Abstract
The Caulobacter cell cycle is regulated by a network of two-component signal transduction proteins. Phosphorylation and stability of the master transcriptional regulator CtrA are controlled by the CckA-ChpT phosphorelay, and CckA activity is modulated by another response regulator, DivK. In a screen to identify suppressors of the cold-sensitive divK341 mutant, we found point mutations in the essential gene divL. DivL is similar to histidine kinases but has a tyrosine instead of a histidine at the conserved phosphorylation site (Y550). Surprisingly, we found that the ATPase domain of DivL is not essential for Caulobacter viability. We show that DivL selectively affects CtrA phosphorylation but not CtrA proteolysis, indicating that DivL acts in a pathway independent of the CckA-ChpT phosphorelay. divL can be deleted in a strain overproducing the phosphomimetic protein CtrAD51E, but unlike ΔctrA cells expressing CtrAD51E, this strain is profoundly impaired in the control of chromosome replication and cell division. Thus, DivL performs a second function in addition to promoting CtrA phosphorylation. DivL is required for bipolar DivK localization and positively regulates DivK phosphorylation. Our results show that DivL controls two key cell cycle regulators, CtrA and DivK, and that phosphoryl transfer is not DivL's essential cellular activity.
Several two-component signal transduction proteins have been identified in genetic screens for Caulobacter crescentus mutants with defects in cell cycle progression, cell division, and polar morphogenesis (reviewed in reference 38). The essential response regulator CtrA directly controls the expression of at least 95 genes (24) and promotes cell division, DNA methylation, and the biogenesis of polar organelles (22). However, CtrA also binds to five sites within the replication origin to inhibit the initiation of chromosome replication (33), and cells with unrestricted CtrA activity arrest in the G1 phase of the cell cycle (6). CtrA activity must therefore be regulated tightly to allow the orderly progression of DNA replication, morphogenesis, and cell division.
The following three mechanisms control CtrA activity as a function of the cell cycle: (i) ctrA expression peaks after DNA replication has begun, due to the transcriptional effects of DnaA and GcrA (14); (ii) CtrA is activated by phosphorylation in swarmer cells, where DNA replication has not yet occurred (G1), and in predivisional cells, where replication has already been initiated (17); and (iii) the CtrA protein is cleared from cells preparing to initiate DNA replication by the ATP-dependent protease ClpXP (6). Combined, these three mechanisms ensure that phosphorylated CtrA (CtrA∼P) is present only early and late in the cell cycle, precisely when CtrA-dependent genes are activated or repressed. They also restrict CtrA phosphorylation and eliminate the protein itself just before S phase to free the origin for the initiation of DNA replication.
CtrA phosphorylation and proteolysis are coordinated by a phosphorelay containing the hybrid histidine kinase CckA and the histidine phosphotransferase ChpT (16). CckA autophosphorylates on a conserved histidine residue. The phosphoryl group is passed to a conserved aspartate in the receiver domain at the C terminus of CckA and from there to a histidine residue in ChpT. ChpT can pass the phosphoryl group to one of two response regulators, either CtrA or CpdR (3). Phosphorylation activates CtrA to regulate transcription and DNA replication, and phosphorylation of CpdR prevents it from promoting CtrA proteolysis (16). Thus, when the CckA-ChpT phosphorelay is active, CtrA is stable and activated, whereas when the phosphorelay is inactive, CtrA becomes dephosphorylated and proteolyzed.
Fluctuations in CtrA activity during the cell cycle are linked to changes in CckA activity, and CckA phosphorylation itself is cell cycle dependent (17). The only factor known to modulate CckA activity is the essential single-domain response regulator DivK. When phosphorylated, DivK downregulates the activity of the CckA-ChpT pathway. In the cold-sensitive divK341 mutant (41) grown at the nonpermissive temperature, excess CckA∼P and CtrA∼P are present (3), CtrA is no longer proteolyzed, and the net result is G1 cell cycle arrest (15). Conversely, DivK overexpression causes a sharp decrease in the phosphorylation of CckA and an accumulation of chromosomal DNA (3). The mechanism by which DivK affects the activity of CckA is currently unknown.
DivK is implicated not only in cell cycle regulation but also in the generation of cellular asymmetry. Each Caulobacter cell division produces two distinct cell types with different morphologies, protein contents, and cell fates (reviewed in reference 7). The swarmer progeny is motile, contains CtrA∼P, and cannot initiate DNA replication, while the stalked progeny is sessile, lacks CtrA, and can immediately begin a new round of DNA replication. DivJ, a histidine kinase that phosphorylates DivK, resides at the stalked pole of the predivisional cell, while PleC, a histidine kinase that dephosphorylates DivK, is positioned at the swarmer or flagellated pole (48). Once a cytoplasmic barrier is created in the late predivisional cell (21), phosphorylated DivK (DivK∼P) is thought to accumulate in the stalked compartment due to the action of DivJ, triggering the downregulation of CckA and the degradation of CtrA. In the swarmer compartment, DivK is dephosphorylated by PleC, which maintains the activity of the CckA-ChpT phosphorelay and preserves CtrA function (27).
To identify components that act in concert with or in parallel to DivK, we performed a screen for temperature-sensitive suppressors of the cold-sensitive divK341 mutant. Since divK341 cells at the nonpermissive temperature fail to eliminate CtrA activity, we expected to find mutations that compromise CtrA activity in ctrA itself and in genes that positively regulate CtrA. Three independent suppressor mutations were identified in divL, which encodes an essential kinase that regulates the Caulobacter cell cycle (51). Depletion of the DivL protein from Caulobacter cells results in cell filamentation and accumulation of additional copies of the chromosome (36). DivL is unusual in that its dimerization and histidine phosphotransfer (DHp) domain contains a tyrosine residue (Y550) at the conserved phosphorylation site. DivL was shown to autophosphorylate on Y550 in vitro and to pass the phosphoryl group to CtrA (51). Conditional divL mutants also contain reduced levels of phosphorylated CtrA (31), indicating that DivL plays a role in CtrA activation in vivo.
Where does DivL fit into the network of two-component signaling proteins that regulate the Caulobacter cell cycle? One possibility is that DivL acts in a pathway parallel to CckA and ChpT to phosphorylate CtrA. However, cckA mutants lack detectable CtrA∼P (17, 18), so DivL and CckA may act in the same signaling pathway. Some results indicate that DivL could interact with DivK and thereby modulate the CckA pathway. First, divL mutations can suppress phenotypic defects in pleC and divJ strains, which contain altered levels of active DivK (31, 41). Second, fragments of DivL were identified along with those of DivJ and PleC in a yeast two-hybrid screen for peptides that physically interact with DivK (30).
