Abstract
Bordetellae are respiratory pathogens that infect both humans and animals. Bordetella bronchiseptica establishes asymptomatic and long-term to life-long infections of animal nasopharynges. While the human pathogen Bordetella pertussis is the etiological agent of the acute disease whooping cough in infants and young children, it is now being increasingly isolated from the nasopharynges of vaccinated adolescents and adults who sometimes show milder symptoms, such as prolonged cough illness. Although it has been shown that Bordetella can form biofilms in vitro, nothing is known about its biofilm mode of existence in mammalian hosts. Using indirect immunofluorescence and scanning electron microscopy, we examined nasal tissues from mice infected with B. bronchiseptica. Our results demonstrate that a wild-type strain formed robust biofilms that were adherent to the nasal epithelium and displayed architectural attributes characteristic of a number of bacterial biofilms formed on inert surfaces. We have previously shown that the Bordetella Bps polysaccharide encoded by the bpsABCD locus is critical for the stability and maintenance of three-dimensional structures of biofilms. We show here that Bps is essential for the formation of efficient nasal biofilms and is required for the colonization of the nose. Our results document a biofilm lifestyle for Bordetella in mammalian respiratory tracts and highlight the essential role of the Bps polysaccharide in this process and in persistence of the nares.
Bacteria belonging to the genus Bordetella cause respiratory tract infections in both humans and animals (42). Bordetella pertussis is the etiological agent of pertussis, cases of which are steadily increasing in number, even in vaccinated populations (9). It has been proposed that the resurgence of pertussis is due in part to carriage within adolescent and adult populations because of waning immunity (3, 4, 9). Bordetella bronchiseptica has a broad host range and naturally infects a wide variety of nonhuman animals. It typically establishes asymptomatic infections but can cause atrophic rhinitis in pigs, kennel cough in dogs, snuffles in rabbits, and bronchopneumonia in guinea pigs (18, 42).
B. bronchiseptica is capable of establishing a chronic and asymptomatic infection and can be harvested from the nasal cavities of rats and mice for extended periods (1, 37). A convincing and frequently proposed hypothesis to explain long-term carriage is the ability of microorganisms to exist as biofilms. Bacterial biofilms are increasingly recognized as important contributors to chronic or persistent diseases. A biofilm is generally defined as a surface-attached population of one or more types of bacteria encased in a polymeric matrix, which can be composed of a number of different macromolecules, including nucleic acids, proteins, and polysaccharides (5). Numerous studies have documented the ability of biofilm bacteria to be recalcitrant to antibiotic treatments and to the host immune system (31, 39, 40, 53).
We and others have recently demonstrated the ability of the three classical Bordetella species (B. pertussis, B. bronchiseptica, and B. parapertussis) to form biofilms on abiotic surfaces (27, 45, 50). It has been hypothesized that Bordetella biofilm formation may play a role in the pathogenic cycle, specifically in persistence within the nasopharynx (29, 46).
Confocal scanning laser microscopy (CSLM) of nasal tissues harvested from mice infected with these bacteria revealed multilayer clusters of sessile bacterial communities that exhibited distinct architectural features. Scanning electron microscopy (SEM) further revealed the presence of multicellular communities adhered to the ciliated epithelium which appeared to be encased in an opaque matrix-like material.
Although extracellular polysaccharides have been shown to be required for one or more of the steps that lead to in vitro biofilm development (5), clear visualization of a biofilm-associated polysaccharide and direct genetic evidence for the involvement of polysaccharides in the respiratory tract are lacking. We have recently demonstrated the involvement of a polysaccharide locus, bpsABCD, in the formation or stabilization of the three-dimensional architecture of mature biofilms formed by B. bronchiseptica (46). The Bps polysaccharide is antigenically and biochemically similar to the poly-β-1,6-N-acetylglucosamine (called PIA, PNAG, or PGA) group of polysaccharides produce by diverse bacterial species (22, 24, 34, 44, 52, 54). We demonstrate here that in vivo, Bordetella biofilms are characterized by extrusion of the Bps polysaccharide. We compared the abilities of a wild-type B. bronchiseptica strain and an isogenic mutant derivative (Δbps) to form biofilms in the nose. In contrast to the wild-type strain, the Δbps strain was able to neither form robust biofilms nor persist within the nasal cavity of mice at a later time point. The data thus demonstrate the in vivo biofilm mode of existence for B. bronchiseptica and implicate the Bps polysaccharide in efficient biofilm formation in the respiratory tract.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The wild-type B. bronchiseptica strain RB50 and the Δbps strain (an isogenic derivative of RB50 containing an in-frame deletion of the bpsABCD locus) have been previously described (46, 48). All strains were maintained on Bordet-Gengou (BG) agar supplemented with 7.5% defibrinated sheep blood. B. bronchiseptica strains were grown in Stainer-Scholte broth at 37°C.
