Abstract
Summary: Membrane proteins responsible for the active efflux of structurally and functionally unrelated drugs were first characterized in higher eukaryotes. To date, a vast number of transporters contributing to multidrug resistance (MDR transporters) have been reported for a large variety of organisms. Predictions about the functions of genes in the growing number of sequenced genomes indicate that MDR transporters are ubiquitous in nature. The majority of described MDR transporters in bacteria use ion motive force, while only a few systems have been shown to rely on ATP hydrolysis. However, recent reports on MDR proteins from gram-positive organisms, as well as genome analysis, indicate that the role of ABC-type MDR transporters in bacterial drug resistance might be underestimated. Detailed structural and mechanistic analyses of these proteins can help to understand their molecular mode of action and may eventually lead to the development of new strategies to counteract their actions, thereby increasing the effectiveness of drug-based therapies. This review focuses on recent advances in the analysis of ABC-type MDR transporters in bacteria.
INTRODUCTION
Bacteria have the incredible ability to develop resistance to the actions of toxic compounds and to adapt rapidly to a changing environment. To a large extent, such developments are due to the enhanced expression of drug resistance systems in these organisms. The introduction of antibiotics in the 20th century was an important step towards the control of infectious diseases. Antimicrobials increased the average life expectancy of humans by drastically reducing the lethal effects of infectious diseases (20). However, quickly after the introduction of antibiotics, bacteria developed various ways to reverse their actions (70, 71, 94, 148). Consequently, diseases which were thought to be under control were again on the upswing. The causative agents of reemerging infectious diseases increasingly withstand the action of drug-based medical treatment by the development of (multi)drug resistance (MDR) mechanisms (20, 70, 71, 94, 137).
Bacteria exhibit a wide range of mechanisms to resist the actions of noxious agents. These systems can be specific for a drug or a group of closely related drugs, such as the enzymatic inactivation of toxic compounds. β-Lactamase, which hydrolyzes the β-lactam ring of antibiotics (43, 56, 146), is a classic example of such a mechanism (for a review, see reference 29). Another mechanism of resistance is the mutational alteration of the drug target to reduce the target's affinity for the drug (43, 56, 146; reviewed in reference 130) or to reduce the permeability of the cell envelope. Bacteria are surrounded by at least one lipid bilayer, the cytoplasmic membrane that, among others, acts as a general barrier to prevent drug influx into the cell. Gram-positive bacteria are surrounded by a thick cell wall made of peptidoglycan which offers only minor resistance to the diffusion of small molecules. Gram-negative bacteria contain an additional membrane, the outer membrane, which is less permeable to lipophilic drugs. Finally, mycobacteria have developed a more effective diffusion barrier consisting of a thick extracellular layer of mycolic acid (reviewed in reference 96). A decrease of the permeability of the cell envelopes of gram-negative bacteria and mycobacteria can be achieved by decreasing the expression of the porins. These proteins serve as entry gates for small molecules, in particular nutrients, but they also facilitate the passage of noxious agents (reviewed in reference 96).
A general strategy to prevent the cellular entry of drugs is their active efflux from the cell or the cytoplasmic membrane (114). These active extrusion mechanisms involve integral membrane proteins that utilize metabolic energy to expel drugs across the cytoplasmic membrane against their concentration gradients. In gram-negative bacteria, active efflux mechanisms can be coupled to extrusion across the outer membrane. In that case, an outer membrane pore is involved, which together with an adaptor protein, forms a bridge between the cytoplasmic membrane and the outer membrane. Active drug efflux mechanisms can be specific for a given drug, so-called single-drug or group-specific efflux systems, or can have a broad substrate specificity covering a wide range of toxic compounds that are structurally and functionally unrelated. The latter process is termed multidrug efflux. Based on bioenergetic criteria, transporters contributing to MDR (MDR transporters) can be classified into two main groups, namely, proton motive force (PMF)- and ATP-dependent transporters. Here we review the current knowledge of bacterial ATP-dependent MDR proteins, with special emphasis on their distribution in bacterial genomes and their functional importance to the MDR phenotype.
What Is MDR?
Antibiotic resistance emerged and was noticed quickly after the introduction of the first antibiotics in medical practice. When other classes of antibiotics were used, the resistant microbes were found to also be resistant to these drugs even though they had never been exposed to them. This indicated that these organisms had developed multiple resistances against a range of drugs. The molecular basis of the MDR phenomenon was first observed in mammalian cells (24, 25, 61, 106). Mammalian cancer cells may develop an MDR phenotype upon exposure to cytotoxic agents used in cancer therapy. The resistance developed protects the cells not only against the drug used in chemotherapy but also against a range of structurally and functionally unrelated toxic agents (74, 136). Obviously, this seriously undermines the success of further drug therapies. The major causes of MDR are efflux pumps such as P-glycoprotein (19; for a review, see reference 6), the MDR-associated protein MRP1 (21; for a review, see reference 66), and the breast cancer resistance protein BCRP (5, 34, 90; for a review, see reference 33), which have been characterized in great detail. During the last decade, a large number of MDR transporters have also been discovered in prokaryotes. The first bacterial drug-specific efflux pump was described by Levy and McMurry in 1978 (72). About 10 years later, a plethora of MDR transporters had been described for bacteria, such as QacA from Staphylococcus aureus (135) and EmrE (79) and AcrAB (85, 86) from Escherichia coli. The multidrug efflux phenomenon is now recognized as an important mechanism contributing to bacterial resistance to valuable clinical antibiotics. MDR bacteria can also display resistance towards cytotoxic agents that they have never encountered, and thus their activity presents a major threat for drug-based clinical treatments.
Classification of MDR Transporters
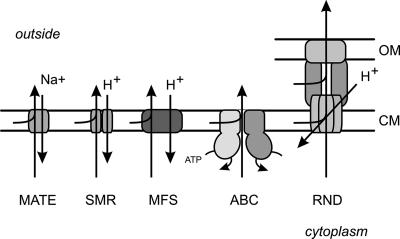
MDR transporters can be classified into the following two main groups according to the mode of energy coupling to drug export across the cytoplasmic and outer membranes (Fig. 1): (i) primary active transporters that belong to the ATP binding cassette (ABC) superfamily and utilize the free energy of ATP hydrolysis to expel the drug from the cell against its concentration gradient and (ii) secondary transporters that utilize the PMF (or sodium motive force) for drug expulsion. The latter systems function as drug/H+ or drug/Na+ antiporters. Secondary MDR transporters can be subdivided further into several families on the basis of their amino acid sequence homology and predicted secondary structure. These are transporters of (i) the major facilitator superfamily (MFS) (87), (ii) the small MDR (SMR) family (108), (iii) the resistance-nodulation-cell division (RND) family (123), and (iv) the multidrug and toxic compound extrusion (MATE) family (16) (Fig. 1). This classification is generally used (73, 114), although other classifications have been proposed as well (120, 122). Several reviews dedicated to the classification and global description of MDR transporter families can be found elsewhere (73, 103, 105, 114, 121, 138). Here we focus on the MDR transporters belonging to the ABC superfamily.
FIG. 1.
Schematic representation of the major families of MDR transporters in prokaryotes. Primary transporters that utilize the energy of ATP hydrolysis belong to the superfamily of ABC transporters. In prokaryotes, these MDR transporters are so-called half-transporters that either homo- or heterodimerize to form a functional unit. Secondary transporters energized by the PMF include MFS members, which are monomeric; SMR family members, which form homo- or hetero-oligomers; and RND family members, which are tripartite systems that consist of an integral membrane transporter domain, a fusion protein (MFP), and an outer membrane protein (OMP). Secondary transporters energized by the sodium motive force include those in the MATE family. OM and CM, outer and cytoplasmic membranes, respectively.
Distribution of MDR Transporters
Historically, the first cloned and characterized MDR transporters were members of the ABC superfamily of eukaryotic origin, such as P-glycoprotein (19) and MRP1 (21). In the early 1990s, the bacterial MDR systems were described for the first time, and these belonged to the group of secondary active transporters (106). The first bacterial ABC-type MDR protein, LmrA from Lactococcus lactis, was described in 1996 (143). The overwhelming majority of reported bacterial MDR systems, however, belong to the group of secondary transporters, and for some time it was believed that primary MDR systems are uncommon in bacteria (73). The recent completion of the genome sequences of many different bacteria, most of which have been poorly studied, now provides a more complete view of the distribution of MDR-like transporters. Saier and colleagues have classified the wealth of this sequence information on membrane transport proteins in terms of transport mode, energy-coupling mechanism, molecular phylogeny, and substrate specificity (120). This information is available at http://www-biology.ucsd.edu/∼msaier/transport/. (The transport classification system has been approved and recommended by the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology.) A relational database (TransportDB) that classifies and predicts the membrane transporters from organisms whose genomes have been sequenced is available at http://membranetransport.org (115).
Sequence analysis of various microbial genomes revealed that both primary and secondary transporters are ubiquitous in bacteria. Their relative presence varies among bacterial species and often correlates with the mode of energy generation. For instance, fermentative bacteria tend to rely more on the primary transporters, whereas aerobic bacteria often contain a larger number of secondary MDR transporters (107, 109). Paulsen performed a comparative genomic analysis of microbial drug efflux systems and concluded that such systems are common in prokaryotes (104). However, the number and type of transporters differ among these microorganisms. For instance, archaea contain a large number of putative MFS-type efflux pumps, whereas Mycoplasma species and other microbial parasites utilize mostly ABC-type drug transporters. Nevertheless, the MFS and ABC types of MDR transporters appear to be the most abundant drug efflux systems in prokaryotes (106). It is important that for only a few of the annotated and presumed MDR transporters have the catalytic activity and involvement in the MDR phenotype been demonstrated. Many of the uncharacterized systems may not actually encode MDR transporters but rather may encode more specialized systems for metabolites or other compounds.
STRUCTURE AND FUNCTION OF PROKARYOTIC MDR ABC-TYPE TRANSPORTERS
The ABC superfamily comprises transporters involved in either uptake or secretion but also includes ABC ATPases that are not involved in transport but instead fulfill roles in cellular processes such as DNA repair, translation, or regulation of gene expression. The functional classification of the ABC systems was recently reviewed by Dassa et al. (14, 27), and a database is available at www.pasteur.fr/recherche/unites/pmtg/abc/index.html. The ABC transporter family is the largest of all paralogous protein families (46). The first described ABC transporter, the histidine permease, was cloned and sequenced in 1982. In 1986, Higgins et al. (47) described a large superfamily of ABC transporters involved in various cellular processes. The diagnostic feature of this family is the motor domain that binds and hydrolyzes ATP. This domain contains a number of characteristic and conserved amino acid sequence motifs. More than 20 years of research have now resulted in the identification of thousands of ABC ATPases (14), and it has become evident that the ABC superfamily is distributed ubiquitously in all three phyla of life (28, 45, 46, 49, 51, 59, 60, 64, 67, 77, 126, 140).