Here we show that DivL positively regulates CtrA phosphorylation without affecting CpdR phosphorylation or the rate of CtrA proteolysis, suggesting that DivL acts selectively on CtrA rather than modulating the CckA-ChpT phosphorelay. DivL also promotes DivK phosphorylation and localization of DivK to the cell poles. Surprisingly, divL alleles that produce proteins incapable of autophosphorylation or phosphoryl transfer can substitute for the wild-type divL gene, causing only moderate defects in cell division. DivL is therefore one of the few histidine kinase homologs known to perform a key cellular function distinct from phosphoryl transfer to a response regulator.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The strains and plasmids used for this study are listed in Table 1. All experiments were performed using derivatives of Caulobacter crescentus strain CB15N grown to mid-logarithmic phase. CB15N strains were grown in peptone-yeast extract (PYE; complex medium), M2G (minimal medium) (9), or M5G [10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), pH 7, 1 mM NaCl, 1 mM KCl, 0.05% NH4Cl, 0.01 mM Fe-EDTA, 0.2% glucose, 0.5 mM MgSO4, 0.5 mM CaCl2, and 0.1 mM phosphate] at the indicated temperatures. Solid and liquid media were supplemented with 3% sucrose, kanamycin (25 μg/ml and 5 μg/ml for solid and liquid media, respectively), chloramphenicol (1 μg/ml), naladixic acid (20 μg/ml), oxytetracyline (2 μg/ml and 1 μg/ml, respectively), or spectinomycin (100 μg/ml and 25 μg/ml, respectively), as required. Escherichia coli strains were grown in Luria broth at 37°C, and solid and liquid media were supplemented with carbenicillin (100 μg/ml and 50 μg/ml, respectively), chloramphenicol (30 μg/ml and 20 μg/ml, respectively), kanamycin (50 μg/ml and 30 μg/ml, respectively), tetracycline (12 μg/ml), or spectinomycin (50 μg/ml), as required.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description or construction | Source or reference |
|---|---|---|
| Strains | ||
| C. crescentus strains | ||
| KR684 | CB15N, from which all Caulobacter strains were derived | 11 |
| CJ396 | ΔcckA ΔctrA/pCtrAD51E | 17 |
| LS2716 | ΔctrA/pCtrAD51E | 17 |
| LS3196 | ΔdivJ | 48 |
| LS3570 | divK341 | 15 |
| NR664 | divL::Tn5 (inserted in codon 636) | This study |
| UJ506 | ΔpleC | 1 |
| KR510 | divK341 divL510 | This study |
| KR635 | divL510 | This study |
| KR648 | divL510/pMR20-divL | This study |
| KR649 | divL510/pMR20 | This study |
| KR674 | divL::eyfp | This study |
| KR748 | ΔpleC/pMR20divK-EGFP | This study |
| KR1609 | CB15N/pMR20-cpdR::3×FLAG | This study |
| KR1612 | divL510/pMR20-cpdR::3×FLAG | This study |
| KR1773 | ΔdivL/pMR20-divL | This study |
| KR1775 | ΔdivL/pMR20-divL(Y550F) | This study |
| KR1776 | ΔdivL/pMR20-divL657 | This study |
| KR1777 | ΔdivL/pMR20-divL635 | This study |
| KR1808 | divL510/pMR20-divJ::yfp | This study |
| KR1809 | CB15N/pMR20-divJ::yfp | This study |
| KR1841 | divK341 divL::eyfp | This study |
| KR1843 | divL::eyfp Pxyl-divK | This study |
| KR1846 | CB15N/pMR20divK-EGFP | 19 |
| KR1848 | divL510/pMR20divK-EGFP | This study |
| KR1985 | Δ-pleC/pMR20-divJ::yfp | This study |
| KR2044 | divL510::eyfp | This study |
| KR2045 | ΔdivL ΔctrA/pCtrAD51E | This study |
| KR2046 | divL::Tn5 (NR664) divK::egfp | This study |
| KR2047 | divK::egfp | This study |
| E. coli strains | ||
| TOP10 | Cloning strain | Invitrogen |
| S17-1 | RP4-2; Tc::Mu Km::Tn7 | 37 |
| CS191 | S17-1/pLW126 | 47 |
| Plasmids | ||
| pMR20 | Broad-host-range, low-copy-number vector | 34 |
| pNPTS138 | Kanr; sacB-containing integration vector | 42 |
| pNPT228 | Kanr integration vector | M. R. K. Alley, unpublished data |
| pJC52 | Vector for fusions to the N terminus of ECFP | 4 |
| pHPV465 | Spec/Strepr suicide vector | 45 |
| pHP45Ω | Plasmid containing Ω spectinomycin/streptomycin resistance cassette | 32 |
| pEYFP | Vector for fusions to the N terminus of EYFP | Clontech |
| pCtrA | pJS14-ctrA, pSAL14 | 6 |
| pCtrAD51E | pJS14-ctrAD51E, pCTD14 | 6 |
| pMR20divK-EGFP | 19 | |
| pHXM-divK | Pxyl-divK in high-copy-number vector pJS71 | 3 |
| pNPTS-cc1063-YFP | pNPTS138-divJ::yfp | M. Laub, unpublished data |
| pKOC3 | Contains frt-flanked Tetr cassette | 40 |
| pLW126 | Vector for inserting Kmr cassette at 3.75 Mb | 47 |
| pKR174 | pMR20-divL | This study |
| pKR196 | pNPTS138-divL::eyfp | This study |
| pKR320 | pNPTS138-divL::tet | This study |
| pSK54 | pMR20-divL(Y550F) | This study |
| pSK118 | pMR20-divL657 | This study |
| pSK123 | pMR20-divL635 | This study |
| pSK128 | pMR20-cpdR::3×FLAG | This study |
| pSK137 | pMR20-divL540::3×FLAG | This study |
| pSK149 | pNPTS138-divL::spec | This study |
| pSK158 | pMR20-divJ::yfp | This study |
| pSK170 | pNPTS-divL510::eyfp | This study |
| pSK171 | pNPT228-divK::egfp | This study |
Site-directed mutagenesis of divL to create divL(Y550F) was carried out using the QuikChange protocol (Stratagene), with pMR20-divL as the template. Truncated divL alleles were generated by PCR, using pKR170 as the template. The 3×FLAG epitope in pSK127 and pSK137 was constructed using an oligonucleotide linker and added the amino acid residues DYKDHDGDYKDHDIDYKDDDDK to the C termini of CpdR and DivL539. For the DivL-enhanced yellow fluorescent protein (DivL-EYFP) fusion, we used PCR to generate an NcoI site at the 3′ end of divL and cloned an ∼880-bp BamHI-NcoI fragment of divL into pEYFP (Clontech). We integrated the divL-eyfp gene into the chromosomal divL locus using the suicide vector pNPTS138. The plasmid carrying divL510::eyfp was generated by site-directed mutagenesis of the divL-eyfp fusion gene on pKR196 to introduce the L740P mutation, creating integration plasmid pSK170. For the DivJ-YFP fusion, we used PCR to amplify divJ from genomic DNA and then performed a three-piece ligation of PCR-amplified divJ digested with SacI and XhoI, pNPTS-cc1063-YFP digested with XhoI and SphI, and pUC19 digested with SacI and SphI. The resulting plasmid, with full-length DivJ fused to YFP, was digested with SacI and HindIII, and the fragment was moved into pMR20 to generate pSK158. For the integration plasmid carrying divK::egfp, pMR20divK-EGFP was digested with SpeI, and the insert was ligated into pNPT228 to generate pSK171. All plasmids were mobilized from E. coli to C. crescentus by conjugation using E. coli strain S17-1 (9). Sequences of all primers used for amplification or mutagenesis are available upon request.
Isolation of suppressors of divK341.
A CB15N strain containing a cold-sensitive mutation in the divK gene (41) was mutagenized with UV radiation and plated in PYE containing 0.3% agar at 20°C. Large swarms were selected from these plates, and the double mutants were screened for the inability to swarm in 0.3% agar at 37°C to identify divK suppressors that conferred a temperature-sensitive phenotype. We complemented the 37°C swarm defect in each suppressor strain using a cosmid library (2). Complementing cosmids were isolated, and the insert ends were sequenced. The complementing cosmid for suppressor strain KR510 included CC3484, encoding the essential tyrosine kinase DivL. A low-copy-number plasmid containing only the wild-type divL gene (pMR20-divL) also complemented KR510. A kanamycin resistance gene was integrated at 3.75 Mb in the genome of KR510, near divL (29, 47). The divL allele in strain KR510 (divL510) was moved into CB15N by cotransduction with the kanamycin resistance gene, using phage ΦCr30 (10).
Two-step gene replacement.
Arms of homology flanking divL were generated by PCR amplification of the regions approximately 1,000 bp upstream and downstream of divL. The left arm included the first three codons of divL, and the right arm included the last three codons of divL. A streptomycin/spectinomycin resistance gene flanked by transcriptional and translational stop signals (32) from pHP45Ω was digested with BamHI and ligated between the two PCR-generated homology arms in pNPTS138 to generate plasmid pSK149. After pSK149 was mobilized into strains carrying various divL alleles on pMR20, first integrants were selected by plating on PYE containing streptomycin, oxytetracycline, and nalidixic acid. Two colonies from each conjugation were inoculated into separate cultures containing PYE with oxytetracycline and streptomycin. After overnight growth, the two cultures for each strain were combined, and serial dilutions were plated for counterselection on PYE containing 3% sucrose, oxytetracycline, and streptomycin. Fifty to 100 of the sucrose-resistant colonies were screened for kanamycin sensitivity and resistance to streptomycin and oxytetracycline to identify colonies in which the wild-type divL allele had been replaced by the ΔdivL mutation. Gene replacements were verified by two PCR tests. First, to verify insertion at the divL locus, we used a forward primer in the streptomycin resistance cassette and a reverse primer placed downstream of the sequence included in the divL integration construct. A second PCR was performed using primers within the sacB gene of pNPTS138 to verify that sucrose resistance resulted from the second recombination event rather than sacB inactivation.
To generate the strain ΔdivL ΔctrA/pCtrAD51E, we amplified the Tetr cassette from pKOC3 (39), using primers that added a BamHI site to each end. We digested this fragment with BamHI and ligated it into the BglII site between the left and right divL homology arms in pNPTS138 to create pKR320. After mobilizing pKR320 into ΔctrA/pCtrAD51E (17), we selected first integrants on PYE-oxytetracycline-nalidixic acid plates. Individual colonies were selected and grown overnight in liquid PYE-oxytetracycline, after which they were plated onto PYE-oxytetracycline containing 3% sucrose for counterselection against the pNPTS138 vector backbone. Sucrose-resistant colonies were screened for kanamycin sensitivity, oxytetracycline resistance, and spectinomycin resistance to identify colonies in which the wild-type divL gene had been deleted. We performed a PCR using primers within the sacB gene of pNPTS138 to verify that sucrose resistance resulted from the second recombination event rather than from sacB inactivation.
Isolation of pleC suppressors.
divL::Tn5 insertions emerged from a genetic screen devised to isolate suppressors of the pleC mutant phenotype that will be published elsewhere (S. K. Rhadakrishnan, S. Pritchard, and P. H. Viollier, in preparation). Briefly, a promoterless nptII gene conferring resistance to kanamycin was transcriptionally fused to the promoter of the pilA gene (PpilA), a promoter that is dependent on PleC for optimal activity (43). The PpilA-nptII transcriptional reporter was integrated at the chromosomal pilA locus in wild-type CB15N and the ΔpleC mutant derivative (5). Of the two resulting reporter strains, the former was resistant to kanamycin (20 μg/ml), while the latter was sensitive. The ΔpleC PpilA-nptII strain was subsequently mutagenized with a Tn5 derivative conferring resistance to tetracycline (20) to isolate mutants that were able to grow in the presence of kanamycin and tetracycline. The six Tn5 insertions that were mapped to divL all occurred downstream of the codon for Y550. The Tn5 elements in strains NR525, NR664, NR1190, NR1193, NR1244, and NR1245 were found to be inserted in the codons for D653, G636, A658, G636, I572, and D604, respectively.
