Abstract
Tellurite exerts a deleterious effect on a number of small molecules containing sulfur moieties that have a recognized role in cellular oxidative stress. Because cysteine is involved in the biosynthesis of glutathione and other sulfur-containing compounds, we investigated the expression of Geobacillus stearothermophilus V cysteine-related genes cobA, cysK, and iscS and Escherichia coli cysteine regulon genes under conditions that included the addition of K2TeO3 to the culture medium. Results showed that cell tolerance to tellurite correlates with the expression level of the cysteine metabolic genes and that these genes are up-regulated when tellurite is present in the growth medium.
Sulfur is an essential element that is required for the biosynthesis of proteins, enzyme cofactors, and other important biomolecules. In bacteria, this element can be assimilated into sulfur-containing amino acids through enzymatic fixation from inorganic sources, such as sulfate and/or thiosulfate (15, 38). Although tellurium shares several chemical properties with sulfur, no biological function for Te is known to date. Conversely, some tellurium compounds, like the oxyanion tellurite (TeO32−), are extremely toxic for most forms of life, especially microorganisms (34).
It has been proposed that K2TeO3 toxicity could be due to the oxidation of cellular thiols such as glutathione (37) or the generation of superoxide radical during tellurite reduction, which would cause a redox imbalance resulting in intracellular oxidative stress (5, 23, 26, 33, 34, 36).
Maintenance of cell redox balance is one of the most important processes involving molecules synthesized from reduced sulfur taken from the environment. Glutathione (GSH) is one of the major nonprotein thiols in living organisms, including humans, yeast, and bacteria (6, 10). GSH has been involved in resistance to osmotic and oxidative stress as well as in Escherichia coli resistance to the toxic effects of electrophiles like methylglyoxal (6, 11, 31). A protective effect of GSH against oxidative stress has also been described for Lactococcus lactis and Rhodobacter capsulatus (17, 18).
Three genes involved in tellurite resistance have been described previously for the thermotolerant gram-positive rod Geobacillus stearothermophilus V (27, 33, 41). The genes that are involved in the metabolism of cysteine are cysK, iscS, and cobA, and they encode a cysteine synthase (CysK), a cysteine desulfurase (IscS), and a uroporphyrinogen-III C-methyltransferase (SUMT), respectively. CysK catalyzes the last step of inorganic sulfur fixation into l-cysteine, while SUMT is involved in the biosynthesis of siroheme, an essential sulfite reductase cofactor that participates in the inorganic assimilation of sulfur (15). We recently demonstrated that cobA and ubiE genes from G. stearothermophilus V confer increased tolerance to oxyanions of selenium and tellurium when expressed in E. coli (1, 32). Finally, IscS, which yields sulfur and l-alanine from l-cysteine, has been shown to be involved, along with IscA and IscU, in the recovery of [Fe-S] clusters (9, 29).
The purpose of this study was to evaluate the responsiveness of cysK, iscS, and cobA from G. stearothermophilus and some genes of the E. coli Cys regulon in medium containing potassium tellurite. Results indicate that bacterial tolerance to tellurite involves, at least in part, several components of the cysteine metabolic pathway.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and stress induction.
E. coli strains and plasmids used in this work are listed in Table 1. Cells were grown routinely in LB medium (28) at 37°C with shaking. Experiments were initiated with the addition of 1:100 dilutions of overnight cultures to the medium. Cell cultures reaching an optical density at 600 nm (OD600) of ∼0.6 were amended with K2TeO3 (0.5 μg/ml), H2O2 (125 μg/ml), diamide (850 μg/ml), or paraquat (250 μg/ml), as required. Controls received equal volumes of sterile water.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| E. coli TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR nupG recA1 araD139 Δ(ara-leu)7697 galU galK rpsL(Strr) endA1 λ− | Invitrogen |
| E. coli BW25113 | laclqrrnBT14 ΔlacZWJ16hsdR514 ΔaraBADAH33 ΔrhaBADLD78 | Datsenko and Wanner (8) |
| JW2415 | E. coli BW25113 ΔcysA | Baba et al. (3) |
| JW2720 | E. coli BW25113 ΔcysC | Baba et al. (3) |
| JW2722 | E. coli BW25113 ΔcysD | Baba et al. (3) |
| JW3582 | E. coli BW25113 ΔcysE | Baba et al. (3) |
| JW3331 | E. coli BW25113 ΔcysG | Baba et al. (3) |
| JW2732 | E. coli BW25113 ΔcysH | Baba et al. (3) |
| JW2407 | E. coli BW25113 ΔcysK | Baba et al. (3) |
| JW2414 | E. coli BW25113 ΔcysM | Baba et al. (3) |
| JW2418 | E. coli BW25113 ΔcysP | Baba et al. (3) |
| JW2416 | E. coli BW25113 ΔcysW | Baba et al. (3) |
| JW3888 | E. coli BW25113 Δsbp | Baba et al. (3) |
| JW2514 | E. coli BW25113 ΔiscS | Baba et al. (3) |
| JW2515 | E. coli BW25113 ΔiscR | Baba et al. (3) |
| E. coli AB734 | F−lac-6(del) | Shapiro and Baneyx (30) |
| E. coli ADA110 | AB734 λφibp::lacZ | Shapiro and Baneyx (30) |
| E. coli ADA310 | AB734 λφcspA::lacZ | Shapiro and Baneyx (30) |
| E. coli ADA410 | AB734 λφP3rpoH::lacZ | Shapiro and Baneyx (30) |
| E. coli ADA510 | AB734 λφsulA::lacZ | Shapiro and Baneyx (30) |
| G. stearothermophilus V | Wild-type Telr | C. Vásquez (39) |
| Plasmids | ||
| pBR322 | Cloning vector, Apr Tetr | Bolivar et al. (4) |
| pBluescript-SK | Cloning vector, Apr | Stratagene |
| pBRcobA | G. stearothermophilus cobA gene cloned in pBR322, Apr | This study |
| pBRcysK | G. stearothermophilus cysK gene cloned in pBR322, Apr | This study |
| pBRiscS | G. stearothermophilus iscS gene cloned in pBR322, Apr | This study |
| pSKcobA | G. stearothermophilus cobA gene cloned in pBluescript-SK, Apr | This study |
| pSKcysK | G. stearothermophilus cysK gene cloned in pBluescript-SK, Apr | This study |
| pSKiscS | G. stearothermophilus iscS gene cloned in pBluescript-SK, Apr | This study |
Telr, tellurite resistance; Tetr, tetracycline resistance; Apr, ampicillin resistance.