Animal experiments.
Five- to 6-week-old female C57BL/6 mice (Jackson Laboratory) were lightly sedated with isoflurane (Butler) and were intranasally inoculated with either 50 μl of sterile phosphate-buffered saline (PBS) alone or with 5 × 105 CFU of RB50 or the Δbps strain. At designated times postinoculation, mice were euthanized, and the nasal septum was excised, fixed in 10% normal buffered formalin, and processed for microscopy as described below.
For quantification of numbers of bacteria from different tissues, groups of six mice were inoculated with different strains as described above. Five weeks postinoculation, excised tissues were homogenized in PBS and plated onto BG blood agar containing streptomycin (50 μg/ml). Colonies were enumerated after 2 days of growth at 37°C. All animal experiments were carried out in accordance with institutional guidelines and were repeated in duplicate. Statistical analysis was carried using an unpaired two-tailed Student t test.
Immunofluorescent labeling of Bordetella biofilms formed in vivo.
After fixation, tissues were washed with PBS and then blocked with 5% normal donkey serum for 30 min. The tissues were then incubated with polyclonal sera (1:1,000; collected from a rat 30 days after inoculation with a Bvg+ phase-locked derivative of RB50) (11) for 2 h at room temperature, washed five times with PBS, and subsequently incubated for 2 h at room temperature with a donkey anti-rat secondary antibody conjugated to Alexa Fluor 488 (1:200). Samples were again washed five times and fixed for 30 min in 10% normal buffered formalin to prevent antibody-antigen dissociation during microscopy. Tissues were washed with PBS, permeabilized with 0.1% Triton X-100, and stained for eukaryotic F-actin with a 1:40 dilution of phalloidin conjugated to Alexa Fluor 633 for 30 min. Samples were visualized using a Zeiss LSM510 confocal scanning laser microscope. Images were analyzed to determine biomass and average thickness using the COMSTAT package (23). Statistical analysis was carried out using an unpaired two-tailed Student t test.
Detection of Bps polysaccharide.
To determine the production of Bps in Bordetella biofilms, nasal septa were processed as described above for the visualization of Bordetella cells by utilizing the anti-Bordetella serum. For detection of Bps, tissues were incubated with affinity-purified goat antibodies specific for deacetylated PNAG (dPNAG) (1:1,000) (35, 41), washed, incubated with secondary donkey anti-goat antibodies conjugated to Texas Red (1:200), and subsequently visualized using a Zeiss LSM510 confocal scanning laser microscope.
SEM of in vivo biofilms.
Samples were collected from PBS-inoculated and Bordetella-infected mice as described above, washed in PBS, processed for SEM as described previously, and viewed with a Philips SEM-515 scanning electron microscope (45).
RESULTS
B. bronchiseptica forms biofilms on murine nasal epithelium.
We hypothesized that the biofilm mode of existence, specifically biofilms in the nasal cavity, promotes persistent Bordetella infection. To investigate this, we inoculated mice intranasally with the wild-type B. bronchiseptica strain RB50. At designated times postinoculation, the nasal septum was excised and stained with rat anti-B. bronchiseptica serum, followed by an anti-rat fluorescent conjugate (Alexa Fluor 488) to detect the bacterial cells, which stained green. In order to better delineate the respiratory epithelium, we stained for eukaryotic actin by utilizing Alexa Fluor 633 conjugated to phalloidin, which stained the epithelium red.
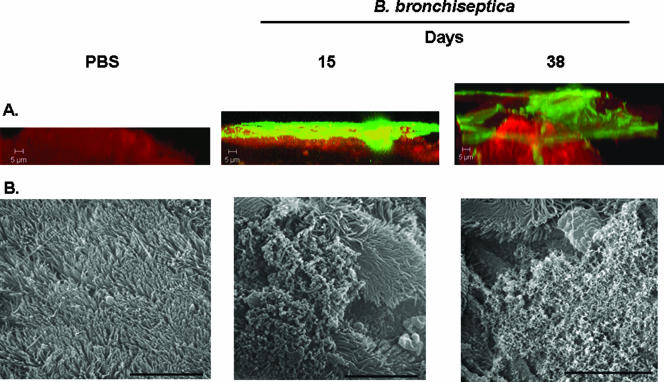
We chose two time points, 15 and 38 days after intranasal inoculation, to demonstrate the presence of biofilms during infection. Within 1 week after inoculation with B. bronchiseptica, the entire respiratory tract is colonized. However by 5 weeks, the concentrations of bacteria decline to comparatively low levels throughout the lower respiratory tract but remain high in the nasal cavity of the animals (1, 37). CSLM analysis of nasal septa harvested 15 days postinoculation revealed that B. bronchiseptica covered the host epithelium as compact layers of cells that resembled a bacterial mat or lawn with some microcolonies (Fig. 1A, middle panel). In contrast, at 38 days postinoculation the bacterial colonies had expanded vertically and were more heterogeneous, with multiple areas of noncolonized epithelium (Fig. 1A, right panel, and 2B). It is possible that the uncolonized epithelium was either composed of nonciliated cells or represented dispersal of a preexisting biofilm. At this later time point, bacteria were present in large foci, and the biofilms formed were thicker than those formed at 15 days (Table 1).