ABC transporters are membrane proteins that are responsible for the uptake and secretion of a wide range of substrates, from ions and small molecules such as amino acids, sugars, xenobiotics, and vitamins up to polymers such as peptides, proteins, and polysaccharides. Because of their wide substrate range, ABC transporters have been implicated in a range of cellular processes, such as nutrition uptake, xenobiotic protection, extrusion of cellular waste products, bacterial immunity and virulence, osmotic stress, lipid transport, and export of macromolecules during biogenesis, differentiation, and pathogenesis (45, 46, 60). The minimal structural organization of an ABC transporter is the presence of four domains, i.e., two nucleotide binding domains (NBDs) and two transmembrane permease domains (TMDs) (48). These four modules are encoded either by four different genes or by fewer genes that specify polypeptides consisting of a fusion of the two NBDs, the two TMDs, one NBD with one TMD, or even the complete ABC transporter. This remarkable modular organization further discriminates uptake from extrusion systems. Uptake systems usually consist of four separate polypeptides that together constitute an active transporter, and in addition, they include an extracellular substrate binding protein that donates the substrate to the permease domain. Extrusion systems are composed of a homo- or heterodimeric organization in which one NBD is fused with one TMD (half-transporter). In eukaryotes, ABC transporters are also found as single proteins in which all domains are present (full transporters).
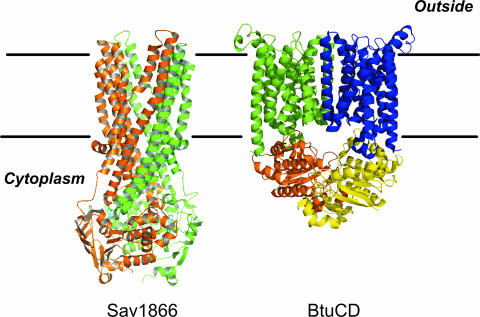
The four-domain organization is both sufficient and necessary to form a functional ABC transporter. Some ABC transporters contain additional domains or auxiliary proteins, such as extracellular binding proteins in bacterial uptake systems (51, 150) or additional transmembrane helices in the TMDs. The TMDs usually consist of six transmembrane α-helices. This topology has been demonstrated biochemically and was confirmed in the crystal structures of the recently described MDR transporters from Staphylococcus aureus Sav1866 (31) (Fig. 2). There is relatively little sequence identity between the TMDs of various ABC transporters, and this is likely a reflection of the large diversity of the substrates transported. The TMDs of some ABC transporters deviate from the 6+6 canonical arrangement, such as the BtuCD subunits of the vitamin B12 uptake system, which comprises 10 transmembrane α-helices per subunit (78) (Fig. 2). Because of the nucleotide binding fold, the NBD is the most conserved domain of the ABC transporters. It acts as the motor domain that binds and hydrolyzes ATP in order to elicit conformational changes in the TMDs to allow transmembrane transport. The NBDs contain highly conserved amino acid sequence motifs that are involved in nucleotide binding and coordination of the Mg2+ ions, i.e., the Walker A (G-X-X-G-X-KS/T) and B (h-h-h-h-D, where “h” is a hydrophobic residue) motifs (145). These motifs are characteristic of P-loop ATPases which, besides membrane transport, are involved in diverse cellular processes, such as receptor signaling, phosphoryl transfer reactions, motility, ATP synthesis, DNA translation, and DNA maintenance and repair (60). In addition, ABC transporters contain a number of unique motifs, such as the C loop or ABC signature (L-S-G-G-Q), which represents a diagnostic motif for the entire superfamily, the Q loop (32), and the H loop (75). A large number of isolated NBDs have been crystallized and their three-dimensional structures determined. The NBD functions as a dimer. Remarkably, isolated NBDs have been crystallized in various dimeric forms (reviewed in references 60 and 64). However, in the physiologically active dimeric state, the ABC signature of one monomer complements the ATP binding site formed by the Walker A and B motifs. This structure was initially observed in the Rad50 structure (52), with several isolated NBDs, and more recently, also with complete transporters. The dimer interface in these structures is in agreement with biochemical data and is therefore considered to represent the physiological arrangement.
FIG. 2.
Structures of prokaryotic ABC-type transporters. (Left) Sav1866 from Staphylococcus aureus (PDB accession no. 2HYD). Sav1866 is a homodimeric ABC transporter, with the individual subunits colored brown and green, respectively. The bound ADP is shown in ball and stick representation. (Right) Vitamin B12 transporter BtuCD of E. coli (PDB accession no. 1L7V). BtuCD belongs to the ABC superfamily of proteins and consists of four separate subunits. The two TMDs of BtuC are colored green and blue, respectively, and the two NBDs are colored orange and yellow, respectively. The figure was prepared using the PyMol program and the respective PDB files.
Most ABC transporters exhibit high specificities for their respective substrates or a group of closely related compounds. However, the MDR ABC transporters are polyspecific and can accommodate a variety of unrelated substrates. Here we discuss several examples of bacterial MDR ABC-type transporters (Table 1). Single-drug-resistance ABC transporters that are specific for a given drug or closely related group of substrates as well as atypical ABC proteins involved in drug resistance but lacking obvious TMDs are not included in this review.
TABLE 1.
Bacterial multidrug efflux systems of the ABC superfamily
| Organism | Protein | Established substratesb | Reference |
|---|---|---|---|
| Lactococcus lactis | LmrA | DA, EB, H, R6G, TPP+, various antibiotics | 143 |
| LmrCDa | DA, EB, H, R6G, CHO, BCECF-AM | 81 | |
| Lactobacillus brevis | HorA | EB, H, hop compounds | 125 |
| Bacillus subtilis | BmrA (YvcC) | H, DO, AD | 131 |
| Enterococcus faecalis | EfrABa | AC, EB, SO, DAPI, DA, DO, NB, AK, DC, NF | 30 |
| Abc7 | DA, DO, EB, OF, CM | ||
| Abc11 | PT, CH | ||
| Abc16 | AZ, EM, CR, | ||
| Abc23 | CL, LM, VM, SC | ||
| Oenococcus oeni | OmrA | SL, CdCl2 | 15 |
| Vibrio cholerae | VcaM | H, TC, CF, NF, OF, DA, DO, DAPI, TTP, R6G, EB, AO | 54 |
| Streptococcus pneumoniae | SP2073/SP2075a | AC, BB, EB, CF, NF, NB, CL | 118 |
Two half-transporters that assemble into a heterodimeric functional unit.
Abbreviations: AC, acriflavine; AD, 7-aminoactinomycin D; AK, arbekacin; AO, acridine orange; AZ, azithromycin; BB, berberine; BCECF-AM, acetoxymethyl ester 2′,7′-bis-(2-carboxyethyl)-5(and 6)-carboxyfluorescein; CF, ciprofloxacin; CH, chlorhexidine; CHO, sodium cholate; CL, clindamycin; CM, chloramphenicol; CR, clarithromycin; DA, daunomycin (daunorubicin); DC, doxycycline; DO, doxorubicin (adriamycin); EB, ethidium bromide; EM, erythromycin; H, Hoechst 33342; LM, lincomycin; NB, novobiocin; NF, norfloxacin; OF, ofloxacin; PT, pentamidine; R6G, rhodamine 6G; SC, Synercid; SL, sodium laureate; SO, safranin O; TC, tetracyclines; TPP+, tetraphenylphosphonium; and VM, virginiamycin M.
ABC-TYPE MDR TRANSPORT SYSTEMS IN GRAM-POSITIVE BACTERIA
ABC-type MDR transporters are relatively poorly characterized for both gram-positive and -negative bacteria. Recent reviews on efflux-based resistance mechanisms in bacteria even highlight the importance of multidrug transport systems energized by the PMF (or sodium motive force) (73, 112). However, functional analysis approaches indicate that the role of ABC MDR transporters in MDR has been underestimated. Most of the described MDR transporters in gram-positive bacteria characterized thus far are secondary MDRs, such as QacA (MFS) (135), NorA (MFS) (149), and Smr (SMR family) (39) from Staphylococcus aureus, the NorA homologs Bmr and Blt (3, 95) from Bacillus subtilis, PmrA from Streptococcus pneumoniae (38), and LmrP from Lactococcus lactis (13). However, several examples of primary MDR pumps, discussed further below, can be found in gram-positive bacteria. It should be stressed that the functional analysis of ABC-type MDR transporters in bacteria is still in its early stage and often incomplete.
Lactococcus lactis
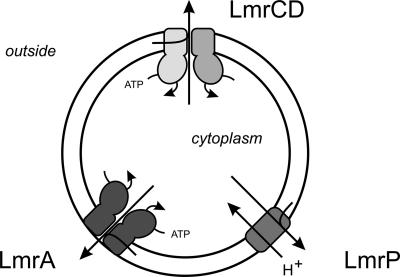
L. lactis is a nonpathogenic bacterium that has served as an excellent model system for studies of MDR. The genome of L. lactis contains 40 putative drug transporter genes, only a few of which have been characterized functionally (83). The first experimental evidence for efflux-based resistance of L. lactis against a variety of toxic compounds dates back more than 10 years. Selection of L. lactis mutants that could grow in the presence of toxic compounds, such as ethidium, rhodamine, and daunomycin, resulted in strains which were not only resistant to the selection drug but also cross-resistant to structurally unrelated drugs (12). The resistance was shown to be due at least partly to active efflux of the drugs. Moreover, bioenergetic studies demonstrated that the efflux activity was either ATP or PMF dependent (12). Studies directed to identify and characterize the molecular basis of the observed efflux activities resulted in the discovery of several MDR transporters in this organism (Fig. 3). Among these systems are the first described ABC-type MDR transporter in prokaryotes, LmrA (143); a member of the MFS, LmrP (13); and the recently described heterodimeric ABC MDR transporter, LmrCD (81). In addition, in silico analysis of the genome of L. lactis IL-1403 revealed the presence of an additional 36 putative MDR transporters (83).
FIG. 3.
Functionally characterized MDR transporters in Lactococcus lactis. Shown are LmrP, a secondary transporter of the MFS; LmrA, a homodimeric member of the ABC family; and LmrCD, a heterodimeric member of the ABC family.
LmrA.
The LmrA protein was termed lactococcal multidrug-resistant protein ATP. The lmrA gene was discovered accidentally, neighboring the apl (alkaline phosphatase-like enzyme) gene on a cloned chromosomal DNA fragment. The lmrA gene specifies a protein that shares structural homology with the well-characterized human P-glycoprotein. Unlike P-glycoprotein, LmrA is a half-transporter which functions as a homodimer. lmrA was expressed in a drug-hypersensitive strain of E. coli CS1562 (tolC) for functional studies. Expression of LmrA renders this E. coli strain resistant to ethidium, daunomycin, rhodamine 6G, and tetraphenylphosphonium (TPP+) (143) and to a multitude of antibiotics, such as aminoglycosides, lincosamides, macrolides, quinolones, streptagramins, tetracyclines, and chloramphenicol (113). Transport of ethidium and daunomycin was demonstrated in cells and inside-out membrane vesicles, respectively (143). Recently, the heterologously expressed LmrA protein was also postulated to confer protection on E. coli strain CS1562 (tolC) against high salt stress, sodium laureate, and ethanol toxicity (15) and against cadmium (1). Similar observations have been made for the Oenococcus oeni OmrA protein, a homolog of LmrA. LmrA has been shown to restore the growth of E. coli WD2, a temperature-sensitive mutant of MsbA, at the nonpermissive temperature (117). Conversely, MsbA expressed in L. lactis conferred resistance to erythromycin and showed elevated levels of Hoechst 33342 and ethidium transport (147). MsbA in E. coli is responsible for the transport of lipid A across the cytoplasmic membrane and thus plays a role in the biogenesis of the outer membrane. A role of MsbA in drug resistance in E. coli has not been documented.