Geobacillus stearothermophilus V was grown in ATTC medium with or without potassium tellurite (50 μg/ml) at 65°C as described previously (39). All experiments were carried out at least in triplicate.
Antimicrobial disk assay and determination of MICs.
Overnight cultures of E. coli or its derivatives were diluted 100-fold with LB medium, and 100-μl aliquots were spread on LB-agar (2%) plates. Ten microliters of K2TeO3 (10 μg), H2O2 (1.25 mg), or paraquat (3.0 mg) was then spotted independently onto sterile filter paper disks that were placed in the center of the plates. Growth inhibition zones were determined after incubation at 37°C for 24 h. To determine MICs, cells were grown with shaking at 37°C in LB medium supplemented with appropriate concentrations of the compounds under study (7).
RNA extraction, plasmid construction, and cell transformation.
RNA purifications used the RNeasy kit (QIAGEN). Briefly, cultures of E. coli K-12 or its derivatives (OD600 of ∼0.6) were split in two and one was amended with 0.5 μg/ml K2TeO3 and incubated for 10 min. Cells were sedimented at 13,000 × g for 3 min and used for RNA extraction. The OD260/280 ratio for the purified RNAs was determined with an Agilent 8453 UV-visible spectrophotometer.
G. stearothermophilus V cobA, cysK, and iscS genes containing 300 bp upstream of their ATG initiation codons were amplified by PCR using primers listed in Table 2. PCR conditions included an initial denaturation at 95°C for 5 min, followed by 30 amplification cycles (95°C for 30 s, 55°C for 30 s, and 72°C for 30 s). A final incubation at 72°C for 10 min was included to ensure complete extension of the amplified fragments. PCR products were cloned in pGEMT-Easy (Invitrogen). Recombinant plasmids were digested with HindIII, and the released fragments were purified and cloned independently into the medium-copy-number vector pBR322 or the high-copy-number vector pBluescript-SK (Table 1).
TABLE 2.
Primers used in PCR and quantitative RT-PCR
| Primer name | Sequence | PCR product |
|---|---|---|
| For G. stearothermophilus V: | ||
| cobAPH | 5′-AAGCTTTTCGCTCCGGATGTTGCCGATTAT-3′ | cobA gene + promoter |
| cobAH3 | 5′-AAGCTTCGGTCCCGGCGATGAAAACGTGATTACCGT-3′ | cobA gene + promoter |
| cysKPH | 5′-AAGCTTCGCTTACAGACTATTCCGCCTGTCT-3′ | cysK gene + promoter |
| cysKH3 | 5′-AAGCTTGTCTTCGAATTGGTAAAGCGGCGTGCTTAAGTAGC-3′ | cysK gene + promoter |
| iscSPH | 5′-AAGCTTTCGCAGACATTACTAGAACGGTTGCTGTAG-3′ | iscS gene + promoter |
| iscSH3 | 5′-AAGCTTATGAATCTTGAACAAATAAGAAAAGATAAC-3′ | iscS gene + promoter |
| gapA3 | 5′-GGTGATGTGTTTACGAGCAG-3 | gapA probe |
| gapA5 | 5′-GTAAAGTTGGTATTAACGGTTTTGG-3′ | gapA probe |
| scobA5 | 5′-GTACCTCTGCTTCTTCCCCG-3′ | cobA probe |
| subiE5 | 5′-CTGCACAAAGCGAATCCGTT-3′ | ubiE probe |
| scysK3 | 5′-ACTCATCGTATCTGGCATGA-3′ | cysK probe |
| siscS3 | 5′-CGAATACACGGTAAAACAAT-3′ | iscS probe |
| For E. coli: | ||
| sgapA3 | 5′-GGTGATGTGTTTACGAGCAG-3′ | gapA probe |
| sgapA5 | 5′-GTAAAGTTGGTATTAACGGTTTTGG-3′ | gapA probe |
| scysA3 | 5′-AACCAGCGGGCATATTCGCTTCCACGGCAC-3′ | cysA probe |
| scysA5 | 5′-CAAGCAGCAGAATTTGCGGTTCCACAGCCA-3′ | cysA probe |
