Abstract
The npd gene cluster, which encodes the enzymes of a p-nitrophenol catabolic pathway from Arthrobacter sp. strain JS443, was cloned and sequenced. Three genes, npdB, npdA1, and npdA2, were independently expressed in Escherichia coli in order to confirm the identities of their gene products. NpdA2 is a p-nitrophenol monooxygenase belonging to the two-component flavin-diffusible monooxygenase family of reduced flavin-dependent monooxygenases. NpdA1 is an NADH-dependent flavin reductase, and NpdB is a hydroxyquinol 1,2-dioxygenase. The npd gene cluster also includes a putative maleylacetate reductase gene, npdC. In an in vitro assay containing NpdA2, an E. coli lysate transforms p-nitrophenol stoichiometrically to hydroquinone and hydroxyquinol. It was concluded that the p-nitrophenol catabolic pathway in JS443 most likely begins with a two-step transformation of p-nitrophenol to hydroxy-1,4-benzoquinone, catalyzed by NpdA2. Hydroxy-1,4-benzoquinone is reduced to hydroxyquinol, which is degraded through the hydroxyquinol ortho cleavage pathway. The hydroquinone detected in vitro is a dead-end product most likely resulting from chemical or enzymatic reduction of the hypothetical intermediate 1,4-benzoquinone. NpdA2 hydroxylates a broad range of chloro- and nitro-substituted phenols, resorcinols, and catechols. Only p-nitro- or p-chloro-substituted phenols are hydroxylated twice. Other substrates are hydroxylated once, always at a position para to a hydroxyl group.
p-Nitrophenol (PNP) is listed as one of 275 hazardous substances which are commonly found at National Priorities List (Superfund) sites and which pose a significant potential threat to human health (38). In the United States the industrial releases of PNP reported to the Environmental Protection Agency totaled 772 lb in 2004 (http://www.epa.gov/tri/tridata/index.htm). In addition to industrial releases, PNP enters the environment through microbial and photochemical degradation of parathion-based insecticides (22, 29, 39).
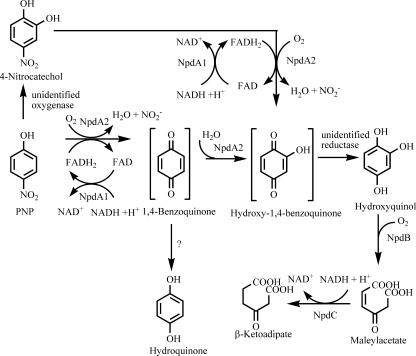
Arthrobacter sp. strain JS443 grows on PNP as a sole source of carbon and energy (20). Jain et al. (20) have reported transient detection of 4-nitrocatechol during degradation of PNP by uninduced whole cells, as well as transformation of m-nitrophenol to nitrohydroquinone by PNP-induced cells. In a 14C trapping experiment, 88% of labeled PNP was transformed to hydroxyquinol (1,2,4-trihydroxybenzene) and 8% was transformed to hydroquinone (1,4-dihydroxybenzene) by PNP-grown whole cells. Extracts of PNP-grown cells catalyzed ortho cleavage of hydroxyquinol but did not oxidize hydroquinone. Based on these results, Jain et al. (20) proposed that PNP is degraded through the nitrocatechol branch of the pathway shown in Fig. 1 and that the first two steps of the pathway involve monooxygenases. The same pathway was proposed for Bacillus sphaericus JS905 (21). An NADH-dependent two-component monooxygenase was found to be responsible for both hydroxylation steps in the transformation of PNP to hydroxyquinol.
FIG. 1.
Proposed pathway for degradation of PNP by Arthrobacter sp. strain JS443. Hypothetical intermediates are indicated by brackets. The pathway encoded by the npd gene cluster proceeds through 1,4-benzoquinone and hydroxy-1,4-benzoquinone, as shown in this study. In strain JS443, an unknown oxygenase diverts an unknown proportion of PNP to 4-nitrocatehcol (20), which reenters the npd pathway after being oxidized by NpdA2. Accumulation of hydroquinone (presumably through reduction of 1,4-benzoquinone) was observed by Jain et al. (20) and in the current study, but its fate is unknown. Hydroquinone is not metabolized by either NpdA2 or NpdB. FADH2, reduced flavin adenine dinucleotide.
PNP degradation pathways have been studied in other bacteria as well, and some of the genes involved in the pathways have been cloned. Moraxella sp. strain 1A degrades PNP through a pathway in which hydroquinone is the ring cleavage substrate (32, 33). The results of nucleic acid hybridization suggest that the genes involved in this pathway are related to the pnp gene cluster of Pseudomonas sp. strain ENV2030 (3). The putative monooxygenase, PnpA, is similar to single-component NAD(P)H-dependent flavoprotein monooxygenases, including PobA (p-hydroxybenzoate hydroxylase) (accession no. NP_248938) (10).
