Abstract
Microcinematography was used to examine fruiting body development of Myxococcus xanthus. Wild-type cells progress through three distinct phases: a quiescent phase with some motility but little aggregation (0 to 8 h), a period of vigorous motility leading to raised fruiting bodies (8 to 16 h), and a period of maturation during which sporulation is initiated (16 to 48 h). Fruiting bodies are extended vertically in a series of tiers, each involving the addition of a cell monolayer on top of the uppermost layer. A pilA (MXAN_5783) mutant produced less extracellular matrix material and thus allowed closer examination of tiered aggregate formation. A csgA (MXAN_1294) mutant exhibited no quiescent phase, aberrant aggregation in phase 2, and disintegration of the fruiting bodies in the third phase.
When nutrients become limiting, swarms of Myxococcus xanthus cells direct their movement into a fruiting body containing approximately 50,000 cells. Inside the nascent fruiting body, cells differentiate into dormant myxospores. Fruiting body morphogenesis requires temporal and spatial coordination of motility. Much of the research in the field is devoted to understanding the signaling mechanisms coordinating development (for reviews, see references 3 and 17). Little is known about the spatial dynamics of fruiting body construction.
Fruiting bodies vary from simple mounds in the case of M. xanthus and Myxococcus fulvus to the branched structures of Stigmatella aurantiaca and Chondromyces crocatus (see pictures in reference 3). Some experiments examining aspects of fruiting body morphogenesis have tracked fluorescently labeled cells within the fruiting body. Most studies focused on the layer of cells in contact with the agar/plastic substrate. One study suggests that developing rods in outer regions of fruiting bodies move with sufficient force to displace sporulating cells to an inner domain, thereby localizing spores in the core of the fruiting body (16). In other studies, streams of M. xanthus cells appear to collide with one another, forming “traffic jams” that create the nucleus for aggregation centers, and continued streaming around the aggregation centers leads to a mound structure (7, 19, 20). One study found that cells entering an aggregation center suffered a reduction in velocity, and mathematical modeling showed that the velocity reduction was sufficient to capture cells in an aggregation focus (18). While these studies offer plausible explanations regarding how aggregation centers become established, none of them observe the vertical formation of a fruiting body. Additionally, it is unclear if the mechanism of aggregation is consistent under different culturing conditions.
This study examined fruiting body formation from the top by using microcinematography. Strains of M. xanthus were grown in Casitone-yeast extract broth (9) at 32°C. Cells were harvested by centrifugation at 12,000 × g for 5 min and resuspended to a density of 5 × 109 cells ml−1 in Casitone-yeast extract broth. A microscope chamber with an air space was prepared. A sterile 0.5-mm-thick silicone rubber gasket (Grace Bio-Labs) was placed on top of a flame-sterilized 25- by 25-mm glass microscope coverslip, creating a small well. The well was filled with 0.4 ml molten TPM agarose (10 mM Tris HCl, 8 mM MgSO4, 1 mM KHPO4-KH2PO4, 1.0% low-melting-point agarose [Bio-Rad], pH 7.6), and a flame-sterilized glass microscope slide was placed on top to create a flat agar surface. Once the agarose cooled, the slide was carefully removed to not disrupt the agar surface, and 0.5 μl of cell culture was spotted on the surface and allowed to dry. The circular spot, approximately 2 mm in diameter, contained roughly 2.5 × 106 cells that formed slightly more than a monolayer. A second gasket was placed on top of a sterilized microscope slide. The two gaskets were gently pressed to seal the chamber. The cell spot was viewed on a Leitz Laborlux D phase-contrast microscope under a magnification of ×160. The chamber temperature was maintained at 32°C using a Biostage 600 stage warmer (20/20 Technology, Inc.). Photographs were taken every 5 min for 48 h with a Spot Insight 2 camera using SPOT software v4.5 (Diagnostic Instruments, Inc.), and movies were compiled from the images using QuickTime Pro (Apple). Each strain in the study was photographed at least twice.