| scysB3 | 5′-CTGCGCGATTAAAGGCAGTATCCAGTTCTG-3′ | cysB probe |
| scysB5 | 5′-ACCTGGCCGGATAAAGGTTCACTGTATATCGC-3′ | cysB probe |
| scysC3 | 5′-TTTAGCGATGCCGATCGTAAAGAGAATATC-3′ | cysC probe |
| scysC5 | 5′-GTTCACCATTGAGATGAATTTCTGCCGATT-3′ | cysC probe |
| scysE3 | 5′-TCTGTGACGTTCCAGGTCGATATTCACCCG-3′ | cysE probe |
| scysE5 | 5′-GCTGGTCCATATCCATTGATGGCTTATCGCTG-3′ | cysE probe |
| scysG3 | 5′-ACCACCACATCTGCCTGCTGAATTTGTTGC-3′ | cysG probe |
| scysG5 | 5′-ACTTGAATCACTGCTGCCGTTACATCTGGG-3′ | cysG probe |
| scysI3 | 5′-GTGAAAACTCGATGGCTTGCGTGTCATTCC-3′ | cysI probe |
| scysI5 | 5′-CCCTATCAGTTCATCAAGCGACGCCAGGAT-3′ | cysI probe |
| scysK3 | 5′-CTTTCAGCAGCTTGCGGCGTTCAATACTCA-3′ | cysK probe |
| scysK5 | 5′-TGAAGATAACTCGCTGACTATCGGTCACACGC-3′ | cysK probe |
| scysM3 | 5′-GGCGAAGGAAAGCTGCTCGATCAGTTCAAT-3′ | cysM probe |
| scysM5 | 5′-GATGAATATCCAGCACCTCATCCACCAGAGAAGC-3′ | cysM probe |
| scbl3 | 5′-GCCCGCAAAGGTTTGCTGGCAGATATTGTA-3′ | cbl probe |
| scbl5 | 5′-TTTCCTCTTCACTGCTTTCCATCACCTGCC-3′ | cbl probe |
E. coli TOP10 cells were used in all transformations. Cells were made competent by electroporation (MicroPulser, Bio-Rad) using 0.2-cm cuvettes and a 2.5-kV pulse.
β-Galactosidase assay.
E. coli cultures (1.5 ml) harvested at different time intervals were sedimented by centrifugation at 13,000 × g for 3 min. Cells were permeabilized by the addition of 1.5 ml ice-cold buffer Z (40 mM Na2HPO4, 60 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 50 mM β-mercaptoethanol, pH 7.5) and assayed for β-galactosidase activity. The o-nitrophenyl-β-d-galactopyranoside (ONPG) substrate was used as described previously by Miller (20). Protein concentration was estimated using the Bradford reagent (Sigma).
Bioinformatics and computation analysis.
Sequence analysis and primer design were performed using Vector NTI 8.0 (InforMax, Inc.). Nucleotide sequences of the G. stearothermophilus V cobA, cysK, and iscS genes and those of the E. coli cys regulon (cysA, cysB, cysC, cysE, cysG, cysI, cysK, cysM, and cbl) were obtained from GenBank (accession numbers AY426747, AF533655, AF198621, and NC_000913). Analysis of variance statistical analyses were performed with a 0.05 level of confidence.
Real-time RT-PCR.
The induction of gene expression was calculated based on the difference between the crossing points of each quantitative reverse transcription-PCR (RT-PCR) determination (Cp − CpTe, where Cp and CpTe are the crossing points in the absence and presence of tellurite, respectively). Crossing points define the cycle at which the amplification becomes exponential, and they are inversely proportional to the amount of the specific RNA template present in the sample.
Approximately 2 μg of RNA from control or tellurite-treated cells was used for real-time RT-PCR experiments using a LightCycler RNA amplification kit (Roche Applied Science) according to instructions of the vendor. The specific oligonucleotide primers used are listed in Table 2. PCR products were visualized and analyzed using a LightCycler 2.0 instrument.
RESULTS
Behavior of E. coli strains carrying G. stearothermophilus genes in tellurite-containing medium.