A PNP-induced culture of Rhodococcus sp. strain PN1 accumulated hydroquinone when it was incubated with PNP, while an uninduced culture accumulated 4-nitrocatechol (35). A DNA fragment encoding the transformation of PNP to 4-nitrocatechol was cloned from this strain and was found to contain two genes, nphA1 and nphA2, which encode the oxygenase and reductase components, respectively, of a two-component flavin-diffusible monooxygenase (TC-FDM). A DNA fragment cloned from another PNP-degrading Rhodococcus strain, Rhodococcus opacus SAO101, also includes genes encoding a TC-FDM oxygenase component and a reductase component (npcA and npcB, respectively), as well as a hydroxyquinol 1,2-dioxygenase gene (24). The monooxygenase is essential for growth on PNP and has been demonstrated to transform PNP and 4-nitrocatechol to hydroxyquinol in Escherichia coli crude cell extracts. 2,4,6-Trichlorophenol is also transformed, although the product(s) has not been identified. It has been proposed that PNP metabolism in R. opacus SAO101 occurs through the nitrocatechol branch of the pathway shown in Fig. 1, the same pathway proposed for JS443, with NpcAB being responsible for both initial hydroxylation steps.
As the above discussion illustrates, bacterial aerobic PNP catabolic pathways are often initiated by monooxygenases belonging to the TC-FDM family. TC-FDMs consist of two components: a reduced flavin-dependent monooxygenase (40) and an NAD(P)H-dependent flavin reductase component, which supplies the monooxygenase with its reduced flavin substrate (11). The monooxygenase component is typically capable of functioning independent of the reductase, provided that an alternate source of reduced flavin is available (11, 13, 26, 40), although exceptions have been reported (24). The majority of TC-FDM monooxygenase components fall into one of two homology groups (24). One group, the phenol 2-monooxygenase group, consists of enzymes that hydroxylate phenols ortho to the original hydroxyl group. The phenol 2-monooxygenase group includes the 4-hydroxyphenylacetate monooxygenase HpaB (accession no. CAA82321) (11), the nitrophenol monooxygenase NphA1 (BAB86378) (35), and the phenol monooxygenase PheA (AAC38324) (8). The second major TC-FDM homology group contains phenol 4-monooxygenases, which hydroxylate phenols para to the original hydroxyl group. This group includes 4-chlorophenol monooxygenase CphC-I (AAN08754) (27), the PNP monooxygenase NpcA (BAD30042) (24), the 2,4,5-trichlorophenol monooxygenase TftD (AAC23548) (18), and the 2,4,6-trichlorophenol monooxygenases TcpA (AAM55214) (26) and HadA (BAA13105) (36). The natural substrates of all the monooxygenases in the phenol 4-monooxygenase group have an electron-withdrawing substituent (nitro or chlorine) in the para position, which is displaced as a result of the monooxygenase-catalyzed hydroxylation. All members of the phenol 4-monooxygenase group, with the possible exception of HadA (36), hydroxylate their natural substrates twice. Xun and Webster (41), working with TcpA, have shown that the second hydroxylation is hydrolytic and dependent upon the presence of the electron-withdrawing substituent.
The goals of the present study were to clone and sequence the genes encoding PNP degradation by Arthrobacter sp. strain JS443, to use heterologous expression studies to more definitively elucidate the initial steps in the pathway, and to study the substrate range and positional specificity of the monooxygenase(s). Cloning and sequencing of the npd gene cluster led to identification of TC-FDM oxygenase and reductase component genes. Evidence is presented that the oxygenase component, NpdA2, is capable of carrying out both of the ring hydroxylation steps in the PNP catabolic pathway and that the product of the first hydroxylation step is 1,4-benzoquinone rather than 4-nitrocatechol.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Arthrobacter sp. strain JS443 and E. coli strains were grown routinely at 30 and 37°C, respectively, on LB medium. For protein purification, Arthrobacter sp. strain JS443 was cultivated on glucose minimal salts broth at 25°C. PNP was added to a final concentration of 400 μM at the beginning of cultivation and after 6, 8, and 9 h of incubation. Glucose minimal salts broth is MSB medium (34) supplemented with 20 mM glucose, 200 μg/ml yeast extract, and 200 μg/ml Casamino Acids. Ampicillin, chloramphenicol, and tetracycline were employed, where appropriate, at concentrations of 100, 33, and 14 μg/ml, respectively.
Chemicals.
Nitrohydroquinone was purchased from Frinton Laboratories, Vineland, NJ. Chlorohydroquinone was purchased from Alfa Aesar, Ward Hill, MA. Other aromatic compounds used in this study were purchased from Sigma-Aldrich, St. Louis, MO, and were the highest purity available. 4-Hydroxy-4-methyl-2,5-cyclohexadien-1-one (HMCH) was synthesized as previously described (1).
Construction of a genomic DNA library from Arthrobacter sp. strain JS443.
