Abstract
The Vibrio fischeri quorum-sensing signal N-3-oxohexanoyl-l-homoserine lactone (3OC6-HSL) activates expression of the seven-gene luminescence operon. We used microarrays to unveil 18 additional 3OC6-HSL-controlled genes, 3 of which had been identified by other means previously. We show most of these genes are regulated by the 3OC6-HSL-responsive transcriptional regulator LuxR directly. This demonstrates that V. fischeri quorum sensing regulates a substantial number of genes other than those involved in light production.
Quorum sensing allows a species to measure its population density and control gene expression in a population density-dependent manner. Bacterial quorum sensing involves cell-cell communication mediated by extracellular signal compounds. Various species of proteobacteria use acyl-homoserine lactones (acyl-HSLs) as quorum-sensing signals (2, 12-15, 36, 42). Acyl-HSL quorum sensing was first described in the marine luminescent bacterium Vibrio fischeri, where it controls transcription of the luminescence (lux) operon (7, 10, 11). The LuxI acyl-HSL synthase and the LuxR transcriptional activator constitute the quorum-sensing system, which controls the lux operon directly. The LuxI-generated signal is N-3-oxohexanoyl-l-HSL (3OC6-HSL), and LuxR activates the lux operon in response to this signal. The LuxR-3OC6-HSL complex binds to a 20-bp inverted repeat centered at −42.5 from the transcriptional start site of the lux operon (for reviews, see references 14, 36, and 41).
Vibrio fischeri occurs at low population densities in seawater and at high densities in specific light organ symbioses with certain fish and squid (3, 21, 29). Quorum sensing allows V. fischeri to discern its existence in the symbiosis and activate transcription of the lux operon (4). Many proteobacteria have acyl-HSL signaling systems, which serve as global regulators of extracellular factor production and are often important for successful interactions with plant or animal hosts (26, 36). Existing evidence shows that besides the lux operon, LuxR and 3OC6-HSL activate five other genes (5). However, the search for quorum-controlled genes in V. fischeri has been limited to proteomic analyses. We recently reported the complete genome sequence of a strain of V. fischeri isolated from a squid light organ (30). Thus, it is now possible to use DNA microarrays to identify LuxR-regulated V. fischeri genes on a global scale. We have designed and constructed a microarray and used it to perform an analysis of 3OC6-HSL-regulated genes in V. fischeri. We furthered this analysis by examining the activity of relevant promoters in response to 3OC6-HSL and LuxR in recombinant Escherichia coli and by analyzing the binding of LuxR to promoter DNA fragments in vitro.
We used E. coli DH12S (Invitrogen, Carlsbad, CA) and V. fischeri ES114 (3). The genome sequence of V. fischeri ES114 is publicly available at http://www.ergo-light.com (30). We used Luria-Bertani broth (31) without added sodium chloride to grow E. coli, at 30°C, and seawater-tryptone broth (3) with seawater salts (300 mM NaCl, 10 mM KCl, 50 mM MgSO4, 10 mM CaCl2) to grow V. fischeri at 28°C. All cultures were grown with shaking at 250 rpm. For LuxR expression in E. coli, we used pHV402, which contains a chloramphenicol resistance gene and contains luxR controlled by its own promoter (18). For cloning of promoter elements, we used pPROBE-gfp[LVA], which contains a promoterless gfp and a kanamycin resistance marker (24). Chloramphenicol and kanamycin were used for plasmid maintenance at 25 and 50 μg/ml, respectively. 3OC6-HSL (Sigma, St. Louis, MO) was added as described elsewhere (33) at a final concentration of 2.3 or 1 μM, as indicated.