Evidence for a role of LmrA in drug transport mostly originates from studies with heterologous systems. Its precise role in drug transport in L. lactis has remained unclear. Cells bearing elevated levels of LmrA have been reported to show enhanced ethidium efflux activity (88), but other studies failed to detect any significant activity above the already high endogenous ethidium extrusion levels (12, 81). Membrane vesicles bearing high levels of LmrA show an ATP-dependent Hoechst 33342 transport activity, but in vivo, LmrA does not protect cells against the toxic effect of Hoechst 33342 (139). A recent study indicated that the LmrA-dependent activity of Hoechst 33342 in membrane vesicles is due to an LmrA-mediated repartitioning of Hoechst 33342 in response to the transmembrane pH gradient generated by the F1Fo ATPase. Hoechst transport was not coupled directly to the hydrolysis of ATP by LmrA (139). LmrA has also been purified and reconstituted in liposomes (88). These proteoliposomes exhibited a very low level of ATP-dependent Hoechst 33342 transport and flip-flop of a fluorescent lipid (88). Although in heterologous systems LmrA renders cells resistant to a remarkably broad range of drugs (113), deletion of the lmrA gene in L. lactis has no effect on the drug resistance profile of L. lactis (30). Likewise, overexpression of LmrA in a drug-sensitive L. lactis ΔlmrA ΔlmrCD strain (see the next section) does not result in protection of the cells against drugs, suggesting that LmrA is not the major MDR transporter in this organism. The lack of a phenotype of the lmrA deletion strain has been attributed to a compensatory increase in the levels of other MDR transporters, but so far, this remains to be investigated. It may well be that the observed drug extrusion activity of LmrA in heterologous systems reflects a minor activity that is observed only upon massive overexpression. So far, drug export and resistance have not been attributed to LmrA expressed at wild-type levels, and its physiological role remains to be elucidated.
LmrA and P-glycoprotein are both structurally and functionally related. LmrA overproduced in human fibroblasts localizes to the plasma membrane and confers MDR on these cells, with a substrate spectrum similar to that of P-glycoprotein (141). This has led to the hypotheses that these proteins not only are distant structural homologs but also share a close functional homology and that ABC-type MDR transporters are conserved from bacteria to humans. LmrA has become a model protein for the study of structural and mechanistic features of ABC MDR transporters. LmrA is a half-transporter with an amino-terminal TMD comprising six transmembrane α-helices and a carboxyl-terminal NBD. Covalent linkage of two LmrA monomers by means of a “linker peptide” derived from P-glycoprotein yields a functional protein. The introduction of a mutation into one of the two monomers caused a negative dominant effect in a lipid flip-flop assay, suggesting that the protein functions as an oligomer, likely a homodimer (142). The molecular mechanism of LmrA-mediated drug transport has been studied in great detail. Photoaffinity labeling and drug equilibrium binding experiments suggest that LmrA contains two cooperative drug binding sites which are allosterically linked (142). This has led to the proposition of a two-cylinder engine mechanism in which the homodimeric LmrA protein contains two substrate binding sites that are cooperatively linked. According to this model, a high-affinity binding site facing the cytoplasm binds the drug molecule, most likely from the inner leaflet of the membrane, whereas the second, low-affinity binding site is exposed to the extracellular milieu. The two-cylinder model proposes a physical movement of the high-affinity binding site from inside to outside the cell and a subsequent change from a high- to a low-affinity binding site, whereupon the substrate is released. This conformational transition is driven by the hydrolysis of ATP by one of the NBDs. This reallocation of the binding site would be accompanied by a reversal of the second binding site, from an outer membrane-facing to an inner membrane-facing state, accompanied by a transition from a low- to a high-affinity drug binding conformation. In a next cycle, ATP hydrolysis by the second NBD would drive the reallocation of the second, drug-loaded binding site from the inside to the outside, whereas the other empty drug binding site would reorient towards the inside of the cell. This model implies a stoichiometry of one molecule transported per ATP hydrolyzed. Strikingly, in the vanadate-trapped ADP-bound state of LmrA, only the low-affinity site was found to be accessible to drugs, whereas the high-affinity drug binding site appeared occluded. This model resembles the alternating catalytic site mechanism of ATP hydrolysis suggested for ABC transporters (128).
Recently, the structure of a bacterial ABC exporter, Sav1866 from Staphylococcus aureus (31), which is homologous to LmrA, was resolved. Sav1866 may specify an MDR transporter, as reconstitution studies demonstrated that its ATPase activity is stimulated by typical MDR transporter substrates. The transporter was crystallized in the presence of ADP sandwiched between the P loop of one subunit and the signature motif of the opposite subunit, yielding an outward-facing conformation of the proposed binding site. The structural study suggests an “alternating access and release” transport mechanism. As with the previously discussed model of LmrA, two binding conformations are proposed, namely, an inwardly facing state which accepts the substrate and an outwardly facing state which releases the substrate outside the cell. The energy required to reorient these two binding sites would be supplied by ATP binding and hydrolysis. However, this model seriously deviates from the “two-cylinder engine model” that implies two reorienting binding sites, as with Sav1866 there is only a single reorienting binding site. An interesting feature of the Sav1866 structure is the tight and intertwined association of the two monomers. It has been suggested that this architecture allows for a close association of the two NBDs, which makes it unlikely that they would completely dissociate during the catalytic cycle. This model again differs from other ATP binding and hydrolysis models, such as the “processive clamp” (55, 92) and “ATP switch” (49) models.
A truncated version of LmrA in which the NBD was genetically removed, thus leaving only the TMD (LmrA-MD), still forms a dimeric active unit. Surprisingly, LmrA-MD expressed in E. coli was reported to mediate the influx of ethidium in symport with protons. It was suggested that the membrane domain of LmrA acts as a secondary transporter, i.e., a H+/drug symporter, which would be reminiscent of an evolutionary ancestor of LmrA that acquired the NBDs as catalytic modules to become a drug efflux system. With intact LmrA, NBD-mediated nucleotide binding and hydrolysis are supposed to reverse the directionality of drug transport as well as of proton movement (144). According to this hypothesis, ATP-driven transport of drugs out of the cells would be accompanied by proton extrusion. Amino acyl residue E314 has been implicated in this proton-coupled ethidium transport by LmrA (129). Remarkably, the proposed LmrA-mediated proton movements are energetically unfavorable and seemingly functionally obsolete. In the absence of the NBDs, LmrA would work as a PMF-driven drug uptake system, while the complete LmrA protein would function as a proton extrusion system that acts against the existing electrochemical gradient of protons. Further studies on the uptake of ethidium suggest that LmrA present at overexpressed levels mediates ethidium influx into cells under nonenergized conditions (7). This process is not dependent on ATP hydrolysis, but instead, ethidium influx was shown to drive ATP synthesis at LmrA, although the levels of ATP synthesis reported were infinitely small (about 1 mol of ATP synthesized per mol of LmrA in a time frame of 30 min; turnover of 0.00056 s−1) and barely above the background levels observed with an NBD-ATPase mutant of LmrA. Typically, ABC transporters show turnover numbers of 10 to 200 s−1, which are many orders of magnitude higher than the ATP synthesis rate reported. Therefore, the significance of this drug-induced ATP synthesis activity by LmrA is elusive.
Typically, both glucose-energized L. lactis wild-type and ΔlmrA cells show a significant ethidium efflux activity that is preceded by an initial Δψ-dependent accumulation of ethidium (12, 13, 125). This suggests the presence of other MDR transporters involved in ethidium efflux. However, in a recent study (129), it was claimed that there is no residual ethidium efflux activity in energized wild-type cells and that ethidium efflux is observed only upon LmrA overexpression. Currently, the discrepancy in these observations is difficult to explain. However, both strong functional and genetic evidence suggests that MDR transporters other than LmrA are involved in endogenous and acquired drug (and ethidium) resistance, as discussed in the next section. Likewise, transcriptome analysis indicates that many MDR transporter-like proteins are endogenously expressed in wild-type cells even when they are not challenged with drugs.
LmrCD.
LmrC and LmrD are two ABC half-transporters whose genes are organized in a small operon (81). These proteins are homologous to other members of the ABC-type MDR family, such as LmrA, human P-glycoprotein, and BmrA from B. subtilis (Table 2). LmrC and LmrD are each composed of a TMD with six transmembrane segments and a carboxyl-terminally located NBD. Biochemical evidence demonstrated that LmrC and LmrD are interacting proteins. They can be isolated as a stoichiometric complex with a high endogenous ATPase activity. In contrast, the separately purified subunits are essentially inactive with regard to ATPase activity (81). L. lactis cells show a high level of drug extrusion upon overexpression of both LmrC and LmrD, whereas no elevated activity is observed when LmrC and LmrD are expressed separately. LmrCD substrates identified thus far are drugs that are typically used in transport assays, such as the fluorescent dyes acetoxymethyl ester 2′,7′-bis-(2-carboxyethyl)-5(and 6)-carboxyfluorescein (BCECF-AM), daunomycin, ethidium, and Hoechst 33342 (81). Likewise, LmrCD mediates Hoechst 33342 export from both cells and membrane vesicles (139). Transport of these drugs is independent of the PMF and requires the ATPase activity of the LmrCD complex. Importantly, an L. lactis strain lacking the lmrCD genes is hypersensitive to a range of toxic compounds, such as ethidium, daunomycin, Hoechst 33342, cholate, and rhodamine 6G, but not to a variety of tested antibiotics or quinine. This sensitivity to toxicity could be rescued by low-level expression of lmrCD but not by mutants of LmrCD that are devoid of ATPase activity (82). Moreover, attempts to reisolate ethidium- and daunomycin-resistant strains from the L. lactis ΔlmrCD strain have been unsuccessful, suggesting that at least for this panel of drugs, there is no functional alternative extrusion route. These data demonstrate that LmrC and LmrD are involved in the intrinsic drug resistance of L. lactis.
TABLE 2.
Amino acid identity between L. lactis LmrCD and MDR transporters of gram-positive human pathogens
| Protein | % Identitya
|
|||||||
|---|---|---|---|---|---|---|---|---|
| LmrC | LmrD | SP2075 | SP2073 | EF0790 | EF0789 | Spy0229 | Spy0230 | |
| LmrC | 22 | 56 | 23 | 25 | 55 | 56 | 23 | |
| LmrD | 22 | 26 | 57 | 60 | 23 | 25 | 60 | |
| SP2075 | 56 | 26 | 25 | 24 | 57 | 65 | 24 | |
| SP2073 | 23 | 57 | 25 | 57 | 26 | 24 | 67 | |
| EF0790 | 25 | 60 | 24 | 57 | 28 | 26 | 62 | |
| EF0789 | 55 | 23 | 57 | 26 | 28 | 57 | 25 | |
| Spy0229 | 56 | 25 | 65 | 24 | 26 | 57 | 24 | |
| Spy0230 | 23 | 60 | 24 | 67 | 62 | 25 | 24 | |
Percentage of identical residues in pairwise alignments, using the ClustalW tool available on the World Wide Web at http://www.ebi.ac.uk/clustalw/.
bData shown are for the following proteins (GenBank accession no.): L. lactis LmrC (AAK04408) and LmrD (AAK04409), Streptococcus pneumoniae SP2075 (AAK76137) and SP2073 (AAK76136), Enterococcus faecalis EF0790 (AAO80604) and EF0789 (AAO80603), and Streptococcus pyogenes Spy0229 (AAK33312) and Spyo0230 (AAK33313).