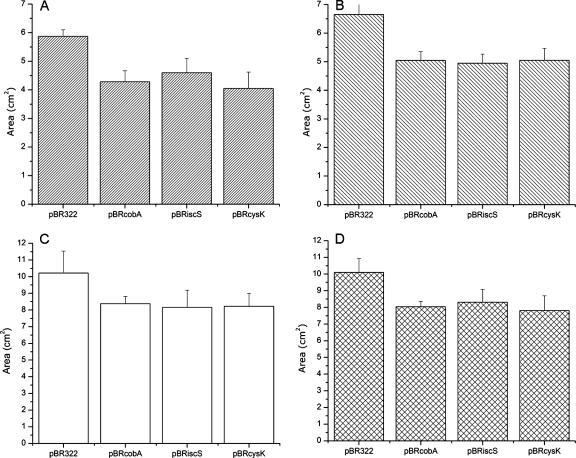
G. stearothermophilus V cobA, cysK, and iscS genes containing their own promoters were separately cloned in the medium- and high-copy-number plasmids pBR322 and pBluescript-SK, respectively. The resulting recombinant plasmids were used to transform Escherichia coli, and bacterial tolerance to K2TeO3 and other oxidative stress inducers was evaluated by measuring growth inhibition zones in tellurite-amended solid medium. Figure 1 shows that pBRcobA, pBRcysK, and pBRiscS increased E. coli resistance approximately 20% for tellurite, 25% for paraquat, 25% for diamide, and 20% for hydrogen peroxide. In turn, E. coli cells carrying pSKcobA, pSKcysK, and pSKiscS exhibited an additional 20% tolerance to K2TeO3 and hydrogen peroxide (see Fig. S1S in the supplemental material), suggesting a gene dosage effect. This behavior was not observed for the thiol reducer diamide (data not shown).
FIG. 1.
Growth inhibition zones of E. coli cells expressing G. stearothermophilus V cobA, cysK, and iscS genes cloned in pBR322 when exposed to the following toxic substances: paraquat (A), diamide (B), hydrogen peroxide (C), and potassium tellurite (D). Cells were grown overnight in plates of LB-ampicillin at 37°C. Error bars represent standard deviations (n = 8). See Materials and Methods for details.
To circumvent the repression of cysteine biosynthesis in rich medium, the same experiments were conducted in M9 minimal plates. Results showed that all strains exhibited increased tolerance levels (∼10 to 20%) to K2TeO3, paraquat, and H2O2 compared to those observed with LB medium (not shown). Differences in tellurite tolerance observed in solid medium were also assessed by MIC determinations (Table 3). Curiously, the MICs for pSKcobA and pBRcobA increase by twofold after 24 h, while the others remain the same. Although we do not have a definitive explanation for this result, it was systematically observed. In this context, a physiological adaptation phenomenon cannot be ruled out.
TABLE 3.
MICs of K2TeO3 for E. coli cells expressing cobA, cysK, and iscS genes from G. stearothermophilus V
| Plasmid | K2TeO3 MIC (μg/ml)a at:
|
|
|---|---|---|
| 12 h | 24 h | |
| pBR322 | 1 | 1 |
| pBluescript SK− | 1 | 1 |
| pSKcobA | 6.25 | 12.5 |
| pSKcysK | 12.5 | 12.5 |
| pSKiscS | 12.5 | 12.5 |
| pBRcobA | 3.125 | 6.25 |
| pBRcysK | 6.25 | 6.25 |
| pBRiscS | 6.25 | 6.25 |
Values are the means of three independent determinations.
Response of E. coli stress reporter strains to potassium tellurite.
E. coli ADA strains induce the expression of the β-galactosidase gene when exposed to different stress conditions (30). These strains carry single-copy fusion genes consisting of the lacZ reporter gene fused to the promoter regions of the highly inducible cytoplasmic small heat shock proteins IbpA and IbpB (ADA110), the major cold shock protein CspA (ADA310), the P3 promoter of the rpoH gene (activated upon periplasmic protein misfolding) (ADA410), or the SOS-inducible sulA promoter (ADA510) (30).
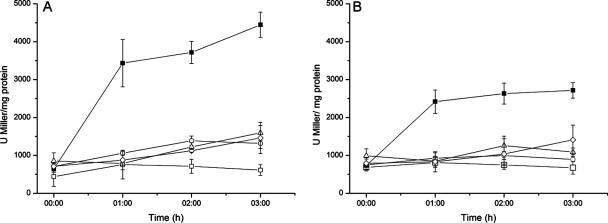
E. coli ADA cells were challenged with potassium tellurite to determine promoter activation in vivo. The parental isogenic E. coli AB734 was used as a control. Sublethal concentrations of K2TeO3 had very little effect on P3rpoH and sulA activation, while E. coli ADA110 and ADA310 showed increased β-galactosidase activities, suggesting that tellurite affects cytoplasmic proteins and has no evident effect on DNA (not shown). When E. coli ADA110 and ADA310 cells carrying G. stearothermophilus V cobA, cysK, or iscS genes were challenged with K2TeO3, β-galactosidase activity was lower than that observed for control cells transformed with pBluescript (Fig. 2A and B), suggesting that expression of cobA, cysK, and iscS protects against the denaturation of cytoplasmic proteins. Similar results were obtained when diamide, paraquat, and hydrogen peroxide were used (not shown).
FIG. 2.
Determination of β-galactosidase activity in E. coli ADA110 (A) and ADA310 (B) in the presence of potassium tellurite. Cells harboring pSKcobA (▵), pSKcysK (○), pSKiscS (⋄), or the control plasmid pBluescript SK− (▪) were grown at 37°C in LB medium until an OD600 of about 0.6 (time zero), when tellurite was amended at 0.5 μg/ml. Control cells were grown in the absence of potassium tellurite (□). Error bars represent standard deviations (n = 9).