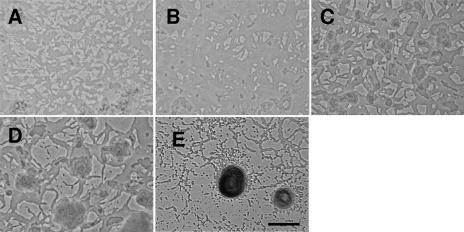
Wild-type M. xanthus DK1622 (6) cells progress through three distinct phases (Fig. 1, and see movie S1 in the supplemental material). In the first phase, cells haphazardly stacked during the drying process (Fig. 1A) rearrange themselves into stable cell layers (Fig. 1B). While some cell motility is observed during this phase, there is little change in the pattern of the cell layers. After approximately 6 to 8 h, the second phase begins with a dramatic burst of cell movement. Cell swarms coalesce, leading to the formation of many small cell towers about three cell layers thick (Fig. 1C). These may represent independent aggregation foci that compete with each other for the remaining cells. Only a fraction of cell towers become fruiting bodies. Successful aggregation centers (Fig. 1D) begin to fill with cells dispersing from the small towers. The mechanism for determining which cell towers remain and which disperse is unknown. Fruiting bodies are extended vertically in a series of tiers, each involving the addition of a cell monolayer on top of the uppermost layer. Cells emerge from a spot in the interior of the uppermost layer of cells and expand over the layer. Even as the new layer is recruiting cells, cells emerge from a similar spot in that layer (now the new bottom layer), again spreading in an even matte. At the same time that new layers form, the bottom layers continue to expand outward so that the entire structure resembles a circular steppe. The tiering can be observed for only a short period of time before individual layers become difficult to discern due to material that encapsulates the fruiting body. This second phase lasts approximately 8 h. During the last phase, the fruiting bodies darken (an indication of sporulation), and there are fewer cells outside the fruiting body (Fig. 1E).
FIG. 1.
Stages of fruiting body formation in wild-type M. xanthus. (A) Initial organization of cells after being spotted on an agarose surface (0 h). (B) Rearrangement of cells into a matte (3 h). (C) Towers of cells only a few cell layers thick (8 h). (D) Aggregation centers are larger towers of cells composed of many cell layers (10 h). (E) Mature fruiting body (48 h). Bar, 0.1 mm. Pictures are derived from two separate movies and are not spatially aligned. This figure contains some frames from Movie S1 in the supplemental material.
Because tier formation can be observed for only a short period of time before cells become obscured, a pilA mutant, which produces much less extracellular matrix (ECM) material but still forms fruiting bodies, was examined. PilA is the major structural protein in the type IV pilus. Pili are used for twitching (S) motility (13, 15), one of two genetically separable motility systems in M. xanthus. Either of the two motility systems is sufficient for fruiting body development. During S motility, the pilus extends, attaches to the ECM of an adjacent cell (14), and retracts to pull the cell forward. pilA (MXAN_5783) mutants lack pili and S motility but form fruiting bodies using adventurous (A) motility. Though pilA mutants are forced to move using only one of the motility systems (and therefore cells of this strain interact in a way that is very different than that of wild-type cells), they are able to form fruiting bodies and sporulate as well as the wild type (2). PilA is also required for ECM production (1).