Flow cytometry.
Samples for flow cytometry were fixed at a final concentration of 70% ethanol, stored at 4°C for 1 day to 2 weeks, stained with SYTOX green nucleic acid stain as described previously (35), and analyzed using an Epics XL-MCL analyzer (Beckman-Coulter).
In vivo phosphorylation, pulse-chase, and immunoblot assays.
In vivo phosphorylation experiments were performed as previously described (6), with the following modifications. Cells to be labeled were grown in M2G overnight, diluted the next day in M5G, diluted after 8 hours of growth in M5G, and grown overnight to an optical density at 660 nm of 0.3. One milliliter of culture was harvested for Western analysis, and another milliliter of culture was labeled for 4 min at 28°C, using 30 μCi [γ-32P]ATP. Each sample was immunoprecipitated with 2.5 μl of anti-CtrA serum (6) along with either 2.5 μl of anti-FLAG serum (Sigma) or 2.5 μl anti-DivK serum (19). Radiolabeled proteins were resolved in sodium dodecyl sulfate (SDS)-polyacrylamide gels and quantified using a Typhoon phosphorimager (Molecular Dynamics). For pulse-chase experiments, CB15N strains grown at 28°C in M2G were shifted to 37°C for 4 h and then pulse labeled with 10 μCi/ml [35S]methionine per ml of culture for 5 min, followed by a chase with 1 mM unlabeled methionine and 0.3% Casamino Acids. Samples (1 ml) were withdrawn from cultures every 15 min for 2 hours and centrifuged to pellet the cells. Cells were lysed using 50 μl SDS buffer (10 mM Tris-Cl, pH 8, 1% SDS, 1 mM EDTA) and diluted with 800 μl chilled wash buffer (50 mM Tris-Cl, pH 8, 150 mM NaCl, 0.5% Triton X-100). After preclearing of the sample with protein A-agarose, anti-CtrA serum (1.5 μl) and protein A-agarose (Roche) were added to each sample and incubated for 1 h at 4°C with rocking. Immunoprecipitates were washed three times with wash buffer and eluted from protein A-agarose using 15 μl 2× Laemmli sample buffer. Samples were separated in 12% SDS-polyacrylamide gels and analyzed using a Typhoon phosphorimager (Molecular Dynamics). Immunoblotting was performed using anti-CtrA serum at 1:10,000 and anti-FLAG serum at 1:5,000.
Differential interference contrast (DIC) and fluorescence microscopy.
Log-phase cells were immobilized on 1% (wt/vol) agarose pads. Images were acquired using a Nikon Eclipse 80i microscope with a PlanApo 100×, 1.40-numerical-aperture objective and a Cascade 512B camera (Roper Scientific). Enhanced green fluorescent protein (EGFP) and EYFP were imaged using Chroma filter sets 41001 and 41028, respectively. Images were acquired using Metavue software (Universal Imaging).
RESULTS
A conditional divL mutation suppresses the phenotype of the divK341 mutant.
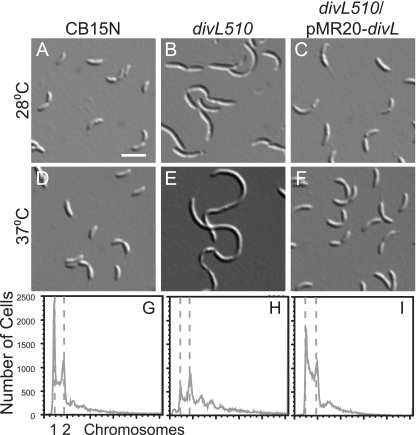
To identify proteins that participate in Caulobacter cell cycle regulation, we screened for mutations that restored swarming to the cold-sensitive divK341 mutant (41) at 20°C. Suppressors were then screened for those that conferred a new growth or motility defect in swarm agar at 37°C. Some temperature-sensitive mutants were complemented by providing the ctrA gene on a low-copy-number plasmid, which was expected because one ctrA mutant previously suppressed the phenotype of the divK341 mutant (50). Other mutants had temperature-sensitive phenotypes not complemented by ctrA, so we attempted to complement each one using a cosmid library containing Caulobacter genomic DNA (2). Three strains were complemented by cosmids containing divL and also by the divL gene alone on a low-copy-number plasmid (pMR20-divL). We integrated a kanamycin resistance gene at a chromosomal locus near divL (3.75 Mb) (29, 47) and transduced one temperature-sensitive allele (divL510) into the wild-type strain CB15N to examine its phenotype in isolation. In contrast to wild-type cells (Fig. 1A), divL510 cells had a range of lengths at 28°C (Fig. 1B). At 37°C, divL510 cells became more filamentous and accumulated DNA in excess of one or two chromosomes (Fig. 1E and H). These mutant cells resembled cells depleted of the DivL protein (36), and their phenotype was complemented by pMR20-divL (Fig. 1C, F, and I), indicating that divL510 is a recessive loss-of-function allele. The divL510 allele contains two point mutations, leading to the amino acid changes L740P and A140V, but the L740P mutation alone is sufficient to cause the observed phenotypes (data not shown).
FIG. 1.
The divL510 mutant is impaired in cell division and regulation of chromosome replication. DIC images of CB15N (A and D), divL510 (B and E), and divL510/pMR20-divL (C and F) cells grown at 28°C or 37°C for 4 h are shown. Bar, 2 μm. The DNA contents of CB15N (G), the divL510 mutant (H), and the divL510/pMR20-divL mutant (I) grown at 37°C for 4 h were measured by flow cytometry of SYTOX green-stained cells. The two dashed lines represent DNA contents of one (left) and two (right) chromosomes.
DivL promotes CtrA phosphorylation without affecting the CckA-ChpT phosphorelay.
The amount of CtrA∼P is reduced in a temperature-sensitive divL mutant (31), and the C-terminal kinase domain of DivL was shown to phosphorylate CtrA in vitro (51). However, the cckATS1 strain contains undetectable levels of CtrA∼P (18), suggesting that no other kinase can generate a significant amount of cellular CtrA∼P independent of CckA. To account for these data, DivL could act between DivK and CckA, blocking DivK-mediated downregulation of the CckA-ChpT phosphorelay. In this model, DivL and CckA both affect CtrA, but each is essential for viability because they act in the same signaling pathway.
