Abstract
Summary: To survive within the host, pathogens such as Mycobacterium tuberculosis and Helicobacter pylori need to evade the immune response and find a protected niche where they are not exposed to microbicidal effectors. The pH of the microenvironment surrounding the pathogen plays a critical role in dictating the organism's fate. Specifically, the acidic pH of the endocytic organelles and phagosomes not only can affect bacterial growth directly but also promotes a variety of host microbicidal responses. The development of mechanisms to avoid or resist the acidic environment generated by host cells is therefore crucial to the survival of many pathogens. Here we review the processes that underlie the generation of organellar acidification and discuss strategies employed by pathogens to circumvent it, using M. tuberculosis and H. pylori as examples.
INTRODUCTION
The biosynthetic pathway consists of an elaborate and highly differentiated membranous network responsible for sorting and delivering newly synthesized (glyco)proteins and lipids to their final destinations. An equally sophisticated network of membrane vesicles and tubules, known as the endocytic pathway, is involved in routing internalized molecules towards recycling or degradation stations. The secretory and endocytic pathways are composed of discrete subcompartments that differ in the compositions of their membrane and luminal contents. It has recently become apparent that the luminal pH is not homogeneous but varies in a controlled and systematic manner along each pathway and, importantly, that maintenance of a defined pH profile is crucial for the normal traffic of cargo.
The luminal pH is also a critical determinant of the function of phagosomes, unique endocytic organelles specialized in the elimination of invading microorganisms and in the presentation of antigens derived thereof. In the case of phagosomes, an acidic luminal pH not only is essential for the optimal activity of a variety of microbicidal agents but also appears to be required to coordinate the fusion of membranes that promote maturation, culminating in the formation of phagolysosomes.
In view of the central role of luminal pH in membrane trafficking, serious alterations in the routing of cargo and in the ability of phagocytes to eliminate pathogens are anticipated when proton homeostasis is disrupted. These predictions have been borne out by experiments using pharmacological agents that interfere with normal pH regulation. More strikingly, bacteria that impair the development of phagosomal acidification often show increased virulence. Clearly, elucidation of the basic mechanisms underlying organellar acidification and of the means whereby it can be disrupted is key to the understanding of the normal innate immune response and the different modes of evasion that certain microbial species have developed. In this review, we initially analyze the determinants of normal pH homeostasis and then proceed to illustrate two instances where microbial pathogenesis is associated with abnormal pH regulation.
SOURCE AND REGULATION OF ORGANELLAR ACIDIFICATION
The lumens of most organelles of the biosynthetic and endocytic pathways are more acidic than the surrounding cytosol. Protons are driven into the lumen against their electrochemical gradient by active pumps, the vacuolar ATPases (V-ATPases) (53, 89). V-ATPases utilize the energy released from ATP hydrolysis to translocate protons across biological membranes (Fig. 1). Their stoichiometry is still the subject of debate and may vary depending on the opposing proton motive force (PMF), but most researchers believe that two or three protons are translocated per ATP hydrolyzed, depending on the pH gradient across the membrane (15, 37). The V-ATPase is a large multisubunit complex that approaches a molecular mass of 103 kDa. Grossly, the structure of V-ATPases can be divided into the following two major functional domains: a 570-kDa peripheral subcomplex, known as V1, that is thought to bind and hydrolyze ATP, and an integral membrane subcomplex, termed V0, that serves as the pore through which protons traverse the bilayer (48, 90, 115). The rate of proton translocation is obviously dictated by the density of functional pumps in the organellar membrane, but other parameters also contribute importantly. Firstly, the activity of the V-ATPases is regulated by a variety of factors, some of which are believed to induce dissociation of the V1 domain from the membrane-associated holoenzyme. The regulation of the V-ATPases is complex and not yet fully understood and is not discussed further here, but more detailed information can be found in previous studies (64, 69).
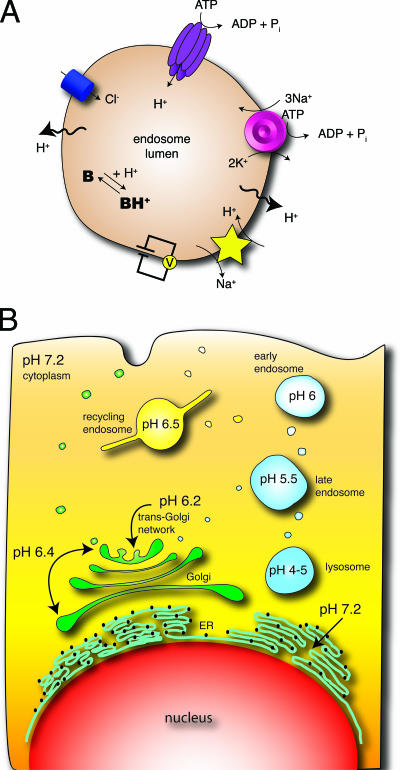
FIG. 1.
Regulation of organellar acidification. (A) Endocytic vesicles quickly acquire V-ATPases through fusion with early endosomes. V-ATPases use the energy of ATP hydrolysis to accumulate protons in the lumen. The concomitant translocation of counterions, e.g., Cl−, via conductive pathways curtails the buildup of a transmembrane electrical potential and allows the luminal accumulation of protons. Proton (equivalent) leakage pathways limit the extent of acidification, while intracellular buffers (B) dictate the rate of acidification. When present, Na+/K+-ATPases can contribute to the electrical potential across the endosomal membrane, tending to reduce the rate of proton pumping. Na+ accumulated in the lumen by the Na+/K+-ATPases could in turn drive Na+/H+ exchange, promoting acidification. (B) Schematic representation of the pHs of the main compartments of the secretory (left) and endocytic (right) pathways. The cytosolic pH is also shown, for comparison.
In addition to agents that regulate the intrinsic activity of the V-ATPases, extrinsic factors also control the rate of pumping. The rate of transport is proportional to the difference between the energy provided by the hydrolysis of ATP and the opposing PMF. The latter consists of two components, including the chemical potential intrinsic to the proton concentration gradient and the electrical transmembrane potential (55). An electrical potential builds up during the course of pumping because the V-ATPase is electrogenic, i.e., translocates positive charges (protons) unaccompanied by a counterion. In the absence of parallel, independent permeation routes for compensatory counterions, proton pumping would rapidly generate a very large membrane potential (lumen positive) that would arrest further pump activity even before a measurable pH gradient was established. Conversely, only a negligible electrical potential will build up across membranes that are freely permeable to counterions. In this instance, the PMF would be dominated by the chemical (concentration) gradient, and a marked luminal acidification would be observed.
Because of its electrogenic nature (115), the V-ATPase is susceptible not only to the voltage that it generates during the course of proton pumping but also to other sources of electrical potential. In some organelles, other ion pumps and channels combine to generate voltages that hinder or facilitate the accumulation of protons. A well-documented case is that of early endosomes, which in some cells bear a significant number of Na+-K+ ATPases (22). Like the V-ATPases, these sodium pumps are electrogenic, since they pump unequal numbers of sodium (three) and potassium (two) ions (17, 92). The net movement of positive charges into the endosome lumen, which is topologically equivalent to the extracellular space, results in the accretion of an inside-positive membrane potential. This positive voltage opposes the electrogenic translocation of protons, curtailing the activity of the V-ATPase and limiting the resulting acidification. Accordingly, it has been shown that the addition of cardiac glycoside inhibitors of the Na+-K+ ATPase enhances endosomal acidification (5, 21, 52, 55). The Na+ accumulated in the lumen by the Na+-K+ ATPase, along with that taken up by fluid-phase endocytosis, can drive inward H+ flux, in principle, via Na+-H+ exchange (20, 122). Other electrogenic systems, such as the electron-transporting NADPH oxidase of phagosomes, are also anticipated to alter the progress of acidification in neutrophils, and seemingly also in dendritic cells (66, 67, 103).
As stated earlier, the electrogenic contribution of the V-ATPase is mitigated by the presence of counterion-conductive pathways, which effectively shunt the voltage. The presence of a sizable counterion conductance and its role in acidification of endomembranes, both in vitro and in vivo, have been documented extensively (16, 51). In isolated endosomes, luminal acidification was measurable only when chloride or potassium was provided (7, 114, 132). As predicted, the counterions were driven across the membrane by the potential generated by the V-ATPase. This could be inferred from the concomitant accumulation of luminal chloride, which was inhibited by the V-ATPase inhibitor bafilomycin A (114). The molecular identity of the chloride (counterion) conductance pathway has not been established definitively and is likely to vary among organelles. Genetic experiments indicated that ablation of the chloride channel isoform ClC-3, ClC-4, or ClC-5 altered the rate or extent of endosomal acidification (58, 59, 68, 86). More recently, the cystic fibrosis transmembrane conductance regulator, a different type of Cl− channel, was implicated in lysosomal acidification (42). A more detailed discussion of chloride transporters and their role in the endocytic pathway is available in a recent review (68). Finally, the role of cations as carriers of charge that could neutralize the movement of protons has largely been ignored but is deserving of consideration.
