Abstract
RegSR-like proteins, members of the family of two-component regulatory systems, are present in a large number of proteobacteria in which they globally control gene expression mostly in a redox-responsive manner. The controlled target genes feature an enormous functional diversity. In Bradyrhizobium japonicum, the facultative root nodule symbiont of soybean, RegSR activate the transcription of the nitrogen fixation regulatory gene nifA, thus forming a RegSR-NifA cascade which is part of a complex regulatory network for gene regulation in response to changing oxygen concentrations. Whole-genome transcription profiling was performed here in order to assess the full regulatory scope of RegSR. The comparative analysis of wild-type and ΔregR cells grown under oxic and microoxic conditions revealed that expression of almost 250 genes is dependent on RegR, a result that underscores the important contribution of RegR to oxygen- or redox-regulated gene expression in B. japonicum. Furthermore, transcription profiling of ΔregR bacteroids compared with wild-type bacteroids revealed expression changes for about 1,200 genes in young and mature bacteroids. Incidentally, many of these were found to be induced in symbiosis when wild-type bacteroids were compared with free-living, culture-grown wild-type cells, and they appeared to encode diverse functions possibly related to symbiosis and nitrogen fixation. We demonstrated direct RegR-mediated control at promoter regions of several selected target genes by means of DNA binding experiments and in vitro transcription assays, which revealed six novel direct RegR target promoters.
Rhizobia are soil bacteria able to establish a nitrogen-fixing symbiosis within the root nodule cells of legume host plants. The transition from the free-living to the symbiotic state is accompanied by drastic changes in bacterial metabolism, eventually leading to the formation of bacteroids specialized for nitrogen fixation and life in the microoxic environment within root nodules. Nitrogen fixation genes (nif and fix) are subject to tight regulation, and they are expressed only under the low-oxygen condition that prevails inside root nodules or is artificially maintained in culture (11).
In Bradyrhizobium japonicum, the root nodule symbiont of soybean, two hierarchically organized regulatory cascades, RegSR-NifA and FixLJ-FixK2, control expression of nitrogen fixation genes and genes required for life under microoxic conditions, respectively (11). RegSR and FixLJ form typical bacterial two-component regulatory systems consisting of a sensory histidine protein kinase and a cytoplasmic response regulator (RegR and FixJ) (for reviews, see references 18, 35, and 53). While the activity of the FixL sensor kinase is controlled by oxygen via a prosthetic heme (see reference 11 and references therein), the nature of the signal sensed by RegS remains to be identified. Similarly, knowledge about the regulatory scope of B. japonicum RegR is limited. RegR was identified in the course of studying transcriptional regulation of the B. japonicum fixR-nifA operon, which is preceded by two overlapping promoters, P1 and P2 (3-5). RegR activates transcription originating from P2 under all oxygen conditions via binding to a DNA element located around position −67 upstream of the transcription start site. Upon a switch to low-oxygen or anoxic conditions, the redox-responsive NifA protein in concert with RNA polymerase containing RpoN (σ54) enhances its own synthesis via activation of the −24/−12-type P1 promoter. This results in maximal expression not only of the fixR-nifA operon but also of other target genes (11, 51). Most recently, the NifA-RpoN regulon of B. japonicum was unraveled by a genome-wide transcriptome analysis, which identified numerous new NifA-RpoN-dependent genes (25).
Phenotypic analysis of the ΔregR strain revealed that although the strain is able to form nodules on soybean, it retains only residual nitrogen fixation activity (2%). Nodules elicited by the regR mutant showed a greenish interior harboring a decreased number of bacteroids, which is indicative of defects in proper nodule development (5). By contrast, mutants of the sensor kinase RegS differed only marginally in their symbiotic properties from the wild type on the same host plant.
Orthologs of the B. japonicum RegSR two-component regulatory proteins are widely distributed among proteobacteria, and they include the well-studied RegBA and PrrBA proteins of the purple nonsulfur bacteria Rhodobacter capsulatus and Rhodobacter sphaeroides, respectively. RegBA (PrrBA) was shown to control diverse cellular processes that either generate or utilize reducing equivalents and thus balance the cellular redox status, e.g., photosynthesis, CO2 fixation, N2 fixation, aerotaxis, and respiration (see references 12 and 31). In addition, RoxSR of Pseudomonas aeruginosa and RegSR of Rhodopseudomonas palustris were shown to regulate expression of a cyanide-insensitive oxidase and the uptake hydrogenase, respectively (9, 50). Finally, ActSR of Sinorhizobium meliloti, originally identified in the context of acid tolerance, also control genes involved in CO2 fixation, nitrate assimilation, and N2 fixation (16, 57). The response regulators of this family display an unusually high degree of conservation in their DNA binding domains. In fact, it was demonstrated for some members that they are functionally exchangeable both in vitro and in vivo, raising the question of whether they also control a similar set of target genes (13, 36).
In B. japonicum, no other direct RegR target genes have been studied in great detail apart from fixR-nifA. Here, we have assigned a large number of genes as novel members of the RegR regulon by comparing the transcriptome of the wild type with that of the ΔregR strain under free-living oxic and microoxic conditions and during symbiosis.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The bacterial strains used in this work are listed in Table 1. Escherichia coli was grown in Luria-Bertani medium (39) containing the following concentrations of antibiotics for plasmid selection (μg ml−1): ampicillin, 200; kanamycin, 30; and tetracycline, 10. B. japonicum strains were cultivated in peptone salts-yeast extract medium (49) supplemented with 0.1% l-arabinose. Aerobic cultures (21% O2) for microarray experiments were grown in 5-liter Erlenmeyer flasks containing 200 ml of medium with rigorous shaking (160 rpm) at 30°C. Microaerobic cultures (0.5% O2 in the gas phase) and anaerobic cultures were grown as described previously (24, 25). When appropriate, antibiotics were used at the following concentrations (μg ml−1): spectinomycin, 100; streptomycin, 50; kanamycin, 100; and tetracycline, 50 (solid medium) and 25 (liquid medium).
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| E.coli | ||
| DH5α | λ− φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | Bethesda Research Laboratories, Inc., Gaithersburg, MD |
| S17-1 | Smr SprhsdR (RP4-2 kan::Tn7 tet::Mu; integrated in the chromosome) | 52 |
| BL21 (DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3) (λcIts857 ind1 Sam7 nin5 lacUV5-T7 gene 1) | 54 |
| B. japonicum | ||
| 110spc4 | Spr wild type | 49 |
| 2426 | Spr SmrregR::Ω | 5 |
| 9537 | Spr Kmr bll2087::aphII (same orientation) | This work |
| 9538 | Spr Kmr bll2087::aphII (opposite orientation) | This work |
| 9552 | Spr Kmr bll2109::aphII (same orientation) | This work |
| 9553 | Spr Kmr bll2109::aphII (opposite orientation) | This work |
| Plasmids | ||
| pBluescript SK+ | Apr cloning vector | Stratagene, La Jolla, CA |
| pBSL15 | Apr Kmr | 1 |
| pBSL86 | Apr Kmr | 1 |
| pGEM-T Easy | Apr TA cloning vector | Promega, Madison, WI |
| pSUP202pol4 | Tcr (pSUP202) oriT of RP4 | 17 |
| pSUP202pol6K | Tcr (pSUP202pol4) KpnI linker inserted into SmaI site | 58 |
| pRJ2809 | Apr (pRJ9519) fixR promoter on a 632-bp SacII-BamHI fragment | R. Emmerich, unpublished |
| pRJ8817 | Apr (pRJ9519) fixGHIS promoter on a 524-bp XbaI-EcoRI fragment | 38 |
| pRJ9519 | Apr (pBluescript SK+) 308-bp BstXI-KpnI fragment containing the B. japonicum rrn terminator cloned into the HincII and KpnI sites | 6 |
| pRJ9537 | Kmr Tcr (pSUP202pol6K) 2.3-kb XbaI-EcoRI fragment | This work |
| pRJ9538 | Kmr Tcr (pSUP202pol6K) 2.3-kb XbaI-EcoRI fragment | This work |
| pRJ9542 | Apr (pRJ9519) bll2087 promoter on a 157-bp EcoRI-BamHI fragment | This work |
| pRJ9547 | Apr (pRJ9519) blr2614 promoter on a 164-bp EcoRI-BamHI fragment | This work |
| pRJ9552 | Kmr Tcr (pSUP202pol4) 2.4-kb NotI-PstI fragment | This work |
| pRJ9553 | Kmr Tcr (pSUP202pol4) 2.4-kb NotI-PstI fragment | This work |
| pRJ9562 | Apr (pGEM-T Easy) 434-bp PCR fragment comprising the blr1515 upstream region | This work |
| pRJ9564 | Apr (pRJ9519) blr1515 promoter on a 394-bp BamHI-EcoRI fragment | This work |
| pRJ9567 | Apr (pRJ9519) bll2109 promoter on a 251-bp BamHI-EcoRI fragment | This work |
| pRJ9569 | Apr (pRJ9519) blr2501 promoter on a 349-bp NotI-PstI fragment | This work |
| pRJ9570 | Apr (pRJ9519) blr1883 promoter on a 429-bp NotI-PstI fragment | This work |
| pRJ9571 | Apr (pRJ9519) bll3193 promoter on a 322-bp BamHI-EcoRI fragment | This work |
| pRJ9573 | Apr (pRJ9519) bll1285 promoter on a 443-bp BamHI-EcoRI fragment | This work |
| pRJ9574 | Apr (pRJ9519) bll4833 promoter on a 435-bp BamHI-EcoRI fragment | This work |
| pRJ9576 | Apr (pRJ9519) blr6267 promoter on a 388-bp BamHI-EcoRI fragment | This work |
| pRJ9577 | Apr (pRJ9519) bll5807 promoter on a 316-bp BamHI-EcoRI fragment | This work |
| pRJ9578 | Apr (pRJ9519) bll6633 promoter on a 277-bp BamHI-EcoRI fragment | This work |
| pRJ9601 | Apr (pBluescript SK+) B. japonicum rrn promoter and rrn terminator on a 468-bp SacI-SmaI fragment | 6 |
Plant growth.
Soybean seeds [Glycine max (L.) Merr. cv. Williams] were surface sterilized as previously described except that treatment with 30% H2O2 for 5 min was used for sterilization (21). Nodules for RNA isolation were harvested 13 and 21 days postinoculation (dpi), immediately frozen in liquid nitrogen, and stored at −80°C until RNA isolation. The symbiotic phenotypes of the B. japonicum mutant strains 9537, 9538, 9552, and 9553 were determined in infection tests using soybean as the host plant, and nitrogenase activity was measured in an acetylene reduction assay (20, 23).
RNA isolation, cDNA synthesis, and microarray analysis.