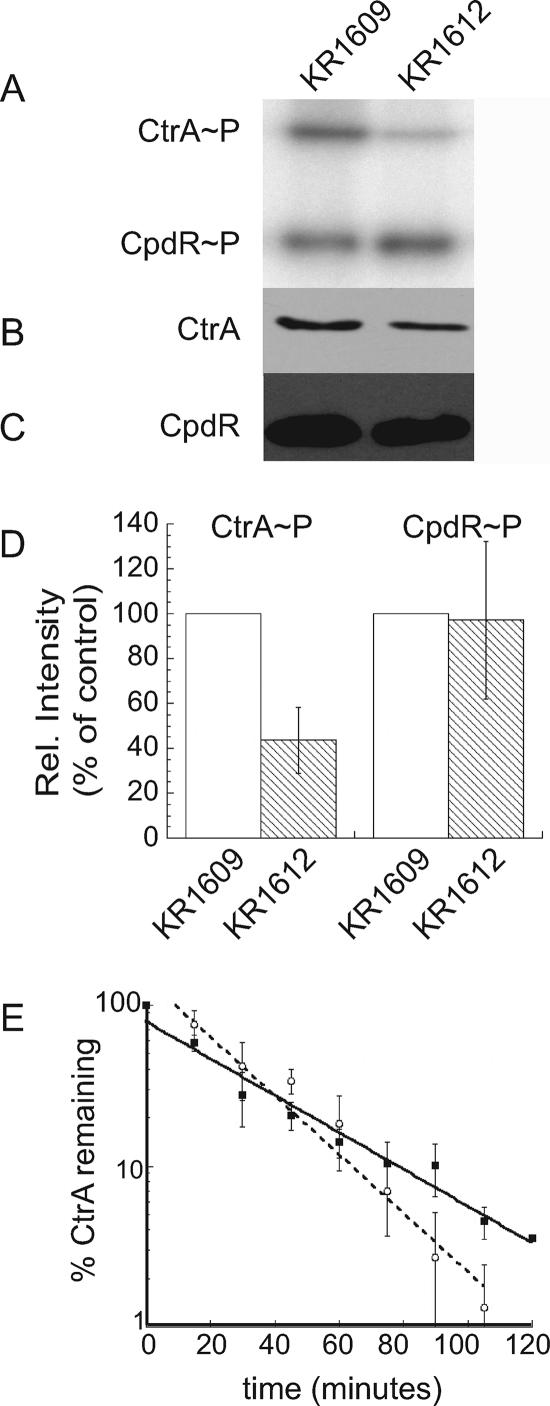
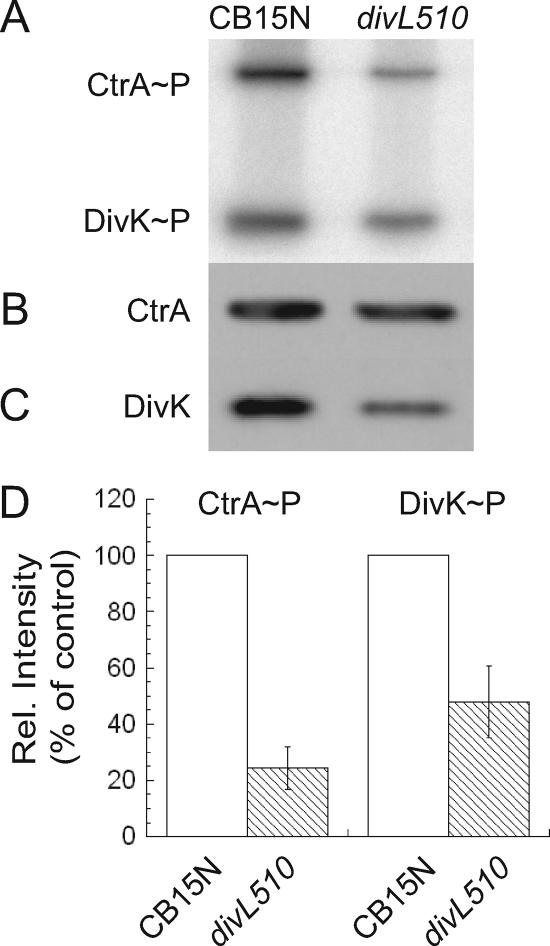
To determine if DivL acts upstream of CckA, we measured the phosphorylation of CtrA and CpdR simultaneously in strain CB15N and in cckATS1 (18) and divL510 mutants. We labeled cultures of each strain with [γ-32P]ATP and immunoprecipitated CtrA and CpdR-3×FLAG, using anti-CtrA (6) and anti-FLAG (Sigma) antibodies, respectively. In agreement with previous results (16, 18), cckATS1 cells contained undetectable levels of CtrA∼P and CpdR∼P, although both proteins were present (data not shown). CtrA∼P was reduced in divL510 cells to ∼40% of the wild-type level (Fig. 2A, B, and D). Surprisingly, however, divL510 cells contained a wild-type level of phosphorylated CpdR (Fig. 2A, C, and D). These data suggest that DivL specifically affects the amount of phosphorylated CtrA rather than modulating the activity of the entire CckA-ChpT phosphorelay.
FIG. 2.
DivL regulates the phosphorylation of CtrA but does not affect CpdR phosphorylation or CtrA stability. (A) In vivo phosphorylation was measured by immunoprecipitation of CtrA and CpdR from cells grown at 28°C and labeled for 4 min with [γ-32P]ATP. CtrA (B) and CpdR (C) protein levels were determined by Western blotting. For panels A to C, anti-CtrA and anti-FLAG sera were used to recognize CtrA and CpdR, respectively. Lane 1, KR1609 (CB15N/pMR20-cpdR::3×FLAG); lane 2, KR1612 (divL510/pMR20-cpdR::3×FLAG). (D) Ratios of phosphorylated to total protein were calculated for three independent experiments with strains KR1609 and KR1612 and normalized to the wild-type strain. Error bars represent standard deviations. (E) CB15N (open circles) and divL510 (closed squares) cells were grown for 4 h at 37°C, labeled for 5 min with [35S]methionine, and chased with excess methionine and Casamino Acids. Samples were withdrawn at the indicated times for immunoprecipitation of CtrA, followed by SDS-polyacrylamide gel electrophoresis and phosphorimager analysis. Three independent experiments were performed for each strain, and error bars represent standard deviations.
To confirm that normal CpdR∼P levels are maintained in divL510 cells, we measured the half-life of the CtrA protein in wild-type and divL510 cultures grown at the nonpermissive temperature (37°C). By phosphorylating CpdR, the CckA-ChpT phosphorelay regulates CtrA proteolysis, limiting it to specific times in the cell cycle. In previous studies, the half-life of CtrA was reduced 2.3-fold in a mutant lacking CckA (17) and 4-fold in a cpdR(D51A) mutant, in which CpdR is present but cannot be phosphorylated (16). In the divL510 strain, the half-life of CtrA was 25 ± 3 min, compared with 18 ± 3 min for the wild-type strain CB15N (Fig. 2E); thus, the rate of CtrA proteolysis is not increased in divL510 cells. Because CpdR phosphorylation is not reduced and CtrA proteolysis is not increased in the divL510 mutant, we infer that DivL selectively affects CtrA phosphorylation.
DivL performs a critical function other than regulating the level of CtrA∼P.
DivL clearly affects the cellular level of CtrA∼P, regardless of the molecular mechanism. Is DivL's effect upon CtrA phosphorylation its only function in Caulobacter? To answer this question, we examined the phenotypes of three mutant strains, the ΔctrA, ΔdivL ΔctrA, and ΔcckA ΔctrA mutants, with each carrying the high-copy-number plasmid pCtrAD51E (6). When overexpressed, the phosphomimetic protein CtrAD51E can replace wild-type CtrA, yielding a mild defect in cell division (17). If the only role of DivL is to regulate CtrA phosphorylation, then we would expect the ΔdivL ΔctrA/pCtrAD51E strain to have a phenotype similar to that of the ΔctrA/pCtrAD51E strain.
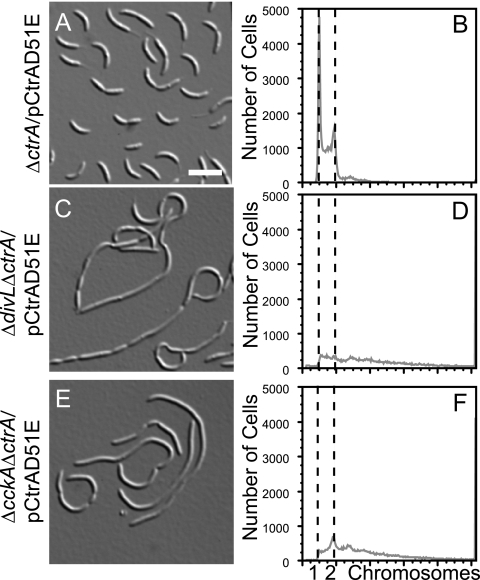
The divL gene could be deleted in a strain overexpressing CtrAD51E but not in a strain harboring pCtrA (6), which overexpresses the wild-type CtrA protein (data not shown). We deleted the wild-type divL gene in the ΔctrA/pCtrAD51E strain to generate the ΔdivL ΔctrA/pCtrAD51E strain, but the resulting cells were filamentous and stalkless and contained excess chromosomal DNA (Fig. 3C and D). This phenotype is much more severe than that of the ΔctrA/pCtrAD51E strain (Fig. 3A and B), indicating that the phosphomimetic protein CtrAD51E can rescue the lethality of the ΔdivL mutant but cannot fully compensate for the absence of the DivL protein. A similar result was observed for the histidine kinase CckA (17), where the ΔcckA ΔctrA/pCtrAD51E strain (Fig. 3E and F) was much more impaired in morphology and DNA content than the ΔctrA/pCtrAD51E strain (Fig. 3A and B). CckA was subsequently found to regulate CtrA stability via the response regulator CpdR, in addition to phosphorylating CtrA via ChpT (17). In the case of DivL, however, the half-life of CtrA was not reduced in the divL510 mutant, and CpdR was phosphorylated normally (Fig. 2). We therefore propose that DivL performs a second function critical for Caulobacter cell cycle progression in addition to promoting CtrA phosphorylation.
FIG. 3.
DivL performs a critical function in addition to regulating CtrA phosphorylation. DIC images and DNA contents of SYTOX green-labeled cells of the ΔctrA/pCtrAD51E (A and B), ΔdivL ΔctrA/pCtrAD51E (C and D), and ΔcckA ΔctrA/pCtrAD51E (E and F) strains are shown. In panels B, D, and F, the two dashed lines represent DNA contents of one (left) and two (right) chromosomes. Bar, 5 μm.
DivL is required for DivK localization.