Induction of G. stearothermophilus V cobA, cysK, and iscS gene promoters by K2TeO3.
Alignment of nucleotide sequences upstream of the ATG initiation codon of cobA, cysK, and iscS showed similarities of 40 to 44%. No binding motifs to known E. coli or Bacillus subtilis sigma factors such as σ70 and σ43 were found.
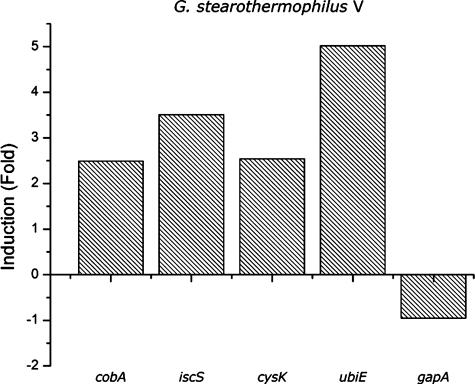
Real-time RT-PCR was used to monitor the functional capability of the G. stearothermophilus V cobA, iscS, and cysK promoters in E. coli grown in the presence or in the absence of K2TeO3. The housekeeping gene gapA was used as a control. Induction of gene expression was expressed as the difference between the crossing points of each RT-PCR determination (Cp − CpTe). Positive numbers reflect larger amounts of the particular, specific mRNA in cells exposed to the toxic condition. The calculated Cp values were 2.49 for cobA, 3.51 for cysK, 2.54 for iscS, 5.02 for ubiE, and −0.95 for gapA. Thus, while expression of the G. stearothermophilus V genes increased in the presence of potassium tellurite, that of the gapA housekeeping gene decreased by approximately 10% (Fig. 3).
FIG. 3.
Expression of G. stearothermophilus V genes in LB medium containing potassium tellurite (50 μg/ml). Cells were grown at 65°C for 24 h as described in Materials and Methods. Values are the means of two independent trials.
Expression of E. coli cysteine metabolism-related genes in cells exposed to potassium tellurite.
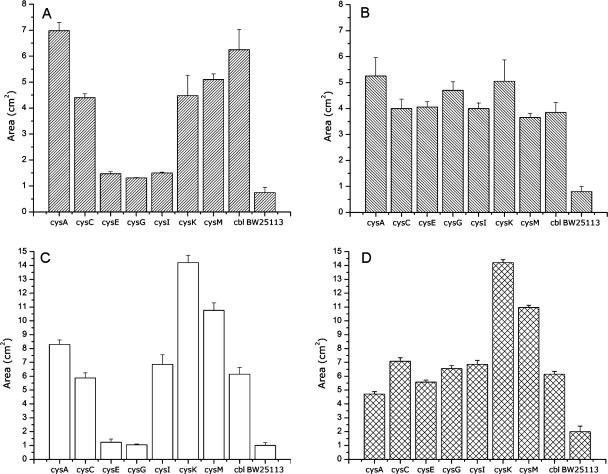
An experimental approach similar to that described in the previous section was followed to evaluate the effect of potassium tellurite on the expression of E. coli cysteine metabolism genes. The participation of Cys regulon genes in tellurite tolerance was investigated using E. coli BW25113 and its isogenic derivatives cysA, cysC, cysE, cysG, cysI, cysK, cysM, and cbl. The loss of cysteine metabolism-related genes is paralleled with an increase of growth inhibition areas for all toxic compounds included in this study. In general, strains ΔcysA, ΔcysK, ΔcysM, Δcbl, and ΔcysC exhibited the highest sensitivity to the reactive oxygen species (ROS) generators paraquat and hydrogen peroxide as well as to potassium tellurite (Fig. 4).
FIG. 4.
Growth inhibition zones of E. coli defective in the indicated cysteine metabolism-related genes. Cells were grown in LB medium in the presence of paraquat (A), diamide (B), hydrogen peroxide (C), and potassium tellurite (D). Results for the parental, isogenic, wild-type BW25113 strain are shown to the right of each histogram. Error bars represent standard deviations (n = 6).
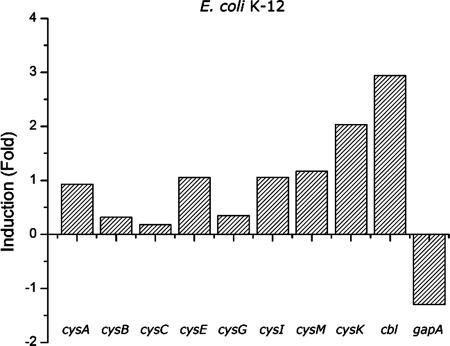
Total RNA from untreated cells and cells treated with potassium tellurite was used, along with specific primers for each gene of the regulon, as templates for performing real-time RT-PCR (Fig. 5). Most of the E. coli cysteine metabolism-related genes showed increased expression when cells were grown in the presence of potassium tellurite. The ΔCp values were 0.92 for cysA, 0.32 for cysB, 0.19 for cysC, 1.06 for cysE, 0.35 for cysG, 1.06 for cysI, 1.17 for cysM, 2.04 for cysK, 2.94 for cbl, and −1.3 for gapA.