Genomic DNA was prepared using the following protocol. Cells were grown in LB medium to an optical density of 1.0 and washed with HTE buffer (50 mM Tris-HCl, 25 mM EDTA; pH 8.0). The pellet from a 100-ml culture was resuspended in 20 ml HTE buffer prewarmed to 65°C and incubated for 45 min to inactivate nucleases. Pellets were incubated at 50°C for 1 h in 100 ml HTE buffer with 100 mg chicken egg white lysozyme and 20% polyethylene glycol 1000. Protoplasts were centrifuged for 10 min at 8,800 × g and resuspended in 18 ml of HTE buffer containing 1% sodium N-lauroylsarcosine and 100 μg/ml pronase. The protoplast suspension was incubated overnight at 50°C with shaking at 50 rpm. Cell debris was removed by centrifugation. Triton X-100 (70 μl) was added to the supernatant, which was subjected to cesium chloride gradient centrifugation (30). Purified genomic DNA was partially digested with MboI, fractionated on a sucrose gradient, and ligated to BamHI-digested pHC79 (17). Recombinant cosmids were transfected into E. coli HB101 (4) using λ packaging extract (Promega Corp., Madison, WI).
RT-PCR.
RNA isolation was carried out with an RNeasy mini kit (QIAGEN Inc., Valencia, CA) and an RNase-free DNase set (QIAGEN Inc., Valencia, CA). Reverse transcription (RT)-PCR was carried out using a QIAGEN One-Step RT-PCR kit.
DNA sequence analysis.
An ABI model 377 DNA sequencer and the AutoAssembler program (Applied Biosystems, Foster City, CA) were employed for DNA sequencing. MapDraw and MegAlign (DNASTAR, Madison, WI) were used to identify and translate open reading frames and to generate multiple-sequence alignments. Online BLAST homology searches were also employed (http://www.ncbi.nlm.nih.gov/BLAST/). Pairwise levels of identity were calculated by EMBOSS using the Needleman-Wunsch global alignment algorithm (http://www.ebi.ac.uk/emboss/align/). Analysis of the 16S rRNA gene was performed using the Ribosomal Database Project release 9.44 online server (5).
Purification and N-terminal sequencing of hydroxyquinol 1,2-dioxygenase from Arthrobacter sp. strain JS443.
A 4-liter culture of induced JS443 cells was grown to an optical density of 1.8 and harvested immediately after the final addition of PNP. Harvested cells (28 g, wet weight) were washed with Tris-sucrose buffer (50 mM Tris-HCl [pH 6.0], 10% sucrose) and stored overnight at −80°C.
Cells were incubated with 500 μg/ml chicken egg white lysozyme in 2 ml buffer (50 mM Tris-HCl[pH 6.0], 10% sucrose, 1 mM EDTA) per g cells for 1 h on ice. The cells were pelleted, subjected to one freeze-thaw cycle, and resuspended in 2 ml lysis buffer (50 mM Na/K phosphate buffer [pH 7.0], 10% glycerol, 174 μg/ml phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 20 μg/ml bovine pancreatic DNase I) per g cells. Cells were broken by four passages through a French pressure cell at 15,000 lb/in2. Debris was removed by centrifugation.
Powdered ammonium sulfate was added to the crude cleared lysate to 35% saturation, and the precipitate was removed by centrifugation. The supernatant was dialyzed three times against 2 liters of 10 mM Tris-HCl (pH 7.0) and purified by column chromatography as follows. (i) Anion-exchange chromatography was performed with a Pharmacia HiLoad 26/10 Q-Sepharose Fast Flow fast protein liquid chromatography column. Protein was eluted in a linear gradient consisting of 150 to 447.5 mM NaCl in 600 ml of 20 mM Tris-HCl (pH 7.0) at a flow rate of 5 ml/min. (ii) Gel filtration was performed using a gravity column (95 by 2 cm) packed with Sephacryl S-100 HR resin. Protein was eluted in 50 mM Tris-HCl (pH 7.6) at an initial flow rate of 0.18 ml/min. (iii) Hydrophobic interaction chromatography was performed using a phenyl Superose HR 5/5 fast protein liquid chromatography column. The sample was applied in 0.8 M ammonium sulfate and eluted with a linear gradient consisting of 0.75 to 0.25 M ammonium sulfate in 50 mM Na/K phosphate buffer (pH 7.0) at a flow rate of 0.5 ml/min. The pooled active fractions were dialyzed twice against 500 ml of 20 mM Tris-HCl, and glycerol was added to a final concentration of 10%.
Hydroxyquinol 1,2-dioxygenase was blotted from a polyacrylamide gel onto a polyvinylidene difluoride membrane using a Bio-Rad mini Trans-Blot protein-blotting cell. The band was excised following visualization with Ponceau S stain. The excised band was destained with 5% acetic acid, washed with ultrapure water, and subjected to automated Edman degradation using a Porton 2090 protein sequencer (Beckman Coulter, Inc., Fullerton, CA).
Construction of expression strains.