The nucleotide binding sites of the heterodimeric LmrCD protein contain partially degenerate sequence motifs. Residues postulated to interact directly or via H2O with the γ-phosphate of a bound nucleotide in the LmrC ATP active site, such as the glutamate following the Walker B motif, a histidine in the H loop, and the first glycine in the signature motif, are replaced by aspartate, glutamine, and valine, respectively. These residues are conserved, however, in the LmrD NBD. It therefore appears that the LmrCD heterodimer contains structurally asymmetric ATP binding/hydrolysis sites (82). The functional state and the contribution of both NBDs to the LmrCD drug transport activity have been examined. The conserved glutamate directly following the Walker B site of LmrD was replaced by a glutamine (“canonical LmrD ATP active site”), and the corresponding aspartate in LmrC was replaced by an asparagine (“noncanonical LmrC ATP active site”). The conserved glutamate was postulated to play an essential role in ATP hydrolysis as a catalytic base positioning the water molecule that attacks the γ-phosphate of ATP or to function in another important step of the ATP hydrolysis cycle (82). Both the double mutant, LmrC(D495N)/LmrD(E587Q), and the single mutant, LmrC/LmrD(E587Q), were found to be defective in ethidium, Hoechst 33342, and BCECF-AM transport, while L. lactis cells expressing the LmrC(D495N)/LmrD mutant showed a transport activity comparable to that of the overexpressed wild-type LmrCD protein (82). LmrCD complexes bearing LmrD(E587Q) were found to be completely defective in ATPase activity, whereas complexes of the LmrC(D495N) mutant appeared unstable but retained significant ATPase activity (82). An asymmetric organization of the NBDs in the LmrCD heterodimer was further supported by azido-ATP photoaffinity studies. Under nonhydrolyzing conditions, this nucleotide analogue labeled mostly the LmrC subunit, while in the presence of AlFx to trap the hydrolyzed azido-ATP at the nucleotide binding site, strong labeling of the LmrD subunit was observed (82). These data suggest that the heterodimer LmrCD transporter harbors two asymmetric nucleotide binding sites, with a canonical LmrD ATP active site which is presumably responsible for high ATPase activity and a slowly hydrolyzing noncanonical LmrC ATP active site.
The two half-transporters LmrC and LmrD in L. lactis interact to form a functional transporter. In eukaryotes, formation of an active transporter from two different ABC half-transporters, for instance, the Drosophila white, brown, and scarlet genes (35) and the fatty acid peroxisomal ABC half-transporters (76), is a more common feature. In those cases, a network of interactions was shown to give rise to various possible heterodimers with different properties. L. lactis contains another ABC half-transporter besides LmrCD, namely, LmrA, which has been implicated as an MDR transporter. One could envision the possibility that these systems may assemble into different heterodimers that differ in their substrate spectra. Although this possibility cannot be excluded fully, it is unlikely for the lactococcal MDR transporters. Namely, analysis of the ΔlmrCD, ΔlmrA, and ΔlmrCD ΔlmrA strains suggested the involvement of only LmrCD in the intrinsic drug resistance of L. lactis. No significant phenotypic changes were recorded for the ΔlmrA strain, while the ΔlmrCD and ΔlmrCD ΔlmrA strains showed identical drug susceptibility phenotypes. Moreover, transcriptome analysis of four different MDR mutants of L. lactis showed strong up-regulation of the LmrCD operon, while the expression of the LmrA gene was not significantly affected. Possibly, the use of mosaic ABC transporters exists in other bacteria, but this remains to be demonstrated.
Other MDR transporters.
The L. lactis genome contains many genes that specify putative MDR systems (83). To address the question of which system(s) is involved in acquired drug resistance, the gene expression profiles of MDR strains of L. lactis MG1363 selected for growth in the presence of high concentrations of ethidium bromide, daunomycin, rhodamine 6G, and cholate were analyzed with DNA microarrays (83). The MDR strains showed high levels of cross-resistance to a wide variety of structurally unrelated toxic compounds, suggesting that a specific set of MDR transporters is responsible for acquired drug resistance (12). Reverse transcription-PCR analysis showed that in all strains, lmrC and lmrD were significantly up-regulated (81). Strikingly, this result was confirmed by DNA microarray analysis, but more importantly, the analysis showed that lmrC and -D are the only MDR transporter genes that are highly up-regulated in all tested MDR strains. It therefore appears that LmrCD is not only an important determinant of the intrinsic drug resistance of L. lactis but also plays an important role in acquired drug resistance. Apparently, L. lactis relies on ABC-type MDR transporters for drug resistance rather than on secondary active transporters, as demonstrated for many other bacteria.
Lactobacillus brevis
HorA is an ABC half-transporter from Lactobacillus brevis which shares 53% identity with LmrA. L. brevis is a beer spoilage bacterium that is able to resist antibacterial hop compounds. The horA gene is plasmid localized in hop-tolerant strains, and when this plasmid is cured, cells become sensitive to hop-like compounds (124). Because of its similarity to LmrA, HorA may be an MDR transporter as well. The protein has been expressed and functionally characterized in L. lactis and was shown to confer resistance to hop compounds. In addition, HorA mediates the excretion of ethidium and Hoechst 33342 from cells, while Hoechst 33342 transport in inside-out membrane vesicles was found to be inhibited by hop compounds (125). Therefore, HorA is an MDR transporter that renders cells resistant to antimicrobial compounds used as food preservatives.
Bacillus subtilis
B. subtilis is a soil bacterium with a very versatile metabolism. The genome of B. subtilis contains many putative MDR transporter genes, but only a few have been characterized. BmrA (formerly known as YvcC) is an ABC half-transporter that shares high homology with LmrA (41.5% identity). Heterologously expressed BmrA was initially characterized in E. coli. In inside-out membrane vesicles, the protein exhibits a high vanadate-sensitive ATPase activity and mediates transport of Hoechst 33342 (131), doxorubicin, and 7-aminoactinomycin D (132). BmrA has been purified, reconstituted, and crystallized in two dimensions. The protein forms ring-shaped structures with a diameter of about 40 nm. A structural model of BmrA suggests a homodimeric organization with a central open chamber between the two subunits and asymmetrically organized NBDs (18). Time-resolved fluorescence resonance energy transfer experiments support the formation of a stable homodimeric protein complex (22). In B. subtilis, the bmrA gene is expressed throughout growth, while its expression is not significantly influenced by the presence of typical MDR substrates. Moreover, the deletion of the bmrA gene did not render cells more sensitive to drugs such as ethidium bromide, although the overexpression of BmrA supports the notion that the protein is involved in ethidium efflux and resistance (132).
The ATPase cycle of BmrA has been studied extensively. The putative catalytic base, the glutamate residue next to the Walker B site, was subjected to site-directed mutagenesis. The mutants were found to be devoid of ATPase activity and showed a vanadate-independent trapping of 8-N3-[α-32P]ATP at the nucleotide binding site. In wild-type BmrA, vanadate traps the nucleotide in its hydrolyzed state. This strongly suggests that the glutamate residue is involved in catalysis (102).
Enterococcus faecalis
Enterococcus faecalis is relatively resistant to a large number of toxic compounds. This has been attributed to the presence of MDR pumps because the genome of E. faecalis contains a large number of putative MDR transporters. Systematic gene inactivation studies with this organism revealed the involvement of ABC-type MDR transporters in drug resistance. EfrAB is a heterodimeric MDR ABC transporter that is structurally and possibly functionally related to LmrCD of L. lactis. Expression in E. coli revealed that efrA and efrB render cells resistant to a range of drugs, such as acriflavine, norfloxacin, ciprofloxacin, doxycycline, 4′,6′-diamidino-2-phenylindole (DAPI), and TPP chloride. EfrAB likely functions as a heterodimer since the elevated drug resistance was observed only when efrA and efrB were coexpressed. Further studies with cells demonstrated that EfrAB mediates the active efflux of acriflavine (69). In addition, another MDR transporter of E. faecalis has been characterized partially, i.e., EmeA, a homolog of NorA from Staphylococcus aureus (57, 68) which is a member of the MFS.
Oenococcus oeni
omrA is a stress response protein from the wine lactic acid bacterium Oenococcus oeni. OmrA shares high sequence similarity with LmrA (54% identical residues) and other members of the ABC-type MDR protein family. The omrA gene is up-regulated by high temperature and osmotic shock. OmrA was further characterized in a drug-hypersensitive E. coli strain and was found to provide resistance to ethanol (but also wine) and sodium laureate. This resistance was reversed by verapamil, a known blocker of ABC-type MDR pumps (15). Furthermore, OmrA but also LmrA and human P-glycoprotein expressed in E. coli rendered cells more resistant to cadmium toxicity, in a verapamil-sensitive manner (1). Cadmium is possibly transported as a glutathione derivative in a similar way to that shown for human MRP1.
ABC-TYPE MDR TRANSPORT SYSTEMS IN GRAM-NEGATIVE BACTERIA
One important difference between gram-negative and gram-positive bacteria is the presence of an outer membrane in the former. This additional membrane constitutes a major barrier for the permeation of lipophilic and amphiphilic compounds. However, the presence of an outer membrane does not sufficiently explain the intrinsic resistance of gram-negative bacteria to drugs (97), and the contribution of MDR efflux pumps to drug resistance in these bacteria is well established. Special mechanisms are required to allow the efficient expulsion of drugs across the cell envelope of the cell. Gram-negative bacteria contain MDR transporters in the cytoplasmic membrane that catalyze the efflux of drugs into the periplasmic space. This involves mostly members of the SMR and MFS transporter families. In addition, cells contain tripartite systems that transport drugs across both the cytoplasmic and the outer membrane. The latter systems constitute a complex of three proteins, i.e., a transporter located in the cytoplasmic membrane, a periplasmic membrane fusion protein, and an outer membrane channel protein (73, 97). These tripartite systems play a major role in both the intrinsic and acquired resistance of gram-negative bacteria (73). The AcrAB-TolC system of E. coli is one of the best-characterized MDR transporters (85, 86). The phenotype caused by its deletion was already described in 1965 as a locus disruption which renders E. coli sensitive to basic dyes, detergents, and hydrophobic antibiotics (37). This system consists of AcrA, the membrane fusion protein; AcrB, a transporter of the RND family; and TolC, the outer membrane protein.
Several members of the RND family have been implicated in fulfilling a dominant role in MDR in gram-negative bacteria (for recent reviews, see references 73 and 111). Also, various transporters of the MATE, MFS, and SMR families, utilizing the PMF or sodium motive force, have been described (for reviews, see references 73 and 111). Interestingly, only a very few members of ABC-type MDR transporter families have been reported to be involved in drug resistance in gram-negative bacteria (73, 111). Systematic gene disruption and expression studies with E. coli did not identify a single ABC-type MDR transporter involved in drug resistance. Nevertheless, a small number of ABC-type MDR transporter-like proteins have been characterized for gram-negative bacteria, and these are discussed in this section.
Vibrio cholerae
VcaM is an ABC half-transporter that was identified during a screen for resistance markers in Vibrio cholerae upon expression in a drug-hypersensitive E. coli strain. VcaM renders E. coli resistant to several unrelated drugs, such as tetracycline, norfloxacin, ciprofloxacin, anthracyclines, DAPI, Hoechst 33342, and others. VcaM was demonstrated to transport Hoechst 33342 and doxorubicin, and this activity was inhibited by reserpine and vanadate (54). Thus, VcaM seems to represent the first example of a true ABC-type MDR transporter from a gram-negative organism. The role of VcaM in its native host, V. cholerae, has not yet been addressed.