In theory, a change in pH equivalent to the free energy of hydrolysis of ATP can be attained across membranes with very large counterion permeability if the pumped protons remain in the luminal space. In practice, however, back-flux of protons occurs across passive “leakage” pathways. The existence of such leaks is readily apparent when V-ATPase inhibitors, such as bafilomycin or concanamycin, are added to organelles that have reached a steady level of acidification. The inhibitors unmask a progressive dissipation of the luminal acidification, whose rate varies among organelles (40, 70, 72). While the loss of the pH gradient is obvious, much less is known about the molecular entities responsible for the leak of proton equivalents. In the Golgi apparatus, the passive back-flux was found to be voltage sensitive and inhibitable by Zn2+ (105), resembling the behavior of a proton conductance detected in the plasmalemma of several cell types by electrophysiological means (24). Other pathways that may contribute to the leakage of proton equivalents include cation-proton exchangers, possibly of the Na+-H+ exchange superfamily; anion exchangers that may promote the uptake of hydroxyl or bicarbonate ions; and transporters that may utilize the proton gradient to extrude or accumulate organic osmolytes.
As a result of this ongoing back-flux, the V-ATPase never reaches thermodynamic equilibrium and the system instead reaches a steady state at the point where the rate of proton pumping is matched by the leakage of proton equivalents. The balance between pumping and leakage will ultimately dictate the pH observed in each organelle at steady state. Systematic analyses have revealed that proton leak permeability decreases progressively along the secretory pathway, being highest in the endoplasmic reticulum, intermediate in the Golgi cisternae and the trans-Golgi network, and lowest in secretory granules (131). Remarkably, the decreasing leakiness is paralleled by an increase in V-ATPase density and, consequently, in pumping activity. The combined effects of more pumping and less leakage render the most distal parts of the secretory pathways very acidic, while the endoplasmic reticulum is essentially neutral, having a high level of leakage and negligible pumping (131). A similar gradation in the balance between pumps and leaks is thought to determine the progressive acidification of the subcompartments of the endocytic pathway, with early endocytic vesicles being slightly acidic (pH, ∼6.4) and lysosomes being profoundly acidic (pH 4 to 5).
ROLE OF ACIDIFICATION IN MEMBRANE TRAFFICKING
Perturbation of the existing organellar acidification has been the preferred strategy for analyzing the role of the pH gradient in cellular function (130). A variety of pharmacological agents have been employed to dissipate the preexisting gradient or to prevent its formation. These can be grouped broadly into the following three categories: (i) inhibitors of the V-ATPase, such as the bafilomycins and the concanamycins, which rapidly and irreversibly inhibit pumping by attaching to the V0 domain (18, 19, 128, 130); (ii) weak bases, which permeate the plasma and organellar membranes in the uncharged, unprotonated form and scavenge luminal protons, elevating the intraorganellar pH (39); and (iii) ionophores that can facilitate the movement of protons down the electrochemical gradient (96, 101, 119). Some of the most frequently used ionophores, such as monensin and nigericin, are in fact electroneutral exchangers of protons for alkali cations that respond to the combined chemical gradients of all the substrate ions. The three classes of agents used to alter pH differ in their modes of action, and therefore their efficacies, reversibility, and specificities are not identical. Notably, all of these agents affect the cell globally, i.e., they perturb the pH of all acidic organelles and, in some cases, even that of other compartments, such as the cytosol. Therefore, nonspecific side effects can be anticipated, which can significantly complicate the interpretation of the data collected. Nonetheless, much has been learned about the role of pH on membrane trafficking by using pump inhibitors, weak bases, and ionophores. A brief overview of this information is presented below.
Early evidence for a role for organellar acidification in membrane targeting and function was obtained by monitoring the fate of receptor-ligand complexes in the endocytic pathway.
In a simplified scenario, ligand binding occurs at the plasma membrane, triggering receptor-ligand internalization. The resultant complex is destined for early endosomes, where complex dissociation can, in some cases, be induced by the mildly acidic (pH, ≈6.4) microenvironment of the lumen (Fig. 1B). In the cases where pH-induced dissociation occurs, ligands and receptors traffic independently henceforth. Typically, ligands are targeted to progressively more acidic compartments, destined for eventual lysosomal degradation. Receptors, on the other hand, are shuttled to specialized endosomal structures for temporary intracellular storage and/or recycling to the plasma membrane (87). The low-density lipoprotein and asialoglycoprotein receptors, which have been studied extensively, conform to this paradigm. Agents that disrupt endosome pH gradients impair ligand dissociation from the low-density lipoprotein receptor (38) and prevent recycling to the plasma membrane, which redistributes the receptors to intracellular pools (12, 56). Interactions between asialoglycoprotein receptors and their ligand, asialo-orosomucoid, are similarly affected by treatment with lysosomotropic (alkalinizing) compounds (100, 108, 109, 137). The equally well-studied transferrin receptor follows a somewhat different pattern. Not only is the binding of the ligand, transferrin, to the receptor dependent on pH, but so is the association of iron with the apoprotein (133). Endosomal acidification promotes the release of iron from the apotransferrin-receptor complex, but the protein ligand remains associated with the receptor. Reexposure to neutral pH upon recycling of the apotransferrin-receptor complex to the surface results in release of the apoprotein (36).
In addition to modulating the association between receptors and their ligands, however, the pH gradient directly controls the traffic of membranes in both the endocytic and secretory pathways. Inhibition of V-ATPase activity impairs the normal routing of membranes and cargo along the endocytic pathway. In HeLa cells, bafilomycin prevented the delivery of human rhinovirus serotype 2 virus particles and of fluid-phase markers to late endosomes (13). Similarly, cationized gold particles, which are normally internalized and directed to lysosomes, failed to do so when the organellar acidification was dissipated (121). A complete and systematic summary of the effects of agents that disrupt pH homeostasis on the endocytic pathway can be found elsewhere (130).
While the essential role of intraorganellar acidification in controlling traffic can be demonstrated readily and is widely accepted, the underlying mechanism(s) is not entirely clear. Useful clues can be derived from the morphological consequences of pH alteration. Extensive tubulation and vesicle enlargement are usually seen (30, 35), suggesting elevated rates of fusion or, more likely, impaired fission. In accordance with the latter hypothesis, early carrier vesicle budding and fission from purified early endosomes were impaired when the V-ATPase was inhibited (13).
The lumens of endocytic and secretory organelles contain a plethora of soluble enzymes, many of which display pH optima tailored to match the prevailing physiological pH. While these enzymes are predicted to function suboptimally when the V-ATPase is impaired, it is difficult to conceive how soluble luminal components may direct membrane fusion and fission events. Instead, membrane-associated molecules are likely to sense the pH, and some of these may be transmembrane proteins capable of relaying the signal inherent to the luminal pH to the cytosolic face of the membrane, where budding and fusion are enacted. Indeed, recent advances have uncovered the participation of molecules that associate with the cytosolic face of endomembranes in a (luminal) pH-dependent manner. The association of coatomer proteins (COPs), namely, β- and ɛ-COP, is necessary for the formation of intermediate transport vesicles believed to deliver cargo from early to late endosomes (6). Coincidentally, both the recruitment of COPs and the subsequent vesicle formation are disrupted when the pH gradient of endosomes is dissipated (6). An inability to form carrier vesicles because of insufficient COP recruitment, a consequence of pH neutralization, may explain not only the observed tubulation of early endosomes (30, 35) but, more importantly, the block in delivery of material from early to late endosomes.
The membrane association of COPs is regulated by small GTPases of the ARF family. As early as 1992, Zeuzem et al. (135) observed that the association of ARF1 with microsomes requires luminal acidification. Maranda and colleagues (79) made similar observations for ARF6 in epithelial cells. Moreover, they realized that the effect was likely mediated by ARNO, the nucleotide exchange factor that activates ARF6. Most interestingly, the same group (63) more recently proposed that the V-ATPase may be engaged directly in binding ARNO and ARF6. The pump may need to be in an active configuration to associate with ARNO/ARF6 and/or may itself sense the transmembrane pH gradient.
Whether ARF- and COP-dependent fission is the sole or even the primary target of the luminal pH remains to be determined. Other sites of action are likely to exist, inasmuch as disruption of traffic also occurs at stages where COPs are not thought to participate.
