurgical psychosocial intervention for breast cancer patients: Psychological distress and the immune response. J Psychosom Res. 2000;48:187–194. doi: 10.1016/s0022-3999(99)00110-5. [DOI] [PubMed] [Google Scholar]
- 78.Richardson MA, Post-White J, Grimm EA, et al. Coping, life attitudes, and immune responses to imagery and group support after breast cancer treatment. Altern Ther Health Med. 1997;3:62–70. [PubMed] [Google Scholar]
- 79.Van der Pompe G, Duivenoorden HJ, Antoni MH, et al. Effectiveness of a short-term group psychotherapy program on endocrine and immune function in breast cancer patients: An exploratory study. J Psychosom Res. 1997;42:453–466. doi: 10.1016/s0022-3999(96)00393-5. [DOI] [PubMed] [Google Scholar]
- 80.Hosaka T, Tokuda Y, Sugiyama Y, et al. Effects of a structured psychiatric intervention on immune function of cancer patients. Tokai J Exp Clin Med. 2000;25:183–188. [PubMed] [Google Scholar]
- 81.Gruber BL, Hersh SP, Hall NRS, et al. Immunological responses of breast cancer patients to behavioral interventions. Biofeedback Self Regul. 1993;18:1–22. doi: 10.1007/BF00999510. [DOI] [PubMed] [Google Scholar]
- 82.Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Edelman S, Bell DR, Kidman AD. A group cognitive behaviour therapy programme with metastatic breast cancer patients. Psychooncology. 1999;8:295–305. doi: 10.1002/(SICI)1099-1611(199907/08)8:4<295::AID-PON386>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 84.Spiegel D, Bloom JR. Group therapy and hypnosis reduce metastatic breast carcinoma pain. Psychosom Med. 1983;45:333–339. doi: 10.1097/00006842-198308000-00007. [DOI] [PubMed] [Google Scholar]
- 85.Richardson JL, Marks G, Johnson CA, et al. Path model of multidimensional compliance with cancer therapy. Health Psychol. 1987;6:183–207. doi: 10.1037//0278-6133.6.3.183. [DOI] [PubMed] [Google Scholar]
- 86.Richardson JL, Marks G, Levine A. The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J Clin Oncol. 1988;6:1746–1752. doi: 10.1200/JCO.1988.6.11.1746. [DOI] [PubMed] [Google Scholar]
- 87.Edmonds CV, Lockwood GA, Cunningham AJ. Psychological response to long-term group therapy: A randomized trial with metastatic breast cancer patients. Psychooncology. 1999;8:74–91. doi: 10.1002/(SICI)1099-1611(199901/02)8:1<74::AID-PON339>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 88.Bordeleau L, Szalai JP, Ennis M, et al. Quality of life in a randomized trial of group psychosocial support in metastatic breast cancer: Overall effects of the intervention and an explanation of missing data. J Clin Oncol. 2003;21:1944–1951. doi: 10.1200/JCO.2003.04.080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Both study arms had excellent retention (Fig 1). Excluding the three cases of recurrence or death (three of 227 patients), there was 94% (210 of 224 patients) retention at 4 months. Notably, six (43%) of the 14 study dropouts also failed to return for their medical follow-up, suggesting nonparticipation was not specific to the trial. Only 12 (5%) of 224 patients missed the 4-month assessment.
Data is analyzed according to intention to treat. Thus, findings include data from 15% of the intervention patients who did not participate but remained in the trial. The overall proportion of patients with valid data for the 4-month assessment is as follows: stress (IES), 87%; emotional distress (POMS), 88%; social adjustment, 88%; health behaviors including food habits, 87%, smoking 85%, and exercise, 96%; and compliance, 100%. Numbers are lower for the 4-month immune assays because 85% of the sample was undergoing chemotherapy; there were difficulties with poor venous access and low cell counts. When samples were insufficient, assays were prioritized. At 4 months, data were available for 83% of the NK cell assays and 75% of the Con A and PHA assays. Finally, data availability was equivalent between study arms for all measures.