FIG. 5.
Expression of E. coli cys genes in medium containing potassium tellurite. Cells were inoculated in LB medium containing 0.5 μg/ml of potassium tellurite and cultivated at 37°C with shaking for 24 h. Values are the means of two independent trials.
DISCUSSION
Living organisms require sulfur for the biosynthesis of proteins and other essential enzymatic cofactors and reducing agents. After incorporation into the cell, sulfur is reduced to sulfide that then reacts with O-acetyl-l-serine to form l-cysteine (15). Cysteine has several vital functions in the catalytic cycle of many enzymes, is part of important reducing agents like glutathione, and is required for the biosynthesis and repair of [Fe-S] centers of several essential proteins, including cytochromes, fumarases, and aconitases.
Cysteine-containing molecules like glutathione and thioredoxin play a major role in maintaining an intracellular reducing environment and protecting the cell from oxidative damage (6, 12, 22). It has been reported that GSH is ubiquitous in most gram-negative bacteria and is absent in most gram-positive organisms examined so far (10, 18). These observations suggest that glutathione is not unique in protecting against oxidative stress or maintaining the redox balance of cells. It has been shown that thioredoxin 1 (TrxA), thioredoxin 2 (TrxB), and thioredoxin reductase (TrxC) are not essential for survival in E. coli. trxA and trxB cells exhibit increased sensitivity to H2O2, a phenotype that is not observed in trxC cells (25). E. coli strains with mutations in the GSH system are also viable. For example, gshA and gshB mutants and gorA mutants, encoding the two enzymes of glutathione biosynthesis and glutathione reductase, respectively, exhibit increased sensitivity to the GSH-oxidizing agent diamide (2). Prinz et al. (24) determined that E. coli requires the thioredoxin or the GSH/Grx system to grow aerobically and that mutants in these systems were incapable of reducing cytoplasmic disulfide bonds.
We have previously identified three G. stearothermophilus V genes (cobA, cysK, and iscS) that encode enzymes involved in cysteine metabolism and whose expression mediates tellurite resistance in E. coli (33, 40, 41). The participation of cysK, cysM, and iscS genes in tellurite resistance as well as in oxidative stress has been also documented with other microorganisms (19, 21, 26).
Tellurite toxicity is thought to result from the oxidizing character of this molecule. More recently, the idea that tellurite would damage the cell through the establishment of oxidative stress has emerged. This stress condition could be a consequence of the drastic decrease of the concentration of cellular antioxidants and/or could be associated with the presence of some kind of ROS. The observation that superoxide generation results from enzymatic tellurite reduction supports this assumption (5, 23).
The above considerations allow the speculation that an increase in cellular antioxidants would result in higher tolerance to ROS elicitors. Expression of G. stearothermophilus V genes containing their own promoters in medium- or high-copy-number plasmids supports this idea (Fig. 1; see Fig S1S in the supplemental material).
To date, most studies concerning bacterial response to oxidative stress have been focused on hydrogen peroxide-, alkylhydroperoxide-, or superoxide-induced stress. Little is known about disulfide stress, a subcategory of oxidative stress that causes the accumulation of nonnative disulfide bonds in the cytoplasm. E. coli cells were also exposed to the thiol-specific oxidant diamide. E. coli cells harboring the G. stearothermophilus V cobA, cysK, and iscS genes exhibited smaller growth inhibition zones than controls that did not express these genes, suggesting that they have a protective effect against the toxic effect of diamide. Leichert et al. (16) reported that B. subtilis isolates independently exposed to hydrogen peroxide or diamide showed similar gene expression profiles, suggesting that they share the same response mechanism. These results agree with our observation that the expression of G. stearothermophilus V genes protects E. coli against the toxic effects of hydrogen peroxide and diamide.
This protective effect was further observed in transformed E. coli reporter strains ADA110 and ADA310 (30), which exhibited important reductions of β-galactosidase activity when exposed to K2TeO3. The levels of β-galactosidase in these E. coli ADA strains expressing Geobacillus genes were indistinguishable from those of controls grown in the absence of tellurite (Fig. 2).
It is well known that living organisms have evolved defense mechanisms to maintain the cell's homeostasis under adverse conditions. In Escherichia coli, for example, temperature upshifts and other kinds of stress induce the synthesis of heat shock proteins when misfolded proteins accumulate in the cytoplasm (13). In this context, gene induction by tellurite poisoning has been reported for E. coli and Proteus mirabilis. The E. coli gutS gene and P. mirabilis terZABCDE operon are positively regulated by tellurite (14, 35). The presence of sequences similar to OxyR binding motifs in the ter operon of P. mirabilis suggested that such induction would be dependent on this transcriptional regulator. However, no OxyR-like binding motifs were found within regulatory regions of the G. stearothermophilus V genes (not shown), suggesting that the K2TeO3-induced positive regulation observed in Fig. 3 is not dependent on this regulator.