FIG. 1.
(A) CSLM of biofilms formed within the murine nasal cavity by B. bronchiseptica. C57BL/6 mice were inoculated with either PBS or B. bronchiseptica RB50. Nasal septa were harvested at 15 or 38 days postinoculation, immediately fixed, and probed with rat anti-Bordetella serum followed by a secondary anti-rat antibody conjugated to Alexa Fluor 488 (which stains bacteria green). To determine the localization of the host epithelium, specimens were stained for F-actin using phalloidin conjugated to Alexa Fluor 633 (which stains the epithelium red) and visualized with CSLM. Each micrograph represents a Z reconstruction. For each specimen, images were obtained from at least five areas of the nasal septum and from at least three independent animals. (B) SEM of B. bronchiseptica biofilm formation on nasal septa. Specimens were collected from animals either 15 days or 38 days postinoculation, directly fixed, and processed for SEM as described previously (45). Scale bars = 10 μm.
TABLE 1.
COMSTAT analysis of CSLM micrographs generated from tissues isolated from mice infected with either the wild-type or Δbps straina
| Day postinfection | Strain | Biomassb
|
Avg thickness
|
||
|---|---|---|---|---|---|
| μm3/μm2 | P valuec | μm | P valuec | ||
| 15 | Wild type | 3.270 (4.581) | 0.741 | 5.527 (8.500) | 0.447 |
| Δbps | 4.494 (9.057) | 2.869 (2.789) | |||
| 38 | Wild type | 3.023 (1.681) | 0.0002 | 12.018 (8.26) | 0.0011 |
| Δbps | 0.065 (0.064) | 0.094 (0.0953) | |||
The values are averages for at least seven images from at least two independent animals. The values in parentheses are standard deviations.
Biomass is expressed in μm3/μm2 of surface area covered by bacteria.
P values were determined using an unpaired two-tailed Student t test.
As a negative control, tissues from PBS-inoculated mice were examined utilizing exactly the same protocol as that used for B. bronchiseptica-infected tissues. As shown in Fig. 1A (left panel) and 2A, the nasal septa from these mice displayed little cross-reactivity with the B. bronchiseptica-specific antiserum. Sera from PBS-infected mice or the secondary antibody alone did not cross-react with nasal tissues (data not shown). Bacteria have been shown to possess distant homologs of F-actin (15). Thus, it is important to note that in vitro-grown B. bronchiseptica did not stain with the antibody conjugated to phalloidin (data not shown).
SEM of nasal tissues.
In addition to CSLM, we utilized SEM as an independent means to confirm the presence of biofilms in respiratory tissues. In the case of B. bronchiseptica, cell clusters were visible at 15 days after inoculation and were adhered to the ciliated epithelium (Fig. 1B, middle panel). By 38 days postinoculation, bacteria were observed to be in a densely packed multicellular community on the ciliated epithelium (Fig. 1B, right panel). Taken together, the results in Fig. 1 and 2 clearly demonstrate that B. bronchiseptica is capable of forming a biofilm in the noses of mammalian hosts.
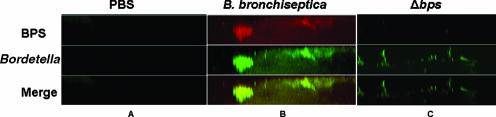
FIG. 2.
Bps production in vivo. Nasal septa were harvested from C57BL/6 mice inoculated with (A) PBS, (B) B. bronchiseptica (RB50), or (C) an isogenic mutant, Δbps. Samples were collected at 38 days postinoculation and were stained for Bordetella (green) as described in the legend to Fig. 1. To detect Bps production (red), specimens were stained using goat anti-dPNAG, followed by anti-goat conjugated to Texas Red. Yellow staining indicates colocalization of Bordetella and Bps. Micrographs are Z reconstructions and are representative of at least three independently harvested tissues.
Production of Bps within the murine nasal cavity.
