Abstract
Summary: Intracellular bacterial pathogens have evolved highly specialized mechanisms to enter and survive within their eukaryotic hosts. In order to do this, bacterial pathogens need to avoid host cell degradation and obtain nutrients and biosynthetic precursors, as well as evade detection by the host immune system. To create an intracellular niche that is favorable for replication, some intracellular pathogens inhibit the maturation of the phagosome or exit the endocytic pathway by modifying the identity of their phagosome through the exploitation of host cell trafficking pathways. In eukaryotic cells, organelle identity is determined, in part, by the composition of active Rab GTPases on the membranes of each organelle. This review describes our current understanding of how selected bacterial pathogens regulate host trafficking pathways by the selective inclusion or retention of Rab GTPases on membranes of the vacuoles that they occupy in host cells during infection.
INTRODUCTION
Intracellular bacterial pathogens have evolved highly specialized mechanisms to enter and survive intracellularly within their eukaryotic hosts. In order to do this, bacterial pathogens need to avoid host cell degradation, obtain nutrients and biosynthetic precursors, as well as evade detection by the host immune system. To accomplish these tasks, intracellular pathogens have been shown to (i) escape the phagosome and replicate within the nutrient-rich cytosol, (ii) inhibit or delay the maturation of the phagosome to restrict fusion with lysosomes, or (iii) exit or inhibit interactions with the endocytic/lysosomal pathway completely (reviewed in references 60, 67, and 174). Pathogens that inhibit the maturation of the phagosome or exit the endocytic pathway do so by modifying the identities of their phagosomes in order to exploit host cell trafficking pathways and create an intracellular niche that favors bacterial replication. Organelle identity is determined, in part, by the composition of active Rab GTPases on the membranes of each organelle (15, 144, 170, 212). This review describes our current understanding of how selected bacterial pathogens regulate host trafficking pathways by the selective exclusion or retention of Rab GTPases on membranes of the vacuoles that they occupy in host cells during infection.
THE Rab GTPase FAMILY
The Rab family constitutes the largest member of the Ras superfamily of small guanosine triphosphatases (GTPases). There are more than 60 members of the Rab family, which are highly conserved among eukaryotic cells (138, 139). Rabs play an essential role in both endocytic and exocytic traffic in eukaryotic cells (177, 183, 212). The fact that the human genome contains nearly fivefold more Rabs than does the Saccharomyces cerevisiae genome suggests that they play a role in specialized trafficking pathways in differentiated cell types. Indeed, the expression of individual Rab family members, as well as their regulators and effectors, is unique to different tissues in mice and humans. This led Gurkan and colleagues to suggest that Rabs coordinate signaling “hubs” through which membrane traffic is regulated (80).
Rab Functions
Rabs are thought to act as molecular switches, being active in their GTP-bound state and inactive in their GDP-bound state. Because Rabs do not have high intrinsic guanine nucleotide exchange or hydrolysis activities, they are regulated by other proteins, guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). In their GDP-bound state, Rabs are typically soluble and bound to guanine nucleotide dissociation inhibitor (GDI). At the acceptor membrane, the Rab-GDI complex is thought to interact with GDI displacement factor, which removes GDI and allows Rab membrane insertion (143). Next, a GEF converts Rab to its GTP-bound, active conformation, allowing it to interact with its downstream effectors. Through their effectors, Rabs control many aspects of membrane traffic, including vesicle formation, vesicle motility along the actin/microtubule cytoskeletons, vesicle tethering, transport, and fusion (80, 177, 212).
Rab Localization
At steady state, Rabs appear to be concentrated to specific subcellular compartments in eukaryotic cells (for a review, see reference 212). Multiple Rabs can be present on single intracellular compartment, each occupying its own distinct “microdomain” (13, 178). Targeting of Rabs to these compartments is thought to be mediated, at least in part, by the posttranslational addition of one or two prenyl modifications of a C-terminal cysteine residue(s) (137). Rab targeting to microdomains within specific organelles is thought to occur via interactions with their effectors and/or lipids present in this compartment (142, 143). Recent studies suggested the importance of both phospholipids {specifically, phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and PI(3,4,5)P3} and membrane surface charge in the targeting of some Rab GTPases to the plasma membrane (91, 211). The role of phospholipids and membrane surface charge in targeting Rabs to other compartments (e.g., endosomes) remains to be determined.
Rab Conversions
Rabs appear to function by coordinating a continuum of membrane traffic. For example, the endosomal system involves a highly coordinated and interdependent network of Rabs that control many aspects of cargo delivery from the cell surface to lysosomes. Many studies focused on the role of Rab5 and Rab7, which are enriched on early and late endosomes, respectively, and thus play key roles in determining organelle identity. Although it is clear that cargo destined for degradation transits from the cell surface through early endosomes to eventually reach lysosomes, the specific mechanism by which this trafficking occurs is still open to debate (78). Two models have been proposed to explain endocytic trafficking. The first model proposes that cargo is delivered from one stable organelle of distinct and defined composition to a second stable organelle of differing identity via vesicles (72, 77, 89). For example, in this model, cargo would be delivered from a stable Rab5-positive early endosome to a stable Rab7-positive late endosome via vesicle-mediated trafficking mechanisms. The second model proposes that organelles are not static and instead mature along with their cargo as it is trafficked to the lysosome (150, 185). In this model, organelles are dynamic structures, and endosomes would mature along with their cargos as they are delivered to the lysosome.
Recent studies by Rink and colleagues using fast live-cell imaging provided convincing data to support the organelle maturation model (150). In that study, by monitoring individual endosomes over time, they demonstrated that endosomes are extremely dynamic, with levels of Rab5 constantly fluctuating. In addition, they observed that cargo destined for degradation is progressively found in fewer, but larger, Rab5-positive organelles. Furthermore, over time, Rab5 is lost, with the concomitant appearance of Rab7 and its effectors (150). Further work demonstrated that the conversion of Rab5-positive vacuoles to Rab7-positive vacuoles was dependent on the class C VPS/HOPS (vacuole protein sorting/homotypic fusion and vacuole protein sorting) complex, which not only binds Rab5-GTP but also displays Rab7-GEF activity (150). Similar types of Rab cascades are also thought to coordinate secretory trafficking pathways (115). The coordination of membrane trafficking by Rab conversion or Rab cascades is likely to be more common than previously thought, as more and more multivalent effectors that link multiple Rab GTPases are being discovered (46, 61, 113, 132, 205, 207).
Rabs Associated with the Phagosome
Phagocytosis is a specialized form of endocytosis that plays a critical role in both innate and adaptive immunity as well as tissue remodeling during development (169). After their formation, phagosomes are thought to undergo a maturation sequence similar to that of endosomes, ultimately resulting in their fusion with degradative lysosomes. Like smaller endosomes, Rab conversion is thought to control the maturation sequence of phagosomes. Initial studies focused on Rab5 and Rab7, which are localized to early and late phagosomes, respectively (50). Vieira and colleagues examined the relationship between these two GTPases and phagosome maturation (204). The expression of dominant negative Rab5 inhibited Rab7 accumulation and phagosome maturation. This is consistent with the Rab conversion model for the endosomal pathway noted above (150). Rab5 is known to coordinate phosphatidylinositol signaling on early endosomes (32, 173). Therefore, Vieira and colleagues studied the effect of wortmannin (an inhibitor of phosphatidylinositol 3-kinase) on phagosome maturation. Wortmannin was found to delay the dissociation of Rab5 from phagosomes but had little effect on the acquisition of Rab7. Importantly, wortmannin effectively blocked phagosome maturation. One important conclusion from those studies is that Rab7 recruitment to phagosomes is not sufficient to promote their maturation. That study suggested that factors other than Rab5 and Rab7 contribute to the control of phagosome maturation.
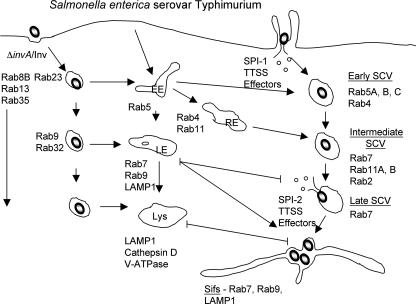
To further characterize phagosome maturation, we examined the association of 48 different Rabs with model phagosomes containing a noninvasive (ΔinvA) mutant of Salmonella enterica serovar Typhimurium (176). These bacteria were engineered to express the Yersinia invasin protein, which promotes bacterial uptake by β1 integrin-mediated phagocytosis (98). This mutant traffics to lysosomes and is degraded, allowing us to determine which Rabs localize to a maturing phagosome. In total, we observed that 18 Rabs associated with phagosomes at some point during their maturation. This number reflects an arbitrary threshold value of 20% phagosomes positive for a given Rab at the time examined. Other Rabs were found to associate with phagosomes to a lesser extent, not meeting the threshold value used here, and may also contribute to phagosome maturation. Remarkably, we observed that up to 12 different Rabs are present on phagosomes at a given stage of maturation. This is consistent with the finding that endosomes are composed of membrane domains coordinated by Rabs (13, 178). These data suggest that a complex network of Rab GTPases regulates phagosome maturation. We hypothesize that this network acts through a series of Rab conversion events (similar to those discussed above) that sequentially control phagosome maturation. The details of how this Rab network is controlled remain unclear.
One of the exciting findings of our “Rab screen” of the phagosome was the identification of novel Rabs present on this compartment. These Rabs included Rab8B, Rab13, Rab23, Rab32, and Rab35, which had not been observed on phagosomes previously (see Fig. 4) (176). The expression of dominant negative mutants of Rab23 and Rab35 was found to significantly impair the fusion of model phagosomes that contained the ΔinvA/Inv mutant of S. enterica serovar Typhimurium with lysosomes preloaded with fluorescent dextran (176). Both Rabs are thought to play roles in endosomal recycling (56, 109). Since endosomal recycling pathways are known to contribute to phagosome maturation (37, 43), it is possible that Rab23 and Rab35 promote phagosome maturation by mediating recycling from this compartment.
FIG. 4.
S. enterica serovar Typhimurium. S. enterica serovar Typhimurium cells replicate in SCVs in host cells. The SCV undergoes a multistep interaction with the host endosomal system but blocks fusion with lysosomes. Up to 18 Rabs are present on the SCV during its maturation sequence. Those Rabs enriched on SCVs containing wild-type bacteria are indicated. Some Rabs are enriched on model phagosomes containing noninvasive bacteria that traffic to and are killed in lysosomes (ΔinvA/Inv). Importantly, these Rabs are excluded from wild-type SCVs, suggesting that the bacteria actively modulate Rab localization to prevent SCV fusion with lysosomes. Several hours after infection, the SCV undergoes extensive fusion with late endosomes. This gives rise to the formation of membrane tubules that extend away from SCVs, known as Sifs. Rab7 and Rab9 are both required for Sif formation. The late SCV may also receive cargo from the trans-Golgi network, possibly providing nutrients for bacteria in vacuoles. EE, early endosome; RE, recycling endosome; LE, late endosome; Lys, lysosome.
Intracellular Bacterial Pathogens and Rab Modulation on Phagosomes
Rab GTPases are central to membrane traffic in eukaryotic cells. Therefore, it is not surprising that intracellular bacterial pathogens target Rab functions to colonize vacuolar compartments (modified phagosomes) in cells of their host. The study of Rabs in cells infected by these pathogens has provided insight into how microorganisms can manipulate host cell function to avoid destruction in lysosomes and to cause disease. Here, we focus on intracellular bacterial pathogens that manipulate/exploit Rab function to colonize cells of their host during infection.
MYCOBACTERIUM TUBERCULOSIS
Mycobacterium tuberculosis is one of the most successful pathogens on the planet, infecting nearly one-third of the human population and killing approximately 1.7 million people each year (158). During infection, these bacteria colonize the lung, predominantly in macrophages. Related mycobacterial species, including M. avium, M. bovis, and M. marinum, are similarly known to colonize macrophages during infection of their hosts and are often used as models of M. tuberculosis infection. These Mycobacterium species share the ability to colonize a nondegradative phagosome in macrophages (8, 41, 186). However, recently reported data demonstrated that M. marinum, M. tuberculosis, and M. leprae also have the ability to escape from phagosomes and grow within the cytosol of host cells (179, 196). Remarkably, M. marinum undergoes actin-based motility in the cytosol, allowing the spread of the organism to neighboring cells (179). Escape of these mycobacteria from the phagosome occurs after a significant delay (48 h postinfection [p.i.] for M. tuberculosis) (196). Therefore, a pathogen-induced block in phagosome-lysosome fusion is an essential prerequisite for escape to the cytosol.
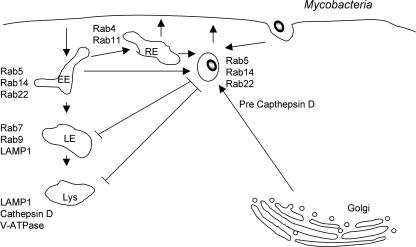
The maturation of mycobacterium-containing phagosomes is blocked at the Rab5-positive stage (Fig. 1). Therefore, they are described as being “four-minute phagosomes,” a reflection on their positioning in the normal phagosome maturation sequence (159). Mycobacterium phagosomes are accessible to early endosomal contents. In fact, the bacterial cell wall component phosphatidylinositol mannoside stimulates the fusion of early endosomes with mycobacterial phagosomes (201). Rab5 facilitates the mycobacterial acquisition of iron within phagosomes by ensuring proper endocytic sorting (105). Despite the retention of Rab5, mycobacterial phagosomes do not recruit Rab7, even at 7 days p.i. (202). In addition, the Rab5 effector early endosomal antigen 1 (EEA1) is also conspicuously absent from these compartments (47). EEA1 binds endosomes by interacting with both Rab5 and phosphatidylinositol 3-phosphate (PI3P). PI3P is generated on early endosomes by the Rab5 effector hVPS34, a type III phosphatidylinositol 3-kinase (32). The use of fluorescent probes and live-cell imaging revealed that PI3P generation on mycobacterial phagosomes is altered compared to that on model phagosomes (33). Two bacterial products generated by M. tuberculosis act on PI3P to block phagosome maturation. First, the cell wall component liparabinomannan prevents PI3P generation on phagosomes (63). Second, the bacterial enzyme SapM acts as a PI3P phosphatase to remove this lipid from phagosomes (200). The generation of PI3P is an essential step in the maturation of both endosomes and phagosomes (79, 203). Therefore, by blocking PI3P action, mycobacteria can effectively block their delivery to lysosomes.