A PCR product containing npdB was amplified using primers ACAAACGCCGGAAGG and ACCGCAAACAACCTC and was cloned into the expression vector pTrcHis2 TOPO (Invitrogen Corp., Carlsbad, CA). The PCR product included the npdB stop codon so that the resulting expression plasmid, pLP237, would yield native NpdB without a His tag.
The npdA1 gene was amplified using primers ATGAGCATCTCCGAC and CCGACGTCCCAGTTC and was cloned into pCRT7/CT-TOPO (Invitrogen Corp., Carlsbad, CA). The resulting plasmid, pLP4145, was introduced into E. coli BL21(DE3)(pLysS) (Invitrogen Corp., Carlsbad, CA) for expression of NpdA1-His.
Plasmid pLP2728 was constructed by amplifying npdA2 using primers ATGAGGACAGGAAAAG and GACTAGTCATGCATTTAGGCCGGTGTG and cloning it in pCRT7/CT TOPO. The stop codon of npdA2 was included so that native NpdA2 would be produced. SpeI and NsiI restriction sites were added downstream from npdA2 to facilitate cloning of additional DNA fragments for use in experiments not reported here. Cleared lysate from E. coli strain BL21(DE3)(pLysS) carrying pLP2728 was used as the source of NpdA2 for all monooxygenase assays. Reaction mixtures also included the test substrate, NpdA1-His, flavin adenine dinucleotide (FAD), NAD, and an NADH regeneration system. Flavin mononucleotide (FMN) could not be substituted for FAD. Transformation of each test substrate was analyzed by both high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS).
Ultrasonic disruption of E. coli expression strains.
Induced cells were concentrated 100-fold in an appropriate enzyme reaction buffer. TOP10 cells were incubated on ice for 30 min with 1 mg/ml chicken egg white lysozyme. BL21(DE3)(pLysS) cells, which express lysozyme, were incubated on ice for 30 min without lysozyme. Cells were subjected to ultrasonic disruption on ice using a 475-W model XL2020 sonicator (Heat Systems-Ultrasonics Inc., Farmingdale, NY) equipped with a standard 0.5-in. tapped titanium disruptor horn with a flat tip. The power output was set to 30%. Ninety 1-s ultrasonic pulses, separated by 1-s intervals, were applied to the cell suspension. The sonication treatment was repeated after the disruptor horn was cooled in ice for 5 min. Cell debris was removed by centrifugation at 4°C for 30 min at 15,800 × g.
Partial purification of NpdA1-His.
Cleared lysate from induced strain BL21(DE3)(pLysS, pLP4145) was purified through Ni-nitrilotriacetic acid agarose (QIAGEN, Valencia, CA) used according to the recommendations of the supplier. The purification buffer contained 50 mM potassium phosphate buffer (pH 8.0), 50 mM KCl, 20% glycerol, and 10 mM β-mercaptoethanol. The column was washed with purification buffer supplemented with 250 mM NaCl and 5 mM imidazole. Bound protein was eluted from the column into purification buffer supplemented with 50 mM NaCl and 200 mM imidazole. All purification steps were carried out at 4°C or on ice. Eluted fractions were stored at −70°C.
Enzyme assays. (i) Hydroxyquinol 1,2-dioxygenase.
Activity against hydroxyquinol was assayed by following the disappearance of hydroxyquinol spectrophotometrically at 289 nm. The assay buffer contained 50 mM morpholineethanesulfonic acid (MES) (pH 6.5) and 100 μM hydroxyquinol. Stock solutions used in the assay were prepared under argon in 10 mM MES buffer (pH 6.5) containing 1 mM dithiothreitol and stored at −20°C in crimp-sealed vials. The molar extinction coefficient of hydroxyquinol at 289 nm is 3.3 × 103 M−1 cm−1 under the conditions used for the assay. The activity of hydroxyquinol 1,2-dioxygenase against catechol was measured as previously described (7). Activity against other substrates was assayed by monitoring changes in the absorption spectrum.
(ii) Flavin reductase.
Flavin reductase activity was assayed in a quartz cuvette sealed with a screw cap and a Teflon/silicone screw cap insert. The assay mixture contained 200 mM HEPES buffer (pH 7.5), 150 μM NAD(P)H, and 60 μM flavin substrate (FAD or FMN). The reaction mixture was sparged with argon for 2 min before the reaction was started by addition of NpdA1-His. The sealed cuvette was mixed by inversion, and the reaction was followed by monitoring the loss of absorbance at 450 nm. Activity was calculated by using 1.13 × 104 M−1 cm−1 as the extinction coefficient for FAD and 1.22 × 104 M−1 cm−1 as the extinction coefficient for FMN (19).
(iii) Monooxygenase.