Cultures of B. japonicum were grown to mid-exponential phase (an optical density at 600 nm of 0.4 to 0.5). Cell harvest, RNA extraction, cDNA synthesis, fragmentation, and labeling were done as described previously (24, 25). Details of the custom-designed Affymetrix B. japonicum gene chip BJAPETHa520090 (Santa Clara, CA) and conditions for microarray hybridization have also been described previously (25). For bacteroid transcriptome analyses, nodules from five plants infected with the wild type or the regR mutant were collected for each hybridization experiment, and RNA was extracted as described previously (45). For each strain grown under free-living and symbiotic conditions, a minimum of five or three biological replicates was analyzed, respectively. Only the probe sets that were called “present” or “marginal” in ≥80% of the replicates of each experiment were considered for further analysis. Details on data processing, normalization, and further analysis including the identification of statistically overrepresented functional categories are described elsewhere (45). We considered genes passing the statistical tests as differentially expressed only if the relative change in expression (n-fold) was ≥2 or ≤−2 when different conditions were compared. Operon predictions were essentially done according to Mwangi and Siggia (40). An operon-like organization of genes (bicistronic or larger) was assumed if they were orientated in the same direction and separated by ≤32 nucleotides (nt). The allowed distance between genes was enlarged to 100 nt if the first three letters in the gene names were identical.
In silico search for RegR binding sites.
Putative promoter regions (500 bp) located upstream of RegR-regulated genes or operons were searched for RegR binding motifs essentially as described previously for NifA and RpoN binding sites (25). A position-specific frequency matrix was generated on the basis of experimentally verified RegR binding sites (see Fig. 4). Predicted RegR binding sites (see Table S2 in the supplemental material) have a score which is higher than that of the motif with the lowest score in the set of known RegR binding sites that was used for generation of the position-specific frequency matrix.
FIG. 4.
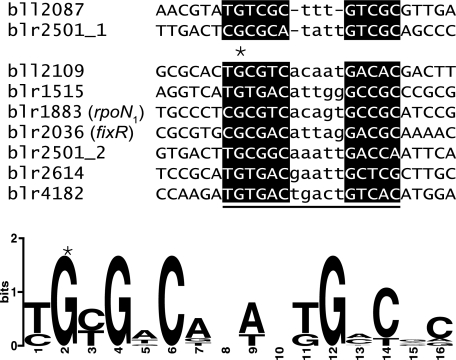
Comparison of DNA sequences included in the oligonucleotides used for EMSAs in this work (Fig. 3). The RegR binding site present in the fixR promoter region is included (15, 56). Shown is the minimal overlap of the oligonucleotides upon alignment with ClustalW plus manual refinements. Nucleotides in reverse capital letters represent the GC-rich putative half-sites of the RegR binding motifs; nucleotides in lowercase letters indicate the AT-rich spacer (variable in number) between the left and right half-sites. Note that gaps were introduced manually in the central portion of the two motifs shown at the top. In the lower part of this figure, the consensus sequence for the RegR binding site is shown based on the underlined positions of the seven ungapped DNA sequences provided in the upper part. The sequence logo was created using WebLogo (10). The conserved G that was mutated to a T in the experiment shown in Fig. 3 is marked with an asterisk in both parts of the figure.
Quantitative real-time PCR.
The expression of genes blr3166, blr3167, and blr3168 was analyzed by quantitative reverse transcription-PCR using a QuantiTect Sybr Green PCR kit (Qiagen, Hilden, Germany) and a Rotor-Gene 3000 thermocycler (Corbett Research, Sydney, Australia). cDNA used as template in combination with primers 3166-5/3166-6 (blr3166), 3167-3/3167-4 (blr3167), and 3168-3/3168-4 (blr3168) corresponded to the cDNA prepared for the gene chip experiments. Sequences of these primers and of all other oligonucleotides used in this work are available from the authors on request. Each PCR contained 10 μl of 2× Sybr Green Master Mix, a 0.2 μM concentration of individual primers, and appropriate dilutions of cDNA in a total volume of 20 μl. Reactions were run in triplicates. Melting curves were generated for verifying the specificity of the amplification. Relative changes in gene expression were calculated as described elsewhere (46). Expression of the primary sigma factor sigA which, based on the microarray data, was found to be unchanged under different conditions was used as a reference for normalization (primers SigA-1069F and SigA-1155R).
Construction of bll2087 and bll2109 deletion mutants.
Plasmids and strains used in this work are listed in Table 1. Deletion mutagenesis was done by marker exchange. Briefly, the 5′ and 3′ flanking regions of bll2087 and bll2109 were PCR amplified and cloned in the pBluescript SK+ vector. A kanamycin resistance cassette (aphII) was inserted between the B. japonicum DNA fragments. Constructs were cloned into the suicide vectors pSUP202pol6K (bll2087) and pSUP202pol4 (bll2109). The resulting plasmids pRJ9537, pRJ9538, pRJ9552, and pRJ9553 were then mobilized by conjugation into B. japonicum strain 110spc4 (wild type) yielding mutant strains 9537, 9538, 9552, and 9553. The correct integration of the aphII cassette in the chromosome by double crossover was verified by PCR.
Overproduction, purification, and phosphorylation of RegR.
His-tagged RegR was overexpressed and purified from E. coli BL21(DE3) as described previously (14). For in vitro phosphorylation, RegR protein (20 μM final concentration) was incubated with 25 mM acetyl phosphate (Fluka, Buchs, Switzerland) in either DNA binding buffer (5) or in vitro transcription buffer (6) for 1 h at 30°C.
In vitro transcription assays.
Plasmids used as templates for in vitro transcription are based on plasmid pRJ9519 which contains the B. japonicum rrn transcriptional terminator (6). They are listed in Table 1. Purification of B. japonicum RNA polymerase and multiple-round in vitro transcription assays were done as previously described (6, 38). Assays were performed in a 20-μl reaction mixture containing in vitro transcription buffer and increasing amounts of RegR protein (untreated or pretreated with acetyl phosphate). Radioactive transcription products were purified by phenol extraction and ethanol precipitation, separated on 6% denaturing polyacrylamide gels, and visualized with a phosphorimager using Quantity One software, version 4.6.1 (Bio-Rad, Reinach, Switzerland).
EMSAs.
Binding of RegR to putative target promoters was initially tested using PCR-amplified DNA fragments. Acetyl phosphate-treated RegR (0 to 1 μM) was incubated with column-purified DNA fragments (3 to 95 nM) in DNA binding buffer in a total volume of 20 μl in the presence of 1 μg of poly(dI-dC) as a nonspecific competitor. After a 5-min incubation at room temperature, samples were mixed with loading dye and separated on 6% nondenaturing polyacrylamide gels in 1× Tris-borate EDTA electrophoresis buffer for 30 min at 180 V. Gels were stained with Sybr Green I according to the instructions of the provider (Invitrogen, Basel, Switzerland) and visualized on a UV transilluminator. Alternatively, electrophoretic mobility shift assays (EMSAs) were performed using radiolabeled PCR fragments or short oligonucleotides (30 to 35 bp). PCR fragments or single-stranded oligonucleotides (30 pmol) were end labeled with [γ-32P]ATP using T4 polynucleotide kinase (MBI Fermentas). The single-stranded oligonucleotides were then incubated with the complementary oligonucleotides (60 pmol) for 10 min at 95°C and slowly cooled down to room temperature to allow hybridization. Finally, both labeled oligonucleotides and PCR fragments were purified over NAP-10 Sephadex G-25 columns (Amersham Biosciences, Buckinghamshire, United Kingdom). EMSAs were done as described above using a 0.25 nM to 1 nM concentration of labeled PCR fragments or 2 nM oligonucleotide probes. Gels were dried, and radioactive bands were visualized with a phosphorimager.
Transcript mapping.
The 5′ end of the blr1515 transcript synthesized in vivo was mapped in a primer extension experiment using oligonucleotide 1515-P1. RNA was isolated from aerobically grown wild-type and the regR mutant strains as described above. Approximately 10 μg of RNA and at least 100,000 cpm of 32P-labeled primer were used per reaction, which was performed as previously described (2). Extension products were loaded on a 6% denaturing polyacrylamide gel adjacent to a sequencing ladder obtained with plasmid pRJ9562 and labeled primer 1515-P1. The 5′ ends of in vitro synthesized RNA from the fixR (P2), bll2087, and blr1515 promoters were mapped using oligonucleotide 9519-1 which hybridizes to a sequence located on vector pRJ9519 used for construction of the template plasmids (38). Sequencing ladders from plasmids pRJ2809, pRJ9542, and pRJ9564 were obtained with the same primer.
Microarray data accession numbers.
The microarray data have been deposited in the NCBI Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo) and are accessible via GEO Series accession numbers GSE9026 and GSE9100.
RESULTS AND DISCUSSION
Transcription profiling of the ΔregR strain grown under free-living conditions.
Comparative analysis of the B. japonicum wild-type and the ΔregR strains grown under oxic conditions revealed 117 genes with differential expression (relative change of at least twofold). Seventy-four genes showed decreased expression in the mutant; i.e., RegR exerts direct or indirect positive control on these genes in the wild type. Of these genes, 25 are members of 19 putative bicistronic or larger operons (for definition of operons, see Materials and Methods). Increased expression in the mutant was observed for 43 genes (24 of them belonging to 13 operons). Under microoxic conditions, expression of 170 genes was altered between the two strains (126 genes with decreased expression [73 genes in 32 operons] and 44 genes with increased expression [18 genes in 11 operons]).
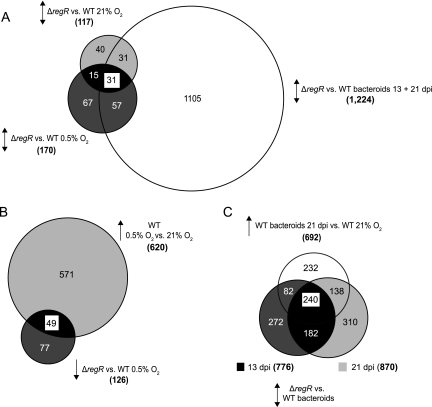
Differentially expressed genes in culture-grown cells were subdivided into three classes, reflecting their differential expression either under both free-living conditions (oxic and microoxic) or under one or the other condition only (Fig. 1A and Table 2). Figure 1A also illustrates the proportion of genes found to be differentially expressed in symbiosis based on a comparison of the transcriptomes of wild-type and ΔregR bacteroids (details given below).
FIG. 1.
Venn diagrams representing the number of differentially expressed genes in transcriptome comparisons of the B. japonicum wild type (WT) and the ΔregR strain grown under free-living and symbiotic conditions. Strains and conditions are indicated alongside the circles. Overlapping sectors are highlighted in different gray tones. Up and down arrows reflect increased and decreased gene expression in microarray analyses, respectively. Numbers in parentheses indicate the total number of differentially expressed genes. (A) Number of genes differentially expressed in the ΔregR strain under oxic (21% O2), microoxic (0.5% O2), and symbiotic conditions (bacteroids at 13 and 21 dpi). See text for a definition of the genes in the overlaps (classes 1, 2, and 3). (B) Number of genes induced upon a switch from oxic to microoxic conditions in the wild type and genes showing a decreased expression in the ΔregR strain under microoxic conditions. The overlap shows the proportion of oxygen-responsive genes activated by RegR. (C) Number of genes differentially expressed in a comparison between wild-type and ΔregR bacteroids at 13 and 21 dpi. Genes induced in wild-type bacteroids compared to free-living, aerobically grown cells are in the circle on top.