Escherichia coli
E. coli contains five putative ABC-type MDR-like transporters. These systems were all cloned and expressed in a drug-sensitive E. coli strain, and the drug resistance phenotypes were investigated. None of these systems provided an appreciable drug resistance to E. coli, except for YbjYZ, which conferred resistance to erythromycin (100). YbjYZ was renamed MacAB (macrolide-specific ABC-type efflux carrier) and characterized as a macrolide-specific transporter conferring resistance to macrolides composed of 14- and 15-membered lactones (65). A homolog of MacAB has been identified and characterized in Neisseria gonorrhoeae (119).
ABC-TYPE MDR TRANSPORT SYSTEMS IN ARCHAEA
Genomic sequences of organisms belonging to the archaea indicate the presence of a multitude of putative MDR transporters, similar to the case for bacteria (115, 116; www.membranetransport.org). However, only a few experimental studies have been performed on multidrug transporters in archaea, even though archaeal genomes contain a greater number of membrane proteins with unknown function than do bacterial genomes (116). HsmR of a Halobacterium sp. is a secondary transporter belonging to the SMR family (99). There is indirect experimental evidence for the presence of a P-glycoprotein-like ABC-type MDR transporter in Haloferax volcanii (91). An anthracycline-resistant mutant of H. volcanii was shown to transport rhodamine 123 more efficiently than the wild type. This efflux activity was reduced by the addition of P-glycoprotein modulators, such as diltiazem, verapamil, or the Ca2+-channel antagonist nifedipine (63, 91).
THE GLOBAL PICTURE OF MDR
With the availability of an increasing number of sequenced genomes, it has become possible to predict the distribution of MDR-like transporters in various organisms for comparative purposes. MDR-like transporters are highly abundant and ubiquitous in nature. They represent, on average, more than 10% of the total number of transporters per organism (104). Several questions arise, as follows. Why are there so many MDR pumps? Do they fulfill redundant or overlapping functions? Which MDR transporters are involved in intrinsic and/or acquired resistance? Do they contribute to clinical resistance? What is the preferred mode of energization? Does this differ for organisms and does it relate to their respective growth habitat and physiology? What is the physiological role of MDR proteins? Do they fulfill tasks other than xenobiotic protection?
Systematic genetic approaches try to answer some of these questions. Gene knockouts and/or overexpression studies, often using drug-hypersensitive strains, have proved to be very useful. In addition, microarray analysis of resistance mutants can shed more light on the factors that influence acquired MDR. Such studies can potentially highlight novel antimicrobial targets or identify MDR transporters which, upon deletion, will yield hypersensitive drug strains that might be valuable tools for cell-based screening of novel antimicrobials (53, 133).
Escherichia coli
The contributions of putative and established MDR proteins to both intrinsic and acquired resistance have been examined in great detail. A comprehensive knockout analysis involving 25 transporters, including members of all known MDR families, was conducted. Knockout strains were tested for susceptibility towards 20 different classes of antimicrobial compounds. This study revealed that the AcrAB-TolC system is the major determinant for drug resistance in E. coli (133). AcrB belongs to the RND family and makes up an MDR transporter that employs the PMF as a driving force. Several other MDR transporters provide resistance to a narrow range of compounds. The only putative ABC-type MDR transporter included in this study, that encoded by ybjYZ, failed to show any functional contribution to the intrinsic drug resistance of E. coli (133).
In addition, complementary studies have been conducted to identify MDR systems that contribute to the acquired resistance of E. coli (100). This analysis involved 37 proteins with a presumed role in drug efflux. All of these proteins were expressed in a drug-hypersensitive strain of E. coli and tested against 26 antimicrobial compounds. The expression of 16 systems rendered E. coli cells resistant to various antimicrobials. This included the AcrAB-TolC transporter, with its broad-substrate spectrum, and other PMF-dependent systems. Of the seven proteins belonging to the ABC type of MDR transporters, only YbjYZ (MacAB) conferred resistance to 14- and 15-membered lactone macrolides (100). These studies clearly demonstrated a central position of AcrAB-TolC in both intrinsic and acquired MDR in E. coli and indicated a minor role for other systems, in particular ABC-type MDR transporters. The latter systems are likely involved in transport of specific subsets of molecules, either drugs or metabolism-derived compounds. Indole, which is metabolically synthesized from tryptophan by tryptophanase in E. coli, was shown to induce the expression of a number of MDR pumps. Indole induces acquired multiple drug resistance in E. coli (50).
Enterococcus faecalis
Bioinformatic analysis revealed the presence in E. faecalis of 34 putative MDR transporters belonging to four different transporter families, with 23 ABC, 9 MFS, 1 MATE, and 1 SMR transporter. Single-gene disruptions were created and characterized in resistance assays. Four ABC-type MDR transporters were identified as contributing significantly to intrinsic drug resistance. Each constitutes a separate transport system, with little overlap in the respective substrate spectra (30). The analysis did not reveal a role for heterodimeric ABC transporters in intrinsic resistance, even though EfrAB was described as an MDR transporter of E. faecalis (69). EfrAB might, however, contribute to acquired drug resistance.
Streptococcus pneumoniae
The genome of S. pneumoniae specifies 14 putative drug efflux pumps. Except for the SP1435 gene, all putative MDR genes were disrupted, and the resulting knockout strains were analyzed for their drug resistance profiles. Only the deletion of two linked genes, the SP2073 and SP2075 genes, each encoding an ABC half-transporter, showed a significant decrease in resistance towards acriflavine, ethidium bromide, berberine, and norfloxacin. The individual knockout strains of SP2073 and SP2075 showed identical patterns, suggesting that these two proteins constitute a heterodimeric ABC-type MDR transporter. Interestingly, SP2075 and SP2073 are close homologs of the well-characterized LmrC and LmrD proteins from L. lactis, bearing 56% and 57% identical residues, respectively, in pairwise alignments (Table 2). Likewise, the deletion of the lmrC and lmrD genes in L. lactis resulted in a drug-sensitive phenotype, suggesting that homologs of LmrCD play an important role in the drug resistance of human pathogens. Importantly, LmrCD homologs can also be identified in Streptococcus pyogenes and Listeria monocytogenes, but their role in drug resistance has not yet been addressed. A knockout mutant of a previously identified MDR transporter, PmrA, belonging to the MFS, did not show altered sensitivity to tested compounds (118). Again, this system may play a more dominant role in acquired resistance.
Studies directed to detect efflux-based resistance have been conducted with various important human pathogens. The involvement of efflux systems in drug resistance has been demonstrated for S. pneumoniae (26, 110), Staphylococcus aureus (62), S. pyogenes (58), and various enterococci (84). Inhibitors of mammalian MDR transporters, such as reserpine, biricodar (VX-710), and timcodar (VX-853), increase the drug sensitivities of these strains. This global approach also demonstrated efflux-based resistance mechanisms to a number of antibiotics for three clinically significant gram-positive pathogens, Staphylococcus aureus, E. faecalis, and S. pneumoniae (93). Again, the molecular details of the resistance remain to be resolved.
Pst TRANSPORT SYSTEM AND MDR
The phosphate-specific transporter (Pst) has been well characterized for E. coli and P. aeruginosa and specifies a high-affinity, ATP-dependent transport system involved in phosphate uptake under starvation conditions, i.e., when the phosphate concentration is below 1 mM (98, 134). The Pst uptake system in Mycobacterium smegmatis has also been implicated in drug efflux (11). Mutants highly resistant to fluoroquinolones (CIPr) overexpress the pstB gene. PstB is the NBD of the ABC transporter involved in phosphate uptake (9). The increased expression level of the pstB gene in a CIPr mutant was due to up-regulation of the entire pst transport system operon (17). Moreover, the CIPr mutant showed an increased uptake of phosphate. Strikingly, inactivation of pstB in the wild-type strain resulted in loss of the high-affinity phosphate uptake activity and caused an increased sensitivity to fluoroquinolones (8, 11). Interestingly, a DNA microarray study of gene expression in a daunomycin cross-resistant L. lactis mutant strain revealed increased expression of the lactococcal pst system (83). These studies suggest a role for phosphate transport in drug resistance, but it is not clear if this relates to a direct involvement of such transporters in drug extrusion or reflects an indirect effect, for instance, by maximizing the generation of ATP due to an increased availability of inorganic phosphate. Therefore, it will be interesting to investigate the exact role of the Pst system in efflux-based MDR.
REGULATION OF MDR ABC TRANSPORTERS
Bacterial MDR transporters are usually subjected to transcriptional regulation, which may involve several local transcriptional regulators, repressors, or activators as well as global transcriptional regulators. Local regulators are often found adjacent to the structural genes of MDR transporters and regulate the expression of the transporters depending on the presence of substrates in the medium. Additionally, MDR transporters are regulated by global modulators in response to a global stress response (41, 42). The complex regulation of MDR pumps may relate to the fact that these transporters can potentially secrete valuable molecules from the cell. Like the case with many other membrane proteins, the excessive overproduction of MDR transporters can be harmful for the cell, but it remains to be determined if this involves specific activity-related defects, such as interference with the integrity of the membrane or the unwanted secretion of important metabolites (41, 42).
The bacterial transcriptional regulators of MDR proteins belong to four regulatory protein families, namely, AraC, MarR, MerR, and TetR. All of these MDR regulators possess the typical helix-turn-helix DNA-binding motif. Classification of regulator families is mainly based on homology in the DNA-binding domains (42). Several local regulators have been characterized in great detail. These include BmrR from B. subtilis, which is an activator of the MerR family that regulates the expression of bmr (2). Binding of drugs such as TPP+ and rhodamine 6G by BmrR results in an increased affinity of BmrR for the bmr promoter and in subsequent transcriptional activation of the expression of structural genes (89). Structural studies in combination with biochemical analysis have shed more light on the molecular basis of drug binding and the molecular mechanism of BmrR (44). QacR is another well-characterized regulator which represses its cognate gene, qacA. Similar to BmrR, QacR interacts with toxic compounds, such as ethidium and rhodamine 6G. However, in this case, drug binding prevents binding of QacR to the target promoter, thereby relieving the repression of qacA expression (40). The structure of QacR has been solved and provides deeper insight into the mechanism of drug recognition (127). Another MarR-type repressor, EmrR, has been reported to regulate the EmrAB efflux transporter of E. coli (80).
The expression of several MDR transporters has been shown to be controlled by global regulators. The main MDR transporter of E. coli, AcrAB, is regulated by the global activators MarA, Rob, and SoxS (4) and the local transcriptional regulator AcrR. Similarly, in B. subtilis, two MDR transporters, Bmr and Blt, are regulated by the local activators BmrR and BltR and, at the global level, by the activator Mta (10). Also, the involvement of two-component systems has been demonstrated. Such systems consist of a histidine kinase that senses drugs extracellularly and phosphorylates the response regulator, which modulates the expression of MDR pumps. Some examples are AlrSR (36), which regulates the expression of NorA of S. aureus, and EvgAS (101), which modulates the expression of the RND transporter YhiUV of E. coli.
Most of the described MDR pumps that are subject to regulation belong to the MFS and RND families, which are energized by the PMF, whereas members of the SMR family are usually constitutively expressed and not subject to transcriptional regulation (42). Since only limited numbers of ABC-type MDR transporters have been studied, so far their regulation is poorly understood. Recent observations demonstrated that LmrCD of L. lactis is regulated by the local repressor YdaF (83).