FIG. 1.
M. tuberculosis. M. tuberculosis cells replicate in a phagosome where the normal maturation process has been blocked at an early stage. The phagosome retains Rab5, Rab14, and Rab22 but does not acquire Rab7 and does not fuse with lysosomes. The phagosome receives iron by continued fusion with early/recycling endosomes and also receives cargo from the trans-Golgi network (such as immature cathepsins). Despite the presence of Rab5 on M. tuberculosis phagosomes, its downstream effector EEA1 was not recruited. Bacterial products target the formation/retention of PI3P on M. tuberculosis phagosomes to block phagosome maturation. EE, early endosome; RE, recycling endosome; LE, late endosome; Lys, lysosome.
Roberts and colleagues further examined how mycobacteria can modify Rabs associated with phagosome maturation. They recently reported that Rab22a, a member of the group V Rabs, accumulates on mycobacterial phagosomes (151). Live mycobacteria retained increasing amounts of Rab22a, while dead mycobacteria and latex bead phagosomes did not. Reduced expression of Rab22a (by small interfering RNA treatment) enhanced the maturation of phagosomes containing live mycobacteria, as evidenced by an increased Rab7 association. In contrast, the overexpression of a constitutively active mutant of Rab22a, Rab22a(Q64L), prevented the maturation of phagosomes containing heat-killed mycobacteria. Those authors concluded that Rab22a is critical for the regulation of Rab7 conversion on phagosomes and, subsequently, phagosomal maturation (151).
The role of Rab14 in mycobacterial phagosome maturation was also addressed by Kyei and colleagues (112). Those authors observed the accumulation of Rab14 on live-mycobacterium-containing phagosomes. The reduced expression of Rab14 (by small interfering RNA treatment) or overexpression of dominant negative Rab14(S25N) promoted the fusion of lysosomes with phagosomes containing live mycobacteria. The expression of constitutively active Rab14(Q70L) prevented the fusion of phagosomes containing dead mycobacteria with lysosomes. A role for Rab14 in stimulating fusion between phagosomes and early endosomes was also suggested. Together, those studies suggested that M. tuberculosis and related mycobacterial species modulate the phagosome-associated Rab network at various levels (Fig. 1). The mechanisms by which Rab14 and Rab22a are maintained on mycobacterial vacuoles remain unclear (112).
COXIELLA BURNETII
Coxiella burnetii, an obligate intracellular bacterium, is the etiologic agent of the zoonotic disease Q fever (116). C. burnetii undergoes a biphasic developmental cycle consisting of two cell types, the non-metabolically-active, environmentally-resistant, infectious small-cell variant and the replicative and more fragile large-cell variant (36). Unlike most intracellular pathogens that actively avoid fusion with lysosomes, C. burnetii requires a low-pH environment to activate its metabolism and thus replicates within an acidified parasitophorous vacuole that shares many characteristics with lysosomes (84, 88).
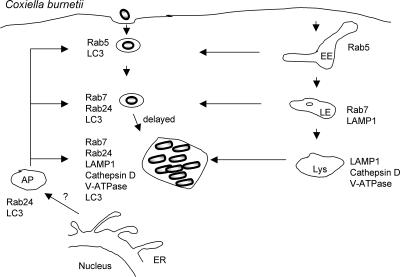
In vivo, C. burnetii has a tropism for mononuclear phagocytic cells such as alveolar macrophages and Kupffer cells in the liver (106, 182). However, epithelial and fibroblast cell lines have been used most often as in vitro model systems to study the intracellular development of this bacterium. In vitro, C. burnetii undergoes a 1- to 2-day lag phase followed by 4 days of exponential growth. By 48 h p.i., C. burnetii replicates within large spacious vacuoles termed the replicative Coxiella vacuole (RCV) (36). The RCV is decorated with the lysosomal glycoproteins LAMP-1 and LAMP-2 (73, 88), LAMP-3 (73), the vacuolar H+ ATPase (73, 88), and Rab7 (17). In addition, the RCV is accessible to fluid-phase markers (88) and is acidified (84) (Fig. 2). Furthermore, the vacuole contains lysosomal hydrolytic enzymes such as acid phosphatase (1, 28, 95), cathepsin D (73, 88), and 5′-nucleotidase (28), suggesting that the RCV is phenotypically similar to lysosomes.
FIG. 2.
C. burnetii. C. burnetii cells replicate in a bacterium-modified vacuole that shares many characteristics with lysosomes as well as with autophagosomes. Although C. burnetii cells passively traffic through the default endocytic pathway, as evidenced by the sequential recruitment of Rab5 and Rab7, their delivery to lysosomes is delayed as a result of interactions with the host autophagy pathway. Interactions with the autophagy pathway, such as the recruitment of LC3 and Rab24, as well as the formation of the spacious replicative vacuole require bacterial protein synthesis. EE, early endosome; LE, late endosome; Lys, lysosome, AP, autophagosome.
Because the RCV shares many traits with lysosomes, Coxiella was initially thought to passively traffic through the default endocytic pathway to eventually fuse with lysosomes (88). However, recent data suggest that although the RCV shares many characteristics with lysosomes, the RCV is not a typical phagolysosome but is rather a bacterium-modified phagolysosome. Even though C. burnetii does not block the acquisition of LAMP-1 (96, 154), the bacterium still actively modifies the parasitophorous vacuole to regulate the formation and maintenance of the spacious RCV (96). Spacious vacuoles fail to form in cells treated with chloramphenicol (a bacterial protein synthesis inhibitor). In contrast, RCV formation is normal in cells treated with penicillin (a cell wall biosynthetic inhibitor) or nalidixic acid (a DNA synthesis inhibitor) (96). These data demonstrate that bacterial protein synthesis, but not replication, is required for the formation of the spacious RCV. In addition, unlike lysosomes, Coxiella-containing vacuoles (CVs) undergo both homo- and heterotypic fusion with a large variety of different compartments, including pinocytic vesicles containing thorium dioxide, horseradish peroxidase, or ferritin (1, 11), as well as phagosomes containing beads (198), yeast (198), zymosan (198), Mycobacterium species (45, 74), or Leishmania amazonensis (199). Finally, as described below, C. burnetii actively promotes interactions with the autophagy pathway (17, 81, 154), which is thought to delay fusion with lysosomes.
Because of the phagolysosomal characteristics of the RCV, the roles of Rab5 and Rab7 in the biogenesis of the RCV have been extensively examined. A kinetic analysis examining early times p.i. demonstrated that the early CV sequentially acquired wild-type green fluorescent protein (GFP)-Rab5 and GFP-Rab7 with kinetics similar to those observed for a typical latex bead-containing phagosome (154). The recruitment of Rab5 was observed as early as 5 min p.i., with maximal recruitment by 20 min p.i. By 60 min, the majority of CVs lacked Rab5. In contrast, a gradual increase in Rab7 recruitment was observed over time, with maximal staining by 40 min. Additionally, Rab7 recruitment was maintained over the course of infection and was present on the large spacious RCV observed at 48 and 72 h p.i. (17, 154). In cells expressing the constitutively active GTPase-defective mutant GFP-Rab5(Q79L) at 60 min p.i., there was an increase in both the number of infected cells and the size of the vacuole (154). In addition, multiple bacteria were present in each vacuole. Since the generation time of Coxiella is at least 10 to 12 h, the increased size of the vacuole is most likely due to the Rab5(Q79L)-mediated induction of homotypic fusion events and not an increase in bacterial replication (155, 184). In contrast, in cells expressing the dominant negative GDP-restricted Rab5(S34N) mutant, there was a decrease in the number of infected cells. Collectively, these data demonstrate that the overexpression of wild-type and constitutively active Rab5 promotes bacterial entry, while the overexpression of Rab5(S34N) inhibits entry. In contrast, at 20 min p.i., similar numbers of infected cells were observed in cells irrespective of the expression of any of the Rab7 guanine nucleotide binding mutants, demonstrating that Rab7 does not play a role in mediating entry. However, similar to what was observed in Rab5(Q79L)-expressing cells, in cells expressing the constitutively active GTPase-defective Rab7(Q67L) mutant, the CV contained multiple bacteria when examined at early times prior to bacterial replication (154).
To further define the role of Rab5 and Rab7 in the maturation of the RCV and to eliminate defects associated with impaired entry, guanine nucleotide binding mutants were introduced after Coxiella infection had been established. In infected Chinese hamster ovary (CHO) cells that were superinfected with Sindbis virus expressing wild-type or constitutively active GTPase-defective forms of Rab5 [Rab5(Q67L)] and Rab7 [Rab7(Q67L)], both wild-type and constitutively active Rab7 were localized to the RCV (17). These data demonstrate that Rab7 can be recruited to mature CVs by either active recruitment or fusion with Rab7-positive compartments (17). In contrast, constitutively active Rab5 but not wild-type Rab5 localized to the RCV. Therefore, unlike wild-type Rab5, the Rab5 mutant is either mistargeted to or remains associated with the RCV. Since the expression of Rab5(Q67L) is thought to promote the aberrant fusion of endosomes with phagosomal or lysosomal compartments (155), it is likely that the expression of Rab5(Q67L) induces the aberrant fusion of endosomes with the RCV, consistent with the presence of Rab5(Q67L) but not wild-type Rab5 on the RCV.
In cells expressing either GFP-Rab5(S34N) or GFP-Rab7(T22N), instead of large spacious vacuoles, small bacterium-containing vacuoles are formed (154). These data suggest that both Rab5 and Rab7 are required for the formation of large spacious RCVs. Because the expression of Rab5(S34N) inhibited RCV formation without the presence of wild-type Rab5 on the RCV, these data suggest that Rab5 is required for continual membrane flow through the endocytic pathway and not directly involved in vacuolar formation. A similar model was proposed for the Rab5(S34N)-mediated inhibition of H. pylori VacA-induced vacuole formation (136). On the other hand, since wild-type Rab7 is localized to the RCV, the inhibition of the maturation of the RCV resulting from the overexpression of GFP-Rab7(T22N) suggests that the large spacious vacuole is formed as a result of Rab7-mediated fusion events between the RCV and late endosomes or lysosomes (26, 57, 206).
Although Coxiella passively transits through the default endocytic pathway, it has recently been shown that Coxiella actively interacts with the autophagy pathway to delay trafficking to the lysosome (17, 81, 154). Autophagy is a normal cellular process that nonselectively degrades organelles and other cellular components in times of nutrient starvation to provide a source of carbohydrates and amino acids (107, 148). As early as 5 min p.i., the nascent CV interacts with the autophagic pathway, as demonstrated by the presence of microtubule-associated protein light chain 3 (LC3), a well-defined marker of autophagic vacuoles, on the CV membrane (154). LC3, the mammalian homologue of yeast Atg8, localizes to and is required for the formation of autophagosomes (101). At later times p.i., mondansylcadaverine (MDC) (18) and Rab24 (81, 129), which are both additional markers of autophagosomes, were localized to the RCV. The localization of MDC and Rab24 to the CV were not analyzed at times earlier than 12 h. From 12 to 48 h, in CHO cells stably expressing GFP-LC3, the construct remained strongly associated with the majority of RCVs (17). However, by 72 h p.i., GFP-LC3 was no longer associated with the RCV.
Autophagy is a constitutive process that can also be upregulated by nutrient limitation or through the use of rapamycin, which inhibits TOR, a kinase critical for autophagy (107). By 12 h p.i., both nutrient limitation and treatment with rapamycin increased the percentage of infected cells, the size of the vacuoles, and Coxiella replication but had no effect on bacterial entry. These data demonstrate that the induction of autophagy promotes Coxiella infection and the formation of the RCV at a postentry stage (81). Furthermore, the pharmacological inhibition of phosphatidylinositol 3-kinase with wortmannin (21) or 3-methyladenine (172), both inhibitors of autophagy, reversed the stimulatory effect of nutrient limitation on Coxiella infection. These data confirm that nutrient limitation promotes Coxiella infection through the induction of the autophagic pathway (81). Similar to nutrient starvation, the overexpression of both GFP-LC3 and GFP-Rab24 has also been shown to increase the number of autophagic vesicles (129). Consistent with a positive role for autophagy during Coxiella infection, in cells overexpressing either GFP-LC3 or GFP-Rab24, an increase in both the number of infected cells and the size of the vacuole was observed by 12 h p.i., a time when vacuoles are normally barely visible. Finally, the induction of autophagy induces the posttranslational modification of LC3. First, immediately after synthesis, a carboxy-terminal fragment is proteolytically cleaved, yielding LC3-I (101). Subsequently, LC3-I is conjugated with phosphatidylethanolamine, generating LC3-II (102). LC3-II is the form of LC3 that is localized to the autophagosome and is essential for autophagosomal maturation. Autophagosomal maturation can also be inhibited by the overexpression of an LC3 mutant, LC3(G120A), that cannot be posttranslationally modified to LC3-II or a nonfunctional allele of Rab24 (129). The formation of the RCV is delayed in cells expressing either of these mutant proteins that are no longer targeted to autophagosomes (81). In summary, although autophagy is induced as a host defense mechanism to eliminate invading pathogens in some cases, in the case of C. burnetii, the induction of autophagy promotes Coxiella infection and the formation of the RCV, while the inhibition of autophagy inhibits Coxiella infection (17).
The data described above demonstrate that the induction of autophagy enhances Coxiella infection, but there is also evidence to suggest that infection by Coxiella induces this innate immune mechanism to promote its intracellular growth. First, in Coxiella-infected cells, both GFP-Rab24 and GFP-LC3 are present in punctate and ring-like vesicular compartments with or without bacteria, which is a pattern of redistribution seen in uninfected cells induced for autophagy (129). Second, similarly to uninfected cells grown under nutrient-limited conditions, LC3-I is processed to LC3-II in cells that have been infected for 72 h or 96 h with Coxiella (154). The redistribution and localization of LC3 to the vacuole, processing of LC3-I to LC3-II, and formation of the spacious vacuole are all dependent on Coxiella protein synthesis (154). Finally, under nutrient-starved conditions, the acquisition of cathepsin D, a lysosomal marker, to the CV is delayed (154). Collectively, these data suggest that Coxiella actively induces and interacts with the autophagy pathway to delay fusion with lysosomes.