In conjunction with Affymetrix (Santa Clara, CA), we designed custom GeneChips (11-μm feature size) representing >99% of the annotated open reading frames in the V. fischeri ES114 genome, as well as 125 intergenic regions greater than 150 bp. GeneChips were designed as described by the manufacturer, except that because the V. fischeri genome has a low G+C content (38 mol%), we used 16 probe pair sets (25-bp oligonucleotide probe length) per gene or intergenic region. Poly(A) control sequences of dap, lys, phe, and trp from Bacillus subtilis were included. We compared transcript profiles of V. fischeri ES114 grown in the presence versus the absence of added 3OC6-HSL (1 μM). Compared to other strains of V. fischeri, strain ES114 produces little 3OC6-HSL in laboratory culture (4). While it produces 3OC6-HSL in amounts insufficient to appreciably induce the lux genes (23, 39), strain ES114 produces larger quantities of a second acyl-HSL, C8-HSL, by using an enzyme called AinS (16, 19). C8-HSL can bind LuxR, but C8-HSL does not appreciably activate LuxR (32, 39). However, C8-HSL can affect lux gene expression in V. fischeri ES114 indirectly (23). Therefore, we were able to add 3OC6-HSL to wild-type cultures and monitor the induction of LuxR-controlled genes. We isolated and purified RNA for GeneChip analysis at culture optical density at 600 nm (OD600) values of 0.3 and 1.5, prepared cDNA, and performed hybridizations and scanning as described elsewhere (www.affymetrix.com). Results are the averages from two independent experiments, and the results were analyzed by using the Affymetrix Microarray Suite v.5 and Cyber-T (http://cybert.microarray.ics.uci.edu) (1). We sorted for genes that showed 2.5-fold or greater differential regulation with a P value of <0.001.
Predicted promoter regions of genes identified as 3OC6-HSL-regulated in transcript analyses were amplified by PCR with V. fischeri genomic DNA as the template. Fusions were constructed by generation of PCR fragments tailed with EcoRI and BamHI sites, digestion of the PCR fragments with EcoRI and BamHI, and ligation of the digested PCR fragments with EcoRI-BamHI-digested pPROBE-gfp[LVA]. The resulting plasmids were used to transform E. coli DH12S with or without pHV402 as previously described (31). Promoter activity was measured as follows. Overnight cultures of recombinant E. coli were used to inoculate fresh cultures at a starting OD600 of 0.1 in medium with or without added 3OC6-HSL (2.3 μM). At an OD600 of 1.2 (±0.2), cells from each culture were pelleted by centrifugation and suspended in phosphate-buffered saline and the fluorescence of the suspension was measured with a GENios Pro 96-well plate reader (TECAN, Research Triangle Park, NC). Results are the averages of three independent experiments.
Global analysis of 3OC6-HSL-regulated gene expression.
Our microarray analysis revealed 30 genes and 2 intergenic regions with transcript levels that were at least 2.5-fold different when 3OC6-HSL-grown cells were compared to cells grown without added 3OC6-HSL (Table 1). One of the 30 genes (VFA0895) showed a minimal activation, and it overlaps and forms a predicted operon with VFA0896 (http://www.ergo-light.com), which was not regulated by 3OC6-HSL in our experiments. We did not study VFA0895 further. Also, VF1247, VF1615, VF2034, VF2035, VF2036, and VFA0834 showed induction levels just above our 2.5-fold cutoff (2.6- to 3.2-fold). We examined transcript levels of these six genes further by real-time PCR using SYBR green PCR master mix (Applied Biosystems, Foster City, CA), 5 ng of cDNA, and 900 nM of each primer. With the exception of VF1247 and VF1615, all genes showed minimal activation by 3OC6-HSL (<2-fold). Thus, we did not examine VF2034 to VF2036 and VFA0834 further.
TABLE 1.
Differential expression of V. fischeri genes in response to 3OC6-HSL
| Gene or intergenic region (gene location)a | Annotation | Fold regulation at ODb660 of:
|
|
|---|---|---|---|
| 0.3 | 1.5 | ||
| VF1161 (1290494-1291648) | Periplasmic component of efflux system | 25.6 | 10.3 |
| VF1162 (1291644-1292921) | Outer membrane efflux protein (TolC) | 12.5 | 7.1 |