One of the defining characteristics of mature bacterial biofilms is the presence of a matrix which is generally composed of proteins, nucleic acids, and/or polysaccharides (5, 30, 38, 53, 54, 56, 58). We have recently demonstrated the involvement of the Bps polysaccharide in B. bronchiseptica biofilm formation in vitro (46). We hypothesized that similar to our previous findings, Bps is produced within B. bronchiseptica biofilms and is required for efficient biofilm development in the mouse nasopharynx. Thus, we stained nasal septum specimens harvested from mice infected with B. bronchiseptica 38 days postinoculation for bacteria (anti-Bordetella serum) and for the Bps polysaccharide (anti-dPNAG). We previously showed that antibodies raised against dPNAG specifically detected Bps in vitro (36, 41, 46). Our results (Fig. 2B) demonstrate that the majority of cells comprising B. bronchiseptica biofilms (green) colocalized with the Bps stain (red), thereby suggesting that there was uniform production of the Bps polysaccharide during biofilm maturation in vivo. Most importantly, the production of an extruded polysaccharide along with the presence of distinct architectural features further confirms the biofilm nature of B. bronchieptica in the respiratory tract.
As a negative control, we stained tissues from a PBS-infected mouse for the presence of both Bordetella and Bps and observed little cross-reactivity (Fig. 2A). Heterologous goat sera did not cross-react with either B. bronchiseptica or the host epithelium (data not shown). Additionally, nasal tissues from mice infected with the Δbps strain showed little cross-reactivity with the dPNAG antibody, demonstrating its specificity for detecting Bps in the respiratory tract (Fig. 2C). The low degree of cross-reactivity that remained associated with the Δbps strain was also seen in vitro, where this phenotype can be complemented by restoring the bpsABCD locus (46). The complementing plasmid is highly unstable in animals, and we have been unable to obtain any chloramphenicol-resistant bacteria from the animals as early as 2 days postinoculation (data not shown). Thus, it was not possible to use a complemented strain in mice.
The bps locus is essential for Bordetella biofilm formation in vivo.
Based on our previous studies that clearly documented that the bps locus is critical for mature biofilm formation on glass surfaces (46), we hypothesized that it is also required for the production of mature biofilms within the nasal cavity. Thus, we compared the characteristic features of the biofilms formed by the wild-type and Δbps strains by CSLM. We also utilized the COMSTAT software to quantitate the differences in biomass and average thickness between the wild-type and mutant strains (Table 1) (23).
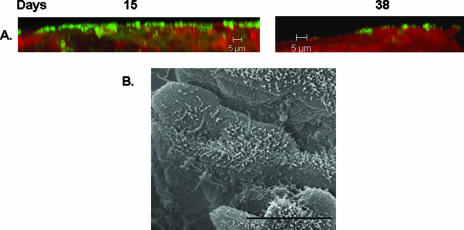
At 15 days, while the wild-type strain was capable of existing as a lawn of cells with some microcolonies, the Δbps strain existed mainly as clusters of cells which covered the majority of the epithelium (compare Fig. 1A, middle panel, to Fig. 3A, left panel). While there appeared to be some differences between the wild-type and Δbps strains microscopically, there were no statistical differences in either the total biomass or the average thickness (Table 1).
FIG. 3.
Involvement of the bpsABCD locus in nasal cavity biofilm formation. (A) CSLM analysis of biofilms formed by the Δbps strain. Samples were collected at 15 and 38 days postinoculation and stained as described in the legend to Fig. 1. (B) SEM of Δbps-infected nasal tissue. Samples were collected at 38 days postinoculation and processed as described in the legend to Fig. 1.
In contrast, at 38 days postinoculation, a severe reduction in the ability of the Δbps strain to form biofilms on the nasal septum was observed. The nasal septa harvested from the Δbps strain were to a large extent devoid of bacterial cells (Fig. 2C and 3A, right panel). In the regions where the Δbps strain did colonize the epithelium, it was present as very thin spikes and in minute clusters (Fig. 2C and 3A, right panel). Similarly, as quantified by COMSTAT, the Δbps strain formed biofilms whose thickness and biomass were considerably reduced (Table 1).
Since our CSLM and COMSTAT analysis revealed a difference only at 38 days, we analyzed biofilms formed by the Δbps strain at this time point by SEM. The results shown in Fig. 3B demonstrate that the Δbps strain did not form large cell clusters at 38 days and existed only as single cells adhered to the nasal septum.
The bps locus promotes persistent colonization of the mouse.