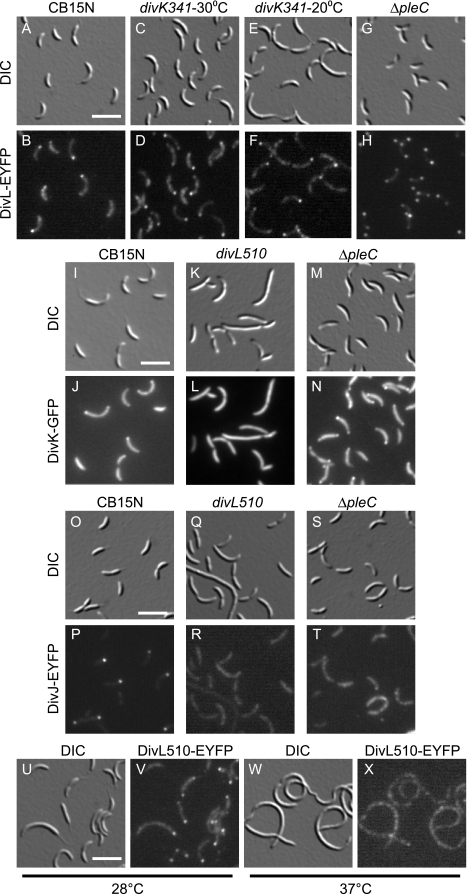
Although DivL seems not to regulate CckA, the isolation of divK suppressors in divL mutants suggests that DivL could still function downstream of DivK in a different signal transduction pathway. In this case, DivL localization may be altered in divK mutants. We fused the 3′ end of the divL coding region to the gene for EYFP (Clontech) and integrated this construct at the divL chromosomal locus so that divL::eyfp was the only source of DivL protein in the cell. As previously observed in wild-type cells (36), DivL-EYFP was located at a single pole or delocalized in swarmer cells and was located at the pole opposite the stalk in stalked and predivisional cells (Fig. 4A and B). In some predivisional cells, a second, dimmer focus of DivL-EYFP was visible at the stalked pole.
FIG. 4.
DivL is required for polar localization of DivK. DIC and fluorescence images of DivL-EYFP expressed from the chromosomal divL locus in CB15N (A and B), divK341 (4 h at 30°C) (C and D), divK341 (4 h at 20°C) (E and F), and ΔpleC (G and H) cells are shown at the top. DIC and fluorescence images of DivK-EGFP expressed from pMR20divK-EGFP in CB15N (I and J), divL510 (K and L), and ΔpleC (M and N) cells are shown next. DIC and fluorescence images of DivJ-YFP expressed from pMR20-divJ::yfp in CB15N (O and P), divL510 (Q and R), and ΔpleC (S and T) cells are shown below these. DIC and fluorescence images of DivL510-EYFP expressed from the chromosomal divL locus in cells grown for 4 h at 28°C (U and V) or 37°C (W and X) are shown at the bottom. Bar, 2 μm.
In the divK341 mutant grown at either the permissive temperature (30°C) (Fig. 4C and D) or the nonpermissive temperature (20°C) (Fig. 4E and F), DivL-EYFP localization was unchanged, with foci occurring primarily at the cell pole opposite the stalk. To determine the effect of DivK overexpression, we transformed the Pxyl-divK plasmid into the strain producing DivL-EYFP. When the cells were grown in medium containing either 0.1% dextrose or 0.3% xylose, the location of DivL-EYFP was unchanged (data not shown). Therefore, the localization of DivL does not depend on DivK.
Because a yeast two-hybrid screen indicated that DivL may interact physically with DivK (30) and because we saw no change in DivL localization in strains with altered DivK activity, we examined the location of DivK-EGFP expressed from pMR20divK-EGFP (19) in wild-type and divL510 cells. In the wild-type strain, DivK shuttles back and forth between the cell poles (27), so all cells contain some diffuse DivK-EGFP. In addition, stalked cells contain a focus of DivK-EGFP at the stalked pole, and predivisional cells contain foci at both poles (19). In contrast, DivK-EGFP was completely delocalized, even at the permissive temperature (28°C), in divL510 cells (Fig. 4K and L). These data suggest that for purposes of localization, DivL is functionally upstream of DivK.
DivL promotes DivK phosphorylation and may act as a polar binding site for DivK.
DivK must be phosphorylated to localize at the Caulobacter cell poles, and localization at the stalked pole specifically requires the histidine kinase DivJ (23). In the divL510 mutant, DivK may be delocalized because it is dephosphorylated, because polar binding proteins for DivK are nonfunctional, or both. To determine how DivL participates in DivK localization, we first measured DivK and CtrA phosphorylation simultaneously in wild-type and divL510 cells. In the divL510 mutant, DivK∼P was reduced to ∼50% of its wild-type level, and CtrA∼P was reduced to ∼25% of its wild-type level (Fig. 5A to D).
FIG. 5.
DivL promotes DivK phosphorylation. (A) In vivo phosphorylation was measured by immunoprecipitation of CtrA and DivK from cells grown at 28°C and labeled for 4 min with [γ-32P]ATP. CtrA (B) and DivK (C) protein levels were determined by Western blotting. For panels A to C, anti-CtrA and anti-DivK sera were used to recognize CtrA and DivK, respectively. Lane 1, KR684 (CB15N); lane 2, KR635 (divL510). (D) Ratios of phosphorylated to total protein were calculated for three independent experiments with KR684 and KR635 and were normalized to the wild-type strain. Error bars represent standard deviations.
We next examined the location of DivJ, a known polar binding protein for DivK, in the divL510 mutant. In wild-type cells, DivJ-YFP expressed from a low-copy-number plasmid was delocalized in swarmer cells and was located at the stalked pole of stalked and predivisional cells (Fig. 4O and P). In divL510 cells at the permissive temperature (28°C), DivJ-YFP was delocalized (Fig. 4Q and R). Wild-type DivL may therefore promote the polar localization of DivK by increasing cellular levels of DivK∼P and by promoting DivJ localization.
Although DivJ is known to recruit DivK to the stalked pole, DivK binding proteins at the pole opposite the stalk have not been identified. The histidine kinase PleC dephosphorylates DivK (27, 48), is located at the pole opposite the stalk (48), and is required to release DivK from that pole following cell division (19). In a ΔpleC mutant, the proportion of cells with bipolar DivK-EGFP was increased (Fig. 4M and N) (19), but DivJ was delocalized (Fig. 4S and T) (48), suggesting that proteins other than DivJ and PleC can interact with DivK at the cell poles. DivL-GFP expressed from a plasmid was reported to localize normally in the pleC301::Tn5 mutant (36). In contrast, we found that ΔpleC cells contained bipolar DivL-EYFP (Fig. 4G and H), while wild-type cells chiefly contained a single focus of DivL-EYFP at the pole opposite the stalk (Fig. 4A and B). The bipolar localization of DivK and DivL in ΔpleC cells, where DivK phosphorylation is increased, PleC is absent, and DivJ is delocalized, suggests that DivL recruits DivK to both poles in this mutant and may bind DivK to the pole opposite the stalk in wild-type cells.
Since DivK is delocalized in divL510 cells, we fused the DivL510 protein to EYFP at the chromosomal divL locus and verified that the resulting strain was temperature sensitive. In contrast to the wild-type protein, DivL510-EYFP at the permissive temperature (28°C) was often located at both cell poles or at the stalked pole only (Fig. 4U and V) rather than at the pole opposite the stalk (Fig. 4A and B). At the nonpermissive temperature (37°C), DivL510-EYFP was located in patches throughout the cell. Thus, if DivL acts as a localization factor for DivK, then for DivL510 at the permissive temperature this function must be perturbed, delocalizing DivK while still allowing DivL510 to reach one or both cell poles.
The phosphorylation site and kinase domain of DivL are not essential for viability.
Since DivL promotes the phosphorylation of CtrA and DivK in vivo and the kinase domain of DivL phosphorylates CtrA in vitro (51), we performed several experiments to determine if the essential function of DivL is its kinase activity. In two previous studies, the role of the phosphorylation site residue Y550 was investigated by replacing this amino acid with phenylalanine [divL(Y550F)] and determining whether this allele complements the growth and cell division defects of the temperature-sensitive divL356 strain (41). Both studies concluded that DivLY550F could not complement the filamentation of the divL356 mutant, but one study found that DivLY550F partially complemented the growth defect of the divL356 mutant at 37°C (51). Histidine kinases dimerize, and each monomer within the dimer phosphorylates the conserved histidine residue of the other monomer (reviewed in reference 46). Partial complementation in the latter experiment could have resulted from heterodimers in which the functional kinase domain of DivLY550F phosphorylated Y550 of the DivL356 protein.
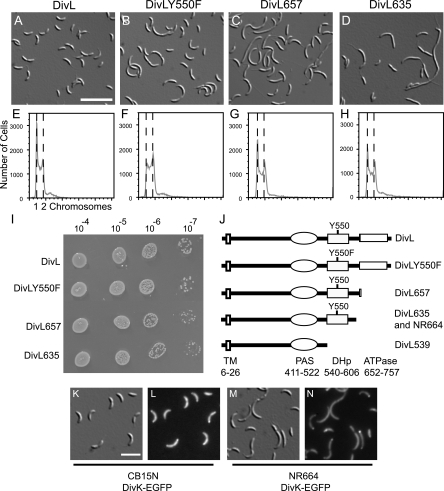
To resolve this issue, we examined the ability of divL(Y550F) to complement a deletion of divL rather than a conditional allele. In CB15N, we expressed DivLY550F from a low-copy-number plasmid using the divL promoter (pMR20-divLY550F) and then attempted to replace the chromosomal copy of divL with a spectinomycin/streptomycin resistance cassette (ΔdivL), using a two-step recombination protocol. In a control strain containing the empty vector pMR20, we were unable to replace the wild-type divL allele with a ΔdivL mutation, in agreement with previous reports showing that divL is essential for Caulobacter viability (51). However, we successfully replaced the chromosomal divL gene in a strain containing pMR20-divLY550F (Fig. 6B). The resulting cells were impaired in cell division, with an average length two to three times greater than that for a control population containing pMR20-divL (Fig. 6A), and were often stalkless. Flow cytometry experiments measuring chromosomal DNA content revealed that cells of both strains contained primarily one or two chromosomes (Fig. 6E and F). In contrast, when cells are depleted of the DivL protein by making its transcription dependent upon a xylose-inducible promoter, the cells become filamentous and accumulate additional DNA (36). Thus, a tyrosine residue at position 550 is necessary for normal cell division and stalk biogenesis but is not strictly required for Caulobacter viability.