CONCLUSIONS
Since the initial discovery of MDR transporters in mammalian cell lines by Dano in 1972 (24), the number of reported MDR transporter-like proteins has expanded tremendously. These systems belong to various structural and functional groups, but historically, most emphasis in functional characterization has been on ion motive force-dependent systems. For a long time, ABC-type transporters were underrepresented in those studies. The genomic era allowed systematic studies of MDR in prokaryotes, and now several ABC-type MDR transporters have been identified as being involved in intrinsic and acquired drug resistance in bacteria. Interestingly, the ABC-type MDR transporters seem to fulfill a major role in drug resistance of pathogenic gram-positive bacteria, such as E. faecalis and S. pneumoniae (118). In these organisms, a homolog of the heterodimeric ABC-type MDR transporter LmrCD of L. lactis seems to be responsible for drug resistance. Homologs of LmrCD are widely distributed among gram-positive bacteria, including many clinically significant pathogens (Table 2). Interestingly, a homolog of LmrCD in S. pyogenes is involved in stress responses and is needed for growth of S. pyogenes at elevated temperatures (23). However, it will be important to determine if this transporter also contributes to virulence and resistance when patients are treated with drugs.
The systematic analysis of MDR transporters has also resulted in the construction of a new generation of drug-hypersensitive strains. Drug-hypersensitive E. coli strains lacking several major MDR transporters have been postulated to represent potentially valuable tools for cell-based screens for antimicrobials (53). Similar strains of gram-positive bacteria would be highly useful for MDR activity studies. In this respect, the L. lactis ΔlmrA ΔlmrCD strain is highly sensitive to several drugs, but since L. lactis contains other uncharacterized putative MDR transporters, exploitation of such a strain for drug screening awaits further gene knockout analysis. Likewise, similar strains of pathogenic prokaryotes can be foreseen and will provide greater insight into the natural role of these systems.
Acknowledgments
This work was supported by the Dutch Cancer Society.
We thank G. Poelarends and P. Mazurkewicz for valuable suggestions.
REFERENCES
- 1.Achard-Joris, M., H. B. van den Berg van Saparoea, A. J. M. Driessen, and J. P. Bourdineaud. 2005. Heterologously expressed bacterial and human multidrug resistance proteins confer cadmium resistance to Escherichia coli. Biochemistry 44:5916-5922. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, M., C. M. Borsch, S. S. Taylor, N. Vazquez-Laslop, and A. A. Neyfakh. 1994. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J. Biol. Chem. 269:28506-28513. [PubMed] [Google Scholar]
- 3.Ahmed, M., L. Lyass, P. N. Markham, S. S. Taylor, N. Vazquez-Laslop, and A. A. Neyfakh. 1995. Two highly similar multidrug transporters of Bacillus subtilis whose expression is differentially regulated. J. Bacteriol. 177:3904-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alekshun, M. N., and S. B. Levy. 1997. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob. Agents Chemother. 41:2067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allikmets, R., L. M. Schriml, A. Hutchinson, V. Romano-Spica, and M. Dean. 1998. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 58:5337-5339. [PubMed] [Google Scholar]
- 6.Ambudkar, S. V., C. Kimchi-Sarfaty, Z. E. Sauna, and M. M. Gottesman. 2003. P-glycoprotein: from genomics to mechanism. Oncogene 22:7468-7485. [DOI] [PubMed] [Google Scholar]
- 7.Balakrishnan, L., H. Venter, R. A. Shilling, and H. W. van Veen. 2004. Reversible transport by the ATP-binding cassette multidrug export pump LmrA: ATP synthesis at the expense of downhill ethidium uptake. J. Biol. Chem. 279:11273-11280. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee, S. K., K. Bhatt, P. Misra, and P. K. Chakraborti. 2000. Involvement of a natural transport system in the process of efflux-mediated drug resistance in Mycobacterium smegmatis. Mol. Gen. Genet. 262:949-956. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee, S. K., P. Misra, K. Bhatt, S. C. Mande, and P. K. Chakraborti. 1998. Identification of an ABC transporter gene that exhibits mRNA level overexpression in fluoroquinolone-resistant Mycobacterium smegmatis. FEBS Lett. 425:151-156. [DOI] [PubMed] [Google Scholar]
- 10.Baranova, N. N., A. Danchin, and A. A. Neyfakh. 1999. Mta, a global MerR-type regulator of the Bacillus subtilis multidrug-efflux transporters. Mol. Microbiol. 31:1549-1559. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt, K., S. K. Banerjee, and P. K. Chakraborti. 2000. Evidence that phosphate specific transporter is amplified in a fluoroquinolone resistant Mycobacterium smegmatis. Eur. J. Biochem. 267:4028-4032. [DOI] [PubMed] [Google Scholar]
- 12.Bolhuis, H., D. Molenaar, G. Poelarends, H. W. van Veen, B. Poolman, A. J. M. Driessen, and W. N. Konings. 1994. Proton motive force-driven and ATP-dependent drug extrusion systems in multidrug-resistant Lactococcus lactis. J. Bacteriol. 176:6957-6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolhuis, H., G. Poelarends, H. W. van Veen, B. Poolman, A. J. M. Driessen, and W. N. Konings. 1995. The lactococcal lmrP gene encodes a proton motive force-dependent drug transporter. J. Biol. Chem. 270:26092-26098. [DOI] [PubMed] [Google Scholar]
- 14.Bouige, P., D. Laurent, L. Piloyan, and E. Dassa. 2002. Phylogenetic and functional classification of ATP-binding cassette (ABC) systems. Curr. Protein Pept. Sci. 3:541-559. [DOI] [PubMed] [Google Scholar]
- 15.Bourdineaud, J. P., B. Nehme, S. Tesse, and A. Lonvaud-Funel. 2004. A bacterial gene homologous to ABC transporters protect Oenococcus oeni from ethanol and other stress factors in wine. Int. J. Food Microbiol. 92:1-14. [DOI] [PubMed] [Google Scholar]
- 16.Brown, M. H., I. T. Paulsen, and R. A. Skurray. 1999. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 31:394-395. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborti, P. K., K. Bhatt, S. K. Banerjee, and P. Misra. 1999. Role of an ABC importer in mycobacterial drug resistance. Biosci. Rep. 19:293-300. [DOI] [PubMed] [Google Scholar]
- 18.Chami, M., E. Steinfels, C. Orelle, J. M. Jault, A. Di Pietro, J. L. Rigaud, and S. Marco. 2002. Three-dimensional structure by cryo-electron microscopy of YvcC, an homodimeric ATP-binding cassette transporter from Bacillus subtilis. J. Mol. Biol. 315:1075-1085. [DOI] [PubMed] [Google Scholar]
- 19.Chen, C. J., J. E. Chin, K. Ueda, D. P. Clark, I. Pastan, M. M. Gottesman, and I. B. Roninson. 1986. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell 47:381-389. [DOI] [PubMed] [Google Scholar]
- 20.Cohen, M. L. 2000. Changing patterns of infectious disease. Nature 406:762-767. [DOI] [PubMed] [Google Scholar]
- 21.Cole, S. P., G. Bhardwaj, J. H. Gerlach, J. E. Mackie, C. E. Grant, K. C. Almquist, A. J. Stewart, E. U. Kurz, A. M. Duncan, and R. G. Deeley. 1992. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258:1650-1654. [DOI] [PubMed] [Google Scholar]
- 22.Dalmas, O., M. A. Do Cao, M. R. Lugo, F. J. Sharom, P. A. Di, and J. M. Jault. 2005. Time-resolved fluorescence resonance energy transfer shows that the bacterial multidrug ABC half-transporter BmrA functions as a homodimer. Biochemistry 44:4312-4321. [DOI] [PubMed] [Google Scholar]
- 23.Dalton, T. L., J. T. Collins, T. C. Barnett, and J. R. Scott. 2006. RscA, a member of the MDR1 family of transporters, is repressed by CovR and required for growth of Streptococcus pyogenes under heat stress. J. Bacteriol. 188:77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dano, K. 1972. Cross resistance between vinca alkaloids and anthracyclines in Ehrlich ascites tumor in vivo. Cancer Chemother. Rep. 56:701-708. [PubMed] [Google Scholar]
- 25.Dano, K. 1973. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim. Biophys. Acta 323:466-483. [DOI] [PubMed] [Google Scholar]
- 26.Daporta, M. T., J. L. Munoz Bellido, G. Y. Guirao, M. S. Hernandez, and J. A. Garcia-Rodriguez. 2004. In vitro activity of older and newer fluoroquinolones against efflux-mediated high-level ciprofloxacin-resistant Streptococcus pneumoniae. Int. J. Antimicrob. Agents 24:185-187. [DOI] [PubMed] [Google Scholar]
- 27.Dassa, E., and P. Bouige. 2001. The ABC of ABCs: a phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 152:211-229. [DOI] [PubMed] [Google Scholar]
- 28.Davidson, A. L., and J. Chen. 2004. ATP-binding cassette transporters in bacteria. Annu. Rev. Biochem. 73:241-268. [DOI] [PubMed] [Google Scholar]
- 29.Davies, J. 1994. Inactivation of antibiotics and the dissemination of resistance genes. Science 264:375-382. [DOI] [PubMed] [Google Scholar]
- 30.Davis, D. R., J. B. McAlpine, C. J. Pazoles, M. K. Talbot, E. A. Alder, C. White, B. M. Jonas, B. E. Murray, G. M. Weinstock, and B. L. Rogers. 2001. Enterococcus faecalis multi-drug resistance transporters: application for antibiotic discovery. J. Mol. Microbiol. Biotechnol. 3:179-184. [PubMed] [Google Scholar]
- 31.Dawson, R. J., and K. P. Locher. 2006. Structure of a bacterial multidrug ABC transporter. Nature 443:180-185. [DOI] [PubMed] [Google Scholar]
- 32.Diederichs, K., J. Diez, G. Greller, C. Muller, J. Breed, C. Schnell, C. Vonrhein, W. Boos, and W. Welte. 2000. Crystal structure of MalK, the ATPase subunit of the trehalose/maltose ABC transporter of the archaeon Thermococcus litoralis. EMBO J. 19:5951-5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyle, L. A., and D. D. Ross. 2003. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 22:7340-7358. [DOI] [PubMed] [Google Scholar]
- 34.Doyle, L. A., W. Yang, L. V. Abruzzo, T. Krogmann, Y. Gao, A. K. Rishi, and D. D. Ross. 1998. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 95:15665-15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ewart, G. D., and A. J. Howells. 1998. ABC transporters involved in transport of eye pigment precursors in Drosophila melanogaster. Methods Enzymol. 292:213-224. [DOI] [PubMed] [Google Scholar]
- 36.Fournier, B., R. Aras, and D. C. Hooper. 2000. Expression of the multidrug resistance transporter NorA from Staphylococcus aureus is modified by a two-component regulatory system. J. Bacteriol. 182:664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.George, A. M. 1996. Multidrug resistance in enteric and other gram-negative bacteria. FEMS Microbiol. Lett. 139:1-10. [DOI] [PubMed] [Google Scholar]
- 38.Gill, M. J., N. P. Brenwald, and R. Wise. 1999. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grinius, L. L., and E. B. Goldberg. 1994. Bacterial multidrug resistance is due to a single membrane protein which functions as a drug pump. J. Biol. Chem. 269:29998-30004. [PubMed] [Google Scholar]
- 40.Grkovic, S., M. H. Brown, N. J. Roberts, I. T. Paulsen, and R. A. Skurray. 1998. QacR is a repressor protein that regulates expression of the Staphylococcus aureus multidrug efflux pump QacA. J. Biol. Chem. 273:18665-18673. [DOI] [PubMed] [Google Scholar]
- 41.Grkovic, S., M. H. Brown, and R. A. Skurray. 2001. Transcriptional regulation of multidrug efflux pumps in bacteria. Semin. Cell Dev. Biol. 12:225-237. [DOI] [PubMed] [Google Scholar]
- 42.Grkovic, S., M. H. Brown, and R. A. Skurray. 2002. Regulation of bacterial drug export systems. Microbiol. Mol. Biol. Rev. 66:671-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes, J. D., and C. R. Wolf. 1990. Molecular mechanisms of drug resistance. Biochem. J. 272:281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heldwein, E. E., and R. G. Brennan. 2001. Crystal structure of the transcription activator BmrR bound to DNA and a drug. Nature 409:378-382. [DOI] [PubMed] [Google Scholar]
- 45.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 46.Higgins, C. F. 2001. ABC transporters: physiology, structure and mechanism—an overview. Res. Microbiol. 152:205-210. [DOI] [PubMed] [Google Scholar]
- 47.Higgins, C. F., P. D. Haag, K. Nikaido, F. Ardeshir, G. Garcia, and G. F. Ames. 1982. Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature 298:723-727. [DOI] [PubMed] [Google Scholar]
- 48.Higgins, C. F., I. D. Hiles, G. P. Salmond, D. R. Gill, J. A. Downie, I. J. Evans, I. B. Holland, L. Gray, S. D. Buckel, A. W. Bell, et al. 1986. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature 323:448-450. [DOI] [PubMed] [Google Scholar]
- 49.Higgins, C. F., and K. J. Linton. 2004. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 11:918-926. [DOI] [PubMed] [Google Scholar]
- 50.Hirakawa, H., Y. Inazumi, T. Masaki, T. Hirata, and A. Yamaguchi. 2005. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol. Microbiol. 55:1113-1126. [DOI] [PubMed] [Google Scholar]
- 51.Holland, I. B., S. P. Cole, K. Kuchler, and C. F. Higgins. 2003. ABC proteins, from bacteria to man. Academic Press, San Diego, CA.