It is not clear why Coxiella delays fusion with lysosomes. One possible model suggests that the delay allows time for the differentiation of the small-cell variant into the large-cell variant. However, the actual timing of the differentiation of the small-cell variant into the large-cell variant is not entirely clear and may occur prior to (95) or after (36) delivery to lysosomes. Alternatively, fusion with autophagosomes may be a mechanism used by Coxiella to obtain nutrients and membrane required for the growth of the spacious vacuolar membrane or to trigger its differentiation or metabolism. Due to the obligate nature of the organism and the lack of a tractable genetic system, the identities of the Coxiella proteins that regulate the maturation of the spacious vacuole or interception of the autophagy pathway have remained elusive. However, a type IV secretion system has been identified in C. burnetii (171). Therefore, type IV effectors secreted into the host cytosol may function to delay maturation of the phagosome, intercept autophagosomes, or alter the fusogenicity of the vacuole.
HELICOBACTER PYLORI
Helicobacter pylori is a gram-negative, spiral-shaped bacterium that infects the human stomach and contributes to the development of gastritis, gastroduodenal ulcers, and stomach adenocarcinoma (54, 188). Although over half of the world's population is infected with H. pylori, only a fraction of those infected develop disease (188). Several factors are thought to be important for the development of disease, including the presence of two virulence factors, VacA and CagA, encoded within the cag pathogenicity island in the infecting bacterium (38, 54, 122, 123), host genetic immune determinants (55, 156), as well as undefined environmental factors. Although H. pylori is generally considered to be an extracellular pathogen that colonizes the mucus layer of the gastric mucosa, there is both in vitro and in vivo data to suggest that H. pylori may also invade and reside within gastric epithelial cells (52).
The identities of the specific bacterial virulence factors and host factors that promote disease are not fully understood. However, VacA, a secreted vacuolating toxin, is considered to be an important virulence factor that is directly involved in the pathogenesis of the organism (39). In vivo, VacA is important for the colonization of the stomach (162). In vitro, many functions have been attributed to VacA (44), including the induction of apoptosis through the release of cytochrome c from mitochondria (65), the inhibition of T-cell development (22, 71), antigen presentation (121), and the induction of cellular vacuolation (9, 39, 40).
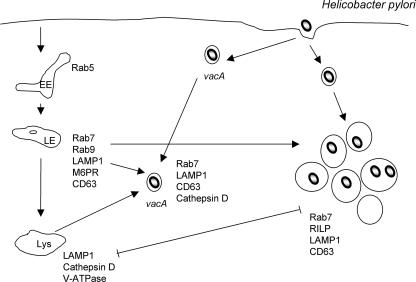
VacA-mediated vacuolation requires the presence of weak anionic bases such as ammonium chloride and the activity of the vacuolar ATPase proton pump (124). These data suggest that vacuolation results from the formation of anion-selective pores, which allow the accumulation of osmotically active, membrane-permeant, weak bases into the vacuoles, resulting in the swelling of the vacuoles. Immunofluorescence microscopic examination of VacA-induced vacuoles, together with the biochemical characterization of purified VacA-induced vacuoles, demonstrated that the vacuoles were enriched for Rab7 and LAMP-1 and were accessible to the fluid-phase marker Lucifer yellow. On the other hand, the early endocytic markers Rab5 and transferrin (Tfn) receptor, the late endosomal marker mannose-6-phosphate receptor, and the lysosomal hydrolase cathepsin D were absent from VacA-induced vacuoles (135). Collectively, these data suggest that VacA induces the accumulation of vacuoles that display characteristics of both late endosomes and lysosomes and, as such, have been described as a postendosomal hybrid of late endosome and lysosomal compartments.
To elucidate the host proteins that were required for the formation of VacA-induced vacuoles, the ability of VacA to induce Rab7-positive vacuoles in HeLa cells transiently expressing nucleotide binding mutants of Rab5, Rab7, and Rab9 was examined (136). VacA-mediated vacuole formation was greatly inhibited by the dominant negative Rab7 mutants Rab7(T22N) and Rab7(N125I) and inhibited to a lesser extent by the dominant negative Rab5 mutants Rab5(S34N) and Rab5(N133I) (136). In addition, the expression of the constitutively active GTPase-defective Rab7 mutant Rab7(Q67L) enhanced the effect of VacA. None of the Rab9 guanine nucleotide binding mutants altered the ability of VacA to induce the formation of vacuoles (136). The absence of Rab5 on the vacuole, together with the inhibitory effect of the dominant negative Rab5 mutants, suggests that continual membrane flow through the endocytic pathway is necessary for vacuole formation. Furthermore, the enrichment of Rab7 on the vacuoles and the inhibitory effect of the dominant negative Rab7 mutants suggest that Rab7 is actively required for vacuole formation. However, since the expression of the constitutively active Rab7 mutant did not mimic the phenotype of VacA-treated cells, Rab7 activation is necessary but not sufficient for VacA-induced vacuole formation, suggesting the involvement of other factors. Several other guanine nucleotide binding proteins have also been shown to localize to VacA-induced vacuoles and to be required for VacA activity, including Rac1 (94) and dynamin (189). However, similar to Rab7, constitutively active mutants of both proteins failed to mimic the activity of VacA.
Late endosomes are also referred to as multivesicular bodies (MVBs) due to the presence of internal membrane-bound vesicles (145). In yeast, two proteins, Vps4 (10) and Fab1p (134), regulate the formation of MVBs. Similarly to VacA, the expression of a dominant negative mutant of PIKfyve, the mammalian homologue of the yeast Fab1p, induced the formation of enlarged vacuoles that colocalized with late endocytic markers (97). Furthermore, the overexpression of wild-type PIKfyve and microinjection of the PIKfyve lipid product PI(3,5)P2 abrogated the ability of VacA to induce vacuoles (97). These data are consistent with the idea that VacA functions to inhibit PIKfyve activity and decrease the levels of PI(3,5)P2 and that this inhibition is essential to the induction of vacuoles. The molecular mechanisms by which VacA might inhibit PIKfyve or how Rab7 is involved in this process remains to be determined.
In order to further characterize the mechanism of VacA-induced vacuole formation, early events in VacA-induced intoxification were examined. In the absence of weak anionic bases, although large vacuoles were not formed, VacA induced the redistribution and clustering of late endocytic compartments enriched for Rab7 and LAMP-1 (120). The addition of ammonium chloride to these VacA-treated cells induced the formation of large Rab7-positive vacuoles from the clustered Rab7-positive compartments (120). Finally, the expression of dominant negative Rab7 but not Rab9 inhibited the VacA-dependent redistribution of late endocytic compartments (120). Collectively, these data suggest that the Rab7-dependent redistribution and clustering of late endocytic compartments precede and are required for the formation of VacA-induced vacuoles and that VacA alters late endocytic trafficking of Rab7- and LAMP-1-positive vesicles.
H. pylori can also invade gastric epithelial cells (52), and recent data suggest that VacA is also required for the enhanced intracellular survival of H. pylori in gastric AGS epithelial cells (191). Although H. pylori can invade AGS cells in a VacA-independent manner, the formation of large H. pylori-containing vacuoles is VacA dependent. In addition, although the intracellular survival rates of vacA mutant and wild-type strains were similar when examined at 24 h p.i., at later times, there were significant differences in CFU between the wild type and the mutant. This suggests that the VacA-mediated formation of large vacuoles contributes to the enhanced survival of the organism. Both vacA and wild-type H. pylori strains invade AGS cells and are contained within vacuoles derived from late endocytic organelles, as demonstrated by the presence of Rab7, LAMP-1, and CD63 (191) (Fig. 3). The decreased survival of the vacA mutant was correlated with the greater retention of cathepsin D and the increased degradative capability of the vacA-derived bacterium-containing vacuoles. Moreover, the addition of purified VacA or conditioned culture supernatant derived from wild-type-infected cells rescued the intracellular survival defect of the vacA mutant and generated spacious vacuoles from VacA-negative tight vacuoles. Finally, wild-type H. pylori, but not vacA mutant bacteria, induced the fusion of bead-containing phagosomes with H. pylori-containing vacuoles (191).
FIG. 3.
H. pylori. H. pylori-containing vacuoles originate from the fusion of late endocytic vesicles in a process mediated by the VacA-dependent retention of active Rab7 and the interaction of Rab7 with RILP. Rab7 retention on the bacterium-containing vacuoles inhibits further maturation of the vacuole, thus limiting the degradative capabilities of the vacuole and enhancing the intracellular survival of the organism. In the absence of VacA, spacious vacuoles fail to form, and vacA-containing vacuoles acquire cathepsin D, resulting in a decrease in the intracellular survival of the bacteria. EE, early endosome; LE, late endosome; Lys, lysosome.
Similarly to VacA-induced vacuolation, the VacA-dependent formation of large spacious bacterium-containing vacuoles was dependent on Rab7 (191). In cells expressing GFP-Rab7(F45A), a lack-of-function mutant that does not associate with endocytic organelles, GFP-Rab7(F45A), failed to localize to H. pylori-containing vacuoles, and its expression had no effect on the formation of spacious vacuoles (191). In addition, GFP-Rab-interacting lysosomal protein (RILP), a Rab7 effector that binds only to GTP-Rab7 (29), was recruited to the VacA-induced bacterial vacuoles, demonstrating that active Rab7 was present on the vacuoles. Fluorescence recovery after photobleaching demonstrated that the rate of fluorescence recovery of GFP-Rab7 on VacA-mediated bacterium-containing vacuoles is much slower than that on endosomes, which suggests that the recycling of Rab7 was slower on the bacterium-containing vacuole (191). These data suggest that the bacterial vacuole retains active Rab7 by either inhibiting the intrinsic GTPase activity of Rab7 or inhibiting a Rab7-GAP.
Not only is RILP recruited to the bacterium-containing vacuole, the interaction between Rab7 and RILP is required to generate the spacious vacuole (191). First, in cells expressing GFP-Rab7(V180A), a mutant that is unable to interact with RILP but is still recruited to bacterium-containing vacuoles, the generation of large vacuoles was inhibited. Second, the expression of myc-tagged RILP(I125A), which is defective in Rab7 binding, in AGS cells also inhibited the formation of large vacuoles and caused a redistribution of Rab7 adjacent to the nucleus. Finally, the formation of large vacuoles but not the recruitment of Rab7 was inhibited by the expression of a truncated RILP mutant, RILPC33-GFP (191). RILPC33-GFP retains the ability to bind to active Rab7 but is unable to bind to microtubule motors and, as a result, inhibits late endosome and/or lysosomal fusion. Collectively, these data suggest that VacA functions to retain active Rab7 at the bacterium-containing vacuole and that RILP/Rab7 interactions are important for the formation of the large spacious vacuole. Finally, the retention of active Rab7 alters endocytic trafficking such that the maturation of the bacterium-containing vacuole is inhibited at a late endocytic stage prior to the delivery of active lysosomal hydrolases.
SALMONELLA ENTERICA SEROVAR TYPHIMURIUM
S. enterica serovar Typhimurium typically causes gastroenteritis in humans and a systemic, typhoid-like disease in genetically susceptible mice (194). These bacteria are facultative intracellular pathogens that can infect a wide variety of cell types in their host during infection. Since they initially interact with intestinal epithelial cells during infection, many in vitro studies of Salmonella pathogenesis have involved polarized or nonpolarized epithelial cell lines. Murine macrophages are also studied since this cell type is the primary cellular niche occupied by the bacteria during systemic infection of mice (149, 163). Most of the research described below involves S. enterica serovar Typhimurium infection of these cell types. Despite differences in cellular trafficking within epithelial cells and macrophages, it is noteworthy that the key phenotypes associated with S. enterica serovar Typhimurium infection in vitro (growth in nondegradative vacuoles and the formation of tubular endosomal structures) (see below) are common to both cell types. This indicates that these bacteria employ a common strategy that allows them to infect both cell types. However, there is also evidence that S. enterica serovar Typhimurium replicates less efficiently in some cell types than in others, in part due to differences in the intracellular trafficking of these cell types (perhaps due to Rab expression) and/or differential virulence gene expression (the reader is directed to reviews in references 24, 66, 92, 108, and 192, which discuss infection of different cell types by S. enterica serovar Typhimurium).
S. enterica serovar Typhimurium invades and replicates within host cells using two different type III secretion systems (T3SS) (64). These needle-like structures deliver bacterial virulence proteins, called effectors, into the host cell. During initial contact with host cells, the first group of effectors is delivered across the plasma membrane to modulate host signal transduction pathways, including the activation of Rho family GTPases, to induce actin rearrangements that drive the ruffling of the cell surface and the uptake of the bacteria into Salmonella-containing vacuoles (SCVs) (166). After entry, the bacteria typically remain in SCVs, although a small population can damage this compartment and either escape (140) or become targeted by the autophagy system of the host cell (20). By modifying the SCV using type III secreted effectors and other virulence factors, the bacteria are able to block SCV fusion with lysosomes (27, 42, 70, 119, 176).
SCVs are thought to undergo a multistep maturation process that is distinct from the normal phagosome maturation pathway (Fig. 4). In the first step (0 to 15 min p.i.), the SCV interacts with early endosomes and acquires the markers Rab4, Rab5, and EEA1 (175, 181). The role of Rab5 in SCV biogenesis was explored by Baldeon and colleagues (12). The overexpression of constitutively active Rab5(Q79L) caused the retention of the early endosomal marker EEA1. This observation is consistent with the ability of Rab5 to promote homotypic early endosome fusion. Rab5(Q79L) expression also promoted the early acquisition of LAMP-2 (12). The accumulation of Rab5 on wild-type SCVs was found to be higher than on model phagosomes containing the ΔinvA/Inv mutant of S. enterica serovar Typhimurium (176). This suggests that wild-type bacteria might be promoting fusion with early endosomes to control the maturation of SCVs and that this may be regulated by bacterial effector proteins delivered into cells by the SPI-1 T3SS (180). Indeed, the SPI-1 effector protein SopB was found to promote SCV maturation (LAMP-1 acquisition). The mechanism(s) by which SopB (and possibly other effectors) contributes to SCV maturation is not clear.
The second step (15 to 60 min p.i.) of SCV maturation involves the recruitment of lysosomal glycoproteins (Lgp) such as LAMP-1 and LAMP-2. In contrast to model phagosomes, SCVs acquire Lgp without interacting with mannose-6-phosphate-containing late endosomes or lysosomes (25, 118). Instead, SCVs are thought to acquire Lgp by interacting with a Rab7-positive/Lgp-positive/M6PR-negative/cathepsin D-negative compartment (118). The expression of dominant negative Rab7(T22N) delayed LAMP-1 acquisition by SCVs (118). Pulse-chase analysis suggested that SCVs acquire LAMP-2 derived from the cell surface rather than from the biosynthetic route (12). In support of this hypothesis, LAMP-2 acquisition on SCVs occurred normally in cells lacking a functional AP3 adaptor complex (12), and LAMP-1 acquisition by SCVs was not affected by blocking protein synthesis (118). During this stage of SCV maturation, active Rab7 recruits its effector RILP and promotes centripetal SCV movement to the microtubule organizing center (MTOC) (85). The overexpression of RILPC33, the truncated form of RILP that lacks the dynein-recruiting domain (29, 99), impaired centripetal SCV movement during the first 2 h p.i. (85). Therefore, Rab7 promotes Lgp acquisition and contributes to the control of SCV positioning within host cells during infection.