ROLE OF ACIDIFICATION IN PHAGOCYTOSIS
Phagocytosis is of paramount importance in the control of invading microorganisms by the innate immune system. The process involves the engulfment of foreign particles into an intracellular vacuole or phagosome, where microbicidal effectors are deployed by a process known as maturation. Maturation involves the gradual remodeling of the phagosomal membrane and contents through a finely coordinated sequence of fusion events with vesicular components of the endocytic and possibly also secretory pathways (14, 41, 81, 95). Despite multiple rounds of fusion, the surface area of the phagosome remains approximately constant (10, 57, 61) by virtue of concomitant fission events that contribute to remodeling.
One component of the microbicidal response is the acidification of the phagosomal lumen, which is brought about by V-ATPases delivered via fusion with membranes of the endocytic pathway (Fig. 2). As in the case of lysosomes, acidification of the phagosomal lumen favors the lytic activity of a variety of degradative enzymes and also promotes the generation of hydrogen peroxide. In addition, as found for the endocytic and secretory systems, there is mounting evidence suggesting that acidification is not only a consequence but also a determinant of phagosomal maturation. Dissipation of the pH gradients inhibits phagolysosome fusion (54) while promoting phagosome-endosome fusion (54, 60). Others have replicated these observations, but a very recent report found that fusion of phagosomes and lysosomes persisted when lysosomal acidification was (partially) impaired. Specifically, Nelson and colleagues (42) found that in alveolar macrophages from cftr−/− mice, the lysosomal pH was abnormally high due to limited counterion permeability of the membrane. Despite the elevated pH, the fusion of lysosomes with phagosomes proceeded normally. Thus, the control of phagolysosome formation by luminal acidification may not be identical in all cells.
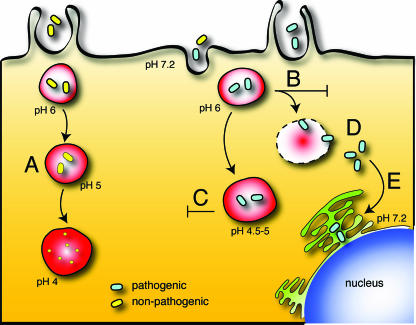
FIG. 2.
Phagosome maturation and mechanisms of evasion. (A) Phagocytosis of nonpathogenic particles follows a pathway that resembles endosomal progression, characterized by progressive acidification of the lumen to a pH of >5 and delivery of lytic enzymes and other microbicidal factors that kill and degrade the contents. (B to E) Some pathogenic bacteria have evolved mechanisms to evade killing. These strategies include inhibition of phagosome maturation and acidification (B); development of resistance to the acidic and lytic environment of phagolysosomes (C); rupture of the phagosome, enabling the pathogens to gain access to the cytoplasm (D); and redirection of phagosomal maturation, with fusion to other, nonmicrobicidal compartments, such as the endoplasmic reticulum or Golgi complex (E).
MICROORGANISMS, PHAGOSOMES, AND pH
Certain pathogens evade the microbicidal response mounted by phagocytes and can therefore survive as intracellular pathogens. Bacteria have evolved the following general strategies to avoid being killed by phagosomes: (i) arrest of phagosomal maturation at an early, nonmicrobicidal stage (Fig. 2B); (ii) development of resistance to the microbicidal arsenal of phagolysosomes (Fig. 2C); (iii) escape from the phagosome into the cytosol (Fig. 2D); and (iv) redirection of phagosomal maturation, with fusion to other organelles devoid of microbicidal components (Fig. 2E). Interference with the pH of the host phagosome is a possible means of arresting or redirecting maturation. In keeping with the central theme of this review, we restrict the following discussion to two exemplary cases where maturation is impaired and pH changes have been implicated. Specifically, we highlight the interactions of Mycobacterium tuberculosis and Helicobacter pylori with host cells.
Mycobacterium tuberculosis
M. tuberculosis is a facultative pathogen that exhibits a strong propensity to infect human macrophages. Mycobacteria do not avoid engulfment but are instead recognized by phagocytic receptors and internalized readily. Upon entering the host phagocyte, the bacilli stow themselves away in a safe intracellular niche where they can survive and even replicate. Their ability to subsist within macrophages is attributed to the striking ability of the mycobacteria to arrest phagosome maturation at an early stage, thereby avoiding the delivery of microbicidal effectors found in mature phagolysosomes. M. tuberculosis-containing phagosomes are characterized by minimal acidification and the notable absence of lysosomal hydrolases.
Several phagocytic receptors have been implicated in the uptake of tubercle bacilli, which can seemingly be internalized with or without opsonization. Receptors reported to mediate mycobacterial uptake include Fcγ (8), mannose (9, 106), fibronectin (91, 99), and scavenger receptors (138). However, members of the complement receptor family, i.e., CR1, CR3, and CR4, have most commonly been observed to facilitate mycobacterial uptake (45, 106, 107). Notably, the mode of entry appears to predetermine the intracellular fate of this pathogen. The more frequently observed internalization via complement or scavenger receptors leads to incomplete maturation and bacterial survival, whereas bacilli opsonized by immunoglobulin G and internalized via Fcγ receptors are efficiently destroyed (8).
As described above, phagosome maturation entails progressive fusion with organelles of the endocytic pathway. For this reason, the precise stage of maturation can be established by analyzing the presence or absence of specific markers, characteristic of individual organelles, on the membrane or among the contents of phagosomes. Nascent phagosomes containing inert latex beads or nonpathogenic bacteria promptly acquire Rab5 and EEA1 and accumulate phosphatidylinositol 3-phosphate [PI(3)P] (111, 127). These markers are lost subsequently, as the phagosome acquires Rab7 and LAMP proteins (111, 127). Phagosomes containing virulent mycobacteria acquire and retain Rab5, but the late endosomal markers Rab7 and LAMP are notably absent, implying maturation arrest (125). These observations, however, are not universally replicated. In some systems, M. tuberculosis-containing vacuoles were reported to contain Rab7 (32, 33) yet were consistently LAMP-1 deficient. Conversely, LAMP-1 but not the V-ATPase was found in mycobacterial phagosomes by others (118). The specific paucity of V-ATPases invariably alters the pH of the phagosomal lumen and could potentially serve as a determinant of the extent of maturation. It appears that while there is agreement that maturation is arrested, the precise stage and mode of arrest are still debated.
The finding that Rab5 persists on mycobacterial phagosomes stimulated further characterization of the vacuole. These studies revealed that most other early endosomal markers, including transferrin and its receptor, Vps34 [and its product, PI(3)P, by inference], were also present but that EEA1 was excluded (49). The absence of EEA1 is peculiar in that its association with early endosomes is anchored in part by Rab5 and PI(3)P (113), both of which were found on mycobacterial phagosomes (29, 49). Furthermore, after binding to its receptors, extracellular transferrin can reach mycobacterial phagosomes (31, 117), implying that early endosome-phagosome interactions are curiously unperturbed. It is unclear how these interactions are directed when EEA1, a tethering molecule required to bridge early endosomes prior to fusion (28), is absent.
Fratti et al. (49, 50) attributed the lack of EEA1 to a mannose-capped lipoarabinomannan (ManLAM) that is released from the bacterial wall and was purported to mimic PI(3)P and/or alter its production, potentially altering binding of physiological effectors such as EEA1. It seems unlikely that ManLAM can efficiently act as a PI(3)P mimetic. Although the two lipids share a similar inositol ring, the one in ManLAM is in all likelihood sterically hindered by the surrounding mannose moieties. Additionally, it is unclear how ManLAM, which is released into the phagosomal lumen, could flip or otherwise traverse the phagosomal bilayer to insert itself in its cytosolic aspect, where recruitment of EEA1 takes place.
An alternative, possibly complementary explanation was recently proposed by the same group. This more recent mechanism involves a secreted bacterial phosphatase called SapM, which was proposed to actively degrade PI(3)P on the phagosomal membrane (124). Elimination of the phosphoinositide would explain the reduced recruitment of EEA1 and may well account for the phagosomal maturation arrest. How SapM exerts action on the cytoplasmic face from the phagosomal lumen remains undefined.
Mycobacteria were also reported to utilize coronin-1 (also referred to as TACO), a host actin-binding protein involved in phagocytosis (134), to evade phagosome maturation and killing. Coronin-1 is normally recruited to the phagocytic cup but resides there only transiently, detaching upon membrane closure. Pathogenic mycobacteria, however, actively promote the retention of coronin-1 around the vacuole membrane, which is believed to interfere with the apposition of lysosomes, impeding their fusion and ultimately enabling the infection to persist (47).