We further studied the involvement of the E. coli Cys regulon genes in tellurite-amended medium. Results showed that strains defective in cysteine metabolism-related genes exhibited higher sensitivity to K2TeO3 than did the isogenic, parental, wild-type strain (Fig. 4), suggesting that most of the elements of the cysteine biosynthetic pathway are required to manage the tellurite-induced stress. In addition, all the Cys regulon genes studied here were turned on in the presence of tellurite even in rich medium like LB, where transcription of the cysB and cysE regulatory genes is repressed (Fig. 5).
In conclusion, we have shown that genes of cysteine biosynthesis are induced in the presence of potassium tellurite. The behavior of the Cys regulon elements could reflect a tellurite-mediated derepression, which in turn could be explained by the depletion of cellular thiols or sulfur-containing molecules such as glutathione.
Acknowledgments
D.E.F. and J.M.P. were supported by doctoral fellowships from MECESUP (Chile), and M.E.C. received a doctoral fellowship from Fondecyt (Chile). This work received financial support from grant 1060022 from Fondecyt and Dicyt, Universidad de Santiago de Chile, to C.C.V. T.G.C. appreciates support from an SHSU Faculty Enhancement Grant and the Robert A. Welch Foundation.
Footnotes
Published ahead of print on 19 October 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Araya, M. A., J. W. Swearingen, Jr., M. F. Plishker, C. P. Saavedra, T. G. Chasteen, and C. C. Vásquez. 2004. Geobacillus stearothermophilus V ubiE gene product is involved in the evolution of dimethyl telluride in Escherichia coli K-12 cultures amended with potassium tellurate but not with potassium tellurite. J. Biol. Inorg. Chem. 9:609-615. [DOI] [PubMed] [Google Scholar]
- 2.Aslund, F., and J. Beckwith. 1999. Bridge over troubled waters: sensing stress by disulfide bond formation. Cell 96:751-753. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. http://www.nature.com/msb/journal/v2/n1/full/msb4100050.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 5.Calderón, I. L., F. A. Arenas, J. M. Pérez, D. E. Fuentes, M. A. Araya, C. P. Saavedra, J. C. Tantaleán, S. E. Pichuantes, P. A. Youderian, and C. C. Vásquez. 2006. Catalases are NAD(P)H-dependent tellurite reductases. PLoS ONE 1:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmel-Harel, O., and G. Storz. 2000. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 54:439-461. [DOI] [PubMed] [Google Scholar]
- 7.Chiong, M., R. Barra, E. González, and C. Vásquez. 1988. Resistance of Thermus spp. to potassium tellurite. Appl. Environ. Microbiol. 54:610-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding, H., K. Harrison, and J. Lu. 2005. Thioredoxin reductase system mediates iron banding in IscA and iron delivery for the iron-sulfur cluster assembly in IscU. J. Biol. Chem. 280:30432-30437. [DOI] [PubMed] [Google Scholar]
- 10.Fahey, R., W. Brown, W. Adams, and M. Worsham. 1978. Occurrence of glutathione in bacteria. J. Bacteriol. 133:1126-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson, G. 1999. Protective mechanisms against toxic electrophiles in Escherichia coli. Trends Microbiol. 7:242-247. [DOI] [PubMed] [Google Scholar]
- 12.Gleason, F., and A. Holmgren. 1988. Thioredoxin and related proteins in procaryotes. FEMS Microbiol. Rev. 4:271-297. [DOI] [PubMed] [Google Scholar]
- 13.Gross, C. 1996. Function and regulation of the heat shock proteins, p. 1382-1399. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 14.Guzzo, J., and M. Dubow. 2000. A novel selenite- and tellurite-inducible gene in Escherichia coli. Appl. Environ. Microbiol. 66:4972-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kredich, N. 1996. Biosynthesis of cysteine, p. 514-527. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 16.Leichert, L., C. Scharf, and M. Hecker. 2003. Global characterization of disulfide stress in Bacillus subtilis. J. Bacteriol. 185:1967-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, K., S. Hein, W. Zou, and G. Klug. 2004. The glutathione-glutaredoxin system in Rhodobacter capsulatus: part of a complex regulatory network controlling defense against oxidative stress. J. Bacteriol. 186:6800-6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, Y., J. Hugenholtz, T. Abee, and D. Molenaar. 2003. Glutathione protects Lactococcus lactis against oxidative stress. Appl. Environ. Microbiol. 69:5739-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lithgow, J., E. Hayhurst, G. Cohen, Y. Aharonowitz, and S. Foster. 2004. Role of a cysteine synthase in Staphylococcus aureus. J. Bacteriol. 186:1579-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 21.O'Gara, J., M. Gomelsky, and S. Kaplan. 1997. Identification and molecular genetic analysis of multiple loci contributing to high-level tellurite resistance in Rhodobacter sphaeroides 2.4.1. Appl. Environ. Microbiol. 63:4713-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penninckx, M., and M. Elskens. 1993. Metabolism and functions of glutathione in micro-organisms. Adv. Microbiol. Physiol. 34:239-301. [DOI] [PubMed] [Google Scholar]