| VF1163 (1292921-1294135) | Export ABC transporter permease protein | 8.9 | 6.4 |
| VF1164 (1294142-1295422) | Export ABC transporter permease protein | 7.2 | 5.4 |
| VF1165 (1295418-1296140) | ABC transporter ATP-binding protein | 7.3 | 5.4 |
| VF1246 (1390844-1389936) | Putative omptin family serine protease | 31.8 | 12.2 |
| VF1247 (1391269-1391697) | Peptidoglycan-binding protein, LysM | 2.6 | 2.7 |
| VF1348 (1493989-1492961) | Hypothetical cytosolic protein | 9.5 | 6.7 |
| VF1349 (1495924-1494185) | Alkaline serine protease | 8.9 | 6.6 |
| VF1725 (1948180-1949709) | Secretory tripeptidyl aminopeptidase | 27.3 | 16.0 |
| VF1977* (2215928-2215569) | Protein YgiW precursor | 6.1 | ND |
| VF1978* (2216620-2215988) | Accessory colonization factor A (AcfA)-like protein | 16.1 | ND |
| VF2034 (2270424-2270026) | Hypothetical polypeptide | 2.6 | ND |
| VF2035 (2270891-2270424) | Hypothetical polypeptide | 2.7 | ND |
| VF2036 (2271435-2270905) | Possible phage regulatory protein | 2.7 | ND |
| VFA0090 (99228-98098) | Astacin peptidase | 29.2 | 9.9 |
| VFA0373 (420748-422019) | Mechanosensitive ion channel | 9.7 | 3.5 |
| VFA0622 (702734-702162) | Hypothetical protein | 7.2 | 3.6 |
| VFA0834 (939286-941157) | NirV precursor | ND | 3.2 |
| VFA0894 (1014502-1016295) | Bacterial immunoglobulin-like protein | 35.9 | 16.7 |
| VFA0895 (1016495-1016854) | Permease (major facilitator superfamily) | 3.3 | 2.6 |
| VFA0918* (1044437-1043730) | luxG | 9.0 | 5.9 |
| VFA0919* (1045577-1044450) | luxE | 10.0 | 6.7 |
| VFA0920* (1046636-1045659) | luxB | 8.1 | 4.9 |
| VFA0921* (1047714-1046650) | luxA | 10.6 | 6.0 |
| VFA0922* (1048660-1047740) | luxD | 8.9 | 5.0 |
| VFA0923* (1050115-1048679) | luxC | 14.4 | 7.4 |
| VFA0924* (1050725-1050156) | luxI | 13.9 | 5.7 |
| VFA1058* (1194722-1195164) | qsrP | 37.2 | 10.9 |
| VF1866 to VF1867 (2105494-2106116) | Intergenic region | ND | 3.8 |
| VFA0643 to VFA0644 (719637-720412) | Intergenic region | 3.5 | 2.4 |
| VF1615 (1814208-1814837) | Hypothetical protein | ND | −2.7 |
Genes marked with an asterisk have been identified as 3OC6-HSL regulated elsewhere. VF designates genes on the large chromosome, and VFA designates genes on the small chromosome.
“Fold regulation” refers to transcript levels in the presence versus absence of 3OC6-HSL. ND, no significant difference. All significant differences show a P value of <0.001.
The 25 3OC6-HSL-controlled genes we continued to study were distributed almost equally between the large and small V. fischeri chromosomes. We did not study expression of the intergenic regions differentially regulated by 3OC6-HSL (Table 1). Twenty-four of the 25 genes were induced and 1 was repressed by the quorum-sensing signal (Table 1). Ten of these genes have been identified previously as 3OC6-HSL controlled: VF1977, VF1978, and VFA1058 (5) and the 7 genes in the lux operon (7, 10, 11). These genes code for functions related to light production or have unknown functions. Activities predicted for the 15 newly discovered genes include protease and peptidase functions, an ABC-type transporter, and a mechanosensitive ion channel.
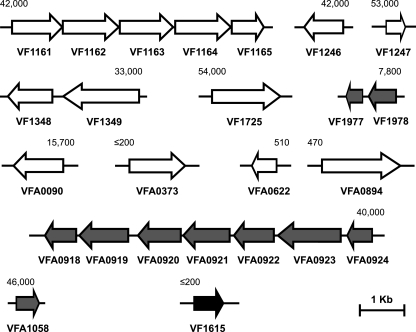
Many of the 3OC6-HSL-regulated genes we discovered are predicted to be in operons (http://www.cifn.unam.mx/moreno/pub/TUpredictions/Predictions/) (25). There are two clusters of linked genes predicted to consist of two transcriptional units each: VFA0924 to VFA0918 and VF1348 and VF1349. The in silico predictions are based primarily on distances between predicted reading frames and are conservative predictors of operons. In fact, we know from previous work that the VFA0924-to-VFA0918 cluster, luxICDABEG, is organized as an operon (10, 11). To determine whether VF1348 and VF1349 might constitute an operon, we performed open reading frame junction-based reverse transcription-PCR, and the analysis supports the contention that these two genes constitute an operon (data not shown). Thus, the 3OC6-HSL-controlled genes identified in the microarray analysis appear to be organized as 13 transcriptional units: 4 multigene operons and 9 single-gene units (Fig. 1).