FIG. 6.
DivL variants impaired in kinase activity or phosphoryl transfer support Caulobacter viability and DivK localization. DIC images (A to D) and DNA contents of SYTOX green-stained cells (E to H) are shown for ΔdivL strains expressing the following proteins from pMR20: DivL (KR1773) (A and E), DivLY550F (KR1775) (B and F), DivL657 (KR1776) (C and G), and DivL635 (KR1777) (D and H). (I) Growth of ΔdivL strains expressing the indicated proteins from pMR20. Serial dilutions of exponential-growth-phase cultures at an optical density at 660 nm of 0.25 were made, and 5 microliters of each indicated dilution was spotted onto a PYE plate and incubated for 2 days at 30°C. Three independent experiments were performed, and a representative plate is shown. (J) Diagram of the DivL protein, including the predicted transmembrane (TM), Per-ARNT-Sim (PAS), DHp, and catalytic (ATPase) domains. Y550 represents the site of phosphorylation, and numbers indicate amino acid residues. Mutated and truncated DivL proteins used in this study are depicted, and the DivL635 protein is truncated at the site of the Tn5 insertion in strain NR664. (K to N) DIC and fluorescence images of DivK-EGFP expressed from the chromosomal divK locus in CB15N (KR2047) (K and L) and NR664 (KR2046) (M and N). Bar, 5 μm (A to D) or 2 μm (K to N).
Conditional alleles of divL have been isolated in screens for point mutations that suppress the motility defect of pleC mutants (41). Since divL is essential for viability, however, we were surprised to obtain motile pleC suppressors containing transposon insertions in the divL open reading frame in a separate screen (see Materials and Methods). In all cases, the transposon insertions interrupted the catalytic domain of DivL downstream of the Y550 phosphorylation site. Spurred by these results, we constructed three divL truncations, expressed each truncated protein from pMR20, and attempted to delete the chromosomal divL gene in these strains. Two of the truncations, divL635 and divL657, corresponded to transposon insertion sites, while the third truncation, divL539, was designed to remove both the DHp and ATPase domains of DivL (Fig. 6J).
We isolated viable strains containing the ΔdivL mutation and expressing the truncated proteins DivL657 and DivL635, but not DivL539. DivL539 contains a C-terminal 3×FLAG tag which can be detected using an anti-FLAG antibody on Western blots (8). CB15N cells containing pMR20-divL539 produced a FLAG-tagged protein of the correct size (data not shown), suggesting that this protein is produced in Caulobacter but is unable to support life in the absence of the full-length divL gene. The divL657 and divL635 mutants supported viability, but each truncation yielded slower-growing cells (Fig. 6I) with a mixture of lengths (Fig. 6C and D). While ΔdivL/pMR20-divLY550F cells were stalkless (Fig. 6B), ΔdivL/pMR20-divL635 cells often had elongated stalks (Fig. 6D). Again, despite the morphology defects, cells expressing each truncated DivL protein had normal DNA contents, containing primarily one or two chromosomes (Fig. 6G and H).
These findings are at odds with a previous study (36) in which a DivL-GFP fusion protein lacking the C-terminal 23 amino acids of DivL was incapable of complementing the temperature-sensitive lethality of the divL346 mutant. Our experiment is different in that the truncated DivL proteins tested were not GFP fusions, and we assayed complementation of a divL deletion rather than a temperature-sensitive mutation. We are confident that the ATPase domain of DivL is dispensable for viability, however, because two separate techniques, transposon mutagenesis and deletion analysis, gave similar results. The phenotypes of our divL alleles indicate that DivL catalytic activity and phosphorylation at Y550 are not required for viability. Thus, DivL performs an essential function other than autophosphorylation or phosphoryl transfer to a response regulator.
Since DivL participates in localizing DivK to the cell poles, we assayed DivK-EGFP localization in the mutant NR664, which contains a Tn5 element inserted in divL after codon 635 and which was the basis for the DivL635 truncation. In the wild-type background, DivK-EGFP expressed from the chromosomal divK locus was delocalized in swarmer cells and present at the stalked pole (31%) or at both poles (65%) of cells with a polar stalk (n = 250) (Fig. 6K and L), in agreement with previous results using DivK-EGFP expressed from a low-copy-number plasmid (19). In the divL::Tn5 mutant NR664, DivK-EGFP was located at the stalked pole (54%) or both poles (34%) of cells that possessed a polar stalk (n = 160) (Fig. 6M and N). Since localization of DivK was shifted toward the stalked pole in NR664 but was not abolished, we concluded that the catalytic domain of DivL, and therefore DivL kinase activity, is not strictly required for polar localization of DivK.
DISCUSSION
Our studies have revealed unexpected phenotypes of divL mutants that prompt reconsideration of the biochemical function of DivL and indicate that DivL performs more than one role in Caulobacter cell cycle regulation. Although divL is essential for viability (51) and depletion of DivL causes chromosome accumulation (36), removal of the DivL catalytic domain or replacement of its phosphorylation site tyrosine residue with phenylalanine (Y550F) caused moderate effects on cell morphology and had no effect on chromosome content. In contrast, replacement of the phosphorylation site histidine residue in PleC or DivJ with alanine mimics the effect of deleting pleC or divJ, respectively (23, 44). DivL is therefore unusual in that it performs a critical function other than phosphoryl transfer to or from a response regulator. The DivL variants that cannot autophosphorylate or participate in phosphoryl transfer are not perfect substitutes for wild-type DivL. At present, we cannot distinguish between two models to explain these results. DivL may phosphorylate CtrA or another response regulator at a low level required for precise control of cell cycle progression, or the catalytic domain and Y550 residue may be required to create an optimal conformation for interaction with other proteins.
Two other systems provide examples of histidine kinases that function solely or partly via protein-protein interactions. First, the NifL protein regulates the expression of genes for nitrogen fixation in response to redox and fixed nitrogen status (reviewed in reference 26). NifL contains the conserved DHp and catalytic domains of histidine kinases and binds adenine nucleotides (40), but amino acid substitutions at the conserved phosphorylation site, H304, do not disable NifL (49). Rather than phosphorylating its partner protein, the transcriptional regulator NifA, NifL blocks the transcription of genes for nitrogen fixation under the appropriate conditions by sequestering NifA in a complex (28). In the second example, the hybrid histidine kinase RpfC of Xanthomonas campestris performs two distinct functions, namely, regulating virulence factor production via phosphoryl transfer to the cognate response regulator RpfG and regulating synthesis of the cell-cell communication signal DSF by a protein-protein interaction between the RpfC receiver domain and the enzyme RpfF (12).
We show that the divL510 mutation reduces CtrA phosphorylation but not CtrA stability, suggesting that DivL affects CtrA by a pathway independent of CckA-ChpT (Fig. 7). The last 299 amino acids of DivL, comprising the DHp and catalytic domains, can phosphorylate CtrA in vitro (51), but the cckATS1 (18) and ctrAΔ3M2 ΔcckA/pCtrAD51E (17) strains contain no residual phosphorylation on the CtrA and CtrAΔ3M2 proteins, respectively. Furthermore, the ΔcckA/pCtrAD51E and ΔctrA ΔcckA/pCtrAD51E strains are equally impaired in cell division, chromosome content, and gene expression, suggesting that in the absence of CckA, the wild-type CtrA protein in the former strain is not phosphorylated to provide additional CtrA function (17). Thus, DivL may provide a low level of CtrA phosphorylation that is below the limit of detection in vivo, but we favor a model in which DivL regulates CtrA phosphorylation indirectly, perhaps by protecting CtrA∼P from dephosphorylation. Regardless of the mechanism by which DivL promotes CtrA phosphorylation, this activity is not DivL's only function in Caulobacter, since the phosphomimetic protein CtrAD51E does not complement the ΔdivL ΔctrA double mutant as well as it complements the ΔctrA mutant alone.
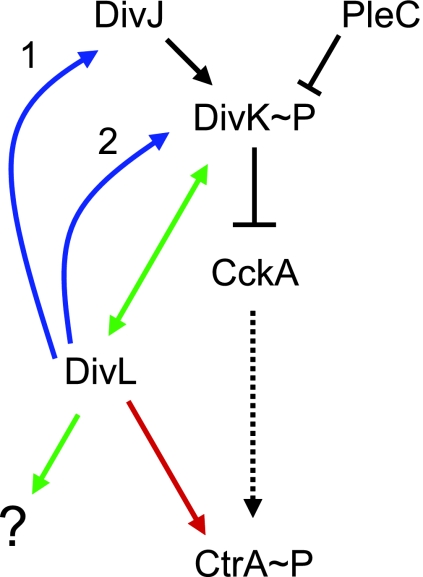
FIG. 7.