TABLE 2.
B. japonicum genes differentially expressed by a factor of ≥2 in the ΔregR strain compared with the wild typea
| Class and gene no.b | Putative operon member (gene no.)b,c | Gene named | Description | Relative change in expression (n-fold) under the indicated conditione
|
|
|---|---|---|---|---|---|
| 21% O2 | 0.5% O2 | ||||
| Class 1 (regulated by RegR under both conditions) | |||||
| bll0693 | Unknown protein | 2.3 | 2.3 | ||
| bll0904 | regR | Two-component response regulator | −111.6 | −84.0 | |
| bll1285 | Unknown protein | −11.4 | −11.2 | ||
| bll1322 | Hypothetical protein | −2.5 | −2.6 | ||
| blr1429 | Unknown protein | −3.5 | −3.2 | ||
| blr1515 | acrA | RND multidrug efflux membrane permease | −22.2 | −21.2 | |
| blr1516 | acrB | RND multidrug efflux transporter | −29.5 | −21.6 | |
| blr1783 | Putative transposase | −2.5 | −2.2 | ||
| blr2036* | fixR | Oxidoreductase | −13.0 | −5.7 | |
| bll2087 | id880 | Unknown protein | −24.3 | −11.2 | |
| bll2268 | Putative xylose operon repressor | −3.1 | −2.8 | ||
| blr2501 | Hypothetical protein | −12.2 | −13.1 | ||
| bsl2596 | Unknown protein | 3.9 | 4.0 | ||
| blr2614 | Hypothetical protein | −4.3 | −3.0 | ||
| blr3161 | Hypothetical protein | −2.4 | −3.5 | ||
| bll3363 | Unknown protein | −2.4 | −2.2 | ||
| bll3753 | Hypothetical protein | 4.3 | 4.4 | ||
| blr3769 | Hypothetical protein | −2.5 | −2.5 | ||
| blr3771 | Hypothetical protein | −3.3 | −4.1 | ||
| blr4031 | Hypothetical glutathione S-transferase-like protein | 2.0 | 2.2 | ||
| bll4130 | Transcriptional regulatory protein LysR family | −2.5 | −2.7 | ||
| bsl4167 | Putative glutamine synthetase translation inhibitor | −3.1 | −2.8 | ||
| blr4182 | Transcriptional regulator | −3.3 | −4.7 | ||
| blr4238 | Hypothetical protein | −3.4 | −2.7 | ||
| blr4257 | Putative hydrolase | 2.4 | 2.3 | ||
| bsr4258 | Hypothetical protein | 2.6 | 3.3 | ||
| blr4259 | Hypothetical protein | 2.4 | 2.0 | ||
| blr4260 | Hypothetical protein | 2.4 | 2.4 | ||
| bll4725 | Unknown protein | 3.8 | 4.8 | ||
| bll4833 | Unknown protein | −3.7 | −3.6 | ||
| blr5220 | hspE | Small heat shock protein | 2.9 | 2.7 | |
| bll5477 | Similar to formate dehydrogenase | −2.5 | −5.2 | ||
| bll5478 | Similar to formate dehydrogenase | −2.2 | −4.6 | ||
| blr5693 | Probable substrate-binding protein | −2.3 | −2.9 | ||
| bll5807 | Hypothetical protein | −8.2 | −5.7 | ||
| blr6210 | Hypothetical protein | −6.5 | −6.1 | ||
| blr6267 | Transcriptional regulator | −3.1 | −2.8 | ||
| bll6513 | Hypothetical protein | −30.2 | −8.1 | ||
| bsl6653 | Unknown protein | −2.8 | −3.0 | ||
| bll6844 | Unknown protein | −2.2 | −5.3 | ||
| bll6850 | fliR | Probable flagellar biosynthetic protein | −2.1 | −2.2 | |
| bll6855 | fliC | Probable flagellar protein | −2.1 | −6.7 | |
| bll6880 | Unknown protein | −2.0 | −3.2 | ||
| blr6918 | Probable substrate-binding protein | −2.8 | −2.2 | ||
| bll7514 | lpxK | Tetraacyldisaccharide 4-kinase | 2.2 | 2.8 | |
| blr7905 | cit | Citrate-proton symporter | 11.9 | 8.9 | |
| Class 2 (regulated by RegR only under microoxic conditions) | |||||
| bll0243 | Hypothetical protein | −2.3 | |||
| blr0305 | Unknown protein | 3.2 | |||
| blr0306 | Hypothetical protein | 3.0 | |||
| blr0366 | Unknown protein | 2.5 | |||
| bll0718 | Putative transporter | 2.4 | |||
| bll0818 | Unknown protein | −5.3 | |||
| blr0907 | Hypothetical protein | 2.2 | |||
| bll1026 | Hypothetical protein | 2.0 | |||
| bll1070 | Probable dioxygenase | −2.0 | |||
| bll1110 | Hypothetical protein | 2.1 | |||
| bll1207 | Hypothetical protein | 2.0 | |||
| bsl1208 | Hypothetical protein | 3.2 | |||
| blr1402 | Hypothetical protein | 2.1 | |||
| bsr1739* | fdxN | Ferredoxin | −6.2 | ||
| blr1743* | nifD | Nitrogenase molybdenum-iron protein alpha chain | −6.6 | ||
| blr1744* | nifK | Nitrogenase molybdenum-iron protein beta chain | −6.4 | ||
| blr1745* | nifE | Nitrogenase molybdenum-cofactor synthesis protein | −4.9 | ||
| blr1746* | nifN | Nitrogenase molybdenum-cofactor synthesis protein | −3.2 | ||
| blr1748* | id79 | Hypothetical protein | −2.3 | ||
| bsr1749* | id80 | Hypothetical protein | −2.0 | ||
| bll1754* | id89 | Hypothetical protein | −3.2 | ||
| blr1755* | Rhizobium etli iscN homolog | −4.5 | |||
| blr1756* | nifS | Nitrogenase metalloclusters biosynthesis protein | −2.8 | ||
| bsr1757* | fixU | Nitrogen fixation protein | −3.2 | ||
| blr1759* | nifB | FeMo cofactor biosynthesis protein | −2.2 | ||
| bsr1760* | frxA | Ferredoxin-like protein | −2.0 | ||
| bll1767* | id121 | Hypothetical protein | −2.4 | ||
| blr1769* | nifH | Dinitrogenase reductase protein | −3.9 | ||
| blr1770* | nifQ | Molybdenum processing protein | −2.6 | ||
| bll1777* | ahpC | Alkyl hydroperoxide reductase | −2.5 | ||
| bll1791 | id172 | Hypothetical protein | 2.2 | ||
| blr1850* | Unknown protein | −2.9 | |||
| bll1872* | Hypothetical protein | −2.5 | |||
| bll1906* | N-acetyltransferase NrgA homolog | −4.7 | |||
| bll1944* | Hypothetical protein | −2.9 | |||
| blr1964* | id568 | Putative sugar hydrolase | −2.1 | ||
| bsr2010* | Unknown protein | −2.4 | |||
| blr2038* | fixA | Electron transfer flavoprotein beta chain | −2.7 | ||
| bll2060* | groES3 | GroES3 chaperonin | −3.1 | ||
| bll2059* | groEL3 | GroEL3 chaperonin | −2.1 | ||
| bll2063* | nrgC | Phenolhydroxylase homolog | −2.5 | ||
| blr2071 | id841 | Similar to inosamine-phosphate amidinotransferase | 2.8 | ||
| blr2106* | ectC | l-Ectoine synthase | −10.0 | ||
| blr2131* | Probable oxygenase | −5.6 | |||
| blr2132* | Unknown protein | −4.1 | |||
| blr2133* | Hypothetical protein | −3.5 | |||
| blr2134* | Hypothetical protein | −2.8 | |||
| blr2135* | Hypothetical protein | −2.4 | |||
| blr2136* | Putative aminotransferase | −2.0 | |||
| blr2144* | cyp112 | Cytochrome P-450 BJ-1 | −5.0 | ||
| blr2145* | cyp114 | Cytochrome P-450 BJ-3 | −2.3 | ||
| blr2147* | cyp117 | Cytochrome P-450 BJ-4 | −2.2 | ||
| blr2148* | Farnesyl diphosphate synthase | −3.0 | |||
| blr2149* | Hypothetical protein | −2.8 | |||
| blr2429 | Unknown protein | −2.0 | |||
| bll2446 | Hypothetical protein | 2.1 | |||
| blr2474 | Hypothetical protein | 2.9 | |||
| blr2475 | tspO | Tryptophan-rich sensory protein | 2.4 | ||
| blr2476 | Hypothetical protein | 2.1 | |||
| blr2696 | Cytochrome c peroxidase | 2.8 | |||
| blr2697 | virA | VirA-like protein | 3.9 | ||
| blr2721 | htrA | Probable serine protease do-like precursor | 2.2 | ||
| bll3807 | msbB | Lipid A biosynthesis lauroyl acyltransferase | 2.1 | ||
| blr3848 | Hypothetical protein | −2.1 | |||
| blr4046 | Unknown protein | 2.4 | |||
| bll4166 | Unknown protein | −2.0 | |||
| bll4282 | Probable acetoacetyl-coenzyme A synthetase | −2.1 | |||
| bll4326 | Putative methyl-accepting chemotaxis protein | −2.5 | |||
| bll4391 | Unknown protein | −2.0 | |||
| blr4450 | Hypothetical protein | −2.3 | |||
| bll4816 | Unknown protein | 2.1 | |||
| blr5025 | Hypothetical protein | −2.0 | |||
| blr5221 | hspF | Small heat shock protein | 2.0 | ||
| blr5346 | Putative hydrolase | 2.2 | |||
| bll5475 | Putative formate dehydrogenase | −3.4 | |||
| bll5476 | Formate dehydrogenase iron-sulfur subunit | −5.3 | |||
| bll5480 | Putative chaperone | −4.1 | |||
| bsl5479 | Hypothetical protein | −4.7 | |||
| blr5556 | Hypothetical protein | −2.4 | |||
| blr5637 | Unknown protein | 2.2 | |||
| blr5712 | Hypothetical protein | 2.2 | |||
| blr5768 | Unknown protein | 2.3 | |||
| blr6103 | Putative oxygenase | 3.0 | |||
| blr6578 | ABC transporter permease protein | 2.7 | |||
| blr6579 | ABC transporter ATP-binding protein | 2.6 | |||
| bsl6617 | Unknown protein | 2.9 | |||
| bll6747 | Unknown protein | −2.5 | |||
| blr6846 | Two-component response regulator | −3.2 | |||
| bll6848 | Hypothetical protein | −2.6 | |||
| bll6847 | Hypothetical protein | −2.4 | |||
| bll6854 | flbT2 | Flagellin synthesis repressor protein | −2.6 | ||
| bll6853 | flgD | Hook formation protein | −3.4 | ||
| bsl6852 | fliQ | Flagellar biosynthetic protein | −2.2 | ||
| bll6858 | flgE | Flagellar hook protein | −3.8 | ||
| bll6857 | flgK | Hook associated protein I homolog | −3.4 | ||
| bll6856 | flgL | Probable flagellar hook-associated protein | −3.7 | ||
| bll6864 | fliF | Flagellar M-ring protein | −2.7 | ||
| bll6863 | Unknown protein | −2.4 | |||
| bll6862 | motB | Probable flagellar motor protein | −3.3 | ||
| bll6861 | motC | Probable chemotaxis protein precursor | −2.7 | ||
| bll6860 | Unknown protein | −2.6 | |||
| bll6859 | Hypothetical protein | −2.7 | |||
| bll6865 | fla | Flagellin | −3.3 | ||
| bll6866 | fla | Flagellin | −2.5 | ||
| bll6867 | fliP | Flagellar biosynthetic protein | −2.1 | ||