The monooxygenase assay was modified from that of Galán et al. (11). The reaction mixture contained 200 mM HEPES buffer (pH 7.5), 0.5 U/ml glucose dehydrogenase (from Bacillus megaterium; Sigma Chemical Co., St. Louis, MO), 150 μM NAD, 50 mM glucose, 120 U/ml catalase (from bovine liver; Sigma Chemical Co., St. Louis, MO), 12 μM FAD, 0.124 U/ml purified NpdA1-His, 1.4 mg protein/ml of cleared lysate from induced E. coli BL21(DE3)(pLysS, pLP2728), and 500 μM monooxygenase substrate.
For time course assays, monooxygenase reactions were carried out in 5-ml reaction mixtures in 70-ml, 4-cm-diameter serum bottles, which were incubated at 25°C with shaking at 125 rpm. Samples were withdrawn, and glacial acetic acid and sodium dithionite were added to final concentrations of 1% and 1 mM, respectively. Precipitated protein was removed by centrifugation prior to analysis by HPLC. Time course assays were performed in triplicate, and each repetition was done with an independent preparation of E. coli BL21(DE3)(pLysS, pLP2728) cleared lysate.
For GC-MS analysis, monooxygenase assays were carried out in 1-ml reaction mixtures in round-bottom test tubes (13 by 2 cm), which were incubated at 25°C with shaking at 50 rpm. Following incubation, phenolic compounds in the reaction mixtures were acetylated with acetic anhydride (14), extracted into 1 ml of pentane, and concentrated by evaporation under nitrogen. The p-cresol transformation was extracted with 1 ml of ethyl acetate instead of pentane and concentrated by evaporation under nitrogen. The acetylation step was omitted.
For each monooxygenase substrate, a control reaction was performed using lysate from E. coli BL21(DE3)(pLysS) carrying the β-galactosidase expression plasmid pCRT7CTLacZ (Invitrogen Corp., Carlsbad, CA). Control reaction mixtures were analyzed by GC-MS.
Analytical methods.
HPLC analysis was carried out with a Beckman System Gold HPLC (Beckman Coulter, Inc., Fullerton, CA) fitted with a diode array detector and a Waters Spherisorb reverse-phase 5-μm C8 analytical column (Alltech Associates, Inc., Deerfield, IL). The dimensions of the analytical column were 25 cm by 4.6 mm. The analytical column was fitted with an All-Guard cartridge system holder containing a Waters Spherisorb 5-μm C8 guard column cartridge (Alltech Associates, Inc., Deerfield, IL). The dimensions of the guard column cartridge were 7.5 by 4.6 mm. The flow rate was 1 ml/min. A gradient separation method was typically employed, in which the mobile phase was changed from 100% solvent A (1% aqueous acetic acid) to 50% solvent A-50% solvent B (1% acetic acid in methanol) at a rate of 2% solvent B/min. The mobile phase was maintained at 50% solvent A-50% solvent B for 10 min. Samples containing 2,4,5-trichlorophenol were separated with a mobile phase gradient of 35 to 70% solvent B, with maintenance of 70% solvent B for 5 min. Samples containing 2,4-dichlorophenol were separated with a mobile phase gradient of 0 to 75% solvent B, followed by 75% solvent B for 7.5 min. Samples containing m-chlorophenol or m-nitrophenol were separated using 40% solvent B.
GC-MS analysis was performed with a Hewlett-Packard 5890 series II gas chromatograph equipped with an HP-5MS (5% diphenylpolysiloxane, 95% dimethylpolysiloxane) capillary column (0.25 mm by 30 m; Hewlett-Packard Corp., Palo Alto, CA) and a Hewlett-Packard 5791 mass spectrometer. Helium was used as the carrier gas. The injection temperature was 250°C. The column temperature was increased from 70 to 210°C at a rate of 12°C/min and kept at the final temperature for 5 min. For analysis of p-cresol transformation, the temperature was increased at a rate of 10°C/min to a final temperature of 142°C.
Nucleotide sequence accession numbers.
The nucleotide sequence of the npd gene cluster has been deposited in the GenBank database under accession number EF052871. The 16S rRNA gene nucleotide sequence of strain JS443 has been deposited in the GenBank database under accession number EF078488.
RESULTS
16S rRNA gene analysis of Arthrobacter sp. strain JS443.
Strain JS443 was isolated and identified as a member of the genus Arthrobacter in 1984 using non-nucleic acid-based criteria (31). In order to obtain molecular confirmation of this assignment, the sequence of the 16S rRNA gene of Arthrobacter sp. strain JS443 was determined and analyzed using the classifier and seqmatch tools at the Ribosomal Database Project release 9.44 online server (5). This analysis placed JS443 within the genus Arthrobacter with 100% confidence. The closest-match type sequence (96.8% identity) is that of Arthrobacter ureafaciens DSM 20126 (GenBank accession number X80744) (25). The closest-match nontype sequence (99.6% identity) is that of Arthrobacter keyseri strain 12B (GenBank accession number AF256196), which carries the phthalate degradation plasmid pRE1 (9).
Purification of hydroxyquinol 1,2-dioxygenase from Arthrobacter sp. strain JS443.