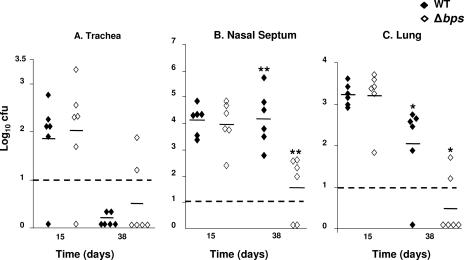
Based on the microscopic evaluations described above, we wished to determine the ability of the B. bronchiseptica wild-type and Δbps strains to colonize the mouse respiratory tract. At 15 days postinoculation, both the wild-type and Δbps strains colonized the nasal septum, trachea, and lungs to the same degree (Fig. 4). However, at 38 days, considerably lower numbers of bacteria were present in the nasal cavities of animals infected with the Δbps strain (P ≤ 0.005) (Fig. 4). While no bacteria were recovered from the lungs of one of the six mice inoculated with the wild-type strain at 38 days, the lungs of four animals inoculated with the Δbps strain were cleared of bacteria. For the rest of the animals that did show evidence of lung colonization with the Δbps strain, fewer bacteria were harvested from their lungs than from the lungs of the animals inoculated with the wild-type strain (P ≤ 0.05) (Fig. 4). Taken together, these results suggest that at least one role for Bps during infection is to allow efficient long-term survival of B. bronchiseptica in the respiratory tract.
FIG. 4.
Colonization of the murine respiratory tract by the wild-type and Δbps B. bronchiseptica strains. Groups of six 6-week-old C57BL/6 mice were intranasally inoculated with 50 μl containing 5 × 105 CFU of either the RB50 (♦) or Δbps (⋄) strain. The trachea (A), the nasal septum (B), and two lobes of the lungs (C) were harvested at 15 and 38 days postinoculation and homogenized, and the resident bacteria were enumerated on BG-blood agar plates containing streptomycin. The horizontal bars indicate the numerical mean for each group. The dashed lines indicate the lower limits of detection. The experiment was repeated in duplicate. A statistical analysis was carried out using an unpaired two-tailed Student t test. One asterisk indicates that the P value is ≤0.05, and two asterisks indicate that the P value is ≤0.005.
DISCUSSION
Biofilm development on inert surfaces by a multitude of organisms has been extensively studied, and it is well recognized that bacteria are generally organized as biofilms in nature (13, 47, 55). Although increasing evidence suggests that biofilms may play a principal role in a variety of chronic microbial infections, limited studies have provided direct evidence of the biofilm mode of existence in vivo (2, 21, 25, 32, 33, 51). Parsek and Singh proposed several criteria to define infections caused by biofilms. These criteria were (i) infecting bacteria should be adherent or attached to the substratum, (ii) there should be direct visualization of either bacterial clusters or microcolonies encased in an extracellular matrix, either self-produced or composed of host components, (iii) infections should be localized to a particular anatomical site, and (iv) there should be evidence of recalcitrance of the bacteria to antibiotics compared to their planktonic counterparts (47). In this work, we obtained experimental evidence that there are B. bronchiseptica biofilms within the upper respiratory tract. By utilizing CSLM and SEM, we observed adherent clusters (criterion i) of B. bronchiseptica that exhibited distinct architectural features (mats, towers, or pillars separated by void spaces) (criterion ii) characteristic of many bacterial biofilms formed in vitro on abiotic surfaces. Notably, the confocal and SEM micrographs of the biofilms observed in vivo were reminiscent of images of in vitro biofilms formed by Bordetella spp. on abiotic surfaces, indicating that in vitro studies may serve as good models for examining the roles of different factors in Bordetella biofilm development (29, 46, 50). We also obtained evidence that these biofilms are characterized by the production of the biofilm-associated Bps polysaccharide and showed that Bps is essential for efficient biofilm development in vivo (criterion ii).
Although very low numbers of the animal pathogen B. bronchiseptica can be isolated at later time points from the lower respiratory tract, this organism mainly establishes asymptomatic and chronic infections of the nose (37). We have been able to isolate B. bronchiseptica from the rat nasopharynx 85 days after inoculation (unpublished results), and this bacterium has previously been reported to be present in the nose for the life of an infected animal (criterion iii). Additionally, while B. pertussis infection of humans is traditionally associated with the acute disease whooping cough in infants and young children, growing numbers of adolescents and adults are showing milder symptoms and evidence of nasopharyngeal colonization by B. pertussis (criterion iii) (10, 20, 49, 57).
Our laboratory has previously shown that compared to their planktonic counterparts, B. bronchiseptica biofilms are up to 1,000-fold more resistant to antibiotics, including the clinically relevant antibiotics erythromycin and ciprofloxacin (45). Recently, it has been shown that despite the finding that planktonic B. bronchiseptica is highly sensitive to antibiotics, elimination of bacteria from the respiratory tract of infected mice with antibiotics could not be achieved (criterion iv) (28). Thus, results presented here, in combination with previously published reports, strongly document the biofilm nature of Bordetella in the respiratory tract.