| bll6876 | flgB | Flagellar basal-body rod protein | −3.4 | ||
| bll6875 | flgC | Flagellar basal-body rod protein | −4.4 | ||
| bll6874 | fliE2 | Flagellar hook-basal body complex protein | −3.3 | ||
| bll6873 | flgG | Flagellar basal-body rod protein | −3.3 | ||
| bll6872 | flgA | Probable flagellar protein | −2.5 | ||
| bll6871 | flgI2 | Flagellar P-ring protein precursor | −4.5 | ||
| bll6870 | Hypothetical protein | −2.1 | |||
| bll6869 | flgH2 | Flagellar L-ring protein precursor | −2.8 | ||
| bll6879 | fliN | Probable flagellar motor switch protein | −4.0 | ||
| bll6878 | fliG | Probable flagellar motor switch protein | −2.5 | ||
| bll6877 | flhB | Flagellar biosynthetic protein | −2.5 | ||
| bll6882 | motA | Chemotaxis protein | −3.0 | ||
| bll6881 | Hypothetical protein | −3.2 | |||
| blr6883 | Hypothetical protein | −3.2 | |||
| blr6951* | modA | Molybdenum ABC transporter; Molybdate-binding protein | −8.9 | ||
| blr6952* | modB | Molybdenum ABC transporter permease protein | −4.9 | ||
| bll7861 | Putative rhizopine catabolism protein | 2.0 | |||
| bll7938 | Hypothetical protein | −3.8 | |||
| bll7952 | Probable selenium-binding protein | −2.5 | |||
| Class 3 (regulated by RegR only under oxic conditions) | |||||
| bll0246 | bam | Indoleacetamide hydrolase | −2.4 | ||
| bsr0247 | Unknown protein | −2.0 | |||
| blr0257 | Two-component sensor histidine kinase | −2.3 | |||
| bll0375 | Probable alginate O-acetyltransferase | −2.1 | |||
| bll0597 | Cytochrome b561 family protein | −2.6 | |||
| bll0805 | Hypothetical protein | −2.2 | |||
| bsr1232 | Hypothetical protein | 2.5 | |||
| blr1307 | Hypothetical protein | −2.0 | |||
| bll1476 | cysD | Sulfate adenylate transferase subunit 2 | 2.8 | ||
| bsr1514 | Unknown protein | −2.3 | |||
| blr2037 | nifA | nif-specific regulatory protein | −3.6 | ||
| blr2074 | id855 | NoeE homolog | 2.1 | ||
| bsl2086 | Unknown protein | −2.7 | |||
| bll2109 | Transcriptional regulatory protein Crp family | −3.1 | |||
| bll2158 | Unknown protein | −2.0 | |||
| blr2815 | Putative transketolase family protein | −2.4 | |||
| blr2891 | Putative phenylacetic acid degradation protein | 2.8 | |||
| bsr2892 | paaB | Phenylacetic acid degradation protein | 3.0 | ||
| blr2893 | paaC | Putative phenylacetic acid degradation protein | 2.9 | ||
| blr2895 | paaE | Putative ferredoxin reductase electron transfer component protein | 2.2 | ||
| blr2896 | paaI | Phenylacetic acid degradation protein | 2.3 | ||
| blr2897 | paaK | phenylacetate-coenzyme A ligase | 2.6 | ||
| blr2976 | Putative methyl accepting chemotaxis protein | −2.2 | |||
| bll3049 | Hypothetical protein | −2.1 | |||
| bll3148 | Hypothetical protein | −2.0 | |||
| bll3150 | Putative Oxalate:formate antiporter | −3.8 | |||
| blr3159 | Hypothetical protein | −2.7 | |||
| blr3166 | gcl | Putative glyoxylate carboligase protein | −12.7 | ||
| blr3167 | hyi | Putative hydroxypyruvate isomerase protein | −36.4 | ||
| blr3168 | Oxidoredutase | −12.0 | |||
| blr3169 | Hypothetical protein | −5.8 | |||
| blr3188 | Unknown protein | 2.3 | |||
| bsr3237 | Unknown protein | 2.6 | |||
| bll3759 | Hypothetical protein | −3.4 | |||
| bll3872 | HlyD family secretion protein | 2.0 | |||
| bll3937 | Hypothetical protein | −2.0 | |||
| blr4028 | Putative RNA polymerase | 2.7 | |||
| blr4176 | Unknown protein | −2.2 | |||
| bll4201 | Unknown protein | 3.1 | |||
| blr4261 | Hypothetical protein | 2.2 | |||
| bll4394 | Hypothetical protein | 2.0 | |||
| blr4695 | kup3 | Potassium uptake protein | 2.3 | ||
| bll4784 | Aldehyde dehydrogenase | −2.2 | |||
| bll5217 | Probable glycosyl transferase | 2.5 | |||
| bll5218 | Unknown protein | 2.5 | |||
| bll5219 | Small heat shock protein | 2.5 | |||
| bsl5225 | Unknown protein | 2.4 | |||
| blr5226 | Heat shock protein | 2.7 | |||
| blr5227 | groEL1 | Heat shock protein | 2.5 | ||
| blr5228 | Unknown protein | 2.4 | |||
| blr5229 | Unknown protein | 5.4 | |||
| blr5231 | rpoH1 | sigma32-like factor | 2.2 | ||
| blr5233 | hspB | Small heat shock protein | 2.9 | ||
| blr5234 | hspC | Small heat shock protein | 2.2 | ||
| blr5730 | Hypothetical protein | −2.3 | |||
| bll5890 | Monocarboxylic acid permease | −3.3 | |||
| bsl5891 | Hypothetical protein | −4.9 | |||
| blr6211 | Unknown protein | −2.3 | |||
| bll6262 | osmC | Probable osmotically inducible protein | −2.2 | ||
| bll6261 | Hypothetical protein | −2.5 | |||
| bll6851 | flhA | Flagellar biosynthesis protein | −2.0 | ||
| blr6885 | fliI | Flagellum-specific ATP synthase | −2.2 | ||
| bll6890 | Unknown protein | −3.2 | |||
| bsl6958 | Hypothetical protein | 2.6 | |||
| blr7064 | Probable ABC transporter substrate-binding protein | −2.2 | |||
| blr7102 | Unknown protein | −2.5 | |||
| blr7589 | Putative oxidoreductase | 3.7 | |||
| blr7780 | Hypothetical protein | −3.6 | |||
| blr7813 | Transcriptional regulatory protein GntR family | 2.1 | |||
| blr7848 | Probable substrate-binding protein | −2.3 | |||
| bll7969 | Putative dihydrodipicolinate synthase | −2.3 | |||
Both the ΔregR and wild-type strains were grown under under free-living, oxic (21% O2) or microoxic (0.5% O2) conditions.
Genes in boldface are induced in the B. japonicum wild-type strain upon a switch from oxic to microoxic conditions; genes in italics are down-regulated (45). Genes labeled with asterisks are known or putative NifA targets (25).
Operon predictions were done as described in Materials and Methods.
Gene names are according to the EMBL-EBI database.
Gene expression changes (n-fold) are average values retrieved by microarray analysis of five biological replicates of the B. japonicum wild type and ΔregR mutant grown under oxic (21% O2) and microoxic (0.5% O2) conditions.
Class 1 is composed of 46 genes showing qualitatively similar differential expression behavior under oxic and microoxic conditions (Table 2). As expected, the previously known RegR-controlled gene fixR is a class 1 member. Likewise, expression levels of the promoter-distal nifA gene of the fixR-nifA operon decreased in regR-deficient cells under both conditions; however, the relative change in expression was above our stringent cutoff value only under oxic conditions. Class 1 also includes the gene bll2087 that was recently discovered as a new RegR target in a pilot microarray study from our group (24). Examples among the novel RegR targets grouped in class 1 are genes coding for putative RND (resistance-nodulation-cell division)-type efflux proteins (Blr1515 and Blr1516) whose amino acid sequences are similar to those of P. aeruginosa MexCD (40% and 50% identity) and E. coli AcrAB (38% and 48% identity) (30, 48). RND-type transporters are generally known for conferring antibiotic resistance, but they are also important for successful colonization by, and persistence of, human pathogenic bacteria (47). The functional characterization of such transport systems in plant-associated bacteria has suggested a role in tolerance against plant-derived secondary metabolites (e.g., flavonoids and isoprenoids), which may facilitate better colonization of the host (7, 19, 43).
Expression of 12 of the 46 class 1 genes was increased in regR mutant cells, e.g., blr7905 (cit) encoding a putative citrate-proton symporter (relative change of more than eightfold). This suggests some kind of direct or indirect negative control of RegR on certain genes in the wild type.
Class 2 contains 124 genes that are RegR controlled only under microoxic but not oxic conditions, including several nif and fix genes (Table 2) which are known to be induced in the wild type by oxygen deprivation and controlled by the RegR-dependent nifA gene product (25, 45).
Class 3 is made up of 71 genes that are differentially expressed under oxic but not microoxic conditions. In class 3, a subset of 20 genes was identified whose expression is down-regulated in wild-type cells grown under microoxic conditions (45). Among these are blr3166 (glc coding for glyoxylate carboligase), blr3167 (hyi, hydroxypyruvate isomerase), and blr3168 (tartronate semialdehyde reductase), which also belong to the genes with the highest decrease in expression in the mutant. These genes are known to encode signature enzymes for the metabolism of C2/C3 carbon compounds in E. coli (8). The observed regulatory pattern suggests that RegR strongly activates expression of these genes only under oxic conditions. Diminished mRNA levels in B. japonicum were confirmed by quantitative reverse transcription-PCR with relative change values of 25.5-fold (blr3166), 31.7-fold (blr3167), and 35.2-fold (blr3168) between wild type and the regR mutant.