Recently, genetic screens of transposon-mutagenized mycobacteria were designed to identify novel genes that may interfere with vacuolar maturation. The two groups that implemented such screens jointly identified about 10 different mutations in genes believed to control phagosome acidification (93, 116). Characterization of the genes involved is still at an early stage, but the initial information indicates that they range from unique unidentified proteins without known homologues (and therefore unpredictable functions) to transporters and lipid-modifying enzymes. More precise mechanistic information on the manner in which these proteins modulate phagosome maturation should be forthcoming in the near future.
Calcium is thought to play a role in phagosome maturation, particularly in phagosome-lysosome fusion (65, 73, 88), and has also been invoked as a factor contributing to the abnormal maturation of mycobacterial phagosomes (77). Maturation is felt to depend on changes in the cytosolic calcium concentration, possibly by recruitment of cytosolic calmodulin to the phagosome membrane and subsequent focal activation of calmodulin-stimulated kinase II. Kusner and his group recently reported that much of this calcium is liberated by sphingosine kinase and that activation of this enzyme is defective when mycobacteria are the phagocytic prey (78). Interference with normal calcium signaling may be caused by ManLAM, which sufficed to block the calcium concentration increase when added to macrophages (123).
The ability of mycobacteria to subvert phagosome maturation is not absolute and depends on the activation state of the infected macrophages. Priming of macrophages with certain cytokines, in particular gamma interferon (IFN-γ), protects them from mycobacteria, promoting phagosomal acidification and subsequent bacterial killing (62, 104, 126). IFN-γ is believed to induce the expression of the antimicrobial enzyme nitric oxide synthase 2 (75), which restricts bacterial growth by generating nitric oxide (23, 44). The protective effects of IFN-γ also include the expression of LRG-47, a GTPase that is recruited to phagosomes and favors their maturation in a poorly understood manner. Accordingly, the ability to sustain phagosome acidification and bacterial killing is lost in LRG-47−/− macrophages, even when the cells are primed with IFN-γ (76).
The pH of mycobacterial phagosomes is less acidic than that of phagosomes containing nonpathogenic bacteria (118), which attain the phagolysosomal stage and therefore a markedly acidic pH akin to that of lysosomes. Is the altered pH a consequence or cause of incomplete maturation? This dilemma was first posed by the classical experiments of Gordon et al. (54) and remains unsolved. It is simplest to assume that maturation arrest precludes the acquisition of a sufficient density of V-ATPases and remodeling of the membrane in order to minimize proton leakage and that the failure to acidify is hence a consequence of the block in membrane trafficking. However, the possibility remains that bacterial products contribute directly to dissipation of the pH gradient and thereby impair fusion with late endosomes and lysosomes.
Helicobacter pylori
H. pylori infection often occurs in early childhood and, without proper treatment, can persist even years after initial exposure. Chronic infection can predispose the individual to the later onset of gastritis, peptic ulcer disease, and some forms of gastric cancer. H. pylori is transmitted by the oral-fecal route and, following ingestion, encounters the extremely low pH of the lumen of the stomach. Remarkably, this organism has evolved adaptive strategies to resist the high acid content, managing not only to survive but even to colonize the gastric lining. These adaptations allow H. pylori to maintain a neutral cytoplasmic pH and the negative membrane potential required for growth while facing extreme acidic conditions.
Acid tolerance and survival are conferred to the bacterium largely by the expression of urease (43), which constitutes as much as 15% of the total protein of H. pylori (102). The enzyme catalyzes the breakdown of urea into NH3 and CO2 (84, 85), which provide both acid-neutralizing and acid-buffering capacities (Fig. 3). Early experiments suggested that urease released upon autolysis of some organisms subsequently bound to the surfaces of neighboring bacteria, generating a protective neutralizing cloud around them (94). Subsequent experiments showed that urease activity in intact bacteria increased as they were cultured in media at progressively lower pHs, without changes to the bacterial cytoplasmic pH, suggesting that urease is a cytoplasmic enzyme. In this event, intracellular catalysis would require entry of the substrate into the cells. Accordingly, Weeks et al. (129) identified a transporter encoded by the ureI gene capable of delivering urea to the cytoplasm, where urease enables neutralization and buffering capacities (112). It is now apparent that the activities of the transporter and enzyme are coupled not only functionally but physically as well.
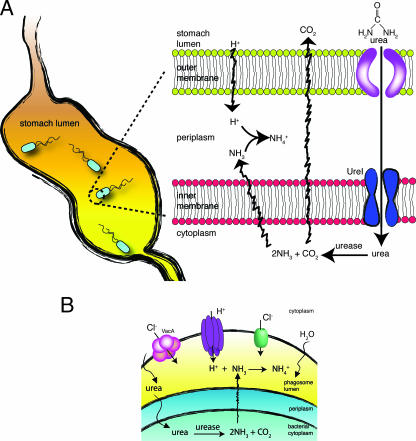
FIG. 3.
Mechanisms of survival and evasion in Helicobacter pylori. (A) Urea, which is present in the stomach, is transported through UreI into the bacterial cytoplasm, where urease converts it into NH3 and CO2. NH3 quickly diffuses into the periplasm, where it prevents the accumulation of protons by generating NH4+, thereby regulating the periplasmic pH. (B) H. pylori also secretes a cytotoxin called VacA that oligomerizes to form a Cl− channel. When inserted into the phagosomal membrane, VacA contributes to the permeation of counterions that serve to collapse the electrical potential generated by the V-ATPase. Dissipation of the electrical potential promotes proton accumulation. Formation of NH3 by the bacterial urease serves to sequester incoming protons by forming NH4+. Together with Cl−, the accumulated NH4+ acts to increase the osmotic content of the phagosomal lumen. This, in turn, drives the influx of osmotically obliged water, causing swelling of phagosomes into megasomes.
The acidic milieu also promotes the expression of an array of bacterial proteins, including arginase and carbonic anhydrase, which together contribute to cytoplasmic and periplasmic pH regulation. Arginase utilizes arginine to produce l-ornithine and urea, thereby contributing to the amount of substrate available to urease (83). Carbonic anhydrase recycles CO2 produced from urease to generate carbonic acid, which in turn dissociates into HCO3−, a periplasmic buffer. Together, NH3 and CO2 (HCO3−), produced by urease in conjunction with its ancillary enzymes, participate in acid acclimation by H. pylori; NH3 sequesters incoming protons and HCO3− buffers the cytoplasm and periplasm (Fig. 3). It must be borne in mind, however, that the generation of HCO3− from CO2 entails the liberation of H+, so extra buffering power is created at the expense of lowering the pH.
The acidic lumen of the stomach represents only the first obstacle to successful colonization; bacteria must also bypass both the adaptive and innate immune responses of the host. Subversion of the adaptive immune response has been reviewed recently (11), and therefore this section focuses only on the methods employed to circumvent phagocytic killing.
Although H. pylori can withstand the extreme conditions that prevail in the lumen of the stomach and are most prevalent in this niche, it will occasionally cross the epithelial barrier. The ability of H. pylori to enter and traverse the gastric epithelium depends on the vacuolating cytotoxin VacA (34). Having traversed the epithelial layer, bacteria must face the rapid onslaught of both macrophages and neutrophils in the basement membrane (1). To counteract the immune effector cells, H. pylori employs antiphagocytic mechanisms; the initial binding to phagocytes does not trigger immediate phagocytosis, as is generally the case, but a delay of variable length ensues (4). Inhibition of uptake was found to be dependent on the bacterial cag pathogenicity island (97). Specifically, the lag requires the virB7 and virB11 genes, which encode components of the type IV secretion system (98). Even without ingesting them, neutrophils can, in principle, respond to adherent bacteria by generating reactive oxygen intermediates, products of the NADPH oxidase. To neutralize the neutrophil respiratory burst, H. pylori can secrete enzymes such as superoxide dismutase and catalase to scavenge superoxide anions and hydrogen peroxide and prevent the formation of other oxidative species (82).
Despite the lag imposed by the effector delivered by the type IV secretion system, bacteria bound to phagocytes via one or more adhesins (25-27, 120) are nevertheless internalized and found in phagosomes (46, 71, 139). Remarkably, fewer than half of the internalized population of unopsonized H. pylori cells are destroyed (1). Killing is greatly enhanced when bacteria are opsonized with immunoglobulin G (3), implying that like the case for mycobacteria, the receptor used for entry dictates the fate of the ingested microorganisms.
Once internalized, viable bacteria accumulate in unusually large phagosomes termed megasomes, which are seemingly the result of homotypic phagosome fusion. These organelles are rich in EEA1 but do not contain LAMP-1 and, importantly, fail to acidify in the way that phagosomes containing inanimate particles or nonpathogenic bacteria eventually do. Accumulation of EEA1 is accompanied by retention of coronin-1, which normally dissociates from nascent phagosomes immediately after they are sealed. As postulated for mycobacteria, the retention of coronin-1 was proposed to prevent phagosome- lysosome fusion, thereby inhibiting phagosome maturation with H. pylori (136).