FIG. 1.
Genetic organization and transcription activation of 3OC6-HSL-controlled genes. White arrows represent 3OC6-HSL-activated genes, and a black arrow represents the 3OC6-HSL-repressed gene. Genes previously reported as 3OC6-HSL controlled are represented by gray arrows. The directions of the arrows indicate the DNA strand. The values for gene activation (relative units of GFP fluorescence per OD unit) are shown above the first gene in each operon and are from the E. coli expression studies described in the text. In all cases, basal levels of expression with LuxR and without 3OC6-HSL or without LuxR and with 3OC6-HSL were <200 fluorescence units. The values are means of three independent experiments, and the ranges were ±28% of the means.
Evidence for direct LuxR activation of 3OC6-HSL-controlled operons.
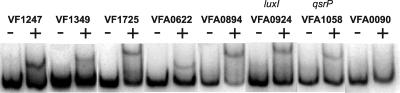
In the first analysis, we examined the dependence of GFP expression driven by the predicted promoter region of each of the 13 3OC6-HSL-regulated V. fischeri transcriptional units in recombinant E. coli. Eleven of the 12 3OC6-HSL-activated promoters showed 3OC6-HSL-LuxR dependence (Fig. 1). The 3OC6-HSL-repressed promoter did not show LuxR-dependent control in E. coli. We confirmed a direct interaction of LuxR with 7 of the 11 promoters activated by LuxR in E. coli (VF1247, VF1349, VF1725, VFA0622, VFA0894, VFA0924, and VFA1058) by showing that they bind to purified LuxR in vitro (Fig. 2). One interpretation of our inability to demonstrate LuxR binding to the other four promoters in vitro is that they are low-affinity LuxR binding targets.
FIG. 2.
Gel shift assays showing direct binding of purified LuxR to promoters of 3OC6-HSL-regulated genes in vitro. Experiments were performed as previously described (28, 37). Reaction mixtures contained 1 fmol of DNA, 6 μM 3OC6-HSL, and either no LuxR (−) or LuxR at a final concentration of 6.2 nM (+). DNA probes were generated by PCR amplification of promoter regions using the transcriptional fusion plasmids as templates. Probes are shown side by side to facilitate comparison, even though sizes are different. VF0090 is shown as an example of a promoter fragment that is not shifted by LuxR, and this serves as a negative control.
Search for LuxR binding sites in 3OC6-HSL-controlled promoter regions.
The LuxR protein interacts with a 20-bp inverted repeat centered at −42.5 from the start of luxICDABEG transcription (6, 8, 9, 37). We sought to assess whether the other LuxR-controlled transcriptional units have similar sequences in their promoter regions by searching for similarities to a relaxed consensus sequence (DVCTGYADKAWNNKACAGKW) based on multiple V. fischeri luxI promoter sequences available from the NCBI database (http://www.ncbi.nlm.nih.gov/) and promoter sequences of qsrP from V. fischeri MJ-100 and ESR1 and ribB from MJ-100 (5) by using the Regulatory Sequence Analysis Tools (http://rsat.ulb.ac.be/rsat/) (38). Out of the 11 promoter regions searched, only 2 showed identifiable lux boxes. The promoter of VFA0924 (luxI) showed the canonical lux box, and the promoter of VFA1058 (qsrP) presented the sequence ACCTGTAATAAACGACAGGA, which was described previously as a potential lux box (5, 28). Our lack of ability to identify lux box elements in the other nine promoters indicates that we do not yet have a clear picture of the elements required for LuxR binding to any given 3OC6-HSL-dependent promoter region. This is not surprising in light of the fact that the Pseudomonas aeruginosa LuxR homolog LasR can bind to lux box-like sequences called las boxes and also to sequences with no obvious similarity to las boxes (35). A considerably more detailed analysis is required to identify the LuxR binding determinants in the newly discovered quorum-sensing controlled promoters.