During these first two stages of maturation, the SCV is subject to endosomal recycling events. This is evidenced by the fact that many cell surface proteins present on early SCVs are subsequently removed during later stages of SCV maturation (68). SCV interactions with various endosomal recycling factors have been observed. For example, SCVs recruit Rab11 and syntaxin 13, with a maximal association at 60 min p.i. (175). Using a dominant negative approach, we determined that Rab11 regulates the recycling of CD44 from SCVs but had no effect on major histocompatibility complex class I recycling. In contrast, syntaxin 13 regulated the recycling of major histocompatibility complex class I but not the recycling of CD44. We also determined that Lgp acquisition by SCVs was slowed when recycling by Rab11 and/or syntaxin 13 was impaired. These findings suggest that protein movement through the endocytic recycling system is regulated through at least two concurrent pathways and that efficient interaction with these pathways is necessary for the maturation of SCVs. Other factors involved in endosomal recycling (e.g., Arf6, Rab4, and Rab10) are also localized to SCVs and may promote the recycling of specific cargo from this compartment (175, 176).
Despite evidence for extensive interactions with the endosomal system, SCVs do not fuse with lysosomes. Our “Rab screen” of the SCV revealed that a number of Rabs enriched on model phagosomes (Rab8b, Rab13, Rab23, Rab32, and Rab35) are excluded from SCVs containing wild-type bacteria (176) (Fig. 4). This suggests that S. enterica serovar Typhimurium can dynamically modify the Rab network associated with phagosome maturation. Bacterial T3SS effectors may play a critical role in this function, but further experimentation is required to test this hypothesis. Several hours after invasion, bacteria induce the expression of the SPI-2-encoded T3SS, which mediates the delivery of a distinct complement of effector proteins into the host cell across the SCV membrane. These effectors modify endosome transport globally in the host cell (195) and promote the formation of Salmonella-induced filaments (Sifs) (25). Sifs associate with microtubules and extend centrifugally away from bacteria, which are localized at the MTOC (69). The function of Sifs is not clear, but the effectors involved in their formation and regulation are all known virulence factors, and their formation is associated with maximal intracellular growth by bacteria (19). Our screen revealed only two Rab GTPases associated with Sifs: Rab7 and Rab9. Dominant negative mutants of these Rabs inhibited Sif formation (176). This finding suggested that late endosomal fusion gives rise to Sif formation. In support of this model, the late endosome markers LBPA, NPC-1, and syntaxin 7 accumulate on late SCVs and on Sifs (25, 176). The SCV may also receive secretory cargo from the Golgi apparatus, possibly allowing the acquisition of nutrients by bacteria in SCVs (110).
Despite the presence of Rab7 on Sifs, these compartments do not colocalize with the Rab7 effector RILP (85). This is consistent with the fact that Sifs extend centrifugally towards the cell periphery: RILP-mediated recruitment of the dynein/dynactin complex would be expected to retract Sifs towards the MTOC where the bacteria are localized. SifA, an effector of the SPI-2 T3SS, was found to be at least partially responsible for uncoupling Rab7 on Sifs from RILP. The expression of a SifA-GFP fusion induced the formation of Lgp-positive “Sif-like tubules” in transfected cells that were positive for Rab7 but negative for RILP (85). In vitro experiments revealed an interaction of SifA with Rab7 (85). Together, these data suggest that by disengaging RILP from Rab7, SifA enables the centrifugal extension of tubules from the SCV (Sif formation). How SifA modulates Rab7's interaction with RILP (and perhaps its other effectors) remains undetermined.
A notable exception to the model described above is presented by the work of Hashim and colleagues (86). Those authors utilized a clinical isolate of S. enterica serovar Typhimurium to infect J774E mouse macrophages and isolated phagosomes using a sucrose density gradient method (86). Phagosomes containing live bacteria were found to retain Rab5 for up to 90 min p.i. and did not acquire Rab7, despite the recruitment of Lgp (86). Their subsequent studies suggested that the SPI-1 T3SS effector SopE interacts with Rab5 and acts as a GEF to promote Rab5 activation and the recruitment of nonprenylated Rab5 to SCVs (128). At present, it is not clear why the results of that group are apparently at odds with those of other investigators. However, it is increasingly apparent that differences in cell type, bacterial strain, or infection conditions can give rise to different outcomes with in vitro bacterial infections. Furthermore, it is now appreciated that intracellular bacterial pathogens can have multiple fates within host cells and that quantitative analysis of different populations is required (20). In a broader context, the nature of SCVs in vivo is not known and needs to be addressed.
CHLAMYDIA SPECIES
Chlamydia species are obligate intracellular bacteria that are important pathogens of ocular, urogenital, and pulmonary mucosal surfaces (165). Of the recognized Chlamydia species, Chlamydia trachomatis, the leading cause of sexually transmitted disease and preventable blindness, and Chlamydia pneumoniae, an important cause of community-acquired pneumonia, are the two major human pathogens (165). The bacterium is characterized by a unique biphasic developmental cycle that alternates between infectious metabolically inactive elementary bodies and noninfectious metabolically active reticulate bodies (126). Differentiation and replication of the bacterium occur solely within a membrane-bound vacuole termed the inclusion. Chlamydiae enter nonprofessional phagocytes by multiple mechanisms, but irrespective of the specific uptake mechanism, chlamydiae actively mediate the remodeling of the nascent vacuole by processes that are dependent on chlamydial gene expression (59, 167). As a result, the inclusion avoids fusion with lysosomes and instead is trafficked to the peri-Golgi region or MTOC in a unique dynein- and microtubule-dependent manner (34, 76) (Fig. 5). Chlamydia-mediated remodeling is absolutely essential for bacterial replication, as inhibiting chlamydial protein synthesis results in the eventual trafficking of the bacterium to the lysosome, where they are ultimately degraded (168). As obligate intracellular bacteria that are sequestered within a vacuole, chlamydiae exploit host trafficking pathways to obtain essential nutrients and biosynthetic precursors (167). Eukaryotic lipids such as sphingomyelin (82, 83), cholesterol (30), and glycerophospholipids (209) are delivered to the inclusion by multiple mechanisms including Golgi apparatus-dependent vesicular trafficking pathways (82, 83), MVB-dependent trafficking pathways (14), and recruitment of lipid bodies (111). In addition, recycling endosomes containing Tfn are recruited to but do not fuse with the inclusion (168, 197). However, the molecular mechanisms by which chlamydiae exploit these host trafficking pathways have not been identified.
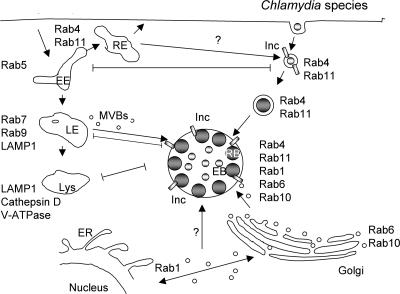
FIG. 5.
Chlamydia species. Chlamydiae replicate within a nonacidified inclusion that is actively remodeled by chlamydiae during the first 2 h p.i. by processes that require chlamydial protein synthesis. As a result of this chlamydia-mediated remodeling, fusion with lysosomes is inhibited, and the inclusion is trafficked to the peri-Golgi region, where it intercepts Golgi apparatus- and MVB-derived vesicles as well as lipid bodies. Multiple Rabs, including both endocytic (Rab4 and Rab11) and secretory (Rab1, Rab6, and Rab10) Rabs, are recruited to the inclusion in a guanine nucleotide-dependent fashion. For at least one Rab, Rab4, recruitment to the inclusion is mediated by a chlamydia-encoded inclusion membrane protein, Inc CT229. Neither the functional role nor the host source of inclusion-localized Rabs has been identified. EE, early endosome; RE, recycling endosome; LE, late endosome; Lys, lysosome.
The potential role of Rabs in the biogenesis or trafficking of the inclusion was investigated by examining the intracellular localization of Rab GTPases in infected HeLa cells transiently expressing a collection of GFP-Rabs (161). From those studies, it was shown that an unexpectedly large number of GFP-Rabs were localized to the 18-h inclusion, including Rab1, Rab4, Rab6, Rab10, Rab11, and Rab14 (161; M. A. Scidmore, unpublished data) (Fig. 5). Even though a large number of Rab GTPases were found to decorate the inclusion, several, including Rab5, Rab7, and Rab9, were shown to be absent from the inclusion, thus demonstrating the specificity of Rab recruitment to the inclusion (161). The absence of Rab5, Rab7, and Rab9 from the inclusion confirms that the inclusion does not interact with the default endocytic/lysosomal pathway. In addition, recruitment of at least one endogenous Rab, Rab14, has been confirmed by laser scanning confocal microscopy, suggesting that the localization of GFP-tagged Rabs reflects the localization of their endogenous counterparts (M. A. Scidmore, unpublished data). Unfortunately, elucidating the functional relevance of the recruitment of Rabs to the inclusion has been difficult. Inclusions develop normally in cells expressing a variety of different guanine nucleotide mutants, including constitutively active GTPase-defective, dominant negative, GDP-restrictive, non-nucleotide-binding, and effector binding Rab mutants (125, 160). The fact that the expression of any single guanine nucleotide binding mutant fails to inhibit inclusion development suggests that, similar to Legionella (51), chlamydiae may target multiple functionally related trafficking pathways and that the inhibition of a single pathway or single step in a pathway may not alter chlamydial development or inclusion formation. Alternatively, chlamydiae may recruit multiple Rabs to the inclusion not to exploit specific trafficking pathways but rather to camouflage the inclusion as either a recycling endosome or secretory vesicle such that it is not targeted to the lysosome for degradation.
Although biological functions of Rabs during chlamydial infection have not been identified, several Rab effectors, including BICD1 (125), have been localized to chlamydial inclusions. Since Rab effectors interact with the GTP-bound active Rabs, the presence of effectors at the inclusion suggests that active GTP-bound Rabs decorate the inclusion membrane and that they are likely performing some function through their interaction with specific effectors. The recycling kinetics of inclusion-localized Rabs have not yet been analyzed, so it is unknown whether chlamydiae secrete GEFs or GAPs that may alter the activation status or recycling kinetics of inclusion-localized Rabs.
The mechanism by which chlamydiae target and recruit Rabs to the inclusion is just beginning to be examined. By analyzing the recruitment of GFP-tagged guanine nucleotide binding mutants, it was shown that only wild-type and constitutively active or GTP-bound Rabs, but not inactive GDP-bound Rabs, localized to the inclusion (125, 160). These data demonstrate that Rabs associate with the inclusion in a manner similar to how they interact with their respective effector molecules. Therefore, the guanine nucleotide-dependent localization to the inclusion suggests that either a Rab effector that is also localized to the inclusion or a chlamydial protein that mimics a Rab effector may recruit Rabs to the inclusion. Chlamydiae secrete both T3SS-dependent proteins (141), including inclusion membrane proteins (Inc) (153), and T3SS-independent proteins into the host cytosol or inclusion membrane. Because of their intracellular localization, some of these secreted proteins may function to recruit or modulate the activity of Rabs at the inclusion membrane. Incs are a large family of highly diverse chlamydia-specific proteins that are incorporated into the growing inclusion membrane (153). Yeast two-hybrid analysis identified a specific guanine nucleotide-dependent interaction between Rab4 and the C. trachomatis-specific Inc CT229 (160). Although loss-of-function mutants cannot be generated in Inc CT229, GFP-Rab4 was shown to colocalize with CT229 at the inclusion membrane, suggesting that CT229 functions to recruit Rab4 to the inclusion (160). A second interaction between the C. pneumoniae inclusion membrane Cpn0585 and multiple Rabs including Rab1, Rab10, and Rab11 has also been reported (B. Wizel and M. A. Scidmore, unpublished data). Collectively, these data demonstrate that chlamydiae modify the inclusion membrane through the secretion of chlamydia-specific Incs to promote interactions with host secretory pathways through the specific recruitment of Rab GTPases.
Although it is known that the inclusion receives cargo from both Golgi apparatus-derived vesicles and MVB-derived vesicles, it is unclear whether Rabs are delivered to the inclusion as a result of fusion with these or other vesicles or, alternatively, are sequestered from a cytosolic pool. However, GFP-Rab6 as well as its effector, BICD1, are still recruited to the inclusion in the presence of brefeldin A (BFA), a fungal metabolite that inhibits endoplasmic reticulum (ER)-to-Golgi apparatus trafficking by inactivating Arf1 (125). These data suggest that neither protein is trafficked to the inclusion through a Golgi apparatus-derived intermediate or via an ER-to-Golgi apparatus-dependent trafficking pathway and are consistent with chlamydiae recruiting at least some Rabs from a cytosolic pool. Without the ability to genetically knock out chlamydial genes that mediate the recruitment of Rabs to the inclusion, the elucidation of the biological role of Rabs will ultimately have to rely on gene-silencing techniques to deplete infected cells of individual Rabs or subsets of Rabs.
LEGIONELLA PNEUMOPHILA
Legionella pneumophila is a gram-negative, facultative, intracellular bacterium that is the causative agent of “Legionnaires' disease” (62, 117). In its natural environment, the bacterium resides within freshwater amoebas such as Acanthamoeba and Hartmenella, but the organism can also infect humans upon the inhalation of contaminated aerosols (2). Invasion and replication in alveolar macrophages can lead to pneumonia or “Legionnaires' disease” in individuals who are immunocompromised or have some underlying conditions that reduce the efficiency of their lung defense mechanisms (2).