Cells grown with PNP were disrupted by sonication, and hydroxyquinol 1,2-dioxygenase was purified from the resulting lysate (Table 1). The specific activity of the purified hydroxyquinol 1,2-dioxygenase was 58 μmol/min/mg protein.
TABLE 1.
Purification of hydroxyquinol 1,2-dioxygenase from Arthrobacter sp. strain JS443
| Purification step | Total protein (mg) | Total activity (μmol min−1) | Sp act (μmol min−1 mg protein−1) | Yield (%) |
|---|---|---|---|---|
| Crude lysate | 620 | 12 | 0.019 | |
| Ammonium sulfate | 610 | 18 | 0.029 | |
| Dialysis | 460 | 200 | 0.43 | 100 |
| Q-Sepharose Fast Flow | 6.8 | 95 | 14 | 48 |
| Sephacryl 100 | 0.95 | 30 | 32 | 15 |
| Phenyl Superose | 0.12 | 6.7 | 58 | 3.3 |
Substrate range of hydroxyquinol 1,2-dioxygenase.
Hydroxyquinol 1,2-dioxygenase is weakly active against catechol but inactive against 4-nitrocatechol, hydroquinone, protocatechuate, and 2,4-dihydroxybenzoate. The rate of catechol transformation by hydroxyquinol 1,2-dioxygenase is 0.73% ± 0.060% of the rate of hydroxyquinol cleavage. During cleavage of catechol, the absorbance maximum at 275 nm decreases and the absorbance at 260 nm increases (data not shown), which is indicative of ortho cleavage (12). There was no evidence of meta cleavage.
Cloning of a gene cluster containing the hydroxyquinol 1,2-dioxygenase gene.
Hydroxyquinol 1,2-dioxygenase was electroblotted from a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel onto a polyvinylidene difluoride membrane, and the amino-terminal sequence, STRPATAISPEQAAVEQQLVD, was determined by automated Edman degradation. A 53-bp oligonucleotide fragment was amplified from JS443 genomic DNA by PCR, using degenerate primers designed to match bases 1 to 17 of the coding strand (including an ATG start codon) and bases 53 to 34 of the noncoding strand of the degenerate sequence. Plasmid pTOPO953 was constructed by ligation of the PCR product into pCRII-TOPO (Invitrogen, Carlsbad, CA). The identity of the cloned PCR product was confirmed by nucleotide sequencing.
A library of Arthrobacter sp. strain JS443 genomic DNA was constructed in the cosmid vector pHC79 and screened by Southern hybridization with a probe derived from pTOPO953. The insert from one positive clone, pLP18B4H, included a 6.2-kb EcoRI fragment that hybridized to the probe. The 6.2-kb EcoRI fragment and an overlapping 7.1-kb NotI-PstI were subcloned and sequenced. The total length of the sequenced region of the JS443 genome is 12,559 bp. This region includes the npd gene cluster, which consists of seven open reading frames, including the hydroxyquinol 1,2-dioxygenase gene npdB.
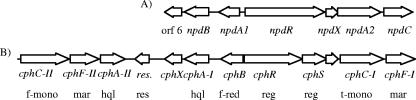
The npd gene cluster is closely related to the cph gene cluster, which encodes the catabolism of 4-chlorophenol in Arthrobacter chlorophenolicus A6 (accession no. AAN08754), (27) (Fig. 2). Little is known about the cph genes, which were identified by hybridization to a hydroxyquinol 1,2-dioxygenase probe. The cph genes can be divided into two groups, cph cluster I, located to the right of the resolvase pseudogene in Fig. 2, and cph cluster II, located to the left of the resolvase pseudogene. A cphA-I insertional mutant accumulates hydroxyquinol and fails to grow on 4-chlorophenol, suggesting that cluster I, but not cluster II, is involved in catabolism of 4-chlorophenol (27). Cluster I is 80.5% identical to the npd gene cluster and has a nearly identical gene organization. Both cph cluster I and the npd gene cluster include a putative flavin reductase gene (npdA1 and cphB) and a gene encoding a putative monooxygenase belonging to the TC-FDM family (npdA2 and cphC-I). NpdA2 is 96% identical to CphC-I (accession no. AAN08754). Sequence similarity between the DNA fragment cloned from strain JS443 (accession no. EF078488) and the DNA fragment cloned from strain A6 (accession no. AAN08754) is restricted to the region between orf6/cphX and npdC/cphF-I. This suggests that the npd gene cluster and cph gene cluster I are at different locations in the genomes to which they belong.
FIG. 2.
Organization of (A) the npd gene cluster from Arthrobacter sp. strain JS443 and (B) the homologous cph gene cluster which encodes 4-chlorophenol catabolism in A. chlorophenolicus A6 (accession number AAN08754) (25). cphR and cphS are putative regulatory genes which are homologous to the 5′ and 3′ and, respectively, of npdR. Putative functions are shown below the cph genes. Abbreviations: f-mono, flavoprotein monooxygenase; mar, maleylacetate reductase; hql, hydroxyquinol 1,2-dioxygenase; res, resolvase pseudogene; f-red, flavin reductase; reg, transcriptional regulator; t-mono, TC-FDM.