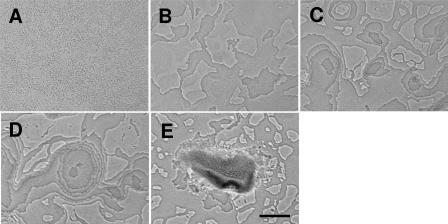
ΔpilA mutants (21) take longer to form fruiting bodies, but the same progression of phases is observed during the development of the ΔpilA mutant DK10410 (Fig. 2, and see movie S2 in the supplemental material). Stable cell mattes form with little coordinated movement (phase 1) (Fig. 2A and B). A burst of motile activity leads to aggregation center formation (phase 2) (Fig. 2C). The burst of activity at approximately 7 to 8 h is subdued compared to the activity of wild-type cells, possibly due to the loss of S motility. The tiering is visible for a longer period of time for the ΔpilA mutant cells, possibly because the ΔpilA mutant produces much less ECM material (1) (Fig. 2D). Cells usually emerge from the lower layer at a single location, though occasionally a second origin point is observed within the same layer (Fig. 2D). The rate of cell accumulation on a new tier is too rapid to be attributed to cell division; even under optimal growth conditions, the doubling time is about 4 h. Rather, it appears as if cells are recruited from a lower layer. Eventually the layers become obscured by ECM material, and the third phase (maturation) begins (Fig. 2E).
FIG. 2.
Stages of fruiting body formation in the pilA mutant. (A) Initial organization of cells after being spotted on agarose (0 h). (B) Rearrangement of cells into a matte (6 h). (C) Towers only three to four cell layers thick (9 h). (D) pilA mutant aggregation centers display tiers for a longer period of time than wild-type cells before becoming obscured by ECM material. Note that an aggregation center has two origins for a new tier, which is not often observed (12 h). (E) Mature fruiting body. pilA mutant fruiting bodies are often less compact and hemispherical than wild-type fruiting bodies (48 h). Bar, 0.1 mm. Pictures are derived from the same movie but not spatially aligned. The original movie on which this figure is based is available as Movie S2 in the supplemental material.
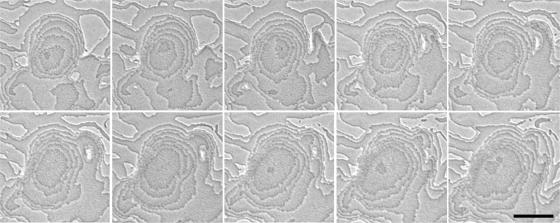
Tiering is observed at a higher resolution with the ΔpilA mutant in Fig. 3. New layers begin near the center of an existing layer, though cell expansion on new layers can be asymmetrical, making it difficult to determine if the point where a new layer emerges is conserved from layer to layer (Fig. 3). As many as nine layers can be observed in the pilA mutant (Fig. 3). The pilA mutant lacks pili and S motility and has less ECM material, so ΔpilA cells interact with one another in ways that are fundamentally different than those of wild-type cells yet produce the same phases and patterns.
FIG. 3.
Tier formation during pilA mutant fruiting body development. Two successive tiers form in the same fruiting body (min 620 to 710 in Movie S2 in supplemental material, 10 min between frames). Pictures are spatially aligned. Bar, 0.1 mm.
The film of pilA mutant development (see movie S2 in the supplemental material) illustrates two additional features of fruiting body formation. First, the process of tiering that is used to construct a fruiting body can be reversed to disassemble a fruiting body. This is most dramatically illustrated in a fruiting body that begins to form in the upper left corner of the field and progresses to a late stage of phase 2 before reversing the process tier by tier. The reverse process is most obvious toward the end of disassembly, when the ECM material disappears. The fact that the sites where new tiers originate are similar between layers suggests that there is a vertical structural component to the aggregate that dictates the origin of a new tier. Since aggregates that abort development use the reverse process to disassemble a fruiting body, the vertical component may be maintained for a large portion of fruiting body formation. Second, maturing aggregates many layers high can move as a group across the agar surface to fuse with another aggregate. This point is illustrated by the pair of aggregates just below the center of the frame where the one on the right moves to the left to join the other aggregate (see movie S2 in the supplemental material).