Within the macrophage, L. pneumophila promotes its own survival by replicating within an ER-derived phagosome that avoids fusion with the default endocytic/lysosomal pathway (93, 190) (Fig. 6). The Icm/Dot-encoded secretion system, which functions to deliver macromolecules (DNA and protein) into the eukaryotic and protozoan host (171), is essential for determining the intracellular fate of the organism as shown by the rapid trafficking of icm/dot-containing phagosomes to late endosomes (16, 100, 157) and the decreased intracellular survival of icm/dot mutants (23). The formation, but not the maintenance, of the Legionella-containing vacuole (LCV) is dependent on the Icm/Dot type IV secretory system (35, 157). Maturation of the nascent LCV into the replicative vacuole (RV) occurs during the first several hours p.i. By 5 min p.i., the vacuole containing wild-type Legionella avoids interactions with the default endocytic/lysosomal pathway. By 15 min p.i., the limiting vacuolar membrane is decorated with smooth vesicles. After 4 h, the vacuolar membrane has thickened and is decorated with ribosomes, at which time the organism has begun to replicate and is thus termed the RV at this stage (93). Within this specialized RV, the bacteria replicate to high densities, resulting in host cell lysis and infection of neighboring macrophages. In primary macrophages derived from bone marrow of the permissive A/J mouse, the interaction with the lysosomal pathway is only delayed, as the RV eventually matures into an acidified vacuole in a process that resembles autophagy (6, 187).
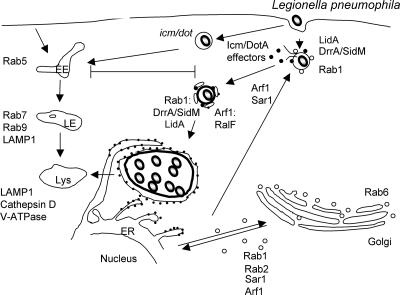
FIG. 6.
L. pneumophila. L. pneumophila cells replicate within an ER-derived vacuole that avoids fusion with the default endocytic pathway. Legionella first recruits and fuses with early secretory vesicles and then subsequently interacts with the ER in an Icm/DotA-dependent manner, resulting in a ribosome-studded RV. In primary macrophages derived from bone marrow of the permissive A/J mouse, after a “pregnant pause,” the RV is delivered to lysosomes. Legionella exploits the early secretory pathway by targeting key regulators of ER-to-Golgi apparatus trafficking, including the small GTPases Rab1, Arf1, and Sar1. Rab1 is recruited to the LCV via a direct interaction with the type IV secreted effector DrrA/SidM, which functions as a Rab1-specific GEF, while LidA plays a cooperative role in recruiting and activating Rab1. Arf1 is recruited to the LCV via a direct interaction with the type IV secreted effector RalF, which functions as an Arf1 GEF. EE, early endosome; LE, late endosome; Lys, lysosome.
Recent studies proposed that the maturation of the LCV into the RV occurs in at least two stages (152). The first stage is the recruitment and fusion of ER-derived early secretory vesicles with the nascent LCV. The second stage, which is dependent on the first stage, involves fusion with the ER to form the RV. In support of this model, coincident with the appearance of vesicles attached to the LCV, proteins that cycle between the ER and the Golgi apparatus are detected at the LCV within the first 30 min p.i. (104). Electron microscopy studies have confirmed the presence of several ER markers including protein disulfide isomerase, KDEL-containing proteins, and glucose-6-phosphatase within vesicles attached to the phagosome by 1 h p.i. (152). In addition, immunofluorescence microscopy studies demonstrated the presence of Arf1 (131), Rab1 (48, 104), and Sec22b (48, 104) by 1 h p.i. as well as the resident ER marker calnexin by 4 h p.i. (103). However, separate studies that examined the presence of resident ER markers on density-purified LCVs by indirect immunofluorescence microscopy demonstrated the recruitment of the resident ER marker calnexin and yellow fluorescent protein-KDEL as early as 30 min p.i. (48). The discrepancies relating to the timing of recruitment of different ER markers may be a result of different sensitivity levels of the assays utilized (48).
How does Legionella target the early secretory pathway? ER-Golgi apparatus trafficking is regulated by Rab1A/Rab1B (133, 208), Rab2 (193), and at least one SNARE complex composed of one v-SNARE (Sec22b) and three cognate t-SNAREs (syntaxin 5, membrin, and Bet1) (87, 210). Rab1 promotes the fusion of ER-derived vesicles with pre-Golgi and Golgi compartments by recruiting effectors, such as p115 (3) and GM130 (127), that are necessary for the tethering and fusion of vesicles and facilitating the pairing of v-SNAREs with cognate t-SNAREs. In addition, the sequential interactions of two additional small GTPases, Sar1 and Arf1, which regulate the formation of COPII- and COPI-coated vesicles, respectively, are also required for the production of early secretory vesicles (7, 53, 164).
Both Rab1 and Arf1, which are found on vesicles that cycle between the ER and the Golgi apparatus, are localized to the LCV by 30 min p.i. (48, 104, 131). In addition, the v-SNARE Sec22b, but not any of its cognate t-SNARES, is also delivered to the LCV (48, 104). Interestingly, prior remodeling of the vacuole with ER-derived vesicles is not required for the delivery of either Rab1 or Arf1 to the vacuole, since both are recruited to the vacuole in the presence of BFA or in cells expressing the dominant interfering Sar1(H179G) mutant (103, 104). These data suggest that Legionella is able to recruit both proteins from a cytosolic pool and activate them at the vacuolar membrane. On the other hand, the delivery of Sec22b is inhibited by BFA and by the expression of dominant interfering Arf1 and Sar1 mutants, demonstrating that ER-Golgi apparatus trafficking is required for the delivery of Sec22b to the LCV (104). In addition, the expression of the dominant negative Rab1 mutant delays the delivery of Sec22b to the vacuole (104). Collectively, these data are consistent with a two-stage maturation model and suggest that Rab1 is required for an early step in remodeling of the LCV and that the subsequent recruitment of Sec22b is dependent on prior Rab1-mediated remodeling of the LCV.
Remodeling of the LCV with ER-derived vesicles is an early event required for the formation of the RV. The expression of dominant interfering mutants in all three small GTPases (Sar1, Rab1, and Arf1) inhibits the formation of the RV and decreases the intracellular survival of the organism (48, 104, 152). Also, time course studies done with BFA demonstrated that BFA blocked the maturation of the vacuole only if it was added prior to or during the first 30 min p.i. (103). The addition of BFA after 30 min had no effect on the formation of the RV, demonstrating that ER-derived vesicle remodeling was required only during the initial stages of infection and that once remodeling has occurred, the inhibition of ER-to-Golgi apparatus trafficking is no longer required to maintain the RV. Collectively, these data suggest that the delivery of ER-derived vesicles is crucial for the remodeling of the LCV and that Legionella recruits these vesicles by targeting key regulatory proteins in this pathway (152). However, in no case was replication inhibited completely (48, 104), consistent with recent data demonstrating that Legionella targets functionally redundant early secretory pathways and that more severe replication defects are observed only when more than one pathway is inhibited (51).
The intracellular survival of the organism, the initial remodeling of the vacuole, and the recruitment of early secretory proteins are all dependent of the Icm/Dot-encoded secretion apparatus. These data suggest that Legionella secretes type IV effectors into the host cell cytosol that function to target specific host trafficking pathways in order to remodel the nascent vacuole (171). Although over 100 potential type IV effectors have been identified, the function of only several effectors has been determined. This includes RalF, which acts as an Arf1-specific GEF that functions to recruit Arf1 to the LCV (131). Additionally, LidA and DrrA/SidM regulate the activity of Rab1 (see below) (114, 130).
As mentioned above, Rab1 is recruited to the LCV in an Icm/Dot-dependent but BFA-independent manner, suggesting that Legionella secretes a type IV effector that plays a role in the cytosolic recruitment and/or modulation of Rab1 activity (48, 104). Consistent with these data, two groups independently identified a type IV effector, DrrA/SidM, which localizes to the cytosolic face of the LCV and functions to recruit and regulate Rab1's activity at the LCV. In one study, SidM (substrate of Icm/Dot) was identified as a result of its ability to bind purified glutathione S-transferase-Rab1 (114), and in the other study, DrrA (defect in Rab recruitment) was identified in a mutant screen designed to identify Legionella proteins that were necessary for the recruitment of Rab1 to the LCV (130). In vitro pull-down assays demonstrated that DrrA/SidM preferentially bound to the GDP-restrictive or no-nucleotide form of Rab1, which is a characteristic of GEFs (114, 130). Consistent with the in vitro binding data, both groups confirmed that DrrA/SidM exhibits a Rab1-specific GEF activity (114, 130), although weak activity for Rab38, a Rab1-related GTPase, was also detected (130).
Although the deletion of DrrA/SidM prevented the recruitment of Rab1 to the LCV, no decrease in bacterial replication resulted from a loss of this protein (114, 130). Again, these data support the hypothesis that Legionella targets multiple functionally redundant trafficking pathways and that the inhibition of only a single trafficking pathway results in little or no intracellular growth defects (51). Ectopic expression of DrrA/SidM in eukaryotic tissue culture cells was shown to interfere with ER-to-Golgi apparatus trafficking and induced the dispersal of the Golgi apparatus (114, 130). In addition, GFP-DrrA localized to and recruited endogenous Rab1 to the plasma membrane (130), suggesting that DrrA/SidM may compete with endogenous GEFs to redirect Rab1 from its normal secretory intracellular localization to plasma membrane-derived vesicles such as the nascent LCV.
A second type IV effector, LidA, which is secreted into the host cytosol and localizes to the cytosolic face of the LCV, was also identified and shown to play a cooperative role in the recruitment and activation of Rab1 (114). Similarly to DrrA/SidM, the ectopic expression of LidA interfered with secretory trafficking, and LidA interacted with Rab1 (49). In contrast to DrrA/SidM, LidA preferentially bound to the GTP-bound form of Rab1 (114). Additionally, LidA was also shown to be more promiscuous in its binding to Rab GTPases, as it also interacted with Rab6 and Rab8 (114). Although lidA mutants still recruited Rab1 to the LCV, these mutants displayed a much more dramatic decrease in intracellular replication than did the drrA/sidM mutant (114). These data may be explained by observation that LidA targets multiple trafficking pathways, as opposed to DrrA, which targets only a single Rab1-dependent trafficking pathway. Although lidA mutants were not defective in Rab1 recruitment, in vitro data suggest that LidA cooperates with DrrA/SidM to recruit ER-derived vesicles. In those in vitro experiments, it was shown that SidM, but not LidA, immobilized to beads facilitated the recruitment of secretory vesicles in a Rab1-dependent manner (114). Interestingly, although LidA was unable to recruit secretory vesicles on its own, the presence of both SidM/DrrA and LidA on the beads increased the efficiency of secretory vesicle recruitment. Collectively, these data suggest that LidA acts with SidM/DrrA in a cooperative manner. Since LidA binds Rab1 but does not display any GEF or GAP activity, LidA may increase the activity of Rab1 by inhibiting the intrinsic GTPase activity of either Rab1 or a Rab1-GAP. Alternatively, LidA may inhibit the membrane recycling of Rab1 by preventing the GDI-mediated removal of Rab1-GDP from the vacuolar membrane.
CONCLUDING REMARKS
Although this review focuses on only a subset of intracellular pathogens, many more are likely to exploit Rab-dependent trafficking pathways to generate replication-competent intracellular niches. For example, similarly to Legionella, Brucella abortus also targets the early secretory pathway by a mechanism that is regulated by the activity of Rab5 (31, 75), while Rhodococcus equi replicates in nondegradative phagosomes that are blocked somewhere between early and late endosomes (58). Listeria monocytogenes grows rapidly in the cytosol (146). However, recent data suggest that these bacteria can modify Rab5 recruitment to phagosomes prior to their escape into the cytosol (90). Conversely, host upregulation of Rab5 may prevent L. monocytogenes escape from phagosomes (4, 5, 147). Further studies are needed to determine whether these and other bacterial pathogens specifically target Rab proteins to modulate the identity of their vacuoles.
As discussed above, bacterium-containing vacuoles retain or exclude Rab proteins to alter their identity. However, except for Legionella, where there is substantial evidence that secreted bacterial proteins interact with and directly regulate the activity of Rab proteins (114, 130), it is unclear whether the other bacterial pathogens use virulence factors to interact directly or indirectly with Rabs. Future challenges in the field include the identification of additional bacterial secreted proteins that interact with or regulate the interaction of the bacterium-containing vacuoles with specific Rab proteins. An open question in cell biology is how Rab proteins are targeted to and maintained at specific intracellular localizations. Future studies of how pathogens recruit or exclude Rabs to compartments that they occupy may provide insights into this problem and may further the understanding of how Rab proteins coordinate distinct trafficking pathways.
Acknowledgments
John H. Brumell holds an Investigators in Pathogenesis of Infectious Disease Award from the Burrough Wellcome Fund. Marci A. Scidmore's research is supported in part by NIH RO1AI52308.
We thank C. Birmingham, V. Braun, S. Shahnazari, J. Szeto, and two anonymous reviewers for constructive criticism of the manuscript.
REFERENCES
- 1.Akporiaye, E. T., J. D. Rowatt, A. A. Aragon, and O. G. Baca. 1983. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect. Immun. 40:1155-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert-Weissenberger, C., C. Cazalet, and C. Buchrieser. 2007. Legionella pneumophila—a human pathogen that co-evolved with fresh water protozoa. Cell. Mol. Life Sci. 64:432-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allan, B. B., B. D. Moyer, and W. E. Balch. 2000. Rab1 recruitment of p115 into cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289:444-448. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Dominguez, C., A. M. Barbieri, W. Berón, A. Wandinger-Ness, and P. D. Stahl. 1996. Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J. Biol. Chem. 271:13834-13843. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Dominguez, C., and P. D. Stahl. 1999. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J. Biol. Chem. 274:11459-11462. [DOI] [PubMed] [Google Scholar]
- 6.Amer, A. O., and M. S. Swanson. 2005. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell. Microbiol. 7:765-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aridor, M., S. I. Bannykh, T. Rowe, and W. E. Balch. 1995. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J. Cell Biol. 131:875-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong, J. A., and P. D. Hart. 1971. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 134:713-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atherton, J. C., T. L. Cover, E. Papini, and J. L. Telford. 2001. Vacuolating cytotoxin, p. 97-110. In H. L. T. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori: physiology and genetics. ASM Press, Washington, DC.