A notable difference between the npd gene cluster and cph gene cluster I concerns the putative regulatory genes. cph gene cluster I contains two putative regulatory genes, cphR and cphS, whose products belong to the MalT and DnrI families of transcriptional regulators, respectively. In the npd gene cluster, these two genes are fused into a single gene, npdR, due to the presence of a CAG codon in place of the TAG codon that terminates translation of cphR. Several other examples of putative regulatory genes with 5′ homology to cphR and 3′ homology to cphS have been found in the GenBank database, including a putative regulatory gene from Symbiobacterium thermophilum IAM 14863 (accession no. BAD39795) (37).
npdA2 and npdB are expressed in Arthrobacter sp. strain JS443 during growth on PNP.
High-quality DNA-free RNA was purified from JS443 cells grown in the presence and absence of PNP. The RNA was used as a template for RT-PCR using primer pairs designed to amplify fragments internal to npdA2 (872 bp) and npdB (882 bp). DNA fragments of the expected sizes were amplified successfully from RNA purified from a JS443 culture grown with PNP but not from a JS443 culture grown without PNP (data not shown). Controls for DNA contamination were negative. This result indicates that both npdA2 and npdB are expressed during growth with PNP.
Expression of npdB in E. coli.
Lysate from induced E. coli TOP10 cells (Invitrogen Corp., Carlsbad, CA) carrying pLP237 contained 0.62 U of hydroxyquinol 1,2-dioxygenase/mg protein and an abundant protein with the same electrophoretic mobility as hydroxyquinol 1,2-dioxygenase purified from Arthrobacter sp. strain JS443 (data not shown). Lysate from E. coli TOP10 cells lacking pLP237 has no detectable hydroxyquinol 1,2-dioxygenase activity.
Expression of npdA1 in E. coli.
NpdA1-His expressed from pLP4145 was purified to near homogeneity by Ni2+-nitrilotriacetic acid agarose affinity chromatography. The specific activity of the partially purified protein was 31 μmol/min/mg protein. NpdA1-His is colorless, indicating the absence of a tightly bound flavin cofactor (23). NpdA1-His is able to reduce both FAD and FMN but has a marked preference for FAD. NADH is the preferred electron donor (Table 2).
TABLE 2.
Substrate specificity of NpdA1-His
| Flavin substrate | Electron donor | Relative activity (%) |
|---|---|---|
| FAD | NADH | 100 ± 6 |
| FMN | NADH | 14 ± 3 |
| FAD | NADPH | 2.2 ± 0.3 |
Transformation of para-substituted phenols by NpdA2.
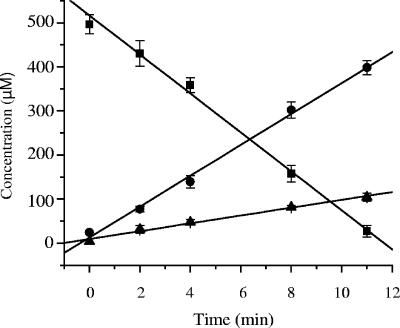
PNP is transformed to hydroquinone and hydroxyquinol by an in vitro reaction system containing NpdA2 (Fig. 3). 4-Nitrocatechol was not detected in PNP transformation assays. The rate of PNP consumption (31 ± 2 nmol/min/mg cleared lysate protein) is equivalent to the combined rates of hydroquinone and hydroxyquinol accumulation (Table 3), indicative of stoichiometric or nearly stoichiometric conversion of PNP to hydroquinone and hydroxyquinol.
FIG. 3.
Transformation of PNP by an E. coli lysate containing NpdA2. The rates of substrate disappearance (PNP [▪]) and product appearance (hydroxyquinol [•] and hydroquinone [▴]) are averages from at least three assays, carried out using independent preparations of E. coli BL21(DE3)(pLysS, pLP261), as described in Materials and Methods. Samples were withdrawn at the indicated time points and treated immediately with glacial acetic acid (1%) and sodium dithionite (1 mM) to stop the reaction and reduce quinones to quinols. Substrates and products were quantified by HPLC.
TABLE 3.