- 23.Pérez, J. M., I. L. Calderón, F. A. Arenas, D. E. Fuentes, G. A. Pradenas, E. L. Fuentes, J. M. Sandoval, M. E. Castro, A. O. Elías, and C. C. Vásquez. 2007. Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS ONE 2:e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prinz, W., F. Aslund, A. Holmgren, and J. Beckwith. 1997. The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. J. Biol. Chem. 272:15661-15667. [DOI] [PubMed] [Google Scholar]
- 25.Ritz, D., H. Patel, B. Doan, M. Zheng, F. Aslund, G. Storz, and J. Beckwith. 2000. Thioredoxin 2 is involved in the oxidative stress response in Escherichia coli. J. Biol. Chem. 275:2505-2512. [DOI] [PubMed] [Google Scholar]
- 26.Rojas, D., and C. C. Vásquez. 2005. Sensitivity to potassium tellurite of Escherichia coli cells deficient in CSD, CsdB and IscS cysteine desulfurases. Res. Microbiol. 156:465-471. [DOI] [PubMed] [Google Scholar]
- 27.Saavedra, C. P., M. V. Encinas, M. A. Araya, J. M. Pérez, J. C. Tantaleán, D. E. Fuentes, I. L. Calderón, S. E. Pichuantes, and C. C. Vásquez. 2004. Biochemical characterization of a thermostable cysteine synthase from Geobacillus stearothermophilus V. Biochimie 86:481-485. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Schwartz, C., O. Djaman, J. Imlay, and P. Kiley. 2000. The cysteine desulfurase, IscS, has a major role in in vivo Fe-S cluster formation in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:9009-9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro, E., and F. Baneyx. 2002. Stress-based identification and classification of antibacterial agents: second-generation Escherichia coli reporter strains and optimization of detection. Antimicrob. Agents Chemother. 46:2490-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smirnova, G., T. Krasnykh, and O. Oktyabrsky. 2001. Role of glutathione in the response of Escherichia coli to osmotic stress. Biochemistry 66:973-978. [DOI] [PubMed] [Google Scholar]
- 32.Swearingen, J. W., Jr., D. E. Fuentes, M. A. Araya, M. F. Plishker, C. P. Saavedra, T. G. Chasteen, and C. C. Vásquez. 2006. The expression of the ubiE gene of Geobacillus stearothermophilus V in Escherichia coli K-12 mediates the evolution of selenium compounds into the headspace of selenite- and selenate-amended cultures. Appl. Environ. Microbiol. 72:963-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tantaleán, J. C., M. A. Araya, C. P. Saavedra, D. E. Fuentes, J. M. Pérez, I. L. Calderón, P. Youderian, and C. C. Vásquez. 2003. The Geobacillus stearothermophilus V iscS gene, encoding cysteine desulfurase, confers resistance to potassium tellurite in Escherichia coli K-12. J. Bacteriol. 185:5831-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor, D. E. 1999. Bacterial tellurite resistance. Trends Microbiol. 7:111-115. [DOI] [PubMed] [Google Scholar]
- 35.Toptchieva, A., G. Sisson, L. Bryden, D. Taylor, and P. Hoffman. 2003. An inducible tellurite-resistance operon in Proteus mirabilis. Microbiology 149:1285-1295. [DOI] [PubMed] [Google Scholar]
- 36.Tremaroli, V., S. Fedi, and D. Zannoni. 2007. Evidence for a tellurite-dependent generation of reactive oxygen species and absence of a tellurite-mediated adaptive response to oxidative stress in cells of Pseudomonas pseudoalcaligenes KF707. Arch. Microbiol. 187:127-135. [DOI] [PubMed] [Google Scholar]
- 37.Turner, R. J., Y. Aharonowitz, J. H. Weiner, and D. E. Taylor. 2001. Glutathione is a target in tellurite toxicity and is protected by tellurite resistance determinants in Escherichia coli. Can. J. Microbiol. 47:33-40. [PubMed] [Google Scholar]
- 38.van der Ploeg, J. R., E. Eichhorn, and T. Leisinger. 2001. Sulfonate-sulfur metabolism and its regulation in Escherichia coli. Arch. Microbiol. 176:1-8. [DOI] [PubMed] [Google Scholar]
- 39.Vásquez, C. 1985. Isolation and partial characterization of BstVI, a thermostable isoschizomer of XhoI. Biochem. Int. 10:655-662. [PubMed] [Google Scholar]
- 40.Vásquez, C. C., C. P. Saavedra, C. A. Loyola, H. Moscoso, and S. E. Pichuantes. 1999. Cloning of a tellurite resistance determinant from Bacillus stearothermophilus V in Escherichia coli. Biochem. Mol. Biol. Int. 47:171-175. [DOI] [PubMed] [Google Scholar]
- 41.Vásquez, C. C., C. P. Saavedra, C. A. Loyola, M. A. Araya, and S. E. Pichuantes. 2001. The product of the cysK gene of Bacillus stearothermophilus V mediates potassium tellurite resistance in Escherichia coli. Curr. Microbiol. 43:418-423. [DOI] [PubMed] [Google Scholar]