- 10.Babst, M., B. Wendland, E. J. Estepa, and S. D. Emr. 1998. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 17:2982-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baca, O. G., Y. P. Li, and Y. Kumar. 1994. Survival of the Q fever agent Coxiella burnetii in the phagolysosome. Trends Microbiol. 2:476-480. [DOI] [PubMed] [Google Scholar]
- 12.Baldeon, M. E., B. P. Ceresa, and J. E. Casanova. 2001. Expression of constitutively active Rab5 uncouples maturation of the Salmonella-containing vacuole from intracellular replication. Cell. Microbiol. 3:473-486. [DOI] [PubMed] [Google Scholar]
- 13.Barbero, P., L. Bittova, and S. R. Pfeffer. 2002. Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells. J. Cell Biol. 156:511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beatty, W. L. 2006. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J. Cell Sci. 119:350-359. [DOI] [PubMed] [Google Scholar]
- 15.Behnia, R., and S. Munro. 2005. Organelle identity and the signposts for membrane traffic. Nature 438:597-604. [DOI] [PubMed] [Google Scholar]
- 16.Berger, K. H., J. J. Merriam, and R. R. Isberg. 1994. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol. Microbiol. 14:809-822. [DOI] [PubMed] [Google Scholar]
- 17.Berón, W., M. G. Gutierrez, M. Rabinovitch, and M. I. Colombo. 2002. Coxiella burnetii localizes in a Rab7-labeled compartment with autophagic characteristics. Infect. Immun. 70:5816-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biederbick, A., H. F. Kern, and H. P. Elsasser. 1995. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur. J. Cell Biol. 66:3-14. [PubMed] [Google Scholar]
- 19.Birmingham, C. L., X. Jiang, M. B. Ohlson, S. I. Miller, and J. H. Brumell. 2005. Salmonella-induced filament formation is a dynamic phenotype induced by rapidly replicating Salmonella enterica serovar Typhimurium in epithelial cells. Infect. Immun. 73:1204-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birmingham, C. L., A. C. Smith, M. A. Bakowski, T. Yoshimori, and J. H. Brumell. 2006. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281:11374-11383. [DOI] [PubMed] [Google Scholar]
- 21.Blommaart, E. F., U. Krause, J. P. Schellens, H. Vreeling-Sindelarova, and A. J. Meijer. 1997. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Cell Biol. 243:240-246. [DOI] [PubMed] [Google Scholar]
- 22.Boncristiano, M., S. R. Paccani, S. Barone, C. Ulivieri, L. Patrussi, D. Ilver, A. Amedei, M. M. D'Elios, J. L. Telford, and C. T. Baldari. 2003. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. J. Exp. Med. 198:1887-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brand, B. C., A. B. Sadosky, and H. A. Shuman. 1994. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol. Microbiol. 14:797-808. [DOI] [PubMed] [Google Scholar]
- 24.Brumell, J. H., A. J. Perrin, D. L. Goosney, and B. B. Finlay. 2002. Microbial pathogenesis: new niches for Salmonella. Curr. Biol. 12:R15-R17. [DOI] [PubMed] [Google Scholar]
- 25.Brumell, J. H., P. Tang, S. D. Mills, and B. B. Finlay. 2001. Characterization of Salmonella-induced filaments (Sifs) reveals a delayed interaction between Salmonella-containing interactions and late endocytic compartments. Traffic 2:643-659. [DOI] [PubMed] [Google Scholar]
- 26.Bucci, C., P. Thomsen, P. Nicoziani, J. McCarthy, and B. van Deurs. 2000. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11:467-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchmeier, N. A., and F. Heffron. 1991. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect. Immun. 59:2232-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton, P. R., N. Kordova, and D. Paretsky. 1971. Electron microscopic studies of the rickettsia Coxiella burnetii: entry, lysosomal response, and fate of rickettsial DNA in L-cells. Can. J. Microbiol. 17:143-150. [DOI] [PubMed] [Google Scholar]
- 29.Cantalupo, G., P. Alifano, V. Roberti, C. B. Bruni, and C. Bucci. 2001. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carabeo, R. A., D. J. Mead, and T. Hackstadt. 2003. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc. Natl. Acad. Sci. USA 100:6771-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaves-Olarte, E., C. Guzmán-Verri, S. Méresse, M. Desjardins, J. Pizarro-Cerdá, J. Badilla, J. P. Gorvel, and E. Moreno. 2002. Activation of Rho and Rab GTPases dissociates Brucella abortus internalization from intracellular trafficking. Cell. Microbiol. 4:663-676. [DOI] [PubMed] [Google Scholar]
- 32.Christoforidis, S., M. Miaczynska, K. Ashman, M. Wilm, L. Zhao, S. C. Yip, M. D. Waterfield, J. M. Backer, and M. Zerial. 1999. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol. 1:249-252. [DOI] [PubMed] [Google Scholar]
- 33.Chua, J., and V. Deretic. 2004. Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles. J. Biol. Chem. 279:36982-36992. [DOI] [PubMed] [Google Scholar]
- 34.Clausen, J. D., G. Christiansen, H. U. Holst, and S. Birklund. 1997. Chlamydia trachomatis utilizes the host cell microtubule network during early events of infection. Mol. Microbiol. 25:441-449. [DOI] [PubMed] [Google Scholar]
- 35.Coers, J., C. Monahan, and C. R. Roy. 1999. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat. Cell Biol. 1:451-453. [DOI] [PubMed] [Google Scholar]
- 36.Coleman, S. A., E. R. Fischer, D. Howe, D. J. Mead, and R. A. Heinzen. 2004. Temporal analysis of Coxiella burnetii morphological differentiation. J. Bacteriol. 186:7344-7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins, R. F., A. D. Schreiber, S. Grinstein, and W. S. Trimble. 2002. Syntaxins 13 and 7 function at distinct steps during phagocytosis. J. Immunol. 169:3250-3256. [DOI] [PubMed] [Google Scholar]
- 38.Cover, T. L., D. E. Berg, M. J. Blaser, and H. L. T. Mobley. 2001. Helicobacter pylori pathogenesis, p. 510-558. In E. A. Groisman (ed.), Principles of bacterial pathogenesis. Academic Press, San Diego, CA.
- 39.Cover, T. L., and S. R. Blanke. 2005. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 3:320-332. [DOI] [PubMed] [Google Scholar]
- 40.Cover, T. L., and M. J. Blaser. 1992. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem. 267:10570-10575. [PubMed] [Google Scholar]
- 41.Crowle, A. J., R. Dahl, E. Ross, and M. H. May. 1991. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect. Immun. 59:1823-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuellar-Mata, P., N. Jabado, J. Liu, W. Furuya, B. B. Finlay, P. Gros, and S. Grinstein. 2002. Nramp1 modifies the fusion of Salmonella typhimurium-containing vacuoles with cellular endomembranes in macrophages. J. Biol. Chem. 277:2258-2265. [DOI] [PubMed] [Google Scholar]
- 43.Damiani, M. T., M. Pavarotti, N. Leiva, A. J. Lindsay, M. McCaffery, and M. I. Colombo. 2004. Rab coupling protein associates with phagosomes and regulates recycling from the phagosomal compartment. Traffic 5:785-797. [DOI] [PubMed] [Google Scholar]
- 44.De Bernard, M., M. Cappon, G. Del Giudice, R. Rappuoli, and C. Montecucco. 2004. The multiple cellular activities of the VacA cytotoxin of Helicobacter pylori. Int. J. Med. Microbiol. 293:589-597. [DOI] [PubMed] [Google Scholar]
- 45.de Chastellier, C., M. Thibon, and M. Rabinovitch. 1999. Construction of chimeric phagosomes that shelter Mycobacterium avium and Coxiella burnetii (phase II) in doubly infected mouse macrophages: an ultrastructural study. Eur. J. Cell Biol. 78:580-592. [DOI] [PubMed] [Google Scholar]
- 46.de Renzis, S., B. Sönnichsen, and M. Zerial. 2002. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat. Cell Biol. 4:124-133. [DOI] [PubMed] [Google Scholar]
- 47.Deretic, V., S. Singh, S. Master, J. Harris, E. Roberts, G. Kyei, A. Davis, S. de Haro, J. Naylor, H. H. Lee, and I. Vergne. 2006. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defense mechanism. Cell. Microbiol. 8:719-727. [DOI] [PubMed] [Google Scholar]
- 48.Derré, I., and R. R. Isberg. 2004. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect. Immun. 72:2048-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derré, I., and R. R. Isberg. 2005. LidA, a translocated substrate of the Legionella pneumophila type IV secretion system, interferes with the early secretory pathway. Infect. Immun. 73:4370-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desjardins, M., J. E. Celis, G. van Meer, H. Dieplinger, A. Jahraus, G. Griffiths, and L. A. Huber. 1994. Molecular characterization of phagosomes. J. Biol. Chem. 269:32194-32200. [PubMed] [Google Scholar]
- 51.Dorer, M. S., D. Kirton, J. S. Bader, and R. R. Isberg. 2006. RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics. PLoS Pathog. 2:315-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubois, A., and T. Boren. 2007. Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell. Microbiol. 9:1108-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duden, R. 2003. ER-to-Golgi transport: COPI and COPII function. Mol. Membr. Biol. 20:197-207. [DOI] [PubMed] [Google Scholar]
- 54.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Omar, E. M., M. Carrington, W. H. Chow, K. E. McColl, J. H. Bream, H. A. Young, J. Herrera, J. Lissowska, C. C. Yuan, N. Rothman, G. Lanyon, M. Martin, J. F. Fraumeni, and C. S. Rabkin. 2000. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404:398-402. [DOI] [PubMed] [Google Scholar]
- 56.Evans, T. M., C. Ferguson, B. J. Wainwright, R. G. Parton, and C. Wicking. 2003. Rab23, a negative regulator of hedgehog signaling, localizes to the plasma membrane and the endocytic pathway. Traffic 12:869-884. [DOI] [PubMed] [Google Scholar]
- 57.Feng, Y., B. Press, and A. Wandinger-Ness. 1995. Rab7: an important regulator of late endocytic membrane traffic. J. Cell Biol. 131:1435-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandez-Mora, E., M. Polidori, A. Lührmann, U. E. Schaible, and A. Haas. 2006. Maturation of Rhodococcus equi-containing vacuoles is arrested after completion of the early endosome stage. Traffic 6:635-653. [DOI] [PubMed] [Google Scholar]
- 59.Fields, K. A., and T. Hackstadt. 2002. The chlamydial inclusion: escape from the endocytic pathway. Annu. Rev. Cell Dev. Biol. 18:221-245. [DOI] [PubMed] [Google Scholar]
- 60.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276:718-725. [DOI] [PubMed] [Google Scholar]
- 61.Fouraux, M. A., M. Deneka, V. Ivan, A. van der Heijden, J. Raymackers, D. van Suylekom, W. J. van Venrooij, P. van der Sluijs, and G. J. M. Pruijn. 2004. Rabip4′ is an effector of rab5 and rab4 and regulates transport through early endosomes. Mol. Biol. Cell 15:611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fraser, D. W., R. R. Tsai, W. Orenstein, W. E. Oarken, H. J. Beechan, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, C. C. Shepard, and P. S. Brachman. 1977. Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297:1189-1197. [DOI] [PubMed] [Google Scholar]
- 63.Fratti, R. A., J. M. Backer, J. Gruenberg, S. Corvera, and V. Deretic. 2001. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154:631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galán, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567-573. [DOI] [PubMed] [Google Scholar]
- 65.Galmiche, A., J. Rassow, A. Doye, S. Cagnol, J. C. Chambard, S. Contamin, V. de Thillot, I. Just, V. Ricci, E. Solcia, E. Van Obberghen, and P. Boquet. 2000. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 19:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.García-del Portillo, F. 2001. Salmonella intracellular proliferation: where, when and how? Microbes Infect. 3:1305-1311. [DOI] [PubMed] [Google Scholar]
- 67.García-del Portillo, F., and B. B. Finlay. 1995. The varied lifestyles of intracellular pathogens within eukaryotic vacuolar compartments. Trends Microbiol. 3:373-380. [DOI] [PubMed] [Google Scholar]
- 68.García-del Portillo, F., M. G. Pucciarelli, W. A. Jeffries, and B. B. Finlay. 1994. Salmonella typhimurium induces selective aggregation and internalization of host cell surface proteins during invasion of epithelial cells. J. Cell Sci. 197:2005-2020. [DOI] [PubMed] [Google Scholar]
- 69.García-del Portillo, F., M. B. Zwick, K. Y. Leung, and B. B. Finlay. 1993. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc. Natl. Acad. Sci. USA 90:10544-10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garvis, S. G., C. R. Beuzón, and D. W. Holden. 2001. A role for the PhoP/Q regulon in inhibition of fusion between lysosomes and Salmonella-containing vacuoles in macrophages. Cell. Microbiol. 3:731-744. [DOI] [PubMed] [Google Scholar]
- 71.Gebert, B., W. Fischer, E. Weiss, R. Hoffmann, and R. Haas. 2003. Helicobacter pylori vacuolating cytotoxin inhibits T cell activation. Science 301:1099-1102. [DOI] [PubMed] [Google Scholar]
- 72.Geuze, H. J., W. Stoorvogel, G. J. Strous, J. W. Slot, J. E. Bleekemolen, and I. Mellman. 1988. Sorting of mannose-6-phosphate receptors and lysosomal membrane proteins in endocytic vesicles. J. Cell Biol. 107:2491-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghigo, E., C. Capo, C. H. Tung, D. Raoult, J. P. Gorvel, and J. L. Mege. 2002. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-γ mediates its restoration and bacterial killing. J. Immunol. 169:4488-4495. [DOI] [PubMed] [Google Scholar]
- 74.Gomes, M. S., S. Paul, A. L. Moreira, R. Appelberg, M. Rabinovitch, and G. Kaplan. 1999. Survival of Mycobacterium avium and Mycobacterium tuberculosis in acidified vacuoles of murine macrophages. Infect. Immun. 67:3199-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorvel, J. P., and E. Moreno. 2002. Brucella intracellular life: from invasion to intracellular replication. Vet. Microbiol. 90:281-297. [DOI] [PubMed] [Google Scholar]
- 76.Grieshaber, S. S., N. A. Grieshaber, and T. Hackstadt. 2003. Chlamydia trachomatis utilizes host cell dynein to traffic to the microtubule-organizing center in a p50 dynamitin-independent process. J. Cell Sci. 116:3793-3802. [DOI] [PubMed] [Google Scholar]
- 77.Griffiths, G., and J. Greunberg. 1991. The argument for pre-existing early and late endosomes. Trends Cell Biol. 1:5-9. [DOI] [PubMed] [Google Scholar]
- 78.Gruenberg, J., and F. R. Maxfield. 1995. Membrane transport in the endocytic pathway. Curr. Biol. 7:552-563. [DOI] [PubMed] [Google Scholar]
- 79.Gruenberg, J., and H. Stenmark. 2004. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 4:317-323. [DOI] [PubMed] [Google Scholar]
- 80.Gurkan, C., H. Lapp, C. Alory, A. I. Su, J. B. Hogenesch, and W. E. Balch. 2005. Large-scale profiling of Rab GTPase trafficking networks: the membrome. Mol. Biol. Cell 16:3847-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gutierrez, M. G., C. L. Vazquez, D. B. Munafó, F. C. M. Zoppino, W. Berón, M. Rabinovitch, and M. I. Colombo. 2005. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell. Microbiol. 7:981-993. [DOI] [PubMed] [Google Scholar]
- 82.Hackstadt, T., D. D. Rockey, R. A. Heinzen, and M. A. Scidmore. 1996. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J. 15:964-977. [PMC free article] [PubMed] [Google Scholar]
- 83.Hackstadt, T., M. A. Scidmore, and D. D. Rockey. 1995. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc. Natl. Acad. Sci. USA 92:4877-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hackstadt, T., and J. C. Williams. 1981. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc. Natl. Acad. Sci. USA 78:3240-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harrison, R. E., J. H. Brumell, A. Khandani, C. Bucci, C. C. Scott, X. Jiang, B. B. Finlay, and S. Grinstein. 2004. Salmonella impairs RILP recruitment to Rab7 during maturation of invasion vacuoles. Mol. Biol. Cell 15:3146-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hashim, S., K. Mukherjee, M. Raje, S. K. Basu, and A. Mukhopadhyay. 2000. Live Salmonella modulate expression of Rab proteins to persist in a specialized compartment and escape transport to lysosomes. J. Biol. Chem. 275:16281-16288. [DOI] [PubMed] [Google Scholar]