FibA is an ECM-associated zinc metalloprotease implicated in lipid chemotaxis (8), a starvation-dependent behavioral response. fibA (MXAN_6106) pilA mutants do not develop, indicating that FibA mediates a developmental process that complements PilA (2). Therefore, the fibA mutant offers a set of compromised signaling pathways that is different than that of the pilA strain. Pattern formation in a fibA in-frame deletion mutant closely resembles that of wild-type M. xanthus (data not shown), demonstrating that FibA-mediated development uses the same tiering mechanism. The three strains tested (wild type and pilA and fibA mutants) all produce aggregates by the same tiering mechanism, even though the mechanism of interaction and/or suite of signaling capacities in each strain is different. This result suggests that tiering is an essential process in fruiting body morphogenesis.
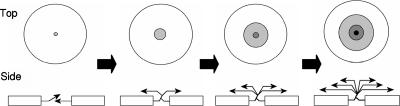
Though the movies in the supplemental material lack enough resolution to determine how tiering begins, previous work helps formulate a plausible hypothesis. In submerged cultures, fruiting bodies form where large swarms moving in opposing directions collide (7). One swarm consequently may develop a slight vertical pitch as it attempts to migrate over another (Fig. 4). The slight vertical pitch of this discontinuity could act as an inclined plane to direct cells upward to form a new tier. If cells enter the inclined plane faster than they can be assimilated by the expanding tier, they could extend the inclined plane to form a new tier, thereby preserving the discontinuity in each layer. If cells from another colliding swarm also develop a slight vertical pitch, the expanding tier could expand symmetrically as depicted in Fig. 4. Both symmetric and asymmetric expansions have been observed. From this hypothesis, the tier origin would be a localized area with a slight vertical pitch that could be perpetuated by recursive traffic. In the reverse process, cells dispersing from an aggregate could follow the trail back down.
FIG. 4.
Model for tier formation in M. xanthus. The panels represent a sequence of events visualized from left to right. The top portion of each panel displays a top-down view of aggregates, while the bottom portion of the panel shows a side view of the aggregate. The blocks in the side view represent cell swarms traveling along the agar surface. In the first panel, colliding swarms moving in opposite directions intersect and cause one of the swarms to orient at a slight vertical pitch to cross over the other swarm (left arrow), generating an inclined plane. As the swarms continue to converge, cells stream along this vertical alignment and then realign horizontally with the cells below them, thereby forming the first tier (second panel from left). More cells stream into the first tier, increasing its surface area. In addition, some cells continue the vertical ascension through the first tier to initiate the second tier (third panel). This process can continue to form multiple tiers (fourth panel).
Tiering is not unique to fruiting body development. A less dramatic form of tiering is observed during movement in vegetative colonies but is confined to one or two tiers above the median cell level. Tier formation during fruiting body morphogenesis differs in that the tiers have boundaries that define the width of the fruiting body and stack to great heights. It is possible that fruiting body morphogenesis is an adaptation of vegetative tier formation that has been embellished to add more tiers and control their dimensions.
Development requires an extracellular signal that is mediated by CsgA and is part of the central timer during development. Low levels of CsgA induce aggregation and early developmental gene expression, whereas high levels induce sporulation and late developmental gene expression (4, 10-12). Though csgA mutants cannot complete the developmental program, on agar surfaces they form unusually large aggregates that never mature into fruiting bodies (12). In order to maintain consistency with the mutants analyzed, the first markerless csgA in-frame deletion mutant was constructed and pattern formation during development was examined by microcinematography to determine how the loss of CsgA impacts the three stages of development. A markerless csgA deletion mutant was constructed using a previously described method (5). The primers (listed 5′ to 3′) for mutagenesis were csgA1F (GGA TCC GCT GGC ACC AGG AAC CCA AAG C), csgA1R (TCT AGA CAT GCA TGG CAT TAA CGC GCT GAA TG), csgA2F (TCT AGA CCG TGC CCT CAT GGG TAG AGC ATC), and csgA2R (AAG CTT GTG CGC CAG CGG CCC CGT CTG G).