A clear role for a Bordetella virulence factor in the colonization of the mammalian nose has not been reported. The majority of Bordetella factors are implicated in the colonization and survival of bacteria in the lower respiratory tract (6, 7, 12, 43). Therefore, it is interesting that despite the presence of numerous virulence factors, deletion of the bpsABCD locus can abrogate biofilm development within the nasal cavity. The only strain described to date which does not exhibit nasal cavity colonization is the Bvg− phase-locked strain which harbors a deletion of the bvgS gene and thus does not express any of the Bvg-activated adhesins and toxins (1, 11). We and other workers have previously shown that the BvgAS two-component system is required for biofilm development (27, 45). We are in the process of determining whether expression of Bps is regulated by BvgAS.
Interestingly, while the ability of the Δbps strain to colonize the lungs was impaired, there was no significant defect in colonization of the trachea. In the upper airways, the mucociliary escalator is involved in the clearance of inhaled particles and bacteria from the airways toward the mouth. However, in the lungs, an army of alveolar macrophages and other phagocytic cells phagocytose particles soon after their deposition. Moreover, there are greater numbers of phagocytic cells in the lungs than in the trachea. Thus, one mechanism by which Bps may enhance survival in the lungs is by antagonizing the function of the phagocytic cells.
The ability to detect biofilms formed by B. bronchiseptica implies that biofilms have a role during both human and animal infections. This aspect of Bordetella physiology and lifestyle has not been examined to date. Several studies have documented the presence of B. pertussis in the nasopharynx of both children and adults (3, 4, 8, 17, 19). It is estimated that 20 to 30% of adolescents and adults who have chronic coughs lasting for more than 1 week are colonized with B. pertussis in the nasopharynx (9, 14, 16, 26). While B. pertussis infections are typically considered acute infections, the course of infection can last for up to 3 months, and a biofilm may allow survival of the bacteria. We hypothesize that the biofilm mode of existence in the respiratory tract, especially in the nasopharynx, allows B. pertussis to escape immune defenses, thereby leading to long-term colonization, and may ultimately serve as a reservoir for transmission of the organism to unvaccinated infants and children. We are currently exploring the ability of B. pertussis to form biofilms within the murine respiratory tract and the role of the Bps polysaccharide during B. pertussis infection.
Acknowledgments
We thank Daniel Wozniak for critical reading of the manuscript. We are grateful to Gerald B. Pier for a generous gift of the dPNAG antibody.
Research in the laboratory of R.D. is supported by funds from Wake Forest University Health Sciences, National Research Initiative of the USDA Cooperative State Research, Education and Extension Service (grant 35604-16874), and the NIH (grant R21 AI071054).
Footnotes
Published ahead of print on 22 June 2007.
REFERENCES
- 1.Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella host interaction. Cell 80:611-620. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 3.Bamberger, E., O. Starets-Haham, D. Greenberg, A. Karidis, N. Porat, G. Bar-Joseph, R. Gershtein, and I. Srugo. 2006. Adult pertussis is hazardous for the newborn. Infect. Control Hosp. Epidemiol. 27:623-625. [DOI] [PubMed] [Google Scholar]
- 4.Birkebaek, N. H., M. Kristiansen, T. Seefeldt, J. Degn, A. Moller, I. Heron, P. L. Andersen, J. K. Moller, and L. Ostergard. 1999. Bordetella pertussis and chronic cough in adults. Clin. Infect. Dis. 29:1239-1242. [DOI] [PubMed] [Google Scholar]
- 5.Branda, S. S., A. Vik, L. Friedman, and R. Kolter. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20-26. [DOI] [PubMed] [Google Scholar]
- 6.Carbonetti, N. H., G. V. Artamonova, C. Andreasen, and N. Bushar. 2005. Pertussis toxin and adenylate cyclase toxin provide a one-two punch for establishment of Bordetella pertussis infection of the respiratory tract. Infect. Immun. 73:2698-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbonetti, N. H., G. V. Artamonova, R. M. Mays, and Z. E. Worthington. 2003. Pertussis toxin plays an early role in respiratory tract colonization by Bordetella pertussis. Infect. Immun. 71:6358-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherry, J. D. 2005. Pertussis vaccines for adolescents and adults. Pediatrics 116:755-756. [DOI] [PubMed] [Google Scholar]
- 9.Cherry, J. D. 2006. Epidemiology of pertussis. Pediatr. Infect. Dis. J. 25:361-362. [DOI] [PubMed] [Google Scholar]