Thirty-one genes of class 3 are more highly expressed in ΔregR cells, e.g., the paa genes (blr2891 to blr2897) encoding enzymes involved in phenylacetic acid degradation. This pathway serves to degrade aromatic compounds in several gram-negative bacteria (26) and might play a role in the catabolism of plant-derived flavonoids in B. japonicum, preferably under low-oxygen conditions where the apparent negative regulatory effect of RegR is abrogated (45).
Figure 1B shows that the 126 genes with decreased expression in ΔregR cells under microoxic conditions include 49 genes that are induced in culture-grown B. japonicum wild-type cells under the same conditions (45), which corresponds to 8% (49/620) of all low-oxygen-responsive genes identified. Notably, 39 of these 49 genes are known or putative targets of the RegR subsidiary NifA protein which activates transcription of σ54 (RpoN)-dependent promoters (marked with asterisks in Table 2). The remaining 10 genes are candidate targets for a redox control mediated either directly via RegR or via regulatory proteins other than NifA, e.g., FixK2.
The identification of RegR-dependent genes that were up-regulated in wild-type B. japonicum cells exposed to either an oxic or a microoxic environment suggests that the RegSR system is somehow involved in sensing different ambient oxygen conditions. Yet this finding does not indicate whether the upregulation is a response to the cellular redox status or to oxygen per se. By analogy with the well-elaborated sensing mechanism of the orthologous two-component regulatory systems RegBA in R. capsulatus or the ArcBA system in E. coli, it seems attractive to speculate that the redox state of the membrane-localized quinone pool is an important cue also for B. japonicum RegSR (32, 33, 55). Alternatively, electron flow through the electron transport chain might play a role in modulating the activity of RegSR similar to the proposed model for control of the PrrBA system in R. sphaeroides by cytochrome cbb3 oxidase (29, 42).
Transcription profiling of the ΔregR strain in symbiosis.
Transcriptome data retrieved from the comparison between ΔregR bacteroids and wild-type bacteroids at 13 and 21 dpi revealed 1,224 genes (511 genes belonging to 311 putative operons) with differential expression at both time points as compiled in Table S1 in the supplemental material and illustrated in Fig. 1A. For comparison, Table S1 in the supplemental material also contains information on (i) whether these genes are induced or repressed in wild-type bacteroids at 21 dpi compared with free-living aerobically grown wild-type cells (45) and (ii) whether these genes were also differentially expressed in the comparison between wild-type and ΔregR cells grown in culture.
The large majority (84%) of the differentially expressed genes in bacteroids are activated by RegR (decreased expression in the mutant). This meets the expectation that response regulators of this global family act predominantly as activators (12). By contrast, a recent microarray analysis of an R. sphaeroides prrA mutant grown under anoxic conditions revealed that 60% of the differentially expressed genes were subject to negative control by PrrA (34). This indicates that there are substantial differences in the regulatory mode of these regulators in B. japonicum and R. sphaeroides.
Only 7% of the 1,224 genes were also controlled by RegR under low-oxygen conditions (Fig. 1A), suggesting that a much wider spectrum of functions is affected by RegR in symbiosis than in free-living conditions. A group of 31 genes (12 genes belonging to 9 putative operons) revealed the same regulatory pattern under all of the conditions investigated in this work (Fig. 1A; see also Table S1 in the supplemental material). As an example of this group, bll2087 (unknown function) was further analyzed by mutagenesis. The bll2087 deletion strains 9537 and 9538 showed a wild-type phenotype with regard to the number and dry weight of nodules and nitrogen fixation (acetylene reduction) activity in symbiosis with soybean (data not shown).
Only about half of the RegR-dependent genes in young (13 dpi) and mature (21 dpi) bacteroids were affected in their expression at both time points (Fig. 1C). Remarkably, 29% of the genes in this overlap map to the so-called 681-kb symbiotic island which comprises 7% of the B. japonicum genome (27). A large number of genes from this region are also found among those genes which are RegR-controlled only in young bacteroids (83/354).
From the set of 692 genes induced in wild-type bacteroids compared to aerobically grown cells (45), 54% (378/692) were identified as members of the RegR regulon at 21 dpi (Fig. 1C). This confirms that RegR is an important regulator of genes related to the symbiotic lifestyle. About one-third of these genes (113/378) were known from previous work to be regulated by NifA and RpoN under anoxic conditions, 51 of which are likely direct targets (25). Assuming that the full regulatory scope of NifA was uncovered in the previous study (25), the remaining 265 (378 minus 113) RegR-dependent bacteroid-induced genes (21 dpi) are controlled directly or indirectly by RegR but independently of NifA. Interestingly, 45% (118/265) of these RegR-dependent genes are at the same time controlled by RpoN in mature bacteroids (45). This coregulation is most likely indirect through RegR-dependent transcription factors other than NifA that interact with the RNA polymerase-σ54 complex in symbiosis. A candidate is the Fis-type transcriptional regulator encoded by blr5735, which harbors a predicted RpoN-interacting domain and is induced in bacteroids.
Of note, a large proportion (55%) of the differentially expressed genes in bacteroids displayed only a moderate regulation by RegR (relative change of two- to threefold) while retaining significant expression in the absence of RegR. This might reflect either constitutive expression or coactivation by other regulators. Given the considerable size and diversity of the RegR regulon, it is not unexpected to encounter coregulation of RegR with other, more specific regulators that function in individual branches of the RegR regulon. Such coregulators also may account for the different expression levels of individual RegR-dependent genes in young and mature bacteroids. In fact, the involvement of additional regulators that integrate signals other than the redox status sensed by RegB seems to be common at RegA-dependent promoters in R. capsulatus (12, 22).
Overrepresented gene categories in the symbiotic RegR regulon.
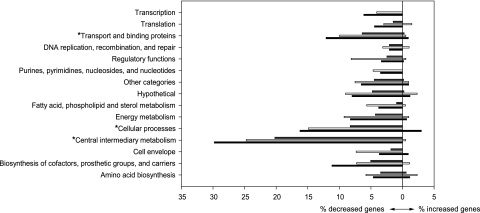
The RegR-controlled genes that were differentially expressed at 13 and 21 dpi were grouped into 15 categories as defined by Kaneko et al. (31) (Fig. 2). Fifty percent of the genes (611/1,224) have no assigned function or encode hypothetical proteins, which does not allow us to draw conclusions about their physiological roles. Among the 613 remaining genes, three functional categories were significantly overrepresented at 13 dpi and/or 21 dpi: (i) cellular processes, (ii) transport and binding proteins, and (iii) central intermediary metabolism.
FIG. 2.
Functional categories of differentially expressed genes between wild-type and regR mutant bacteroids according to the Kazusa annotation (27). Percentages of genes with decreased or increased expression in comparisons between wild-type and ΔregR bacteroids at 13 dpi (black columns), 21 dpi (white columns), or both time points during symbiosis (gray columns) were calculated by dividing the number of differentially expressed genes in each category by the total number of genes in each category. Overrepresented categories are labeled with asterisks.
(i) Cellular processes.
Genes belonging to the cellular processes category were overrepresented in young bacteroids. For example, genes encoding heat shock proteins (hspABC) or enzymes involved in detoxification of reactive oxygen species (ahpCD) were expressed at reduced levels in ΔregR bacteroids. These genes are strongly induced in wild-type bacteroids compared to free-living cells, possibly in response to oxidative stress generated in nodules (44, 45).
(ii) Transport and binding proteins.
Transport and binding proteins are overrepresented only in the data set retrieved from young bacteroids. Seventy-five percent of the RegR-dependent transport and binding proteins encode components of ABC-type transport systems, several of which are strongly induced in the wild type during symbiosis.
(iii) Central intermediary metabolism.
The category of central intermediary metabolism genes includes nitrogen fixation genes and hydrogenase genes which showed reduced expression in ΔregR bacteroids. Expression of the genes encoding the high-affinity cbb3-type terminal oxidase (fixNOQP) and rpoN1, both known targets of FixLJ-FixK2 (41), was RegR dependent at 13 dpi, pointing to a hitherto undescribed link between the RegSR-NifA and FixLJ-FixK2 regulatory cascades. Notably, also in S. meliloti, where the nitrogen fixation regulatory genes nifA and fixK are under control of the FixLJ proteins (11), ActR sets an additional level of control over these genes in response to low pH and low-oxygen conditions (57).
More than 60 genes which are RegR-dependently expressed in bacteroids are involved in transcription regulation, >30 of which are specific for the RegR regulon in mature bacteroids. This points toward an extensive expansion of the RegR regulon concept. New hierarchical cascades, similar to the RegSR-NifA cascade, in which additional environmental stimuli are sensed and transduced to specific subgroups of target genes are highly conceivable. For example, the Crp-like protein encoded by bll2109 might be a member of such a cascade. Interestingly, knockout mutations in the bll2109 gene (strains 9552 and 9553) caused a two-day delay in anaerobic growth of B. japonicum with nitrate as the terminal electron acceptor (data not shown). Since denitrification genes in B. japonicum are governed by the FixLJ-FixK2-NnrR cascade (37), the peculiar bll2109 mutant phenotype could be interpreted as a cross-pathway coregulation of the latter cascade via the RegR-dependent control of bll2109.
RegR binds directly to the promoter regions of new target genes.
Because microarray analysis does not allow differentiation between directly and indirectly controlled genes, we performed DNA binding studies to identify direct RegR target genes. In addition to 14 other promoters, we selected for further studies 16 promoters (belonging either to single genes or operons) from the group of 31 RegR-controlled genes which are differentially expressed under all conditions (Fig. 1A; see also Table S1 in the supplemental material). The latter group was assumed to contain a maximal number of direct RegR targets.
Under our experimental conditions, RegR bound to 23 of 30 investigated promoter regions that were amplified by PCR, albeit with different apparent affinities (Table 3). Notably, except for one gene (blr3769), the genes that are RegR controlled under all environmental conditions displayed consistent RegR binding to their upstream DNA regions, including the promoter region of blr7905 whose expression is negatively affected by RegR. To narrow the regions of RegR-DNA interaction, we tested RegR binding to 32P-labeled double-stranded oligonucleotides (30 to 35 bp) derived from 13 promoters (out of 23 RegR binders), comprising DNA sequences potentially recognized by RegR (RegR binding boxes [RBB]) (16). Binding was observed to DNA probes originating from promoter regions of the positively controlled genes: blr1515, blr2614, bll2109, blr1883 (rpoN1), blr4182, blr2501, and bll2087 (data not shown). RegR binding to the latter gene (bll2087) confirms a previous, preliminary result (24). Two RegR binding sites were mapped upstream of blr2501 (RBB sites 1 and 2 of blr2501, designated RBB2501-1 and RBB2501-2, respectively).
TABLE 3.