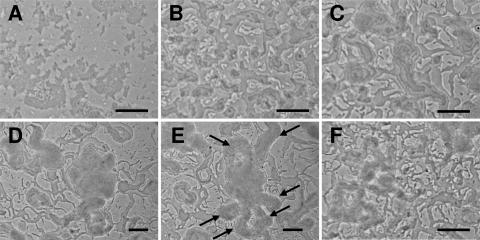
The behavior of the csgA mutant during development is hyperactive (see Movie S3 in the supplemental material). The haphazardly stacked cells (Fig. 5A) never organize into stable mattes as do cells of other strains (Fig. 1B and 2B) but begin forming small cell towers immediately (Fig. 5B). The cell towers constantly dissolve and reform. After approximately 6 h, a change in activity results in the formation of large aggregates. While tiering is evident, the tiering is not spatially confined and takes place in moving swarms (phase 2) (Fig. 5C). Swarms enter the aggregation center to increase the size of the fruiting body (Fig. 5D). However, large swarms also leave the fruiting body (Fig. 5E), leading to fruiting body dissolution (Fig. 5F). The results suggest that the csgA deletion causes a loss of control over swarm behavior, not necessarily tier formation.
FIG. 5.
Fruiting body formation in the csgA mutant. (A) Initial organization of cells after being spotted on agarose (0 h). (B) Instead of cells arranging into a stable matte, tower formation is observed (3 h). (C) Despite the absence of an initial quiescent phase, aggregates are formed at approximately the same time as the wild type (6 h). (D) Unusually large aggregates are formed (16 h). (E) Cells swarm away from large aggregates, often to form new aggregates which also dissolve (17 h). Arrows indicate large flares of cells swarming out of the aggregation center shown in panel D. (F) Constant swarming and reforming of aggregates disrupt fruiting body structure and maturation (45 h). Pictures are derived from the same movie, but with the exception of panels D and E, the pictures are not spatially aligned. Bars, 0.1 mm. The original movie on which this figure is based is available as Movie S3 in the supplemental material.
The three phases suggest that motility is carefully choreographed in the developmental program. The M. xanthus fruiting body is formed initially by stacking many horizontal cell layers. This process is reversible, and fruiting bodies can deconstruct by the reverse mechanism (see Movie S2 in the supplemental material). Large aggregates of cells many tiers high have also been observed to migrate across the agar and fuse with nearby aggregates to form much larger fruiting bodies (for an example, see Movie S2 in the supplemental material). Aggregate fusion is rare in wild-type cells and pilA mutants relative to tier formation. We have documented for the first time two types of directed movement during fruiting body formation, tiering and aggregate fusion, both of which appear to be reversible.
While tiering can explain the initial vertical ascension of fruiting bodies, it does not explain the ultimate spherical shape of the fruiting body, which may be defined by factors other than motility. One possibility is that the ECM material that obscures the tiering in wild-type cells may act as a mesh that determines the volume and shape of the fruiting body. As cells enter the fruiting body from the bottom, they fill up the mesh like a balloon. The covering is reduced in the pilA strain, which may explain why fruiting bodies of the pilA strain are less symmetrical and spherical than those of the wild type. Analysis of other strains deficient in ECM production may test this hypothesis.
Tiering is a methodical way of raising the fruiting body whose genetic components can likely be teased out by microcinematographic studies of developmental mutants. While none of the three mutants examined in this study were defective in tier formation per se, analysis of mutants deficient in aggregation may illuminate the mechanism of tiering during fruiting body formation.
Acknowledgments
We thank Aurelio Moraleda-Munoz for construction of the csgA mutant.
This material is based on work supported by the National Science Foundation under grant 0343874.