- 52.Hopfner, K. P., A. Karcher, D. S. Shin, L. Craig, L. M. Arthur, J. P. Carney, and J. A. Tainer. 2000. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101:789-800. [DOI] [PubMed] [Google Scholar]
- 53.Hsieh, P. C., S. A. Siegel, B. Rogers, D. Davis, and K. Lewis. 1998. Bacteria lacking a multidrug pump: a sensitive tool for drug discovery. Proc. Natl. Acad. Sci. USA 95:6602-6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huda, N., E. W. Lee, J. Chen, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. Molecular cloning and characterization of an ABC multidrug efflux pump, VcaM, in non-O1 Vibrio cholerae. Antimicrob. Agents Chemother. 47:2413-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janas, E., M. Hofacker, M. Chen, S. Gompf, D. C. van der Does, and R. Tampé. 2003. The ATP hydrolysis cycle of the nucleotide-binding domain of the mitochondrial ATP-binding cassette transporter Mdl1p. J. Biol. Chem. 278:26862-26869. [DOI] [PubMed] [Google Scholar]
- 56.Jenkinson, H. F. 1996. Ins and outs of antimicrobial resistance: era of the drug pumps. J. Dent. Res. 75:736-742. [DOI] [PubMed] [Google Scholar]
- 57.Jonas, B. M., B. E. Murray, and G. M. Weinstock. 2001. Characterization of emeA, a NorA homolog and multidrug resistance efflux pump, in Enterococcus faecalis. Antimicrob. Agents Chemother. 45:3574-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones, H. E., N. P. Brenwald, K. A. Owen, and M. J. Gill. 2003. A multidrug efflux phenotype mutant of Streptococcus pyogenes. J. Antimicrob. Chemother. 51:707-710. [DOI] [PubMed] [Google Scholar]
- 59.Jones, P. M., and A. M. George. 1999. Subunit interactions in ABC transporters: towards a functional architecture. FEMS Microbiol. Lett. 179:187-202. [DOI] [PubMed] [Google Scholar]
- 60.Jones, P. M., and A. M. George. 2004. The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol. Life Sci. 61:682-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Juliano, R. L., and V. Ling. 1976. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 455:152-162. [DOI] [PubMed] [Google Scholar]
- 62.Kaatz, G. W., V. V. Moudgal, and S. M. Seo. 2002. Identification and characterization of a novel efflux-related multidrug resistance phenotype in Staphylococcus aureus. J. Antimicrob. Chemother. 50:833-838. [DOI] [PubMed] [Google Scholar]
- 63.Kaidoh, K., S. Miyauchi, A. Abe, S. Tanabu, T. Nara, and N. Kamo. 1996. Rhodamine 123 efflux transporter in Haloferax volcanii is induced when cultured under ‘metabolic stress’ by amino acids: the efflux system resembles that in a doxorubicin-resistant mutant. Biochem. J. 314:355-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerr, I. D. 2002. Structure and association of ATP-binding cassette transporter nucleotide-binding domains. Biochim. Biophys. Acta 1561:47-64. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi, N., K. Nishino, and A. Yamaguchi. 2001. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J. Bacteriol. 183:5639-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kruh, G. D., and M. G. Belinsky. 2003. The MRP family of drug efflux pumps. Oncogene 22:7537-7552. [DOI] [PubMed] [Google Scholar]
- 67.Lage, H. 2003. ABC-transporters: implications on drug resistance from microorganisms to human cancers. Int. J. Antimicrob. Agents 22:188-199. [DOI] [PubMed] [Google Scholar]
- 68.Lee, E. W., J. Chen, M. N. Huda, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. Functional cloning and expression of emeA, and characterization of EmeA, a multidrug efflux pump from Enterococcus faecalis. Biol. Pharm. Bull. 26:266-270. [DOI] [PubMed] [Google Scholar]
- 69.Lee, E. W., M. N. Huda, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. EfrAB, an ABC multidrug efflux pump in Enterococcus faecalis. Antimicrob. Agents Chemother. 47:3733-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levy, S. B. 1998. The challenge of antibiotic resistance. Sci. Am. 278:46-53. [DOI] [PubMed] [Google Scholar]
- 71.Levy, S. B., and B. Marshall. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10:S122-S129. [DOI] [PubMed] [Google Scholar]
- 72.Levy, S. B., and L. McMurry. 1978. Plasmid-determined tetracycline resistance involves new transport systems for tetracycline. Nature 276:90-92. [DOI] [PubMed] [Google Scholar]
- 73.Li, X. Z., and H. Nikaido. 2004. Efflux-mediated drug resistance in bacteria. Drugs 64:159-204. [DOI] [PubMed] [Google Scholar]
- 74.Ling, V. 1997. Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother. Pharmacol. 40(Suppl.):S3-S8. [DOI] [PubMed] [Google Scholar]
- 75.Linton, K. J., and C. F. Higgins. 1998. The Escherichia coli ATP-binding cassette (ABC) proteins. Mol. Microbiol. 28:5-13. [DOI] [PubMed] [Google Scholar]
- 76.Liu, L. X., K. Janvier, V. Berteaux-Lecellier, N. Cartier, R. Benarous, and P. Aubourg. 1999. Homo- and heterodimerization of peroxisomal ATP-binding cassette half-transporters. J. Biol. Chem. 274:32738-32743. [DOI] [PubMed] [Google Scholar]
- 77.Locher, K. P. 2004. Structure and mechanism of ABC transporters. Curr. Opin. Struct. Biol. 14:426-431. [DOI] [PubMed] [Google Scholar]
- 78.Locher, K. P., A. T. Lee, and D. C. Rees. 2002. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science 296:1091-1098. [DOI] [PubMed] [Google Scholar]
- 79.Lomovskaya, O., and K. Lewis. 1992. Emr, an Escherichia coli locus for multidrug resistance. Proc. Natl. Acad. Sci. USA 89:8938-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lomovskaya, O., K. Lewis, and A. Matin. 1995. EmrR is a negative regulator of the Escherichia coli multidrug resistance pump EmrAB. J. Bacteriol. 177:2328-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lubelski, J., P. Mazurkiewicz, R. van Merkerk, W. N. Konings, and A. J. M. Driessen. 2004. ydaG and ydbA of Lactococcus lactis encode a heterodimeric ATP-binding cassette-type multidrug transporter. J. Biol. Chem. 279:34449-34455. [DOI] [PubMed] [Google Scholar]
- 82.Lubelski, J., R. van Merkerk, W. N. Konings, and A. J. M. Driessen. 2006. Nucleotide-binding sites of the heterodimeric LmrCD ABC-multidrug transporter of Lactococcus lactis are asymmetric. Biochemistry 45:648-656. [DOI] [PubMed] [Google Scholar]
- 83.Lubelski, J., A. de Jong, R. van Merkerk, H. Agustiandari, O. P. Kuipers, J. Kok, and A. J. M. Driessen. 2006. LmrCD is a major multidrug resistance transporter in Lactococcus lactis. Mol. Microbiol. 61:771-781. [DOI] [PubMed] [Google Scholar]
- 84.Lynch, C., P. Courvalin, and H. Nikaido. 1997. Active efflux of antimicrobial agents in wild-type strains of enterococci. Antimicrob. Agents Chemother. 41:869-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1993. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J. Bacteriol. 175:6299-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45-55. [DOI] [PubMed] [Google Scholar]
- 87.Marger, M. D., and M. H. Saier, Jr. 1993. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem. Sci. 18:13-20. [DOI] [PubMed] [Google Scholar]
- 88.Margolles, A., M. Putman, H. W. van Veen, and W. N. Konings. 1999. The purified and functionally reconstituted multidrug transporter LmrA of Lactococcus lactis mediates the transbilayer movement of specific fluorescent phospholipids. Biochemistry 38:16298-16306. [DOI] [PubMed] [Google Scholar]
- 89.Markham, P. N., M. Ahmed, and A. A. Neyfakh. 1996. The drug-binding activity of the multidrug-responding transcriptional regulator BmrR resides in its C-terminal domain. J. Bacteriol. 178:1473-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyake, K., L. Mickley, T. Litman, Z. Zhan, R. Robey, B. Cristensen, M. Brangi, L. Greenberger, M. Dean, T. Fojo, and S. E. Bates. 1999. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 59:8-13. [PubMed] [Google Scholar]
- 91.Miyauchi, S., M. Komatsubara, and N. Kamo. 1992. In archaebacteria, there is a doxorubicin efflux pump similar to mammalian P-glycoprotein. Biochim. Biophys. Acta 1110:144-150. [DOI] [PubMed] [Google Scholar]
- 92.Moody, J. E., L. Millen, D. Binns, J. F. Hunt, and P. J. Thomas. 2002. Cooperative, ATP-dependent association of the nucleotide binding cassettes during the catalytic cycle of ATP-binding cassette transporters. J. Biol. Chem. 277:21111-21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mullin, S., N. Mani, and T. H. Grossman. 2004. Inhibition of antibiotic efflux in bacteria by the novel multidrug resistance inhibitors biricodar (VX-710) and timcodar (VX-853). Antimicrob. Agents Chemother. 48:4171-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neu, H. C. 1992. The crisis in antibiotic resistance. Science 257:1064-1073. [DOI] [PubMed] [Google Scholar]
- 95.Neyfakh, A. A., V. E. Bidnenko, and L. B. Chen. 1991. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc. Natl. Acad. Sci. USA 88:4781-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nikaido, H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382-388. [DOI] [PubMed] [Google Scholar]
- 97.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nikata, T., Y. Sakai, K. Shibat, J. Kato, A. Kuroda, and H. Ohtake. 1996. Molecular analysis of the phosphate-specific transport (pst) operon of Pseudomonas aeruginosa. Mol. Gen. Genet. 250:692-698. [DOI] [PubMed] [Google Scholar]
- 99.Ninio, S., and S. Schuldiner. 2003. Characterization of an archaeal multidrug transporter with a unique amino acid composition. J. Biol. Chem. 278:12000-12005. [DOI] [PubMed] [Google Scholar]