- 87.Hay, J. C., D. S. Chao, C. S. Kuo, and R. H. Scheller. 1997. Protein interactions regulating vesicle transport between the endoplasmic reticulum and the Golgi apparatus in mammalian cells. Cell 89:149-158. [DOI] [PubMed] [Google Scholar]
- 88.Heinzen, R. A., M. A. Scidmore, D. D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64:796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Helenius, A., I. Mellman, D. A. Wall, and A. Hubbard. 1983. Endosomes. Trends Biochem. Sci. 8:245-250. [Google Scholar]
- 90.Henry, R., L. Shaugnessy, M. J. Loesnner, C. Alberti-Segui, D. E. Higgins, and J. A. Swanson. 2006. Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes. Cell. Microbiol. 8:107-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heo, W. D., T. Inoue, W. S. Park, M. L. Kim, B. O. Park, T. J. Wandless, and T. Meyer. 2006. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to plasma membrane. Science 314:1458-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holden, D. W. 2002. Trafficking of the Salmonella vacuole in macrophages. Traffic 3:161-169. [DOI] [PubMed] [Google Scholar]
- 93.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hotchin, N. A., T. L. Cover, and N. Akhtar. 2000. Cell vacuolation induced by the VacA cytotoxin of Helicobacter pylori is regulated by the Rac1 GTPase. J. Biol. Chem. 275:14009-14012. [DOI] [PubMed] [Google Scholar]
- 95.Howe, D., and R. A. Heinzen. 2000. Coxiella burnetii exhibits morphological change and delays phagolysosomal fusion after internalization by J774A. 1 cells. Infect. Immun. 68:3815-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Howe, D., J. Melnicakova, I. Barak, and R. A. Heinzen. 2003. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell. Microbiol. 5:469-480. [DOI] [PubMed] [Google Scholar]
- 97.Ikonomov, O. C., D. Sbrissa, T. Yoshimori, T. L. Cover, and A. Shisheva. 2002. PIKfyve kinase and SKD1 AAA ATPase define distinct endocytic compartments. J. Biol. Chem. 277:46785-46790. [DOI] [PubMed] [Google Scholar]
- 98.Isberg, R. R., and J. M. Leong. 1990. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60:861-871. [DOI] [PubMed] [Google Scholar]
- 99.Jordens, I., M. Fernandez-Borja, M. Marsman, S. Dusseljee, L. Janssen, J. Calafat, H. Jannsen, R. Wubbots, and J. Neefjes. 2001. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11:1680-1685. [DOI] [PubMed] [Google Scholar]
- 100.Joshi, A. D., S. Sturgill-Koszycki, and M. S. Swanson. 2001. Evidence that Dot-dependent and -independent factors isolate the Legionella pneumophila phagosome from the endocytic network in mouse macrophages. Cell. Microbiol. 3:99-114. [DOI] [PubMed] [Google Scholar]
- 101.Kabeya, Y., N. Mizushima, T. Ueno, A. Yamamoto, T. Kirisako, T. Noda, E. Kominami, Y. Ohsumi, and T. Yoshimori. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized to autophagosome membranes after processing. EMBO J. 19:5720-5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kabeya, Y., N. Mizushima, A. Yamamoto, S. Oshitani-Okamoto, Y. Ohsumi, and T. Yoshimori. 2004. LC3, GABARAP and GATE 16 localize to autophagosomal membranes depending on form-II formation. J. Cell Sci. 117:2805-2812. [DOI] [PubMed] [Google Scholar]
- 103.Kagan, J. C., and C. R. Roy. 2002. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 4:945-954. [DOI] [PubMed] [Google Scholar]
- 104.Kagan, J. C., M.-P. Stein, M. Pypaert, and C. R. Roy. 2004. Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J. Exp. Med. 9:1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kelley, V. A., and J. S. Schorey. 2004. Modulation of cellular phosphatidylinositol 3-phosphate levels in primary macrophages affects heat-killed but not viable Mycobacterium avium's transport through the phagosome maturation process. Cell. Microbiol. 6:973-985. [DOI] [PubMed] [Google Scholar]
- 106.Khavkin, T., and S. S. Tabibzadeh. 1988. Histologic, immunofluorescence, and electron micrographic study of the infectious process in mouse lung after intranasal challenge with Coxiella burnetii. Infect. Immun. 56:1792-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klionsky, D. J., and S. D. Emr. 2000. Autophagy as a regulated pathway of cellular degradation. Science 290:171-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Knodler, L. A., and O. Steele-Mortimer. 2003. Taking possession: biogenesis of the Salmonella-containing vacuole. Traffic 4:587-599. [DOI] [PubMed] [Google Scholar]
- 109.Kouranti, I., M. Sachse, N. Arouche, B. Goud, and A. Echard. 2006. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr. Biol. 16:1719-1725. [DOI] [PubMed] [Google Scholar]
- 110.Kuhle, V., G. L. Abrahams, and M. Hensel. 2006. Intracellular Salmonella enterica redirect exocytic transport processes in a Salmonella pathogenicity island 2-dependent manner. Traffic 7:716-730. [DOI] [PubMed] [Google Scholar]
- 111.Kumar, Y., J. Cocchiaro, and R. H. Valdivia. 2006. The obligate intracellular pathogen Chlamydia trachomatis targets host lipid droplets. Curr. Biol. 16:1646-1651. [DOI] [PubMed] [Google Scholar]
- 112.Kyei, G. B., I. Vergne, J. Chua, E. Roberts, J. Harris, J. R. Junutula, and V. Deretic. 2006. Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. EMBO J. 25:5250-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lindsay, A. J., A. G. Hendrick, G. Cantalupo, F. Senic-Matuglia, B. Goud, C. Bucci, and M. W. McCaffrey. 2002. Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J. Biol. Chem. 277:12190-12199. [DOI] [PubMed] [Google Scholar]
- 114.Machner, M. P., and R. R. Isberg. 2006. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev. Cell 11:47-56. [DOI] [PubMed] [Google Scholar]
- 115.Markgraf, D. F., K. Peplowska, and C. Ungermann. 2007. Rab cascades and tethering factors in the endomembrane system. FEBS Lett. 581:2125-2130. [DOI] [PubMed] [Google Scholar]
- 116.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McDade, J. E., C. C. Shepard, D. W. Fraser, R. R. Tsai, M. A. Redus, and W. R. Dowdle. 1977. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory diseases. N. Engl. J. Med. 297:1197-1203. [DOI] [PubMed] [Google Scholar]
- 118.Méresse, S., O. Steele-Mortimer, B. B. Finlay, and J. P. Gorvel. 1999. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 18:4394-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mills, S. D., and B. B. Finlay. 1998. Isolation and characterization of Salmonella typhimurium and Yersinia pseudotuberculosis-containing phagosomes from infected mouse macrophages: Y. pseudotuberculosis traffics to terminal lysosomes where they are degraded. Eur. J. Cell Biol. 77:35-47. [DOI] [PubMed] [Google Scholar]
- 120.Molinari, M., C. Galli, N. Norais, J. L. Telford, R. Rappuoli, J. P. Luzio, and C. Montecucco. 1997. Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. J. Biol. Chem. 272:25339-25344. [DOI] [PubMed] [Google Scholar]
- 121.Molinari, M., M. Salio, C. Galli, N. Norais, R. Rappuoli, A. Lanzzvecchia, and C. Montecucco. 1998. Selective inhibition of li-dependent antigen presentation by Helicobacter pylori toxin VacA. J. Exp. Med. 187:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Montecucco, C., and M. De Bernard. 2003. Molecular and cellular mechanisms of action of the vacuolating cytotoxin (VacA) and neutrophil-activating protein (HP-NAP) virulence factors of Helicobacter pylori. Microbes Infect. 5:715-721. [DOI] [PubMed] [Google Scholar]
- 123.Montecucco, C., E. Papini, M. De Bernard, and M. Zoratti. 1999. Molecular and cellular activities of Helicobacter pylori pathogenic factors. FEBS Lett. 452:16-21. [DOI] [PubMed] [Google Scholar]
- 124.Montecucco, C., and R. Rappuoli. 2001. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat. Rev. Mol. Cell. Biol. 2:457-466. [DOI] [PubMed] [Google Scholar]
- 125.Moorhead, A. R., K. A. Rzomp, and M. A. Scidmore. 2007. The Rab6 effector Bicaudal D1 associates with Chlamydia trachomatis inclusions in a biovar-specific manner. Infect. Immun. 75:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moulder, J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55:143-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moyer, B. D., B. B. Allan, and W. E. Balch. 2001. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic 2:268-276. [DOI] [PubMed] [Google Scholar]
- 128.Mukherjee, K., S. Parashurmaman, M. Raje, and A. Mukhopadhyay. 2001. SopE acts as a Rab5-specific nucleotide exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. J. Biol. Chem. 276:23606-23615. [DOI] [PubMed] [Google Scholar]
- 129.Munafó, D. B., and M. I. Colombo. 2002. Induction of autophagy causes dramatic changes in the subcellular distribution of GFP-Rab24. Traffic 3:472-482. [DOI] [PubMed] [Google Scholar]
- 130.Murata, T., A. Delprato, A. Ingmundson, D. K. Toomre, D. G. Lambright, and C. R. Roy. 2006. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat. Cell Biol. 8:971-978. [DOI] [PubMed] [Google Scholar]
- 131.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679-682. [DOI] [PubMed] [Google Scholar]
- 132.Nagelkerken, B., E. Van Anken, M. Van Raak, L. Gerez, K. Morhmann, N. Van Uden, J. Holthuizen, L. Pelkmans, and P. Van Der Sluijs. 2000. Rabaptin4, a novel effector of the small GTPase rab4A, is recruited to perinuclear recycling vesicles. Biochem. J. 346:593-601. [PMC free article] [PubMed] [Google Scholar]
- 133.Nuoffer, C., H. W. Davidson, J. Matteson, J. L. Meinkoth, and W. E. Balch. 1994. A GDP-bound form of Rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J. Cell Biol. 125:225-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Odorizzi, G., M. Babst, and S. D. Emr. 1998. Fab1p PtdIns(3)P 5-kinase function is essential for protein sorting in the multivesicular body. Cell 95:847-858. [DOI] [PubMed] [Google Scholar]
- 135.Papini, E., M. De Bernard, E. Milia, M. Bugnoli, M. Zerial, R. Rappuoli, and C. Montecucco. 1994. Cellular vacuoles induced by Helicobacter pylori originate from late endosomal compartments. Proc. Natl. Acad. Sci. USA 91:9720-9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Papini, E., B. Satin, C. Bucci, M. De Bernard, J. L. Telford, R. Manetti, R. Rappuoli, M. Zerial, and C. Montecucco. 1997. The small GTP binding protein rab7 is essential for cellular vacuolation induced by Helicobacter pylori cytotoxin. EMBO J. 16:15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pechlivanis, M., and J. Kuhlmann. 2006. Hydrophobic modifications of Ras proteins by isoprenoid groups and fatty acids—more than just membrane anchoring. Biochim. Biophys. Acta 1764:1914-1931. [DOI] [PubMed] [Google Scholar]
- 138.Pereira-Leal, J. B., and M. C. Seabra. 2001. Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 313:889-901. [DOI] [PubMed] [Google Scholar]
- 139.Pereira-Leal, J. B., and M. C. Seabra. 2000. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 301:1077-1087. [DOI] [PubMed] [Google Scholar]
- 140.Perrin, A. J., X. Jiang, C. L. Birmingham, N. S. So, and J. H. Brumell. 2004. Recognition of bacteria in the cytosol of mammalian cells by the ubiquitin system. Curr. Biol. 114:806-811. [DOI] [PubMed] [Google Scholar]
- 141.Peters, J., D. P. Wilson, G. Myers, P. Timms, and P. M. Bavoil. 2007. Type III secretion a la Chlamydia. Trends Microbiol. 15:241-251. [DOI] [PubMed] [Google Scholar]
- 142.Pfeffer, S. 2003. Membrane domains in the secretory and endocytic pathways. Cell 112:507-517. [DOI] [PubMed] [Google Scholar]
- 143.Pfeffer, S., and D. Aivazian. 2004. Targeting Rab GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell Biol. 5:886-896. [DOI] [PubMed] [Google Scholar]
- 144.Pfeffer, S. R. 2001. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11:487-491. [DOI] [PubMed] [Google Scholar]
- 145.Piper, R. C., and J. P. Luzion. 2001. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic 2:612-621. [DOI] [PubMed] [Google Scholar]
- 146.Pizarro-Cerda, J., and P. Cozzart. 2006. Subversion of cellular functions by Listeria monocytogenes. J. Pathol. 208:215-223. [DOI] [PubMed] [Google Scholar]
- 147.Prada-Delgado, A., E. Carrasco-Marín, C. Peña-Macarro, E. Del Cerro-Vadillo, M. Fresno-Escudero, F. Leyva-Cobián, and C. Alvarez-Dominguez. 2005. Inhibition of Rab5a exchange activity is a key step for Listeria monocytogenes survival. Traffic 6:252-265. [DOI] [PubMed] [Google Scholar]
- 148.Reggiori, F., and D. J. Klionsky. 2002. Autophagy in the eukaryotic cell. Eukaryot. Cell 1:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Richter-Dahlfors, A., A. M. Buchan, and B. B. Finlay. 1997. Murine salmonellosis studies by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 186:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rink, J., E. Ghigo, Y. Kalaidzidis, and M. Zerial. 2005. Rab conversion as a mechanism of progression from early to late endosomes. Cell 122:735-749. [DOI] [PubMed] [Google Scholar]
- 151.Roberts, E. A., J. Chua, G. Kyei, and V. Deretic. 2006. Higher order Rab programming in phagolysosome biogenesis. J. Cell Biol. 174:923-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Robinson, C. G., and C. R. Roy. 2006. Attachment and fusion of endoplasmic reticulum with vacuoles containing Legionella pneumophila. Cell. Microbiol. 8:793-805. [DOI] [PubMed] [Google Scholar]
- 153.Rockey, D. D., M. A. Scidmore, J. P. Bannantine, and W. J. Brown. 2002. Proteins in the chlamydial inclusion membrane. Microbes Infect. 4:333-340. [DOI] [PubMed] [Google Scholar]
- 154.Romano, P. S., M. G. Gutierrez, W. Berón, M. Rabinovitch, and M. I. Colombo. 2007. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell. Microbiol. 9:891-909. [DOI] [PubMed] [Google Scholar]
- 155.Rosenfeld, J. L., R. H. Moore, K. P. Zimmer, E. Alpizar-Foster, W. Dai, M. N. Zarka, and B. J. Knoll. 2001. Lysosome proteins are redistributed during expression of a GTP-hydrolysis-defective rab5a. J. Cell Sci. 114:4499-4508. [DOI] [PubMed] [Google Scholar]
- 156.Rosenstiel, P., S. Hellmig, J. Hampe, S. Ott, A. Till, W. Fischbach, H. Sahly, R. Lucius, U. R. Folsch, D. Phillpott, and S. Schreiber. 2006. Influence of polymorphisms in the NOD1/CARD4 and NOD2/CARD15 genes on the clinical outcome of Helicobacter pylori infection. Cell. Microbiol. 8:1188-1198. [DOI] [PubMed] [Google Scholar]
- 157.Roy, C. R., K. H. Berger, and R. R. Isberg. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663-674. [DOI] [PubMed] [Google Scholar]
- 158.Russell, D. G. 2007. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 5:39-47. [DOI] [PubMed] [Google Scholar]
- 159.Russell, D. G., G. E. Purdy, R. M. Owens, K. H. Rohde, and R. M. Yates. 2005. Mycobacterium tuberculosis and the four-minute phagosome. ASM News 71:459-463. [Google Scholar]
- 160.Rzomp, K. A., A. R. Moorhead, and M. A. Scidmore. 2006. The GTPase Rab4 interacts with the Chlamydia trachomatis inclusion membrane protein CT229. Infect. Immun. 74:5362-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rzomp, K. A., L. D. Scholtes, B. J. Briggs, G. R. Whittaker, and M. A. Scidmore. 2003. Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infect. Immun. 71:5855-5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Salama, N. R., G. Otto, L. Tompkins, and S. Falkow. 2001. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect. Immun. 69:730-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Salcedo, S. P., M. Noursadeghi, J. Cohen, and D. W. Holden. 2001. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell. Microbiol. 3:587-597. [DOI] [PubMed] [Google Scholar]
- 164.Scales, S. J., R. Pepperkok, and T. E. Kreis. 1997. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 90:1137-1148. [DOI] [PubMed] [Google Scholar]
- 165.Schachter, J. 1999. Infection and disease epidemiology, p. 139-169. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, DC.