Megasome formation is directly correlated with bacterial survival and, importantly, was found to be dependent on urease expression (110). Mutants lacking both urease subunits are destroyed in single, smaller phagosomes that acidify and recruit LAMP-1. Thus, urease is not only essential for H. pylori survival and growth in the acidic gastric milieu but also critical for its intracellular survival (110). The function of urease within the phagosome is likely analogous to its role in the stomach, i.e., to generate ammonium from urea (Fig. 3B). It is not clear whether ammonium functions in conjunction with VacA to produce megasomes by neutralizing the luminal acidity and/or by providing an osmolyte that accumulates and promotes osmotic swelling. A model proposed recently posits that VacA functions as a counterion (chloride) channel that facilitates the activity of the V-ATPase (34). In the absence of urease, the proton pump would build up a pH gradient that would, in turn, reduce pumping activity and increase the leak, eventually attaining a steady state, as detailed above. However, if both urease and urea are present, ammonia can form and become protonated and trapped in the phagosome (Fig. 3B). This has two immediate consequences, as follows: (i) the consumption of protons neutralizes the luminal acidification, reactivating the V-ATPase; and (ii) ammonium accumulates, forcing the uptake of osmotically obliged water and inducing swelling of phagosomes to megasomes.
One aspect that has not been addressed sufficiently is the possibility that by impairing acidification, urease/urea directly precludes the transition from early to late phagosomes, in a manner akin to that revealed by Gordon et al. (54). This possibility has not attracted the attention it deserves, in all likelihood because the molecular mechanism underlying the effects of luminal acidification remains obscure. As discussed earlier, while ARF- and COP-dependent processes are affected by pH, other processes are most likely also involved. These are currently unknown but may include the induction of multivesicular structures, as shown recently in vitro for pure lipid systems containing lysobisphosphatidic acid (80). Alternatively, a luminal pH may be required for accumulation of calcium, which may itself be required for fusion of phagosomes and lysosomes. In Saccharomyces cerevisiae, localized release of luminal calcium is necessary for fusion of the vacuoles, which are equivalent to mammalian lysosomes (74). Certainly, the resistance strategies used to evade the innate immune response are multifaceted and include manipulation of the phagocyte NADPH oxidase, neutrophil activation, and inflammation responses, which without a doubt complement each other to ensure survival within a hostile environment (2).
CLOSING REMARKS
Classically, acidification was believed to be a consequence of endosome progression and phagosome maturation. However, the data reviewed here support the emerging concept that both the phagosomal and endosomal pHs can dictate vesicular fission and/or fusion and thereby control the rate and extent of maturation. Remarkably, these effects of luminal acidification occur at least in part by directing the recruitment or activation of effectors on the cytosolic face of the membranes involved, implying the existence of membrane-spanning transducers that convey the information across the bilayer. Indeed, the V-ATPase itself has recently been implicated as a pH sensor (63) and may serve to translate luminal acidification in the lumen into a signaling cascade in the cytoplasmic aspect of the vacuolar membrane.
In view of the intimate relationship between membrane traffic and luminal acidity, it is not surprising that pathogenic bacteria that evade killing by phagosomes have been linked to alterations in pH homeostasis. As illustrated by the species exemplified here, M. tuberculosis and H. pylori, the distinction between cause and effect is not always made easily. In the case of H. pylori, pathogenesis appears to require effectors, such as urease, that modulate the pH. But even in this case, it is not entirely clear whether the main target is the pH of the bacterium itself or that of the host compartments where the bacterium resides. For M. tuberculosis, it is simplest to assume that normal phagosomal acidification fails to develop because the bacterium interferes with maturation. However, this simplistic hypothesis will only be confirmed when the mycobacterial effectors that arrest maturation are identified and their mode of action elucidated. The possibility remains that molecules such as ESAT-6 and CFP-10, which are thought to be required for mycobacterial virulence, may act primarily by interference with phagosomal pH homeostasis.
Acknowledgments
Original work in our laboratory is supported by grants from the Canadian Cystic Fibrosis Foundation and the Canadian Institutes for Health Research (CIHR). K.K.H. is the recipient of a CIHR Graduate Studentship. S.G. is the current holder of the Pitblado Chair in Cell Biology.
REFERENCES
- 1.Allen, L. A. 1999. Intracellular niches for extracellular bacteria: lessons from Helicobacter pylori. J. Leukoc. Biol. 66:753-756. [DOI] [PubMed] [Google Scholar]
- 2.Allen, L. A. 2007. Phagocytosis and persistence of Helicobacter pylori. Cell. Microbiol. 9:817-828. [DOI] [PubMed] [Google Scholar]
- 3.Allen, L. A., and J. A. Allgood. 2002. Atypical protein kinase C-zeta is essential for delayed phagocytosis of Helicobacter pylori. Curr. Biol. 12:1762-1766. [DOI] [PubMed] [Google Scholar]
- 4.Allen, L. A., L. S. Schlesinger, and B. Kang. 2000. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 191:115-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anbari, M., K. V. Root, and R. W. Van Dyke. 1994. Role of Na, K-ATPase in regulating acidification of early rat liver endocytic vesicles. Hepatology 19:1034-1043. [PubMed] [Google Scholar]
- 6.Aniento, F., F. Gu, R. G. Parton, and J. Gruenberg. 1996. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol. 133:29-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arai, H., S. Pink, and M. Forgac. 1989. Interaction of anions and ATP with the coated vesicle proton pump. Biochemistry 28:3075-3082. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong, J. A., and P. D. Hart. 1975. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J. Exp. Med. 142:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astarie-Dequeker, C., E. N. N′Diaye, V. Le Cabec, M. G. Rittig, J. Prandi, and I. Maridonneau-Parini. 1999. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect. Immun. 67:469-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajno, L., X. R. Peng, A. D. Schreiber, H. P. Moore, W. S. Trimble, and S. Grinstein. 2000. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J. Cell Biol. 149:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldari, C. T., A. Lanzavecchia, and J. L. Telford. 2005. Immune subversion by Helicobacter pylori. Trends Immunol. 26:199-207. [DOI] [PubMed] [Google Scholar]
- 12.Basu, S. K., J. L. Goldstein, R. G. Anderson, and M. S. Brown. 1981. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell 24:493-502. [DOI] [PubMed] [Google Scholar]
- 13.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beron, W., C. Alvarez-Dominguez, L. Mayorga, and P. D. Stahl. 1995. Membrane trafficking along the phagocytic pathway. Trends Cell Biol. 5:100-104. [DOI] [PubMed] [Google Scholar]
- 15.Beyenbach, K. W., and H. Wieczorek. 2006. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 209:577-589. [DOI] [PubMed] [Google Scholar]
- 16.Biwersi, J., N. Emans, and A. S. Verkman. 1996. Cystic fibrosis transmembrane conductance regulator activation stimulates endosome fusion in vivo. Proc. Natl. Acad. Sci. USA 93:12484-12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boldyrev, A. A., O. D. Lopina, and N. U. Fedosova. 1990. The regulation of Na, K-ATPase activity by the substrate. Nauchnye Doki Vyss Shkoly Biol. Nauki 1990:106-120. [PubMed] [Google Scholar]
- 18.Bowman, B. J., M. E. McCall, R. Baertsch, and E. J. Bowman. 2006. A model for the proteolipid ring and bafilomycin/concanamycin-binding site in the vacuolar ATPase of Neurospora crassa. J. Biol. Chem. 281:31885-31893. [DOI] [PubMed] [Google Scholar]