- 10.Cloud, J. L., W. Hymas, and K. C. Carroll. 2002. Impact of nasopharyngeal swab types on detection of Bordetella pertussis by PCR and culture. J. Clin. Microbiol. 40:3838-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal-transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotter, P. A., M. H. Yuk, S. Mattoo, B. J. Akerley, J. Boschwitz, D. A. Relman, and J. F. Miller. 1998. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect. Immun. 66:5921-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards, K., and D. M. Freeman. 2006. Adolescent and adult pertussis: disease burden and prevention. Curr. Opin. Pediatr. 18:77-80. [DOI] [PubMed] [Google Scholar]
- 15.Esue, O., M. Cordero, D. Wirtz, and Y. Tseng. 2005. The assembly of MreB, a prokaryotic homolog of actin. J. Biol. Chem. 280:2628-2635. [DOI] [PubMed] [Google Scholar]
- 16.Fry, N. K., O. Tzivra, Y. T. Li, A. McNiff, N. Doshi, P. A. C. Maple, N. S. Crowcroft, E. Miller, R. C. George, and T. G. Harrison. 2004. Laboratory diagnosis of pertussis infections: the role of PCR and serology. J. Med. Microbiol. 53:519-525. [DOI] [PubMed] [Google Scholar]
- 17.Gilberg, S., E. Njamkepo, C. Du, I. H. Partouche, P. Gueirard, C. Ghasarossian, M. Schlumberger, and N. Guiso. 2002. Evidence of Bordetella pertussis infection in adults presenting with persistent cough in a French area with very high whole-cell vaccine coverage. J. Infect. Dis. 186:415-418. [DOI] [PubMed] [Google Scholar]
- 18.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg, D. P. 2005. Pertussis in adolescents: increasing incidence brings attention to the need for booster immunization of adolescents. Pediatr. Infect. Dis. J. 24:721-728. [DOI] [PubMed] [Google Scholar]
- 20.Hallander, H. O., E. Reizenstein, B. Renemar, G. Rasmuson, L. Mardin, and P. Olin. 1993. Comparison of nasopharyngeal aspirates with swabs for culture of Bordetella pertussis. J. Clin. Microbiol. 31:50-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall-Stoodley, L., F. Z. Hu, A. Gieseke, L. Nistico, D. Nguyen, J. Hayes, M. Forbes, D. P. Greenberg, B. Dice, A. Burrows, P. A. Wackym, P. Stoodley, J. C. Post, G. D. Ehrlich, and J. E. Kerschner. 2006. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296:202-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Gotz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 23.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 24.Hinnebusch, B. J., R. D. Perry, and T. G. Schwan. 1996. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science 273:367-370. [DOI] [PubMed] [Google Scholar]
- 25.Hong, W., K. Mason, J. Jurcisek, L. Novotny, L. O. Bakaletz, and W. E. Swords. 2007. Phosphorylcholine decreases early inflammation and promotes the establishment of stable biofilm communities of nontypeable Haemophilus influenzae strain 86-028NP in a chinchilla model of otitis media. Infect. Immun. 75:958-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu, J. J., C. Y. Lu, L. Y. Chang, C. H. Huang, C. C. Chou, F. Y. Huang, C. Y. Lee, and L. M. Huang. 2006. Survey of pertussis in patients with prolonged cough. J. Microbiol. Immunol. Infect. 39:54-58. [PubMed] [Google Scholar]
- 27.Irie, Y., S. Mattoo, and M. H. Yuk. 2004. The Bvg virulence control system regulates biofilm formation in Bordetella bronchiseptica. J. Bacteriol. 186:5692-5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irie, Y., G. A. O'Toole, and M. H. Yuk. 2005. Pseudomonas aeruginosa rhamnolipids disperse Bordetella bronchiseptica biofilms. FEMS Microbiol. Lett. 250:237-243. [DOI] [PubMed] [Google Scholar]
- 29.Irie, Y., A. Preston, and M. H. Yuk. 2006. Expression of the primary carbohydrate component of the Bordetella bronchiseptica biofilm matrix is dependent on growth phase but independent of Bvg regulation. J. Bacteriol. 188:6680-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson, K. D., M. Starkey, S. Kremer, M. R. Parsek, and D. J. Wozniak. 2004. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 186:4466-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jesaitis, A. J., M. J. Franklin, D. Berglund, M. Sasaki, C. I. Lord, J. B. Bleazard, J. E. Duffy, H. Beyenal, and Z. Lewandowski. 2003. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 171:4329-4339. [DOI] [PubMed] [Google Scholar]