RegR-controlled B. japonicum genes whose promoter region was tested for RegR binding in electrophoretic mobility shift assays
| Gene no.a | Gene name | Description | Genomic regionb | Shiftc | Putative operon membersd |
|---|---|---|---|---|---|
| bll0597 | Cytochrome b561 family protein | −150 to +179 | + | ||
| bll1285* | Unknown protein | −211 to −59 | ++ | ||
| bll1858 | Hypothetical protein | −217 to +14 | − | ||
| bll2109 | Transcriptional regulator CRP family | −55 to +179 | ++ | ||
| bll2125 | Taurine dioxygenase | −313 to −15 | − | ||
| bll2268 | Putative xylose operon repressor | −168 to +32 | ++ | ||
| bll3193 | Transcriptional regulator CRP family | −323 to −8 | + | ||
| bll3363 | Unknown protein | −146 to +60 | + | ||
| bll4130 | Transcriptional regulator LysR family | −323 to +20 | + | ||
| bll4833* | Unknown protein | −186 to +92 | ++ | ||
| bll5480 | Putative chaperone | −216 to +109 | − | bsl5479, bll5478, bll5477, bll5476, bll5475 | |
| bll5805 | Transcriptional regulator CRP family | −278 to +66 | − | ||
| bll5807* | Hypothetical protein | −191 to +235 | ++ | ||
| bll6513 | Hypothetical protein | −184 to −24 | ++ | ||
| bll6633 | Unknown protein | −212 to +67 | ++ | ||
| blr1515* | acrA | RND multidrug efflux membrane permease | −167 to +17 | ++ | blr1516 |
| blr1853 | Cytochrome P450 family protein | −264 to +21 | − | ||
| blr1883 | rpoN1 | RNA polymerase sigma factor | −231 to −17 | ++ | |
| blr2501* | Unknown protein | −235 to +9 | ++ | ||
| blr2614 | Sodium/hydrogen exchanger | −172 to +15 | ++ | ||
| blr3166 | gcl | Glyoxylate carboligase | −463 to +2 | − | blr3167, blr3168 |
| blr3769 | Hypothetical protein | −212 to +8 | − | blr3770, blr3771 | |
| blr4182 | Transcriptional regulator | −230 to +34 | ++ | ||
| blr5693 | Probable substrate-binding protein | −163 to +36 | ++ | ||
| blr6267 | Transcriptional regulator | −233 to +48 | ++ | ||
| blr6918 | Probable substrate-binding protein | −230 to +60 | + | ||
| blr7589 | Indolepyruvate ferredoxin oxidoreductase | −354 to −18 | + | ||
| blr7905 | cit | Citrate-proton symporter | −163 to +11 | ++ | |
| bsl4167 | Putative glutamine synthetase translation inhibitor | −601 to +27 | ++ | ||
| bll2087*e | Unknown protein | −82 to +23 | ++ |
Genes whose promoter regions were tested for RegR binding. Genes in boldface are differentially expressed by a factor ≥2 in the comparison of the wild type with the ΔregR strain under all conditions analyzed in this work (free-living and symbiotic growth). All other genes were differentially expressed in the comparison under either free-living or symbiotic conditions. Genes for which in vitro transcription was shown using purified B. japonicum RNAP and RegR protein (Fig. 6) are marked with asterisks.
Genomic region included in the PCR fragment used for EMSAs. Coordinates refer to the first nucleotide position of the annotated translation start site of the gene listed in column 1.
Indicates qualitatively whether RegR binding was strong (++), weak (+), or absent (−).
Operon predictions were done as described in Materials and Methods. Genes in boldface are differentially expressed under all conditions analyzed in this work (free-living and symbiotic), and the promoters of the genes listed in column 1 were tested for RegR binding.
RegR binding to the promoter region of bll2087 was shown in a previous work (24).
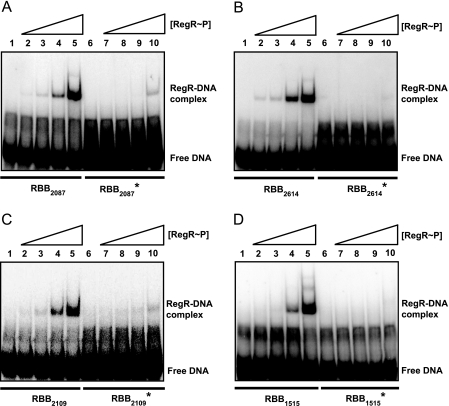
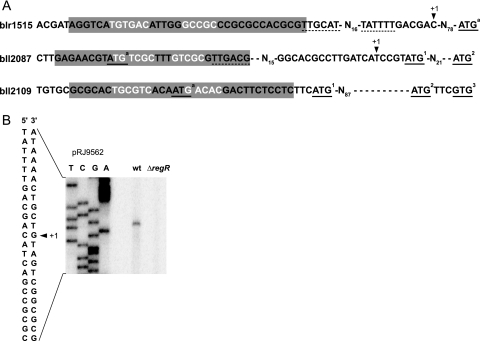
In-depth analyses were subsequently performed for four genes in order to determine RegR binding specificity to the respective DNA targets (Fig. 3). While a shifted band was already detectable for RBB2087, RBB2614, and RBB2109 upon addition of a 67- to 200-fold molar excess (0.07 to 0.2 μM) of phosphorylated RegR (RegR∼P) (Fig. 3A, B, and C, lanes 2 and 3), a shift for RBB1515 was observed only upon addition of 600-fold excess (0.6 μM) of protein (Fig. 3D, lane 4). By analogy with a previous study (15), the implication of a conserved guanine residue in these RBBs (Fig. 4) for RegR binding was investigated with point mutations (G-to-T exchange). When mutant derivatives RBB2087*, RBB2614*, RBB2109*, and RBB1515* were used as targets, RegR binding was strongly diminished (Fig. 3A to D, lanes 6 to 10), demonstrating that this G residue is indeed critical for RegR-DNA interaction.
FIG. 3.
Analysis of RegR binding to RBBs in promoter regions of RegR-controlled genes using EMSAs. Increasing amounts of purified RegR and RegR∼P were incubated with constant amounts of double-stranded 32P-labeled oligonucleotides carrying RBBs derived from promoter regions of genes bll2087 (A), blr2614 (B), bll2109 (C), and blr1515 (D). RBBs with wild-type sequences (RBBx, lanes 1 to 5) and RBBs with a G-to-T exchange (RBBx*, lanes 6 to 10) (compare with Fig. 4 and 5) were applied each at a concentration of approximately 1 nM. RegR protein concentrations were 0.07 μM (lanes 2 and 7), 0.2 μM (lanes 3 and 8), 0.6 μM (lanes 5 and 9), and 1.8 μM (lanes 6 and 10). No RegR protein was added to the control reactions in lanes 1 and 6. Samples were run on 6% nondenaturing polyacrylamide gels and visualized with a phosphorimager.
An alignment of the eight experimentally determined RBBs together with the previously identified fixR upstream activation sequence (15, 56) is shown in Fig. 4. A common element in the DNA sequences is an imperfect inverted repeat consisting of (T/C)G(C/T)GNC and GNCNC, which is separated by an AT-rich spacing of 3 to 5 nt. This element differs from the RegR box [NGNG(A/G)C(A/G)TTNNGNCGC] that had previously been elaborated with a SELEX binding-site selection assay in our group (15) because a variable spacing and a more degenerate right-half site (GNCNC) had to be taken into account. It is, however, rather similar to the (C/T)(G/C)CGG(C/G)-N0-10-G(T/A)C(G/A)(C/A) motif that has been proposed for the PrrA transcriptional regulator based on a bioinformatics approach (34).
The similarly high G+C content of the RegR binding site and the B. japonicum genome sequence (64.1%) plus the variably spaced half-sites of RBBs impair in silico motif searches in the B. japonicum genome. Nevertheless, we have used a matrix based on the seven experimentally verified RBBs which share the 5-nt spacing between the half-sites (Fig. 4) to search for similar motifs in putative promoter regions of the entire B. japonicum genome (see Materials and Methods). The cutoff threshold was defined by RBB2614 which showed the lowest score of all RBBs used for generating the matrix. Using this strategy, putative RegR binding motifs were identified within 500 bp upstream of 226 individual genes or operons. Remarkably, 47 of them (41 new plus 6 previously known promoters) are associated with genes or operons whose expression is positively regulated by RegR at least under one of the conditions tested in this study (see Table S2 in the supplemental material). The fact that we identified only a minor fraction of RegR-regulated promoters can be explained by (i) our stringent definition of the cutoff value, (ii) the restriction to motifs with a 5-nt spacer, and (iii) indirect control via RegR-dependent regulators (e.g., NifA).
Location of RegR binding sites in individual promoter regions.
The new RegR binders determined above were examined more closely with respect to the distance between RBB and the annotated translation start site. In six of the eight cases (RBB1515, RBB1883, RBB2614, RBB4182, RBB2501-1, and RBB2501-2), the RBBs are located in a reasonable distance relative to the putative start codon (−45 to −223 bp) so that a promoter sequence can be accommodated in between and that the RBB can function as an upstream activating sequence for transcriptional regulation of the downstream gene. We sought for confirmation of this inference in at least one case by mapping the transcription start site of blr1515 (Fig. 5A and B). A prominent cDNA as the primer extension product was obtained with wild-type RNA but not with RNA extracted from ΔregR cells (Fig. 5B), thus validating the RegR-dependent expression seen in microarrays. The mapped blr1515 transcription start site is positioned 53 nucleotides downstream of the center of RBB1515. Putative −35/−10 promoter elements were also identified (Fig. 5A).
FIG. 5.
Analysis of the promoter regions of selected RegR-dependent genes. (A) DNA sequence comparison of the blr1515, bll2087, and bll2109 promoter regions. Gray boxes mark the oligonucleotide sequences used in EMSAs (Fig. 3), and nucleotides that form the putative RegR binding sites are shown in white letters. Transcriptional start sites (+1) are indicated by an arrowhead above the blr1515 and bll2087 promoter sequences. Putative −10 and −35 promoter elements are underscored by dashed lines. Start codons annotated in the B. japonicum genome database (http://www.kazusa.or.jp/rhizobase) are shown as ATGa, whereas underlined codons with superscripts 1, 2, or 3 denote putative alternative translation start sites. (B) Transcription start site mapping of blr1515. RNA for primer extension was isolated from aerobically grown B. japonicum wild-type (wt) and ΔregR cells. Extension products obtained with the 32P-labeled primer 1515-P1 were separated on a 6% denaturing polyacrylamide gel loaded next to a sequencing ladder generated with plasmid pRJ9562 and the same primer. The relevant sequence of the blr1515 promoter region is shown on the left with the transcription start site emphasized (+1, arrowhead).
The location of RegR binding sites was exceptional in two cases (RBB2087 and RBB2109) where they overlapped with the annotated translation start sites of bll2087 and bll2109 (Fig. 5A). In fact, we had previously mapped the transcription start site of bll2087 downstream of the annotated start codon (24), which then placed RBB2087 at position −44 relative to the transcription start site and helped identify an alternative start codon for bll2087 (Fig. 5A).