- 19.Bowman, E. J., A. Siebers, and K. Altendorf. 1988. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 85:7972-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brett, C. L., D. N. Tukaye, S. Mukherjee, and R. Rao. 2005. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol. Biol. Cell 16:1396-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cain, C. C., D. M. Sipe, and R. F. Murphy. 1989. Regulation of endocytic pH by the Na+,K+-ATPase in living cells. Proc. Natl. Acad. Sci. USA 86:544-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casciola-Rosen, L. A., and A. L. Hubbard. 1992. Lumenal labeling of rat hepatocyte early endosomes. Presence of multiple membrane receptors and the Na+,K(+)-ATPase. J. Biol. Chem. 267:8213-8221. [PubMed] [Google Scholar]
- 23.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherny, V. V., and T. E. DeCoursey. 1999. pH-dependent inhibition of voltage-gated H(+) currents in rat alveolar epithelial cells by Zn(2+) and other divalent cations. J. Gen. Physiol. 114:819-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chmiela, M., E. Czkwianianc, T. Wadstrom, and W. Rudnicka. 1997. Role of Helicobacter pylori surface structures in bacterial interaction with macrophages. Gut 40:20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chmiela, M., J. Lelwala-Guruge, and T. Wadstrom. 1994. Interaction of cells of Helicobacter pylori with human polymorphonuclear leucocytes: possible role of haemagglutinins. FEMS Immunol. Med. Microbiol. 9:41-48. [DOI] [PubMed] [Google Scholar]
- 27.Chmiela, M., A. Ljungh, W. Rudnicka, and T. Wadstrom. 1996. Phagocytosis of Helicobacter pylori bacteria differing in the heparan sulfate binding by human polymorphonuclear leukocytes. Zentbl. Bakteriol. 283:346-350. [DOI] [PubMed] [Google Scholar]
- 28.Christoforidis, S., H. M. McBride, R. D. Burgoyne, and M. Zerial. 1999. The Rab5 effector EEA1 is a core component of endosome docking. Nature 397:621-625. [DOI] [PubMed] [Google Scholar]
- 29.Chua, J., and V. Deretic. 2004. Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles. J. Biol. Chem. 279:36982-36992. [DOI] [PubMed] [Google Scholar]
- 30.Clague, M. J., S. Urbe, F. Aniento, and J. Gruenberg. 1994. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem. 269:21-24. [PubMed] [Google Scholar]
- 31.Clemens, D. L., and M. A. Horwitz. 1996. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J. Exp. Med. 184:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2000. Mycobacterium tuberculosis and Legionella pneumophila phagosomes exhibit arrested maturation despite acquisition of Rab7. Infect. Immun. 68:5154-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2002. The Mycobacterium tuberculosis phagosome in human macrophages is isolated from the host cell cytoplasm. Infect. Immun. 70:5800-5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cover, T. L., and S. R. Blanke. 2005. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 3:320-332. [DOI] [PubMed] [Google Scholar]
- 35.D'Arrigo, A., C. Bucci, B. H. Toh, and H. Stenmark. 1997. Microtubules are involved in bafilomycin A1-induced tubulation and Rab5-dependent vacuolation of early endosomes. Eur. J. Cell Biol. 72:95-103. [PubMed] [Google Scholar]
- 36.Dautry-Varsat, A., A. Ciechanover, and H. F. Lodish. 1983. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 80:2258-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies, J. M., I. Hunt, and D. Sanders. 1994. Vacuolar H(+)-pumping ATPase variable transport coupling ratio controlled by pH. Proc. Natl. Acad. Sci. USA 91:8547-8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis, C. G., J. L. Goldstein, T. C. Sudhof, R. G. Anderson, D. W. Russell, and M. S. Brown. 1987. Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature 326:760-765. [DOI] [PubMed] [Google Scholar]
- 39.de Duve, C., T. de Barsy, B. Poole, A. Trouet, P. Tulkens, and F. Van Hoof. 1974. Lysosomotropic agents. Biochem. Pharmacol. 23:2495-2531. [DOI] [PubMed] [Google Scholar]
- 40.Demaurex, N., W. Furuya, S. D'Souza, J. S. Bonifacino, and S. Grinstein. 1998. Mechanism of acidification of the trans-Golgi network (TGN). In situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J. Biol. Chem. 273:2044-2051. [DOI] [PubMed] [Google Scholar]
- 41.Desjardins, M., N. N. Nzala, R. Corsini, and C. Rondeau. 1997. Maturation of phagosomes is accompanied by changes in their fusion properties and size-selective acquisition of solute materials from endosomes. J. Cell Sci. 110:2303-2314. [DOI] [PubMed] [Google Scholar]
- 42.Di, A., M. E. Brown, L. V. Deriy, C. Li, F. L. Szeto, Y. Chen, P. Huang, J. Tong, A. P. Naren, V. Bindokas, H. C. Palfrey, and D. J. Nelson. 2006. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 8:933-944. [DOI] [PubMed] [Google Scholar]
- 43.Eaton, K. A., C. L. Brooks, D. R. Morgan, and S. Krakowka. 1991. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect. Immun. 59:2470-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ehrt, S., D. Schnappinger, S. Bekiranov, J. Drenkow, S. Shi, T. R. Gingeras, T. Gaasterland, G. Schoolnik, and C. Nathan. 2001. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194:1123-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Etr, S. H., and J. D. Cirillo. 2001. Entry mechanisms of mycobacteria. Front. Biosci. 6:D737-D747. [DOI] [PubMed] [Google Scholar]
- 46.El-Zimaity, H. M., and D. Y. Graham. 2001. Ultrastructural evidence of in vivo phagocytosis of Helicobacter pylori. Ultrastruct. Pathol. 25:159. [PubMed] [Google Scholar]
- 47.Ferrari, G., H. Langen, M. Naito, and J. Pieters. 1999. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97:435-447. [DOI] [PubMed] [Google Scholar]
- 48.Forgac, M. 1998. Structure, function and regulation of the vacuolar (H+)-ATPases. FEBS Lett. 440:258-263. [DOI] [PubMed] [Google Scholar]
- 49.Fratti, R. A., J. M. Backer, J. Gruenberg, S. Corvera, and V. Deretic. 2001. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154:631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fratti, R. A., J. Chua, I. Vergne, and V. Deretic. 2003. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. USA 100:5437-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuchs, R., P. Male, and I. Mellman. 1989. Acidification and ion permeabilities of highly purified rat liver endosomes. J. Biol. Chem. 264:2212-2220. [PubMed] [Google Scholar]
- 52.Fuchs, R., S. Schmid, and I. Mellman. 1989. A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification. Proc. Natl. Acad. Sci. USA 86:539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Futai, M., T. Oka, G. Sun-Wada, Y. Moriyama, H. Kanazawa, and Y. Wada. 2000. Luminal acidification of diverse organelles by V-ATPase in animal cells. J. Exp. Biol. 203:107-116. [DOI] [PubMed] [Google Scholar]
- 54.Gordon, A. H., P. D. Hart, and M. R. Young. 1980. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature 286:79-80. [DOI] [PubMed] [Google Scholar]
- 55.Grabe, M., and G. Oster. 2001. Regulation of organelle acidity. J. Gen. Physiol. 117:329-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grant, K. I., L. A. Casciola, G. A. Coetzee, D. A. Sanan, W. Gevers, and D. R. van der Westhuyzen. 1990. Ammonium chloride causes reversible inhibition of low density lipoprotein receptor recycling and accelerates receptor degradation. J. Biol. Chem. 265:4041-4047. [PubMed] [Google Scholar]
- 57.Hackam, D. J., O. D. Rotstein, C. Sjolin, A. D. Schreiber, W. S. Trimble, and S. Grinstein. 1998. v-SNARE-dependent secretion is required for phagocytosis. Proc. Natl. Acad. Sci. USA 95:11691-11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hara-Chikuma, M., Y. Wang, S. E. Guggino, W. B. Guggino, and A. S. Verkman. 2005. Impaired acidification in early endosomes of ClC-5 deficient proximal tubule. Biochem. Biophys. Res. Commun. 329:941-946. [DOI] [PubMed] [Google Scholar]
- 59.Hara-Chikuma, M., B. Yang, N. D. Sonawane, S. Sasaki, S. Uchida, and A. S. Verkman. 2005. ClC-3 chloride channels facilitate endosomal acidification and chloride accumulation. J. Biol. Chem. 280:1241-1247. [DOI] [PubMed] [Google Scholar]