Acyl-HSL quorum sensing was discovered in V. fischeri, where it regulates the seven-gene lux operon (7, 10, 11). Studies of other proteobacteria have revealed that acyl-HSL signaling often regulates multiple genes that are distributed throughout the genome (for example, see references 34 and 40). In P. aeruginosa, a number of acyl-HSL-controlled genes code for the production of extracellular virulence factors (20, 34, 40). A few V. fischeri 3OC6-HSL-controlled genes in addition to the lux genes have been described previously (5, 28). We used a V. fischeri DNA microarray to identify 14 previously unreported 3OC6-HSL-activated genes and 1 3OC6-HSL-repressed gene. The annotation of the V. fischeri genome indicates four of the 3OC6-HSL-activated genes we identified code for proteases or peptidases (Table 1). Protease and peptidase genes are regulated by acyl-HSL quorum sensing in other bacteria (34, 40). We know that V. fischeri can acquire amino acids from the squid host during growth in the light organ (17). These 3OC6-HSL-induced proteases and peptidases may thus have significant roles in the symbiosis, aiding in nutrient acquisition. The activated genes also include an intimin-like adhesin (VFA0894). In E. coli, an intimin is involved in virulence by mediating bacterial attachment to mammalian host cells (22). Therefore, it is easy to imagine that the V. fischeri intimin-like protein could be involved in the light organ symbiosis. A gene annotated as coding for an accessory colonization factor A-like protein is also activated by 3OC6-HSL. This gene shows significant sequence similarity to the V. cholerae acfA gene, which is required for efficient intestinal colonization by this pathogen (27). Previous studies showed that a LuxR mutant derived from V. fischeri ES114 colonized light organs at a reduced level compared to its parent. The colonization defect could be compensated for by provision of the lux genes under control of a LuxR-independent promoter (39). These experiments on initial light organ colonization suggest that if any of the genes we have uncovered are involved in symbiotic competence, the involvement is likely to be subsequent to initial colonization.
Microarray data accession number.
The microarray data have been deposited in the GEO database (http://www.ncbi.nlm.nih.gov/geo) under accession no. GSE7485.
Acknowledgments
We thank Sudha Chugani for help with real-time PCR experiments and analysis of microarray results. The cDNA hybridization and microarray scanning were performed by the University of Washington Center for Expression Arrays.
This work was supported by a grant from the W. M. Keck Foundation (E.P.G.) and a subcontract from NIH grant GM066786 (A.M.S.).
Footnotes
Published ahead of print on 7 September 2007.
REFERENCES
- 1.Baldi, P., and A. D. Long. 2001. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17:509-519. [DOI] [PubMed] [Google Scholar]
- 2.Bassler, B. L., and R. Losick. 2006. Bacterially speaking. Cell 125:237-246. [DOI] [PubMed] [Google Scholar]
- 3.Boettcher, K. J., and E. G. Ruby. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 172:3701-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boettcher, K. J., and E. G. Ruby. 1995. Detection and quantification of Vibrio fischeri autoinducer from symbiotic squid light organs. J. Bacteriol. 177:1053-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callahan, S. M., and P. V. Dunlap. 2000. LuxR- and acyl-homoserine-lactone-controlled non-lux genes define a quorum-sensing regulon in Vibrio fischeri. J. Bacteriol. 182:2811-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devine, J. H., G. S. Shadel, and T. O. Baldwin. 1989. Identification of the operator of the lux regulon from the Vibrio fischeri strain ATCC7744. Proc. Natl. Acad. Sci. USA 86:5688-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberhard, A., A. L. Burlingame, C. Eberhard, G. L. Kenyon, K. H. Nealson, and N. J. Oppenheimer. 1981. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 20:2444-2449. [DOI] [PubMed] [Google Scholar]
- 8.Egland, K. A., and E. P. Greenberg. 2000. Conversion of the Vibrio fischeri transcriptional activator, LuxR, to a repressor. J. Bacteriol. 182:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egland, K. A., and E. P. Greenberg. 1999. Quorum sensing in Vibrio fischeri: elements of the luxI promoter. Mol. Microbiol. 31:1197-1204. [DOI] [PubMed] [Google Scholar]
- 10.Engebrecht, J., K. Nealson, and M. Silverman. 1983. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32:773-781. [DOI] [PubMed] [Google Scholar]
- 11.Engebrecht, J., and M. Silverman. 1984. Identification of genes and gene products necessary for bacterial bioluminescence. Proc. Natl. Acad. Sci. USA 81:4154-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuqua, C., and E. P. Greenberg. 2002. Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 3:685-695. [DOI] [PubMed] [Google Scholar]
- 13.Fuqua, C., and E. P. Greenberg. 1998. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1:183-189. [DOI] [PubMed] [Google Scholar]