- 166.Schlumberger, M. C., and W. D. Hardt. 2006. Salmonella type III effectors: pulling the host cell's strings. Curr. Opin. Microbiol. 9:46-54. [DOI] [PubMed] [Google Scholar]
- 167.Scidmore, M. A. 2007. Chlamydial exploitation of host signaling and trafficking pathways, p. 255-295. In P. M. Bavoil and P. B. Wyrick (ed.), Chlamydia: genomics and pathogenesis. Horizon Scientific Press, Norfolk, United Kingdom.
- 168.Scidmore, M. A., D. D. Rockey, E. R. Fischer, R. A. Heinzen, and T. Hackstadt. 1996. Vesicular interactions of the Chlamydia trachomatis inclusion are determined by chlamydial early protein synthesis rather than route of entry. Infect. Immun. 64:5366-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Scott, C. C., R. J. Botelho, and S. Grinstein. 2003. Phagosome maturation: a few bugs in the system. J. Membr. Biol. 193:137-152. [DOI] [PubMed] [Google Scholar]
- 170.Seabra, M. C., and C. Wasmeier. 2004. Controlling the location and activation of Rab GTPases. Curr. Opin. Cell Biol. 16:451-457. [DOI] [PubMed] [Google Scholar]
- 171.Segal, G., M. Feldman, and T. Zusman. 2005. The Icm/Dot type-IV secretion systems of Legionella pneumophila and Coxiella burnetii. FEMS Microbiol. Rev. 29:65-81. [DOI] [PubMed] [Google Scholar]
- 172.Seglen, P. O., and P. B. Gordon. 1982. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc. Natl. Acad. Sci. USA 79:1889-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shin, H. W., M. Hayashi, S. Christoforidis, S. Lacas-Gervais, S. Hoepfner, M. R. Wenk, J. Modregger, S. Uttenweiler-Joseph, M. Wilm, A. Nystuen, W. N. Frankel, M. Solimena, P. De Camilli, and M. Zerial. 2005. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J. Cell Biol. 170:607-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sinai, A. P., and K. A. Joiner. 1997. Safe haven: the cell biology of nonfusogenic pathogen vacuoles. Annu. Rev. Microbiol. 51:415-462. [DOI] [PubMed] [Google Scholar]
- 175.Smith, A. C., J. T. Cirulis, J. E. Casanova, M. A. Scidmore, and J. H. Brumell. 2005. Interaction of the Salmonella-containing vacuole with the endocytic recycling system. J. Biol. Chem. 280:24634-24641. [DOI] [PubMed] [Google Scholar]
- 176.Smith, A. C., W. D. Heo, V. Braun, X. Jiang, C. Macrae, J. E. Casanova, M. A. Scidmore, S. Grinstein, T. Meyer, and J. H. Brumell. 2007. A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J. Cell Biol. 176:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Somsel Rodman, J., and A. Wandinger-Ness. 2000. Rab GTPases coordinate endocytosis. J. Cell Sci. 113:183-192. [DOI] [PubMed] [Google Scholar]
- 178.Sonnichsen, B., S. De Renzis, E. Nielsen, J. Rietdorf, and M. Zerial. 2000. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, Rab11. J. Cell Biol. 149:901-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stamm, L. M., J. H. Morisaki, L. Y. Gao, R. L. Jeng, K. L. McDonald, R. Roth, S. Takeshita, J. Heuser, M. D. Welch, and E. J. Brown. 2003. Mycobacterium marinum escapes from the phagosomes and is propelled by actin-based motility. J. Exp. Med. 198:1361-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Steele-Mortimer, O., J. H. Brumell, L. A. Knodler, S. Méresse, A. Lopez, and B. B. Finlay. 2002. The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell. Microbiol. 4:43-54. [DOI] [PubMed] [Google Scholar]
- 181.Steele-Mortimer, O., S. Méresse, J. P. Gorvel, B. H. Toh, and B. B. Finlay. 1999. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell. Microbiol. 1:33-49. [DOI] [PubMed] [Google Scholar]
- 182.Stein, A., C. Louveau, H. Lepidi, F. Ricci, P. Baylac, B. Davoust, and D. Roult. 2005. Q fever pneumonia: virulence of Coxiella burnetii pathovars in a murine model of aerosol infection. Infect. Immun. 73:2469-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stenmark, H., and V. M. Olkkone. 2001. The Rab GTPase family. Genome Biol. 2:reviews3007.1-reviews3007.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stenmark, H., R. G. Parton, O. Steele-Mortimer, A. Lutcke, J. Gruenberg, and M. Zerial. 1994. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13:1287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stoorvogel, W., G. J. Strous, H. J. Geuze, V. Oorschot, and A. L. Schwartz. 1991. Late endosomes derive from early endosomes by maturation. Cell 65:417-427. [DOI] [PubMed] [Google Scholar]
- 186.Sturgill-Koszycki, S., P. H. Schlesinger, P. Chakraboty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russell. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678-681. [DOI] [PubMed] [Google Scholar]
- 187.Sturgill-Koszycki, S., and M. S. Swanson. 2000. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 9:1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175-1186. [DOI] [PubMed] [Google Scholar]
- 189.Suzuki, J., H. Ohnsihi, H. Shibata, A. Wada, T. Hirayama, T. Iiri, N. Ueda, C. Kanamaru, T. Tsuchida, H. Mashima, H. Yasuda, and T. Fujita. 2001. Dynamin is involved in human epithelial cell vacuolation caused by the Helicobacter pylori-produced cytotoxin VacA. J. Clin. Investig. 107:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Terebiznik, M. R., C. L. Vazquez, K. Torbicki, D. Banks, T. Wang, W. Hong, S. R. Blanke, M. I. Colombo, and N. L. Jones. 2006. Helicobacter pylori VacA toxin promotes bacterial intracellular survival in gastric epithelial cells. Infect. Immun. 74:5699-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tierrez, A., and F. García-del Portillo. 2005. New concepts in Salmonella virulence: the importance of reducing the intracellular growth rate in the host. Cell. Microbiol. 7:901-909. [DOI] [PubMed] [Google Scholar]
- 193.Tisdale, E. J., J. R. Bourne, R. Khosravi-Far, C. J. Der, and W. E. Balch. 1992. GTP-binding mutants of Rab1 and Rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 119:749-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tsolis, R. M., R. A. Kingsley, S. M. Townsend, T. A. Ficht, L. G. Adams, and A. J. Baumler. 1999. Of mice, calves and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv. Exp. Med. Biol. 473:21-274. [PubMed] [Google Scholar]
- 195.Uchiya, K., M. A. Barbieri, K. Funato, A. H. Shah, P. D. Stahl, and E. A. Groisman. 1999. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 18:3924-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van der Wel, N., D. Hava, D. Houben, D. Fluitsma, M. van Zon, J. Pierson, M. Brenner, and P. J. Peters. 2007. M. tuberculosis and M. leprae translocate from the phagosome to the cytosol in myeloid cells. Cell 129:1287-1298. [DOI] [PubMed] [Google Scholar]
- 197.van Ooij, C., G. Apodaca, and J. Engel. 1997. Characterization of the Chlamydia trachomatis vacuole and its interaction with the host endocytic pathway in HeLa cells. Infect. Immun. 65:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Veras, P. S., C. de Chastellier, M. F. Moreau, V. Villiers, M. Thibon, D. Mattei, and M. Rabinovitch. 1994. Fusion between large phagocytic vesicles: targeting of yeast and other particles to phagolysosomes that shelter the bacterium Coxiella burnetii or the protozoan Leishmania amazonensis in Chinese hamster ovary cells. J. Cell Sci. 197:3065-3076. [DOI] [PubMed] [Google Scholar]
- 199.Veras, P. S., C. Moulia, C. Dauget, C. T. Tunis, M. Thibon, and M. Rabinovitch. 1995. Entry and survival of Leishmania amazonensis amastigotes within phagosome-like vacuoles that shelter Coxiella burnetii in Chinese hamster ovary cells. Infect. Immun. 63:3502-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vergne, I., J. Chua, H. H. Lee, M. Lucas, J. Belisle, and V. Deretic. 2005. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 102:4033-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vergne, I., R. A. Fratti, P. J. Hill, J. Chua, J. Belisle, and V. Deretic. 2004. Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol. Biol. Cell 15:751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Via, L. E., D. Deretic, R. J. Ulmer, N. S. Hibler, L. A. Huber, and V. Deretic. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272:13326-13331. [DOI] [PubMed] [Google Scholar]
- 203.Vieira, O. V., R. J. Botelho, L. Rameh, S. M. Brachmann, T. Matsuo, H. W. Davidson, A. D. Schreiber, J. M. Backer, L. C. Cantley, and S. Grinstein. 2001. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 155:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vieira, O. V., C. Bucci, R. E. Harrison, W. S. Trimble, L. Lanzetti, J. Gruenberg, A. D. Schreiber, P. D. Stahl, and S. Grinstein. 2003. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol. Cell. Biol. 23:2501-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vitale, G., V. Rybin, S. Christoforidis, P. Thornqvist, M. McCaffery, H. Stenmark, and M. Zerial. 1998. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 17:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vitelli, R., M. Santillo, D. Lattero, M. Chiarello, M. Bifulco, C. B. Bruni, and C. Bucci. 1997. Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 272:4391-4397. [DOI] [PubMed] [Google Scholar]
- 207.Wang, W., and S. Ferro-Novick. 1992. A Ypt32 exchange factor is a putative effector of Ypt1p. Mol. Biol. Cell 13:3336-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wilson, B. S., C. Nuoffer, J. L. Meinkoth, M. McCaffery, J. R. Feramisco, W. E. Balch, and M. G. Farquhar. 1994. A Rab1 mutant affecting guanine nucleotide exchange promotes disassembly of the Golgi apparatus. J. Cell Biol. 125:557-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wylie, J. L., G. M. Hatch, and G. McClarty. 1997. Host cell phospholipids are trafficked to and then modified by Chlamydia trachomatis. J. Bacteriol. 179:7233-7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xu, D., A. P. Joglekar, J. C. Williams, and J. C. Hay. 2000. Subunit structure of a mammalian ER/Golgi SNARE complex. J. Biol. Chem. 275:39631-39639. [DOI] [PubMed] [Google Scholar]
- 211.Yeung, T., M. Terebiznik, L. Yu, J. Silvius, W. M. Abidi, M. Philips, T. Levine, A. Kapus, and S. Grinstein. 2006. Receptor activation alters inner surface potential during phagocytosis. Science 313:347-351. [DOI] [PubMed] [Google Scholar]
- 212.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107-117. [DOI] [PubMed] [Google Scholar]