Model of DivL and DivK activities in Caulobacter cell cycle regulation. Black connectors represent interactions shown in other studies. The dotted arrow connecting CckA and CtrA represents the branched phosphorelay including ChpT and CpdR. Colored connectors represent interactions inferred from this work. DivJ phosphorylates DivK and binds to DivK at the stalked pole of the cell. PleC resides at the flagellated pole of the cell and dephosphorylates DivK. When phosphorylated, DivK decreases cellular CtrA activity by downregulating the CckA-ChpT phosphorelay, leading to CtrA dephosphorylation and proteolysis. DivL positively regulates CtrA phosphorylation by a mechanism independent of CckA and ChpT (red arrow). DivL promotes DivK localization by regulating DivJ localization and activity (blue arrow 1) and/or by binding directly to DivK at the flagellated pole (blue arrow 2). In concert with DivK, DivL regulates cell cycle processes other than CtrA activity (green arrows).
Our protein localization and in vivo phosphorylation results indicate that DivK function is also compromised in the divL510 mutant. Even at the permissive temperature, DivK phosphorylation is reduced and DivK-EGFP is delocalized in divL510 cells. DivL could affect DivK by a combination of mechanisms. First, it is formally possible that DivL phosphorylates DivK directly. In this scenario, the relative dephosphorylation of DivK in divL510 cells causes its delocalization. However, in a previous in vitro study, the DivL kinase domain preferentially phosphorylated CtrA over DivK in reaction mixtures containing all three proteins (51). Furthermore, since the ATPase domain of DivL is not absolutely required for DivK localization but phosphorylation of DivK is itself needed for localization (23), it is unlikely that DivL is a significant source of DivK∼P in the cell. Second, DivL could modulate DivK function by promoting the polar localization and activity of the DivJ histidine kinase (Fig. 7, blue arrow 1). DivJ is normally located at the stalked pole but is delocalized in divL510 cells. Since DivJ phosphorylates DivK and recruits DivK to the stalked pole (23, 48), its impaired function could account for the observed effects on DivK phosphorylation and, thus, localization. At present, however, we cannot determine if phosphoryl transfer from DivJ to DivK is impaired in divL510 cells. Finally, DivL may function as a binding site for DivK at the pole opposite the stalk (Fig. 7, blue arrow 2). In wild-type predivisional cells, foci of DivK are present at both poles. DivJ is thought to bind DivK at the stalked pole (24), but a comparable DivK binding protein at the flagellated pole has not been identified. DivL could perform this function, since it is located at the flagellated pole. In our model, DivK does not concentrate at the flagellated pole of divL510 cells because its interaction with DivL510 is impaired, nor is DivK sequestered at the stalked pole because DivJ is also delocalized. Consistent with this idea, in ΔpleC cells, where DivK∼P is elevated and DivJ is delocalized (48), bipolar DivK localization may be mediated by DivL, whose location is shifted from the flagellated pole to both poles.
How does the divL510 mutation suppress the temperature-sensitive growth and motility defects of divK341 cells? We infer that divK341 is a loss-of-function mutation because it is recessive (13) and its G1 cell cycle arrest at the nonpermissive temperature (15) is distinct from the chromosome accumulation caused by divK overexpression (3). Since DivK activity is also reduced in divL510 cells, the divL510 mutation is not likely to suppress divK341 by a direct action on DivK. Instead, the divL510 mutation could suppress divK341 by reducing the amount of CtrA∼P in the cell, acting parallel to DivK (Fig. 7, red arrow).
It is paradoxical that DivL promotes DivK phosphorylation and localization yet does not appear to modulate the CckA-ChpT phosphorelay. How can DivL alter DivK activity without affecting downstream events? We propose a branch point in the signal transduction network, at which DivK can either downregulate the CckA-ChpT pathway (Fig. 7, black bar) or act in concert with DivL to affect other cellular processes (Fig. 7, green arrows). Alternatively, it is possible that the reduction in DivK∼P in divL510 cells is too modest to affect CckA.
Finally, it is unusual that mutations in divL have been isolated as suppressors of both divJ and pleC mutants (31, 41; this work), when DivJ and PleC have opposing effects on DivK phosphorylation. One model to explain these results is that distinct divL mutations increase or decrease DivL's propensity to interact with DivK∼P. Histidine kinase dimers are believed to cycle between two states to accommodate dual activities, i.e., autophosphorylation and phosphoryl transfer (25). During autophosphorylation, the DHp domain (including the phosphorylation site histidine residue) of one monomer interacts with the ATPase domain of the other monomer. During phosphoryl transfer, the ATPase domain must move to allow interaction between the phosphorylated histidine and a response regulator receiver domain. Since the DHp domain of DivL specifically interacted with DivK in a yeast two-hybrid screen (30), various divL mutations could promote or block an interaction between this domain and DivK. This model predicts that divL mutants isolated as pleC suppressors will not suppress divJ and divK mutant phenotypes, and vice versa. It also predicts that purified DivL proteins with different amino acid substitutions or truncations will have different affinities for DivK in vitro. We are pursuing studies to characterize the interaction between DivL and DivK and to identify additional processes regulated by these essential signal transduction proteins.
Acknowledgments
We thank Michael Laub and Jeff Skerker for pNPTS-cc1063-YFP, helpful discussions, and critical readings of the manuscript, and we thank Christine Jacobs-Wagner for DivK antiserum.
This work was supported by NIH grant GM032506 to Lucy Shapiro, by startup funds provided by Case Western Reserve University School of Medicine and the Mount Sinai Health Care Foundation to P.V., and by NSF grant 0543801 to K.R.
Footnotes
Published ahead of print on 7 September 2007.
REFERENCES
- 1.Aldridge, P., R. Paul, P. Goymer, P. Rainey, and U. Jenal. 2003. Role of the GGDEF regulator PleD in polar development of Caulobacter crescentus. Mol. Microbiol. 47:1695-1708. [DOI] [PubMed] [Google Scholar]
- 2.Alley, M. R., S. L. Gomes, W. Alexander, and L. Shapiro. 1991. Genetic analysis of a temporally transcribed chemotaxis gene cluster in Caulobacter crescentus. Genetics 129:333-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biondi, E. G., S. J. Reisinger, J. M. Skerker, M. Arif, B. S. Perchuk, K. R. Ryan, and M. T. Laub. 2006. Regulation of the bacterial cell cycle by an integrated genetic circuit. Nature 444:899-904. [DOI] [PubMed] [Google Scholar]
- 4.Chen, J. C., and J. Beckwith. 2001. FtsQ, FtsL and FtsI require FtsK, but not FtsN, for colocalization with FtsZ during Escherichia coli cell division. Mol. Microbiol. 42:395-413. [DOI] [PubMed] [Google Scholar]
- 5.Chen, J. C., A. K. Hottes, H. H. McAdams, P. T. McGrath, P. H. Viollier, and L. Shapiro. 2006. Cytokinesis signals truncation of the PodJ polarity factor by a cell cycle-regulated protease. EMBO J. 25:377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domian, I. J., K. C. Quon, and L. Shapiro. 1997. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell 90:415-424. [DOI] [PubMed] [Google Scholar]
- 7.Ebersbach, G., and C. Jacobs-Wagner. 2007. Exploration into the spatial and temporal mechanisms of bacterial polarity. Trends Microbiol. 15:101-108. [DOI] [PubMed] [Google Scholar]
- 8.Einhauer, A., and A. Jungbauer. 2001. The FLAG peptide, a versatile fusion tag for the purification of recombinant proteins. J. Biochem. Biophys. Methods 49:455-465. [DOI] [PubMed] [Google Scholar]
- 9.Ely, B. 1991. Genetics of Caulobacter crescentus. Methods Enzymol. 204:372-384. [DOI] [PubMed] [Google Scholar]
- 10.Ely, B., and R. C. Johnson. 1977. Generalized transduction in Caulobacter crescentus. Genetics 87:391-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evinger, M., and N. Agabian. 1977. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J. Bacteriol. 132:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He, Y.-W., C. Wang, L. Zhou, H. Song, J. M. Dow, and L.-H. Zhang. 2006. Dual signaling functions of the hybrid sensor kinase RpfC of Xanthomonas campestris involve either phosphorelay or receiver domain-protein interaction. J. Biol. Chem. 281:33414-33421. [DOI] [PubMed] [Google Scholar]
- 13.Hecht, G. B., T. Lane, N. Ohta, J. M. Sommer, and A. Newton. 1995. An essential single domain response regulator required for normal cell division and differentiation in Caulobacter crescentus. EMBO J. 14:3915-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtzendorff, J., D. Hung, P. Brende, A. Reisenauer, P. H. Viollier, H. H. McAdams, and L. Shapiro. 2004. Oscillating global regulators control the genetic circuit driving a bacterial cell cycle. Science 304:983-987. [DOI] [PubMed] [Google Scholar]