- 14.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 15.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50:727-751. [DOI] [PubMed] [Google Scholar]
- 16.Gilson, L., A. Kuo, and P. V. Dunlap. 1995. AinS and a new family of autoinducer synthesis proteins. J. Bacteriol. 177:6946-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graf, J., and E. G. Ruby. 1998. Host-derived amino acids support the proliferation of symbiotic bacteria. Proc. Natl. Acad. Sci. USA 95:1818-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray, K. M., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1994. Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. J. Bacteriol. 176:3076-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo, A., N. V. Blough, and P. V. Dunlap. 1994. Multiple N-acyl-l-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri. J. Bacteriol. 176:7558-7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. Stewart, A. Lazdunski, and P. Williams. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 21.Lee, K.-H., and E. G. Ruby. 1992. Detection of the light organ symbiont, Vibrio fischeri, in Hawaiian seawater by using lux gene probes. Appl. Environ. Microbiol. 58:942-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo, Y., E. A. Frey, R. A. Pfuetzner, A. L. Creagh, D. G. Knoechel, C. A. Haynes, B. B. Finlay, and N. C. Strynadka. 2000. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature 405:1073-1077. [DOI] [PubMed] [Google Scholar]
- 23.Lupp, C., M. Urbanowski, E. P. Greenberg, and E. G. Ruby. 2003. The Vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for persistence in the squid host. Mol. Microbiol. 50:319-331. [DOI] [PubMed] [Google Scholar]
- 24.Miller, W. G., J. H. Leveau, and S. E. Lindow. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant-Microbe Interact. 13:1243-1250. [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Hagelsieb, G., and J. Collado-Vides. 2002. A powerful non-homology method for the prediction of operons in prokaryotes. Bioinformatics 18(Suppl. 1):S329-S336. [DOI] [PubMed] [Google Scholar]
- 26.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson, K. M., and J. J. Mekalanos. 1988. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect. Immun. 56:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin, N., S. M. Callahan, P. V. Dunlap, and A. M. Stevens. 2007. Analysis of LuxR regulon gene expression during quorum sensing in Vibrio fischeri. J. Bacteriol. 189:4127-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruby, E. G., and K. H. Nealson. 1976. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica: a model of symbiosis based on bacterial studies. Biol. Bull. 151:574-586. [DOI] [PubMed] [Google Scholar]
- 30.Ruby, E. G., M. Urbanowski, J. Campbell, A. Dunn, M. Faini, R. Gunsalus, P. Lostroh, C. Lupp, J. McCann, D. Millikan, A. Schaefer, E. Stabb, A. Stevens, K. Visick, C. Whistler, and E. P. Greenberg. 2005. Complete genome sequence of Vibrio fischeri: a symbiotic bacterium with pathogenic congeners. Proc. Natl. Acad. Sci. USA 102:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 32.Schaefer, A. L., B. L. Hanzelka, A. Eberhard, and E. P. Greenberg. 1996. Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J. Bacteriol. 178:2897-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaefer, A. L., B. L. Hanzelka, M. R. Parsek, and E. P. Greenberg. 2000. Detection, purification, and structural elucidation of the acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 305:288-301. [DOI] [PubMed] [Google Scholar]
- 34.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster, M., M. L. Urbanowski, and E. P. Greenberg. 2004. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc. Natl. Acad. Sci. USA 101:15833-15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, D., J. H. Wang, J. E. Swatton, P. Davenport, B. Price, H. Mikkelsen, H. Stickland, K. Nishikawa, N. Gardiol, D. R. Spring, and M. Welch. 2006. Variations on a theme: diverse N-acyl homoserine lactone-mediated quorum sensing mechanisms in gram-negative bacteria. Sci. Prog. 89:167-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urbanowski, M. L., C. P. Lostroh, and E. P. Greenberg. 2004. Reversible acyl-homoserine lactone binding to purified Vibrio fischeri LuxR protein. J. Bacteriol. 186:631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Helden, J. 2003. Regulatory sequence analysis tools. Nucleic Acids Res. 31:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visick, K. L., J. Foster, J. Doino, M. McFall-Ngai, and E. G. Ruby. 2000. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol. 182:4578-4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waters, C. M., and B. L. Bassler. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21:319-346. [DOI] [PubMed] [Google Scholar]
- 42.Williams, P., K. Winzer, W. C. Chan, and M. Camara. 2007. Look who's talking: communication and quorum sensing in the bacterial world. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362:1119-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]