Unfortunately, we did not succeed in mapping the bll2109 transcription start site. Yet from β-galactosidase activity assays with a set of translational bll2109′-′lacZ fusions, we obtained strong evidence that translation of bll2109 is initiated at one of the two alternative start codons, ATG2 and GTG3 (superscripts indicate alternative start codons) (Fig. 5A), located >100 bp downstream of the originally annotated start codon (data not shown). Hence, the distant location of RBB2109 to either ATG2 or GTG3 is more likely of functional relevance.
In vitro transcription of RegR target genes.
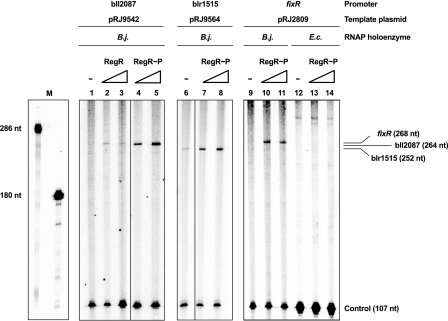
To test direct target gene activation by RegR, we performed in vitro transcription assays with purified B. japonicum RNA polymerase (RNAP) and RegR protein. When a promoter-containing bll2087 fragment was used as a template, RNAP alone was unable to synthesize detectable amounts of specific transcript from this promoter (Fig. 6, lane 1), whereas the addition of purified, His-tagged RegR (Fig. 6, lanes 2 [1 μM RegR] and 3 [3 μM RegR]) resulted in detectable transcription, yielding a transcript of the expected 264-nt length. The use of equal amounts of RegR that had been phosphorylated with acetyl phosphate (RegR∼P) led to a noticeable enhancement of the bll2087 transcript level (lanes 4 and 5), demonstrating that phosphorylation stimulates transcription activation activity of RegR. In all cases, transcript formation from the RNA I control promoter (107 nt) was synthesized independently of RegR.
FIG. 6.
In vitro transcription activated by RegR. Transcripts from the template plasmids pRJ9542, pRJ9564, and pR2809 comprising the bll2087, blr1515, and fixR promoter regions, respectively, were synthesized by multiple-round in vitro transcription using purified B. japonicum (B.j.) or E. coli (E.c.) RNAP holoenzyme and either untreated RegR or RegR∼P, as indicated. RegR(∼P) concentrations were as follows: no protein (lanes 1, 6, 9, and 12), 1 μM (lanes 2, 4, 7, 10, and 13), and 3 μM (lanes 3, 5, 8, 11, and 14). Transcripts were separated on 6% polyacrylamide gels and visualized with a phosphorimager. RNA size markers (M) were generated as described in Materials and Methods. The expected sizes of specific transcripts and of the control transcript are indicated on the right. The transcription products generated from the individual template plasmids were run on separate gels.
In contrast to the bll2087 template, the blr1515 template was already transcribed at a basal level by RNAP in the absence of RegR (Fig. 6, lane 6); however, transcription was stimulated upon addition of RegR, and it was maximal when RegR∼P was used, demonstrating that RegR acts as a direct transcriptional activator also at the blr1515 promoter (Fig. 6, lanes 7 and 8; also data not shown).
The third example shown in Fig. 6 is the well-known RegR target fixR: no in vitro transcript was detectable without RegR, whereas a transcript of the expected length (268 nt) was synthesized in the presence of RegR∼P (Fig. 6, lanes 9 to 11). This demonstrates for the first time that RegR is sufficient to activate transcription from the fixR (P2) promoter. Applying primer extension, the transcription start points of the in vitro synthesized transcripts from the bll2087, blr1515, and fixR promoters were the same as determined in vivo for all three genes (data not shown).
Not shown in Fig. 6 is the equally efficient RegR-dependent in vitro activation of 4 out of 10 additionally tested promoters (those of bll1285, blr2501, bll4833, and bll5807). The absence of detectable transcripts synthesized from the blr1883 (rpoN1), bll2109, blr2614, bll3193, blr6267, and bll6633 promoters might be explained by the requirement for either an alternative transcription factor, whose synthesis in vivo would then depend on RegR, or another factor not present in the in vitro reactions. Also, it cannot be ruled out that in these six cases the promoters are too weak to result in efficient transcription. Indeed, microarray signal intensities for transcripts originating from these promoters are lower than those for which in vitro transcription was easily detectable. Alternatively, the (cryptic) promoters might not be entirely present on the fragment cloned in the template plasmids.
The presence of putative −35/−10 promoter elements in the fixR promoter (3) and the bll2087 and blr1515 promoters (Fig. 5A) suggests that they are recognized by the B. japonicum primary sigma factor (σ80) which is dominant in the RNAP preparation. In a previous work it was shown that promoter elements recognized by the B. japonicum RNAP-σ80 complex are similar to those recognized by E. coli RNAP-σ70 (6). Accordingly, E. coli RNAP-σ70 was able to transcribe from the B. japonicum rrn housekeeping promoter. However, as exemplified by the fixR promoter (Fig. 6, lanes 12 to 14), no transcripts corresponding to the transcripts produced by the B. japonicum RNAP were retrieved from all of the seven RegR-dependent promoters when E. coli RNAP was used in the in vitro assays. E. coli RNAP may not, therefore, be able to recognize these promoters and/or is unable to interact with RegR. In contrast, Karls and coworkers (28) have reported that a constitutively active mutant variant of RegA (RegA*) was able to activate in vitro transcription from the cycA P2 promoter, using either the R. capsulatus or the E. coli RNAP containing σ70. The mutation is supposed to change RegA* conformation such that it mimics the phosphorylated state of wild-type RegA; however, it cannot be ruled out that the mutation facilitates RegA* interaction with E. coli RNAP, which may not work productively with RegA∼P.
Concluding remarks.
The results reported here have put forth a number of important facets of the global regulatory role played by the RegSR system in B. japonicum. We identified several genes encoding proteins involved in oxidative and reductive pathways as members of the RegR regulon. Yet there are RegR targets like the large group of transporters, which cannot be placed easily into the context of redox-related functions. Apparently, functional diversity of target genes is a common attribute of RegR-type regulators in the different proteobacterial species. Our data also demonstrate that RegSR is an important regulatory system for the B. japonicum-soybean symbiosis. Moreover, numerous genes were identified which are not at the same time dependent on the subordinate NifA protein, which strongly suggests that the symbiotic phenotype of the regR mutant cannot solely be attributed to its control of nifA expression. Such genes will be attractive targets for deletion-insertion mutagenesis in future work. Given the high number of regulatory genes that were identified as members of the RegR regulon, we now have to envisage a much greater complexity of regulatory networks in which RegR is integrated. Finally, a significant advancement was made in this study by the demonstration of direct activation of the fixR-nifA promoter as well as several novel promoters by RegR in vitro. The systematic application of these techniques to other candidates found by microarray analysis to be RegR dependent will presumably expand the bona fide RegR regulon even further.
Acknowledgments
We thank Christian H. Ahrens, Andrea Patrignani, Hubert Rehrauer, Ulrich Wagner and Ralph Schlapbach (Functional Genomics Center Zürich) for advice and assistance in the microarray experiments. Socorro Mesa is greatly acknowledged for advice in transcription experiments.
Financial support for this work was provided by the Swiss National Foundation for Scientific Research and the ETH, Zürich, Switzerland, through research programs for the Functional Genomics Center Zürich.
Footnotes
Published ahead of print on 19 October 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alexeyev, M. F. 1995. Three kanamycin resistance gene cassettes with different polylinkers. BioTechniques 18:52-54. [PubMed] [Google Scholar]
- 2.Babst, M., H. Hennecke, and H. M. Fischer. 1996. Two different mechanisms are involved in the heat-shock regulation of chaperonin gene expression in Bradyrhizobium japonicum. Mol. Microbiol. 19:827-839. [DOI] [PubMed] [Google Scholar]
- 3.Barrios, H., H. M. Fischer, H. Hennecke, and E. Morett. 1995. Overlapping promoters for two different RNA polymerase holoenzymes control Bradyrhizobium japonicum nifA expression. J. Bacteriol. 177:1760-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrios, H., R. Grande, L. Olvera, and E. Morett. 1998. In vivo genomic footprinting analysis reveals that the complex Bradyrhizobium japonicum fixR-nifA promoter region is differently occupied by two distinct RNA polymerase holoenzymes. Proc. Natl. Acad. Sci. USA 95:1014-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer, E., T. Kaspar, H. M. Fischer, and H. Hennecke. 1998. Expression of the fixR-nifA operon in Bradyrhizobium japonicum depends on a new response regulator, RegR. J. Bacteriol. 180:3853-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck, C., R. Marty, S. Kläusli, H. Hennecke, and M. Göttfert. 1997. Dissection of the transcription machinery for housekeeping genes of Bradyrhizobium japonicum. J. Bacteriol. 179:364-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burse, A., H. Weingart, and M. S. Ullrich. 2004. The phytoalexin-inducible multidrug efflux pump AcrAB contributes to virulence in the fire blight pathogen, Erwinia amylovora. Mol. Plant-Microbe Interact. 17:43-54. [DOI] [PubMed] [Google Scholar]
- 8.Chang, Y. Y., A. Y. Wang, and J. E. Cronan, Jr. 1993. Molecular cloning, DNA sequencing, and biochemical analyses of Escherichia coli glyoxylate carboligase. J. Biol. Chem. 268:3911-3919. [PubMed] [Google Scholar]
- 9.Comolli, J. C., and T. J. Donohue. 2002. Pseudomonas aeruginosa RoxR, a response regulator related to Rhodobacter sphaeroides PrrA, activates expression of the cyanide-insensitive terminal oxidase. Mol. Microbiol. 45:755-768. [DOI] [PubMed] [Google Scholar]
- 10.Crooks, G. E., G. Hon, J. M. Chandonia, and S. E. Brenner. 2004. WebLogo: a sequence logo generator. Genome Res. 14:1188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon, R., and D. Kahn. 2004. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2:621-631. [DOI] [PubMed] [Google Scholar]