- 60.Hart, P. D., and M. R. Young. 1991. Ammonium chloride, an inhibitor of phagosome-lysosome fusion in macrophages, concurrently induces phagosome-endosome fusion, and opens a novel pathway: studies of a pathogenic mycobacterium and a nonpathogenic yeast. J. Exp. Med. 174:881-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holevinsky, K. O., and D. J. Nelson. 1998. Membrane capacitance changes associated with particle uptake during phagocytosis in macrophages. Biophys. J. 75:2577-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hostetter, J. M., E. M. Steadham, J. S. Haynes, T. B. Bailey, and N. F. Cheville. 2002. Cytokine effects on maturation of the phagosomes containing Mycobacteria avium subspecies paratuberculosis in J774 cells. FEMS Immunol. Med. Microbiol. 34:127-134. [DOI] [PubMed] [Google Scholar]
- 63.Hurtado-Lorenzo, A., M. Skinner, J. El Annan, M. Futai, G. H. Sun-Wada, S. Bourgoin, J. Casanova, A. Wildeman, S. Bechoua, D. A. Ausiello, D. Brown, and V. Marshansky. 2006. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat. Cell Biol. 8:124-136. [DOI] [PubMed] [Google Scholar]
- 64.Inoue, T., Y. Wang, K. Jefferies, J. Qi, A. Hinton, and M. Forgac. 2005. Structure and regulation of the V-ATPases. J. Bioenerg. Biomembr. 37:393-398. [DOI] [PubMed] [Google Scholar]
- 65.Jaconi, M. E., D. P. Lew, J. L. Carpentier, K. E. Magnusson, M. Sjogren, and O. Stendahl. 1990. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J. Cell Biol. 110:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jancic, C., A. Savina, C. Wasmeier, T. Tolmachova, J. El-Benna, P. M. Dang, S. Pascolo, M. A. Gougerot-Pocidalo, G. Raposo, M. C. Seabra, and S. Amigorena. 2007. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat. Cell Biol. 9:367-378. [DOI] [PubMed] [Google Scholar]
- 67.Jankowski, A., C. C. Scott, and S. Grinstein. 2002. Determinants of the phagosomal pH in neutrophils. J. Biol. Chem. 277:6059-6066. [DOI] [PubMed] [Google Scholar]
- 68.Jentsch, T. J. 2007. Chloride and the endosomal/lysosomal pathway—emerging roles of CLC chloride transporters. J. Physiol. 578:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kane, P. M., and A. M. Smardon. 2003. Assembly and regulation of the yeast vacuolar H+-ATPase. J. Bioenerg. Biomembr. 35:313-321. [DOI] [PubMed] [Google Scholar]
- 70.Kim, J. H., C. A. Lingwood, D. B. Williams, W. Furuya, M. F. Manolson, and S. Grinstein. 1996. Dynamic measurement of the pH of the Golgi complex in living cells using retrograde transport of the verotoxin receptor. J. Cell Biol. 134:1387-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kist, M., C. Spiegelhalder, T. Moriki, and H. E. Schaefer. 1993. Interaction of Helicobacter pylori (strain 151) and Campylobacter coli with human peripheral polymorphonuclear granulocytes. Zentbl. Bakteriol. 280:58-72. [DOI] [PubMed] [Google Scholar]
- 72.Llopis, J., J. M. McCaffery, A. Miyawaki, M. G. Farquhar, and R. Y. Tsien. 1998. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA 95:6803-6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lundqvist-Gustafsson, H., M. Gustafsson, and C. Dahlgren. 2000. Dynamic Ca(2+)changes in neutrophil phagosomes: a source for intracellular Ca(2+)during phagolysosome formation? Cell Calcium 27:353-362. [DOI] [PubMed] [Google Scholar]
- 74.Luzio, J. P., B. M. Mullock, P. R. Pryor, M. R. Lindsay, D. E. James, and R. C. Piper. 2001. Relationship between endosomes and lysosomes. Biochem. Soc. Trans. 29:476-480. [DOI] [PubMed] [Google Scholar]
- 75.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 76.MacMicking, J. D., G. A. Taylor, and J. D. McKinney. 2003. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 302:654-659. [DOI] [PubMed] [Google Scholar]
- 77.Malik, Z. A., G. M. Denning, and D. J. Kusner. 2000. Inhibition of Ca(2+) signaling by Mycobacterium tuberculosis is associated with reduced phagosome-lysosome fusion and increased survival within human macrophages. J. Exp. Med. 191:287-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malik, Z. A., C. R. Thompson, S. Hashimi, B. Porter, S. S. Iyer, and D. J. Kusner. 2003. Cutting edge: Mycobacterium tuberculosis blocks Ca2+ signaling and phagosome maturation in human macrophages via specific inhibition of sphingosine kinase. J. Immunol. 170:2811-2815. [DOI] [PubMed] [Google Scholar]
- 79.Maranda, B., D. Brown, S. Bourgoin, J. E. Casanova, P. Vinay, D. A. Ausiello, and V. Marshansky. 2001. Intra-endosomal pH-sensitive recruitment of the Arf-nucleotide exchange factor ARNO and Arf6 from cytoplasm to proximal tubule endosomes. J. Biol. Chem. 276:18540-18550. [DOI] [PubMed] [Google Scholar]
- 80.Matsuo, T., K. Hazeki, O. Hazeki, T. Katada, and M. Ui. 1996. Specific association of phosphatidylinositol 3-kinase with the protooncogene product Cbl in Fc gamma receptor signaling. FEBS Lett. 382:11-14. [DOI] [PubMed] [Google Scholar]
- 81.Mayorga, L. S., F. Bertini, and P. D. Stahl. 1991. Fusion of newly formed phagosomes with endosomes in intact cells and in a cell-free system. J. Biol. Chem. 266:6511-6517. [PubMed] [Google Scholar]
- 82.McGee, D. J., and H. L. Mobley. 1999. Mechanisms of Helicobacter pylori infection: bacterial factors. Curr. Top. Microbiol. Immunol. 241:155-180. [DOI] [PubMed] [Google Scholar]
- 83.McGee, D. J., F. J. Radcliff, G. L. Mendz, R. L. Ferrero, and H. L. Mobley. 1999. Helicobacter pylori rocF is required for arginase activity and acid protection in vitro but is not essential for colonization of mice or for urease activity. J. Bacteriol. 181:7314-7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mobley, H. L., L. T. Hu, and P. A. Foxal. 1991. Helicobacter pylori urease: properties and role in pathogenesis. Scand. J. Gastroenterol. 187(Suppl.):39-46. [PubMed] [Google Scholar]
- 85.Mobley, H. L., M. D. Island, and R. P. Hausinger. 1995. Molecular biology of microbial ureases. Microbiol. Rev. 59:451-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohammad-Panah, R., R. Harrison, S. Dhani, C. Ackerley, L. J. Huan, Y. Wang, and C. E. Bear. 2003. The chloride channel ClC-4 contributes to endosomal acidification and trafficking. J. Biol. Chem. 278:29267-29277. [DOI] [PubMed] [Google Scholar]
- 87.Mukherjee, S., R. N. Ghosh, and F. R. Maxfield. 1997. Endocytosis. Physiol. Rev. 77:759-803. [DOI] [PubMed] [Google Scholar]
- 88.Myers, J. T., and J. A. Swanson. 2002. Calcium spikes in activated macrophages during Fcgamma receptor-mediated phagocytosis. J. Leukoc. Biol. 72:677-684. [PubMed] [Google Scholar]
- 89.Nelson, N., N. Perzov, A. Cohen, K. Hagai, V. Padler, and H. Nelson. 2000. The cellular biology of proton-motive force generation by V-ATPases. J. Exp. Biol. 203:89-95. [DOI] [PubMed] [Google Scholar]
- 90.Nishi, T., and M. Forgac. 2002. The vacuolar (H+)-ATPases—nature's most versatile proton pumps. Nat. Rev. Mol. Cell. Biol. 3:94-103. [DOI] [PubMed] [Google Scholar]
- 91.Pasula, R., P. Wisniowski, and W. J. Martin II. 2002. Fibronectin facilitates Mycobacterium tuberculosis attachment to murine alveolar macrophages. Infect. Immun. 70:1287-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pavlov, K. V., and V. S. Sokolov. 2000. Electrogenic ion transport by Na+,K+-ATPase. Membr. Cell Biol. 13:745-788. [PubMed] [Google Scholar]
- 93.Pethe, K., D. L. Swenson, S. Alonso, J. Anderson, C. Wang, and D. G. Russell. 2004. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc. Natl. Acad. Sci. USA 101:13642-13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phadnis, S. H., M. H. Parlow, M. Levy, D. Ilver, C. M. Caulkins, J. B. Connors, and B. E. Dunn. 1996. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect. Immun. 64:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pitt, A., L. S. Mayorga, A. L. Schwartz, and P. D. Stahl. 1992. Transport of phagosomal components to an endosomal compartment. J. Biol. Chem. 267:126-132. [PubMed] [Google Scholar]
- 96.Pohlmann, R., S. Kruger, A. Hasilik, and K. von Figura. 1984. Effect of monensin on intracellular transport and receptor-mediated endocytosis of lysosomal enzymes. Biochem. J. 217:649-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramarao, N., S. D. Gray-Owen, S. Backert, and T. F. Meyer. 2000. Helicobacter pylori inhibits phagocytosis by professional phagocytes involving type IV secretion components. Mol. Microbiol. 37:1389-1404. [DOI] [PubMed] [Google Scholar]