- 32.Jurcisek, J., L. Greiner, H. Watanabe, A. Zaleski, M. A. Apicella, and L. O. Bakaletz. 2005. Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear. Infect. Immun. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jurcisek, J. A., and L. O. Bakaletz. 2007. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J. Bacteriol. 189:3868-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan, J. B., K. Velliyagounder, C. Ragunath, H. Rohde, D. Mack, J. K. Knobloch, and N. Ramasubbu. 2004. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J. Bacteriol. 186:8213-8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly-Quintos, C., L. A. Cavacini, M. R. Posner, D. Goldmann, and G. B. Pier. 2006. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect. Immun. 74:2742-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly-Quintos, C., A. Kropec, S. Briggs, C. L. Ordonez, D. A. Goldmann, and G. B. Pier. 2005. The role of epitope specificity in the human opsonic antibody response to the staphylococcal surface polysaccharide poly N-acetyl glucosamine. J. Infect. Dis. 192:2012-2019. [DOI] [PubMed] [Google Scholar]
- 37.Kirimanjeswara, G. S., P. B. Mann, and E. T. Harvill. 2003. Role of antibodies in immunity to Bordetella infections. Infect. Immun. 71:1719-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ledeboer, N. A., and B. D. Jones. 2005. Exopolysaccharide sugars contribute to biofilm formation by Salmonella enterica serovar Typhimurium on HEp-2 cells and chicken intestinal epithelium. J. Bacteriol. 187:3214-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leid, J. G., M. E. Shirtliff, J. W. Costerton, and A. P. Stoodley. 2002. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect. Immun. 70:6339-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mah, T. F. C., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 41.Maira-Litran, T., A. Kropec, D. A. Goldmann, and G. B. Pier. 2005. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect. Immun. 73:6752-6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattoo, S., and J. D. Cherry. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18:326-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattoo, S., J. F. Miller, and P. A. Cotter. 2000. Role of Bordetella bronchiseptica fimbriae in tracheal colonization and development of a humoral immune response. Infect. Immun. 68:2024-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKenney, D., J. Hubner, E. Muller, Y. Wang, D. A. Goldmann, and G. B. Pier. 1998. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect. Immun. 66:4711-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra, M., G. Parise, K. D. Jackson, D. J. Wozniak, and R. Deora. 2005. The BvgAS signal transduction system regulates biofilm development in Bordetella. J. Bacteriol. 187:1474-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parise, G., M. Mishra, Y. Itoh, T. Romeo, and R. Deora. 2007. Role of a putative polysaccharide locus in Bordetella biofilm development. J. Bacteriol. 189:750-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677-701. [DOI] [PubMed] [Google Scholar]
- 48.Relman, D. A., M. Domenighini, E. Tuomanen, R. Rappuoli, and S. Falkow. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc. Natl. Acad. Sci. USA 86:2637-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlapfer, G., H. P. Senn, R. Berger, and M. Just. 1993. Use of the polymerase chain reaction to detect Bordetella pertussis in patients with mild or atypical symptoms of infection. Eur. J. Clin. Microbiol. Infect. Dis. 12:459-463. [DOI] [PubMed] [Google Scholar]
- 50.Serra, D., A. Bosch, D. M. Russo, M. E. Rodriguez, A. Zorreguieta, J. Schmitt, D. Naumann, and O. Yantorno. 2007. Continuous nondestructive monitoring of Bordetella pertussis biofilms by Fourier transform infrared spectroscopy and other corroborative techniques. Anal. Bioanal. Chem. 387:1759-1767. [DOI] [PubMed] [Google Scholar]
- 51.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 52.Vuong, C., J. B. Kidder, E. R. Jacobson, M. Otto, R. A. Proctor, and G. A. Somerville. 2005. Staphylococcus epidermidis polysaccharide intercellular adhesin production significantly increases during tricarboxylic acid cycle stress. J. Bacteriol. 187:2967-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vuong, C., S. Kocianova, J. M. Voyich, Y. Yao, E. R. Fischer, F. R. DeLeo, and M. Otto. 2004. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 279:54881-54886. [DOI] [PubMed] [Google Scholar]
- 54.Wang, X., J. F. Preston, and T. Romeo. 2004. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 186:2724-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitchurch, C. B., T. Tolker-Nielsen, P. C. Ragas, and J. S. Mattick. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. [DOI] [PubMed] [Google Scholar]
- 57.Yeh, S. H. 2003. Pertussis: persistent pathogen, imperfect vaccines. Expert Rev. Vaccines 2:113-127. [DOI] [PubMed] [Google Scholar]
- 58.Ziebuhr, W., C. Heilmann, F. Gotz, P. Meyer, K. Wilms, E. Straube, and J. Hacker. 1997. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 65:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]