- 12.Elsen, S., L. R. Swem, D. L. Swem, and C. E. Bauer. 2004. RegB/RegA, a highly conserved redox-responding global two-component regulatory system. Microbiol. Mol. Biol. Rev. 68:263-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emmerich, R., H. Hennecke, and H. M. Fischer. 2000. Evidence for a functional similarity between the two-component regulatory systems RegSR, ActSR, and RegBA (PrrBA) in α-Proteobacteria. Arch. Microbiol. 174:307-313. [DOI] [PubMed] [Google Scholar]
- 14.Emmerich, R., K. Panglungtshang, P. Strehler, H. Hennecke, and H. M. Fischer. 1999. Phosphorylation, dephosphorylation and DNA-binding of the Bradyrhizobium japonicum RegSR two-component regulatory proteins. Eur. J. Biochem. 263:455-463. [DOI] [PubMed] [Google Scholar]
- 15.Emmerich, R., P. Strehler, H. Hennecke, and H. M. Fischer. 2000. An imperfect inverted repeat is critical for DNA binding of the response regulator RegR of Bradyrhizobium japonicum. Nucleic Acids Res. 28:4166-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenner, B. J., R. P. Tiwari, W. G. Reeve, M. J. Dilworth, and A. R. Glenn. 2004. Sinorhizobium medicae genes whose regulation involves the ActS and/or ActR signal transduction proteins. FEMS Microbiol. Lett. 236:21-31. [DOI] [PubMed] [Google Scholar]
- 17.Fischer, H. M., M. Babst, T. Kaspar, G. Acuña, F. Arigoni, and H. Hennecke. 1993. One member of a groESL-like chaperonin multigene family in Bradyrhizobium japonicum is co-regulated with symbiotic nitrogen fixation genes. EMBO J. 12:2901-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, R., T. R. Mack, and A. M. Stock. 2007. Bacterial response regulators: versatile regulatory strategies from common domains. Trends Biochem. Sci. 32:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Pasayo, R., and E. Martinez-Romero. 2000. Multiresistance genes of Rhizobium etli CFN42. Mol. Plant-Microbe Interact. 13:572-577. [DOI] [PubMed] [Google Scholar]
- 20.Göttfert, M., P. Grob, and H. Hennecke. 1990. Proposed regulatory pathway encoded by the nodV and nodW genes, determinants of host specificity in Bradyrhizobium japonicum. Proc. Natl. Acad. Sci. USA 87:2680-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Göttfert, M., S. Hitz, and H. Hennecke. 1990. Identification of nodS and nodU, two inducible genes inserted between the Bradyrhizobium japonicum nodYABC and nodIJ genes. Mol. Plant-Microbe Interact. 3:308-316. [DOI] [PubMed] [Google Scholar]
- 22.Gregor, J., T. Zeller, A. Balzer, K. Haberzettl, and G. Klug. 2007. Bacterial regulatory networks include direct contact of response regulator proteins: interaction of RegA and NtrX in Rhodobacter capsulatus. J. Mol. Microbiol. Biotechnol. 13:126-139. [DOI] [PubMed] [Google Scholar]
- 23.Hahn, M., and H. Hennecke. 1984. Localized mutagenesis in Rhizobium japonicum. Mol. Gen. Genet. 193:46-52. [Google Scholar]
- 24.Hauser, F., A. Lindemann, S. Vuilleumier, A. Patrignani, R. Schlapbach, H. M. Fischer, and H. Hennecke. 2006. Design and validation of a partial-genome microarray for transcriptional profiling of the Bradyrhizobium japonicum symbiotic gene region. Mol. Genet. Genomics 275:55-67. [DOI] [PubMed] [Google Scholar]
- 25.Hauser, F., G. Pessi, M. Friberg, C. Weber, N. Rusca, A. Lindemann, H. M. Fischer, and H. Hennecke. 2007. Dissection of the Bradyrhizobium japonicum NifA+σ54 regulon, and identification of a ferredoxin gene (fdxN) for symbiotic nitrogen fixation. Mol. Genet. Genomics 278:255-271. [DOI] [PubMed] [Google Scholar]
- 26.Ismail, W., M. El-Said Mohamed, B. L. Wanner, K. A. Datsenko, W. Eisenreich, F. Rohdich, A. Bacher, and G. Fuchs. 2003. Functional genomics by NMR spectroscopy. Phenylacetate catabolism in Escherichia coli. Eur. J. Biochem. 270:3047-3054. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko, T., Y. Nakamura, S. Sato, K. Minamisawa, T. Uchiumi, S. Sasamoto, A. Watanabe, K. Idesawa, M. Iriguchi, K. Kawashima, M. Kohara, M. Matsumoto, S. Shimpo, H. Tsuruoka, T. Wada, M. Yamada, and S. Tabata. 2002. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9:189-197. [DOI] [PubMed] [Google Scholar]
- 28.Karls, R. K., J. R. Wolf, and T. J. Donohue. 1999. Activation of the cycA P2 promoter for the Rhodobacter sphaeroides cytochrome c2 gene by the photosynthesis response regulator. Mol. Microbiol. 34:822-835. [DOI] [PubMed] [Google Scholar]
- 29.Kim, Y.-J., I.-J. Ko, J.-M. Lee, H.-Y. Kang, Y. M. Kim, S. Kaplan, and J.-I. Oh. 2007. Dominant role of the cbb3 oxidase in regulation of photosynthesis gene expression through the PrrBA system in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 189:5617-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45-55. [DOI] [PubMed] [Google Scholar]
- 31.Mackenzie, C., J. M. Eraso, M. Choudhary, J. H. Roh, X. Zeng, P. Bruscella, A. Puskas, and S. Kaplan. 2007. Post-genomic adventures with Rhodobacter sphaeroides. Annu. Rev. Microbiol. 61:283-307. [DOI] [PubMed] [Google Scholar]
- 32.Malpica, R., B. Franco, C. Rodriguez, O. Kwon, and D. Georgellis. 2004. Identification of a quinone-sensitive redox switch in the ArcB sensor kinase. Proc. Natl. Acad. Sci. USA 101:13318-13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malpica, R., G. R. Sandoval, C. Rodriguez, B. Franco, and D. Georgellis. 2006. Signaling by the Arc two-component system provides a link between the redox state of the quinone pool and gene expression. Antioxid. Redox Signal. 8:781-795. [DOI] [PubMed] [Google Scholar]
- 34.Mao, L., C. Mackenzie, J. H. Roh, J. M. Eraso, S. Kaplan, and H. Resat. 2005. Combining microarray and genomic data to predict DNA binding motifs. Microbiology 151:3197-3213. [DOI] [PubMed] [Google Scholar]
- 35.Mascher, T., J. D. Helmann, and G. Unden. 2006. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70:910-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masuda, S., Y. Matsumoto, K. V. Nagashima, K. Shimada, K. Inoue, C. E. Bauer, and K. Matsuura. 1999. Structural and functional analyses of photosynthetic regulatory genes regA and regB from Rhodovulum sulfidophilum, Roseobacter denitrificans, and Rhodobacter capsulatus. J. Bacteriol. 181:4205-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mesa, S., E. J. Bedmar, A. Chanfon, H. Hennecke, and H. M. Fischer. 2003. Bradyrhizobium japonicum NnrR, a denitrification regulator, expands the FixLJ-FixK2 regulatory cascade. J. Bacteriol. 185:3978-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mesa, S., Z. Ucurum, H. Hennecke, and H. M. Fischer. 2005. Transcription activation in vitro by the Bradyrhizobium japonicum regulatory protein FixK2. J. Bacteriol. 187:3329-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 40.Mwangi, M. M., and E. D. Siggia. 2003. Genome wide identification of regulatory motifs in Bacillus subtilis. BMC Bioinformatics 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nellen-Anthamatten, D., P. Rossi, O. Preisig, I. Kullik, M. Babst, H. M. Fischer, and H. Hennecke. 1998. Bradyrhizobium japonicum FixK2, a crucial distributor in the FixLJ-dependent regulatory cascade for control of genes inducible by low oxygen levels. J. Bacteriol. 180:5251-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh, J. I., I. J. Ko, and S. Kaplan. 2004. Reconstitution of the Rhodobacter sphaeroides cbb3-PrrBA signal transduction pathway in vitro. Biochemistry 43:7915-7923. [DOI] [PubMed] [Google Scholar]
- 43.Palumbo, J. D., C. I. Kado, and D. A. Phillips. 1998. An isoflavonoid-inducible efflux pump in Agrobacterium tumefaciens is involved in competitive colonization of roots. J. Bacteriol. 180:3107-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pauly, N., C. Pucciariello, K. Mandon, G. Innocenti, A. Jamet, E. Baudouin, D. Herouart, P. Frendo, and A. Puppo. 2006. Reactive oxygen and nitrogen species and glutathione: key players in the legume-Rhizobium symbiosis. J. Exp. Bot. 57:1769-1776. [DOI] [PubMed] [Google Scholar]
- 45.Pessi, G., C. Ahrens, H. Rehrauer, A. Lindemann, F. Hauser, H. M. Fischer, and H. Hennecke. 2007. Genome-wide transcript analysis of Bradyrhizobium japonicum bacteroids in soybean root nodules. Mol. Plant-Microbe Interact. 20:1353-1363. [DOI] [PubMed] [Google Scholar]
- 46.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:2002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piddock, L. J. 2006. Multidrug-resistance efflux pumps—not just for resistance. Nat. Rev. Microbiol. 4:629-636. [DOI] [PubMed] [Google Scholar]
- 48.Poole, K., K. Krebes, C. McNally, and S. Neshat. 1993. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Regensburger, B., and H. Hennecke. 1983. RNA polymerase from Rhizobium japonicum. Arch. Microbiol. 135:103-109. [DOI] [PubMed] [Google Scholar]
- 50.Rey, F. E., Y. Oda, and C. S. Harwood. 2006. Regulation of uptake hydrogenase and effects of hydrogen utilization on gene expression in Rhodopseudomonas palustris. J. Bacteriol. 188:6143-6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sciotti, M. A., A. Chanfon, H. Hennecke, and H. M. Fischer. 2003. Disparate oxygen responsiveness of two regulatory cascades that control expression of symbiotic genes in Bradyrhizobium japonicum. J. Bacteriol. 185:5639-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon, R., U. Priefer, and A. Pühler. 1983. Vector plasmids for in vivo and in vitro manipulation of gram-negative bacteria, p. 98-106. In A. Pühler (ed.), Molecular genetics of the bacteria-plant interaction. Springer-Verlag, Heidelberg, Germany.
- 53.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 54.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 55.Swem, L. R., X. Gong, C. A. Yu, and C. E. Bauer. 2006. Identification of a ubiquinone-binding site that affects autophosphorylation of the sensor kinase RegB. J. Biol. Chem. 281:6768-6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thöny, B., D. Anthamatten, and H. Hennecke. 1989. Dual control of the Bradyrhizobium japonicum symbiotic nitrogen fixation regulatory operon fixR nifA: analysis of cis- and trans-acting elements. J. Bacteriol. 171:4162-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiwari, R. P., W. G. Reeve, B. J. Fenner, M. J. Dilworth, A. R. Glenn, and J. G. Howieson. 2004. Probing for pH-regulated genes in Sinorhizobium medicae using transcriptional analysis. J. Mol. Microbiol. Biotechnol. 7:133-139. [DOI] [PubMed] [Google Scholar]
- 58.Zufferey, R., O. Preisig, H. Hennecke, and L. Thöny-Meyer. 1996. Assembly and function of the cytochrome cbb3 oxidase subunits in Bradyrhizobium japonicum. J. Biol. Chem. 271:9114-9119. [DOI] [PubMed] [Google Scholar]