- 15.Hung, D., and L. Shapiro. 2002. A signal transduction protein cues proteolytic events critical to Caulobacter cell cycle progression. Proc. Natl. Acad. Sci. USA 99:13160-13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iniesta, A. A., P. T. McGrath, A. Reisenauer, H. H. McAdams, and L. Shapiro. 2006. A phospho-signaling pathway controls the localization and activity of a protease complex critical for bacterial cell cycle progression. Proc. Natl. Acad. Sci. USA 103:10935-10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs, C., N. Ausmees, S. J. Cordwell, L. Shapiro, and M. T. Laub. 2003. Functions of the CckA histidine kinase in Caulobacter cell cycle control. Mol. Microbiol. 47:1279-1290. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs, C., I. J. Domian, J. R. Maddock, and L. Shapiro. 1999. Cell cycle-dependent polar localization of an essential bacterial histidine kinase that controls DNA replication and cell division. Cell 97:111-120. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs, C., D. Hung, and L. Shapiro. 2001. Dynamic localization of a cytoplasmic signal transduction response regulator controls morphogenesis during the Caulobacter cell cycle. Proc. Natl. Acad. Sci. USA 98:4095-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs, M. A., A. Alwood, I. Thaipisuttikul, D. Spencer, E. Haugen, R. Kaul, C. Raymond, R. Levy, L. Chun-Rong, D. Guenther, D. Bovee, M. V. Olson, and C. Manoil. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 100:14339-14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judd, E. M., K. R. Ryan, W. E. Moerner, L. Shapiro, and H. H. McAdams. 2003. Fluorescence bleaching reveals asymmetric compartment formation prior to cell division in Caulobacter. Proc. Natl. Acad. Sci. USA 100:8235-8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly, A. J., M. J. Sackett, N. Din, E. Quardokus, and Y. V. Brun. 1998. Cell cycle-dependent transcriptional and proteolytic regulation of FtsZ in Caulobacter. Genes Dev. 12:880-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam, H., J.-Y. Matroule, and C. Jacobs-Wagner. 2003. The asymmetric spatial distribution of bacterial signal transduction proteins coordinates cell cycle events. Dev. Cell 5:149-159. [DOI] [PubMed] [Google Scholar]
- 24.Laub, M. T., S. L. Chen, L. Shapiro, and H. H. McAdams. 2002. Genes directly controlled by CtrA, a master regulator of the Caulobacter cell cycle. Proc. Natl. Acad. Sci. USA 99:4632-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marina, A., C. D. Waldburger, and W. A. Hendrickson. 2005. Structure of the entire cytoplasmic portion of a sensor histidine-kinase protein. EMBO J. 24:4247-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Argudo, I., R. Little, N. Shearer, P. Johnson, and R. Dixon. 2004. The NifL-NifA system: a multidomain transcriptional regulatory complex that integrates environmental signals. J. Bacteriol. 186:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matroule, J.-Y., H. Lam, D. T. Burnette, and C. Jacobs-Wagner. 2004. Cytokinesis monitoring during development: rapid pole-to-pole shuttling of a signaling protein by localized kinase and phosphatase in Caulobacter. Cell 118:579-590. [DOI] [PubMed] [Google Scholar]
- 28.Money, T., T. Jones, R. Dixon, and S. Austin. 1999. Isolation and properties of the complex between the enhancer binding protein NIFA and the sensor NIFL. J. Bacteriol. 181:4461-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nierman, W. C., T. V. Feldblyum, M. T. Laub, I. T. Paulsen, K. E. Nelson, J. Eisen, J. F. Heidelberg, M. R. Alley, N. Ohta, J. R. Maddock, I. Potocka, W. C. Nelson, A. Newton, C. Stephens, N. D. Phadke, B. Ely, R. T. DeBoy, R. J. Dodson, A. S. Durkin, M. L. Gwinn, D. H. Haft, J. F. Kolonay, J. Smit, M. B. Craven, H. Khouri, J. Shetty, K. Berry, T. Utterback, K. Tran, A. Wolf, J. Vamathevan, M. Ermolaeva, O. White, S. L. Salzberg, J. C. Venter, L. Shapiro, and C. M. Fraser. 2001. Complete genome sequence of Caulobacter crescentus. Proc. Natl. Acad. Sci. USA 98:4136-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohta, N., and A. Newton. 2003. The core dimerization domains of histidine kinases contain recognition specificity for the cognate response regulator. J. Bacteriol. 185:4424-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce, D. L., D. S. O'Donnol, R. C. Allen, J. W. Javens, E. M. Quardokus, and Y. V. Brun. 2006. Mutations in DivL and CckA rescue a divJ null mutant of Caulobacter crescentus by reducing the activity of CtrA. J. Bacteriol. 188:2473-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 33.Quon, K. C., B. Yang, I. J. Domian, L. Shapiro, and G. T. Marczynski. 1998. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc. Natl. Acad. Sci. USA 95:120-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts, R. C., C. D. Mohr, and L. Shapiro. 1996. Developmental programs in bacteria. Curr. Top. Dev. Biol. 34:207-257. [DOI] [PubMed] [Google Scholar]
- 35.Sciochetti, S. A., T. Lane, N. Ohta, and A. Newton. 2002. Protein sequences and cellular factors required for polar localization of a histidine kinase in Caulobacter crescentus. J. Bacteriol. 184:6037-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sciochetti, S. A., N. Ohta, and A. Newton. 2005. The role of polar localization in the function of an essential Caulobacter crescentus tyrosine kinase. Mol. Microbiol. 56:1467-1480. [DOI] [PubMed] [Google Scholar]
- 37.Simon, R., U. Prieffer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology 1:784-790. [Google Scholar]
- 38.Skerker, J. M., and M. T. Laub. 2004. Cell-cycle progression and the generation of asymmetry in Caulobacter crescentus. Nat. Rev. Microbiol. 2:325-337. [DOI] [PubMed] [Google Scholar]
- 39.Skerker, J. M., M. S. Prasol, B. S. Perchuk, E. G. Biondi, and M. T. Laub. 2005. Two-component signal transduction pathways regulating growth and cell cycle progression in a bacterium: a systems-level analysis. PLoS Biol. 3:e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soderback, E., F. Reyes-Ramirez, T. Eydmann, S. Austin, S. Hill, and R. Dixon. 1998. The redox- and fixed nitrogen-responsive regulatory protein NIFL from Azotobacter vinelandii comprises discrete flavin and nucleotide-binding domains. Mol. Microbiol. 28:179-192. [DOI] [PubMed] [Google Scholar]
- 41.Sommer, J. M., and A. Newton. 1991. Pseudoreversion analysis indicates a direct role of cell division genes in polar morphogenesis and differentiation in Caulobacter crescentus. Genetics 129:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spratt, B. G., P. J. Hedge, S. te Heesen, A. Edelman, and J. K. Broome-Smith. 1986. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene 41:337-342. [DOI] [PubMed] [Google Scholar]
- 43.Viollier, P. H., and L. Shapiro. 2003. A lytic transglycosylase homologue, PleA, is required for the assembly of pili and the flagellum at the Caulobacter crescentus cell pole. Mol. Microbiol. 49:331-345. [DOI] [PubMed] [Google Scholar]
- 44.Viollier, P. H., N. Sternheim, and L. Shapiro. 2002. A dynamically localized histidine kinase controls the asymmetric distribution of polar pili proteins. EMBO J. 21:4420-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viollier, P. H., M. Thanbichler, P. T. McGrath, L. West, M. Meewan, H. H. McAdams, and L. Shapiro. 2004. Rapid and sequential movement of individual chromosomal loci to specific subcellular locations during bacterial DNA replication. Proc. Natl. Acad. Sci. USA 101:9257-9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West, A. H., and A. M. Stock. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26:369-376. [DOI] [PubMed] [Google Scholar]
- 47.West, L., D. Yang, and C. Stephens. 2002. Use of the Caulobacter crescentus genome sequence to develop a method for systematic genetic mapping. J. Bacteriol. 184:2155-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wheeler, R. T., and L. Shapiro. 1999. Differential localization of two histidine kinases controlling bacterial cell differentiation. Mol. Cell 4:683-694. [DOI] [PubMed] [Google Scholar]
- 49.Woodley, P., and M. Drummond. 1994. Redundancy of the conserved His residue in Azotobacter vinelandii NifL, a histidine autoinase homologue which regulates transcription of nitrogen fixation genes. Mol. Microbiol. 13:619-626. [DOI] [PubMed] [Google Scholar]
- 50.Wu, J., N. Ohta, and A. Newton. 1998. An essential, multicomponent signal transduction pathway required for cell cycle regulation in Caulobacter. Proc. Natl. Acad. Sci. USA 95:1443-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu, J., N. Ohta, J.-L. Zhao, and A. Newton. 1999. A novel bacterial tyrosine kinase essential for cell division and differentiation. Proc. Natl. Acad. Sci. USA 96:13068-13073. [DOI] [PMC free article] [PubMed] [Google Scholar]