- 100.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishino, K., and A. Yamaguchi. 2002. EvgA of the two-component signal transduction system modulates production of the yhiUV multidrug transporter in Escherichia coli. J. Bacteriol. 184:2319-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orelle, C., O. Dalmas, P. Gros, A. Di Pietro, and J. M. Jault. 2003. The conserved glutamate residue adjacent to the Walker-B motif is the catalytic base for ATP hydrolysis in the ATP-binding cassette transporter BmrA. J. Biol. Chem. 278:47002-47008. [DOI] [PubMed] [Google Scholar]
- 103.Pao, S. S., I. T. Paulsen, and M. H. Saier, Jr. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paulsen, I. T. 2003. Multidrug efflux pumps and resistance: regulation and evolution. Curr. Opin. Microbiol. 6:446-451. [DOI] [PubMed] [Google Scholar]
- 105.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paulsen, I. T., and K. Lewis. 2001. Microbial multidrug efflux: introduction. J. Mol. Microbiol. Biotechnol. 3:143-144. [PubMed] [Google Scholar]
- 107.Paulsen, I. T., L. Nguyen, M. K. Sliwinski, R. Rabus, and M. H. Saier, Jr. 2000. Microbial genome analyses: comparative transport capabilities in eighteen prokaryotes. J. Mol. Biol. 301:75-100. [DOI] [PubMed] [Google Scholar]
- 108.Paulsen, I. T., R. A. Skurray, R. Tam, M. H. Saier, Jr., R. J. Turner, J. H. Weiner, E. B. Goldberg, and L. L. Grinius. 1996. The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol. Microbiol. 19:1167-1175. [DOI] [PubMed] [Google Scholar]
- 109.Paulsen, I. T., M. K. Sliwinski, and M. H. Saier, Jr. 1998. Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J. Mol. Biol. 277:573-592. [DOI] [PubMed] [Google Scholar]
- 110.Piddock, L. J., and M. M. Johnson. 2002. Accumulation of 10 fluoroquinolones by wild-type or efflux mutant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Poole, K. 2004. Efflux-mediated multiresistance in gram-negative bacteria. Clin. Microbiol. Infect. 10:12-26. [DOI] [PubMed] [Google Scholar]
- 112.Poole, K. 2005. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 56:20-51. [DOI] [PubMed] [Google Scholar]
- 113.Putman, M., H. W. van Veen, J. E. Degener, and W. N. Konings. 2000. Antibiotic resistance: era of the multidrug pump. Mol. Microbiol. 36:772-773. [DOI] [PubMed] [Google Scholar]
- 114.Putman, M., H. W. van Veen, and W. N. Konings. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ren, Q., K. H. Kang, and I. T. Paulsen. 2004. TransportDB: a relational database of cellular membrane transport systems. Nucleic Acids Res. 32:D284-D288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ren, Q., and I. T. Paulsen. 2005. Comparative analyses of fundamental differences in membrane transport capabilities in prokaryotes and eukaryotes. PLoS Comput. Biol. 1:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reuter, G., T. Janvilisri, H. Venter, S. Shahi, L. Balakrishnan, and H. W. van Veen. 2003. The ATP binding cassette multidrug transporter LmrA and lipid transporter MsbA have overlapping substrate specificities. J. Biol. Chem. 278:35193-35198. [DOI] [PubMed] [Google Scholar]
- 118.Robertson, G. T., T. B. Doyle, and A. S. Lynch. 2005. Use of an efflux-deficient Streptococcus pneumoniae strain panel to identify ABC-class multidrug transporters involved in intrinsic resistance to antimicrobial agents. Antimicrob. Agents Chemother. 49:4781-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rouquette-Loughlin, C. E., J. T. Balthazar, and W. M. Shafer. 2005. Characterization of the MacA-MacB efflux system in Neisseria gonorrhoeae. J. Antimicrob. Chemother. 56:856-860. [DOI] [PubMed] [Google Scholar]
- 120.Saier, M. H., Jr. 2000. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 64:354-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saier, M. H., Jr., J. T. Beatty, A. Goffeau, K. T. Harley, W. H. Heijne, S. C. Huang, D. L. Jack, P. S. Jahn, K. Lew, J. Liu, S. S. Pao, I. T. Paulsen, T. T. Tseng, and P. S. Virk. 1999. The major facilitator superfamily. J. Mol. Microbiol. Biotechnol. 1:257-279. [PubMed] [Google Scholar]
- 122.Saier, M. H., Jr., and I. T. Paulsen. 2001. Phylogeny of multidrug transporters. Semin. Cell Dev. Biol. 12:205-213. [DOI] [PubMed] [Google Scholar]
- 123.Saier, M. H., Jr., R. Tam, A. Reizer, and J. Reizer. 1994. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol. Microbiol. 11:841-847. [DOI] [PubMed] [Google Scholar]
- 124.Sakamoto, K., and W. N. Konings. 2003. Beer spoilage bacteria and hop resistance. Int. J. Food Microbiol. 89:105-124. [DOI] [PubMed] [Google Scholar]
- 125.Sakamoto, K., A. Margolles, H. W. van Veen, and W. N. Konings. 2001. Hop resistance in the beer spoilage bacterium Lactobacillus brevis is mediated by the ATP-binding cassette multidrug transporter HorA. J. Bacteriol. 183:5371-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schmitt, L., and R. Tampé. 2002. Structure and mechanism of ABC transporters. Curr. Opin. Struct. Biol. 12:754-760. [DOI] [PubMed] [Google Scholar]
- 127.Schumacher, M. A., M. C. Miller, S. Grkovic, M. H. Brown, R. A. Skurray, and R. G. Brennan. 2001. Structural mechanisms of QacR induction and multidrug recognition. Science 294:2158-2163. [DOI] [PubMed] [Google Scholar]
- 128.Senior, A. E., and D. C. Gadsby. 1997. ATP hydrolysis cycles and mechanism in P-glycoprotein and CFTR. Semin. Cancer Biol. 8:143-150. [DOI] [PubMed] [Google Scholar]
- 129.Shilling, R., L. Federici, F. Walas, H. Venter, S. Velamakanni, B. Woebking, L. Balakrishnan, B. Luisi, and H. W. van Veen. 2005. A critical role of a carboxylate in proton conduction by the ATP-binding cassette multidrug transporter LmrA. FASEB J. 19:1698-1700. [DOI] [PubMed] [Google Scholar]
- 130.Spratt, B. G. 1994. Resistance to antibiotics mediated by target alterations. Science 264:388-393. [DOI] [PubMed] [Google Scholar]
- 131.Steinfels, E., C. Orelle, O. Dalmas, F. Penin, B. Miroux, A. Di Pietro, and J. M. Jault. 2002. Highly efficient over-production in E. coli of YvcC, a multidrug-like ATP-binding cassette transporter from Bacillus subtilis. Biochim. Biophys. Acta 1565:1-5. [DOI] [PubMed] [Google Scholar]
- 132.Steinfels, E., C. Orelle, J. R. Fantino, O. Dalmas, J. L. Rigaud, F. Denizot, A. Di Pietro, and J. M. Jault. 2004. Characterization of YvcC (BmrA), a multidrug ABC transporter constitutively expressed in Bacillus subtilis. Biochemistry 43:7491-7502. [DOI] [PubMed] [Google Scholar]
- 133.Sulavik, M. C., C. Houseweart, C. Cramer, N. Jiwani, N. Murgolo, J. Greene, B. DiDomenico, K. J. Shaw, G. H. Miller, R. Hare, and G. Shimer. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Surin, B. P., H. Rosenberg, and G. B. Cox. 1985. Phosphate-specific transport system of Escherichia coli: nucleotide sequence and gene-polypeptide relationships. J. Bacteriol. 161:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tennent, J. M., B. R. Lyon, M. Midgley, I. G. Jones, A. S. Purewal, and R. A. Skurray. 1989. Physical and biochemical characterization of the qacA gene encoding antiseptic and disinfectant resistance in Staphylococcus aureus. J. Gen. Microbiol. 135:1-10. [DOI] [PubMed] [Google Scholar]
- 136.Tiirikainen, M. I., and T. Krusius. 1991. Multidrug resistance. Ann. Med. 23:509-520. [DOI] [PubMed] [Google Scholar]
- 137.Travis, J. 1994. Reviving the antibiotic miracle? Science 264:360-362. [DOI] [PubMed] [Google Scholar]
- 138.van Bambeke, F., E. Balzi, and P. M. Tulkens. 2000. Antibiotic efflux pumps. Biochem. Pharmacol. 60:457-470. [DOI] [PubMed] [Google Scholar]
- 139.van den Berg van Saparoea, H., J. Lubelski, R. van Merkerk, P. S. Mazurkiewicz, and A. J. M. Driessen. 2005. Proton motive force-dependent Hoechst 33342 transport by the ABC transporter LmrA of Lactococcus lactis. Biochemistry 44:16931-16938. [DOI] [PubMed] [Google Scholar]
- 140.van der Does, C., and R. Tampe. 2004. How do ABC transporters drive transport? Biol. Chem. 385:927-933. [DOI] [PubMed] [Google Scholar]
- 141.van Veen, H. W., R. Callaghan, L. Soceneantu, A. Sardini, W. N. Konings, and C. F. Higgins. 1998. A bacterial antibiotic-resistance gene that complements the human multidrug-resistance P-glycoprotein gene. Nature 391:291-295. [DOI] [PubMed] [Google Scholar]
- 142.van Veen, H. W., A. Margolles, M. Muller, C. F. Higgins, and W. N. Konings. 2000. The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. EMBO J. 19:2503-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van Veen, H. W., K. Venema, H. Bolhuis, I. Oussenko, J. Kok, B. Poolman, A. J. Driessen, and W. N. Konings. 1996. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc. Natl. Acad. Sci. USA 93:10668-10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Venter, H., R. A. Shilling, S. Velamakanni, L. Balakrishnan, and H. W. van Veen. 2003. An ABC transporter with a secondary-active multidrug translocator domain. Nature 426:866-870. [DOI] [PubMed] [Google Scholar]
- 145.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Walsh, C. 2000. Molecular mechanisms that confer antibacterial drug resistance. Nature 406:775-781. [DOI] [PubMed] [Google Scholar]
- 147.Woebking, B., G. Reuter, R. A. Shilling, S. Velamakanni, S. Shahi, H. Venter, L. Balakrishnan, and H. W. van Veen. 2005. Drug-lipid A interactions on the Escherichia coli ABC transporter MsbA. J. Bacteriol. 187:6363-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wood, M. J., and R. C. Moellering, Jr. 2003. Microbial resistance: bacteria and more. Clin. Infect. Dis. 36:S2-S3. [DOI] [PubMed] [Google Scholar]
- 149.Yoshida, H., M. Bogaki, S. Nakamura, K. Ubukata, and M. Konno. 1990. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J. Bacteriol. 172:6942-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Young, J., and I. B. Holland. 1999. ABC transporters: bacterial exporters—revisited five years on. Biochim. Biophys. Acta 1461:177-200. [DOI] [PubMed] [Google Scholar]