Footnotes
Published ahead of print on 5 October 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Black, W. P., Q. Xu, and Z. Yang. 2006. Type IV pili function upstream of the Dif chemotaxis pathway in Myxococcus xanthus EPS regulation. Mol. Microbiol. 61:447-456. [DOI] [PubMed] [Google Scholar]
- 2.Bonner, P. J., W. P. Black, Z. Yang, and L. J. Shimkets. 2006. FibA and PilA act cooperatively during fruiting body formation of Myxococcus xanthus. Mol. Microbiol. 61:1283-1293. [DOI] [PubMed] [Google Scholar]
- 3.Dworkin, M. 1996. Recent advances in the social and developmental biology of the myxobacteria. Microbiol. Rev. 60:70-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jelsbak, L., and L. Sogaard-Andersen. 2002. Pattern formation by a cell surface-associated morphogen in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 99:2032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Julien, B., A. D. Kaiser, and A. Garza. 2000. Spatial control of cell differentiation in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 97:9098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaiser, D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 76:5952-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaiser, D., and R. Welch. 2004. Dynamics of fruiting body morphogenesis. J. Bacteriol. 186:919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearns, D. B., P. J. Bonner, D. R. Smith, and L. J. Shimkets. 2002. An extracellular matrix-associated zinc metalloprotease is required for dilauroyl phosphatidylethanolamine chemotactic excitation in Myxococcus xanthus. J. Bacteriol. 184:1678-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kearns, D. B., and L. J. Shimkets. 1998. Chemotaxis in a gliding bacterium. Proc. Natl. Acad. Sci. USA 95:11957-11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, S. K., and D. Kaiser. 1991. C-factor has distinct aggregation and sporulation thresholds during Myxococcus development. J. Bacteriol. 173:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruse, T., S. Lobedanz, N. M. Berthelsen, and L. Sogaard-Andersen. 2001. C-signal: a cell surface-associated morphogen that induces and co-ordinates multicellular fruiting body morphogenesis and sporulation in Myxococcus xanthus. Mol. Microbiol. 40:156-168. [DOI] [PubMed] [Google Scholar]
- 12.Li, S., B. U. Lee, and L. J. Shimkets. 1992. csgA expression entrains Myxococcus xanthus development. Genes Dev. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 13.Li, Y., R. Lux, A. E. Pelling, J. K. Gimzewski, and W. Shi. 2005. Analysis of type IV pilus and its associated motility in Myxococcus xanthus using an antibody reactive with native pilin and pili. Microbiology 151:353-360. [DOI] [PubMed] [Google Scholar]
- 14.Li, Y., H. Sun, X. Ma, A. Lu, R. Lux, D. Zusman, and W. Shi. 2003. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 100:5443-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu, A., K. Cho, W. P. Black, X. Y. Duan, R. Lux, Z. Yang, H. B. Kaplan, D. R. Zusman, and W. Shi. 2005. Exopolysaccharide biosynthesis genes required for social motility in Myxococcus xanthus. Mol. Microbiol. 55:206-220. [DOI] [PubMed] [Google Scholar]
- 16.Sager, B., and D. Kaiser. 1993. Spatial restriction of cellular differentiation. Genes Dev. 7:1645-1653. [DOI] [PubMed] [Google Scholar]
- 17.Shimkets, L. J. 1999. Intercellular signaling during fruiting-body development of Myxococcus xanthus. Annu. Rev. Microbiol. 53:525-549. [DOI] [PubMed] [Google Scholar]
- 18.Sliusarenko, O., D. R. Zusman, and G. Oster. 2007. Aggregation during fruiting body formation in Myxococcus xanthus is driven by reducing cell movement. J. Bacteriol. 189:611-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sozinova, O., Y. Jiang, D. Kaiser, and M. Alber. 2005. A three-dimensional model of myxobacterial aggregation by contact-mediated interactions. Proc. Natl. Acad. Sci. USA 102:11308-11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sozinova, O., Y. Jiang, D. Kaiser, and M. Alber. 2006. A three-dimensional model of myxobacterial fruiting-body formation. Proc. Natl. Acad. Sci. USA 103:17255-17259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu, S. S., J. Wu, and D. Kaiser. 1997. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol. Microbiol. 23:109-121. [DOI] [PubMed] [Google Scholar]