- 98.Ramarao, N., and T. F. Meyer. 2001. Helicobacter pylori resists phagocytosis by macrophages: quantitative assessment by confocal microscopy and fluorescence-activated cell sorting. Infect. Immun. 69:2604-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rao, S. P., K. Ogata, and A. Catanzaro. 1993. Mycobacterium avium-M. intracellulare binds to the integrin receptor alpha v beta 3 on human monocytes and monocyte-derived macrophages. Infect. Immun. 61:663-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reif, J. S., A. L. Schwartz, and R. J. Fallon. 1991. Low concentrations of primaquine inhibit degradation but not receptor-mediated endocytosis of asialoorosomucoid by HepG2 cells. Exp. Cell Res. 192:581-586. [DOI] [PubMed] [Google Scholar]
- 101.Reijngoud, D. J., P. S. Oud, and J. M. Tager. 1976. Effect of ionophores on intralysosomal pH. Biochim. Biophys. Acta 448:303-313. [DOI] [PubMed] [Google Scholar]
- 102.Sachs, G., D. L. Weeks, Y. Wen, E. A. Marcus, D. R. Scott, and K. Melchers. 2005. Acid acclimation by Helicobacter pylori. Physiology (Bethesda) 20:429-438. [DOI] [PubMed] [Google Scholar]
- 103.Savina, A., C. Jancic, S. Hugues, P. Guermonprez, P. Vargas, I. C. Moura, A. M. Lennon-Dumenil, M. C. Seabra, G. Raposo, and S. Amigorena. 2006. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126:205-218. [DOI] [PubMed] [Google Scholar]
- 104.Schaible, U. E., S. Sturgill-Koszycki, P. H. Schlesinger, and D. G. Russell. 1998. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J. Immunol. 160:1290-1296. [PubMed] [Google Scholar]
- 105.Schapiro, F. B., and S. Grinstein. 2000. Determinants of the pH of the Golgi complex. J. Biol. Chem. 275:21025-21032. [DOI] [PubMed] [Google Scholar]
- 106.Schlesinger, L. S. 1993. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 150:2920-2930. [PubMed] [Google Scholar]
- 107.Schlesinger, L. S., C. G. Bellinger-Kawahara, N. R. Payne, and M. A. Horwitz. 1990. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 144:2771-2780. [PubMed] [Google Scholar]
- 108.Schwartz, A. L. 1991. Trafficking of asialoglycoproteins and the asialoglycoprotein receptor. Targeted Diagn. Ther. 4:3-39. [PubMed] [Google Scholar]
- 109.Schwartz, A. L., A. Bolognesi, and S. E. Fridovich. 1984. Recycling of the asialoglycoprotein receptor and the effect of lysosomotropic amines in hepatoma cells. J. Cell Biol. 98:732-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwartz, J. T., and L. A. Allen. 2006. Role of urease in megasome formation and Helicobacter pylori survival in macrophages. J. Leukoc. Biol. 79:1214-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scott, C. C., R. J. Botelho, and S. Grinstein. 2003. Phagosome maturation: a few bugs in the system. J. Membr. Biol. 193:137-152. [DOI] [PubMed] [Google Scholar]
- 112.Scott, D. R., E. A. Marcus, D. L. Weeks, A. Lee, K. Melchers, and G. Sachs. 2000. Expression of the Helicobacter pylori ureI gene is required for acidic pH activation of cytoplasmic urease. Infect. Immun. 68:470-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simonsen, A., R. Lippe, S. Christoforidis, J. M. Gaullier, A. Brech, J. Callaghan, B. H. Toh, C. Murphy, M. Zerial, and H. Stenmark. 1998. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394:494-498. [DOI] [PubMed] [Google Scholar]
- 114.Sonawane, N. D., J. R. Thiagarajah, and A. S. Verkman. 2002. Chloride concentration in endosomes measured using a ratioable fluorescent Cl− indicator: evidence for chloride accumulation during acidification. J. Biol. Chem. 277:5506-5513. [DOI] [PubMed] [Google Scholar]
- 115.Stevens, T. H., and M. Forgac. 1997. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu. Rev. Cell Dev. Biol. 13:779-808. [DOI] [PubMed] [Google Scholar]
- 116.Stewart, G. R., J. Patel, B. D. Robertson, A. Rae, and D. B. Young. 2005. Mycobacterial mutants with defective control of phagosomal acidification. PLoS Pathog. 1:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sturgill-Koszycki, S., U. E. Schaible, and D. G. Russell. 1996. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 15:6960-6968. [PMC free article] [PubMed] [Google Scholar]
- 118.Sturgill-Koszycki, S., P. H. Schlesinger, P. Chakraborty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russell. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678-681. [DOI] [PubMed] [Google Scholar]
- 119.Tartakoff, A. M. 1983. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell 32:1026-1028. [DOI] [PubMed] [Google Scholar]
- 120.Teneberg, S., H. Miller-Podraza, H. C. Lampert, D. J. Evans, Jr., D. G. Evans, D. Danielsson, and K. A. Karlsson. 1997. Carbohydrate binding specificity of the neutrophil-activating protein of Helicobacter pylori. J. Biol. Chem. 272:19067-19071. [DOI] [PubMed] [Google Scholar]
- 121.van Deurs, B., P. K. Holm, and K. Sandvig. 1996. Inhibition of the vacuolar H(+)-ATPase with bafilomycin reduces delivery of internalized molecules from mature multivesicular endosomes to lysosomes in HEp-2 cells. Eur. J. Cell Biol. 69:343-350. [PubMed] [Google Scholar]
- 122.Van Dyke, R. W. 1995. Na+/H+ exchange modulates acidification of early rat liver endocytic vesicles. Am. J. Physiol. 269:C943-C954. [DOI] [PubMed] [Google Scholar]
- 123.Vergne, I., J. Chua, and V. Deretic. 2003. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J. Exp. Med. 198:653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vergne, I., J. Chua, H. H. Lee, M. Lucas, J. Belisle, and V. Deretic. 2005. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 102:4033-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Via, L. E., D. Deretic, R. J. Ulmer, N. S. Hibler, L. A. Huber, and V. Deretic. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272:13326-13331. [DOI] [PubMed] [Google Scholar]
- 126.Via, L. E., R. A. Fratti, M. McFalone, E. Pagan-Ramos, D. Deretic, and V. Deretic. 1998. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 111:897-905. [DOI] [PubMed] [Google Scholar]
- 127.Vieira, O. V., R. J. Botelho, and S. Grinstein. 2002. Phagosome maturation: aging gracefully. Biochem. J. 366:689-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang, Y., T. Inoue, and M. Forgac. 2005. Subunit a of the yeast V-ATPase participates in binding of bafilomycin. J. Biol. Chem. 280:40481-40488. [DOI] [PubMed] [Google Scholar]
- 129.Weeks, D. L., S. Eskandari, D. R. Scott, and G. Sachs. 2000. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science 287:482-485. [DOI] [PubMed] [Google Scholar]
- 130.Weisz, O. A. 2003. Acidification and protein traffic. Int. Rev. Cytol. 226:259-319. [DOI] [PubMed] [Google Scholar]
- 131.Wu, M. M., M. Grabe, S. Adams, R. Y. Tsien, H. P. Moore, and T. E. Machen. 2001. Mechanisms of pH regulation in the regulated secretory pathway. J. Biol. Chem. 276:33027-33035. [DOI] [PubMed] [Google Scholar]
- 132.Xie, X. S., D. K. Stone, and E. Racker. 1983. Determinants of clathrin-coated vesicle acidification. J. Biol. Chem. 258:14834-14838. [PubMed] [Google Scholar]
- 133.Yamashiro, D. J., and F. R. Maxfield. 1984. Acidification of endocytic compartments and the intracellular pathways of ligands and receptors. J. Cell Biochem. 26:231-246. [DOI] [PubMed] [Google Scholar]
- 134.Yan, M., R. F. Collins, S. Grinstein, and W. S. Trimble. 2005. Coronin-1 function is required for phagosome formation. Mol. Biol. Cell 16:3077-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zeuzem, S., P. Feick, P. Zimmermann, W. Haase, R. A. Kahn, and I. Schulz. 1992. Intravesicular acidification correlates with binding of ADP-ribosylation factor to microsomal membranes. Proc. Natl. Acad. Sci. USA 89:6619-6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng, P. Y., and N. L. Jones. 2003. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell. Microbiol. 5:25-40. [DOI] [PubMed] [Google Scholar]
- 137.Zijderhand-Bleekemolen, J. E., A. L. Schwartz, J. W. Slot, G. J. Strous, and H. J. Geuze. 1987. Ligand- and weak base-induced redistribution of asialoglycoprotein receptors in hepatoma cells. J. Cell Biol. 104:1647-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zimmerli, S., S. Edwards, and J. D. Ernst. 1996. Selective receptor blockade during phagocytosis does not alter the survival and growth of Mycobacterium tuberculosis in human macrophages. Am. J. Respir. Cell Mol. Biol. 15:760-770. [DOI] [PubMed] [Google Scholar]
- 139.Zu, Y., N. D. Cassai, and G. S. Sidhu. 2000. Light microscopic and ultrastructural evidence of in vivo phagocytosis of Helicobacter pylori by neutrophils. Ultrastruct. Pathol. 24:319-323. [DOI] [PubMed] [Google Scholar]