Transformation rates for E. coli lysate containing NpdA2
| Substrate | Degradation rate (nmol/min/mg lysate protein) | Product | Accumulation rate (nmol/min/mg lysate protein) |
|---|---|---|---|
| 4-Chlorocatechol | 14.0 ± 0.7 | Hydroxyquinol | 7.8 ± 0.9 |
| Differencea | 6 ± 1 | ||
| Chlorohydroquinone | NDb | Hydroxyquinol | ND |
| m-Chlorophenol | ND | Chlorohydroquinone | ND |
| p-Chlorophenol | 18 ± 2 | Hydroquinone | 4 ± 1 |
| Hydroxyquinol | 15 ± 1 | ||
| 4-Chlororesorcinol | 18 ± 2 | Hydroxyquinol | 16.6 ± 0.9 |
| m-Cresol | ND | Methylhydroquinone | ND |
| p-Cresol | ND | HMCH | ND |
| 2,4-Dichlorophenol | 14.5 ± 0.9 | Chlorohydroquinone | 3.1 ± 0.2 |
| Hydroxyquinol | 7 ± 2 | ||
| Difference | 4.1 ± 0.9 | ||
| 2,4-Dinitrophenol | ND | Nitrohydroquinone | ND |
| 4-Methylcatechol | ND | 5-Methylhydroxy-quinol | ND |
| 4-Nitrocatechol | 15.7 ± 0.9 | Hydroxyquinol | 15.8 ± 0.3 |
| m-Nitrophenol | 10 ± 1 | Nitrohydroquinone | 9 ± 1 |
| PNP | 31 ± 2 | Hydroquinone | 7 ± 2 |
| Hydroxyquinol | 25 ± 0.9 | ||
| Phenol | ND | Hydroquinone | ND |
| 2,4,5-Trichlorophenol | ND | 2,5-Dichloro-hydroquinone | ND |
| 5-Chlorohydroxy-quinol | ND | ||
| 2,4,6-Trichlorophenol | ND | 2,6-Dichlorohydro-quinone | ND |
| 6-Chlorohydroxy-quinol | ND | ||
| Unidentified | ND |
Difference between rate of substrate disappearance and sum of the rates of product accumulation. Some or all of the difference is due to accumulation of a product which has been identified as 5-chlorohydroxyquinol based on MS characteristics, but for which an authentic standard with a known concentration is unavailable.
ND, not determined.
Because of the structural similarity between PNP and p-chlorophenol, NpdA2 was tested for the ability to transform p-chlorophenol. p-Chlorophenol, like p-nitrophenol, is transformed stoichiometrically to hydroxyquinol and hydroquinone. p-Chlorophenol is consumed at a rate of 18 ± 2 nmol/min/mg cleared lysate protein, which is appreciably less than the rate of PNP transformation.
The effects of additional chlorine or nitro groups beyond the para substituent were evaluated using 2,4-dinitrophenol, 2,4-dichlorophenol, 2,4,5-trichlorophenol, and 2,4,6-trichlorophenol as test substrates. This was done in order to evaluate the potential of NpdA2 to degrade these compounds, some of which are degraded through pathways involving monooxygenases related to NpdA2, and also to determine whether the second hydroxylation occurs preferentially at a substituted position. The results of these assays showed that NpdA2 transforms 2,4-dinitrophenol poorly and yields only one product, nitrohydroquinone. By contrast, 2,4-dichlorophenol is transformed efficiently into both chlorohydroquinone and hydroxyquinol. This transformation is not stoichiometric (Table 3), but no unidentified product peaks were observed that might account for the discrepancy between the substrate disappearance rate and the product accumulation rates.
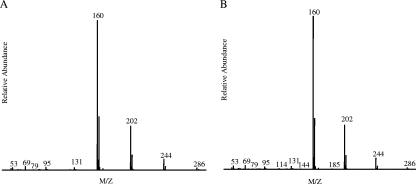
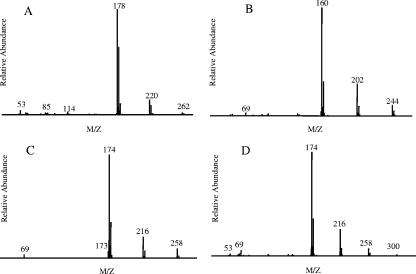
2,4,5-Trichlorophenol is transformed slowly into two products. The mass spectrum and GC-MS retention time of the acetylated derivative of one of the products are identical to those of the acetylated derivative of authentic 2,5-dichlorohydroquinone. The acetylated derivative of the second product has a molecular ion peak at 286 mass units and major fragments at 244, 202, and 160 mass units (due to the sequential loss of three acetyl groups) (Fig. 4A). The ratio of the abundance of the 160-mass unit fragment to the abundance of the 162-mass unit fragment indicates that a single chlorine atom is present. These features are consistent with identification of the compound as 5-chlorohydroxyquinol, for which an authentic standard was not available. However, due to the positions of the substituents of 2,4,5-trichlorophenol, replacement of two of the three chlorine substituents with hydroxyl groups can result only in 5-chlorohydroxyquinol, not one of its isomers. Therefore, it is highly likely that the transformation product is 5-chlorohydroxyquinol. 5-Chlorohydroxyquinol has also been identified as a product of 2,4,5-trichlorophenol transformation by the NpdA2 homolog TftD (13).
FIG. 4.
Mass spectra of the acetylated derivatives of the NpdA2 transformation product identified as 5-chlorohydroxyquinol. Transformations were carried out using cleared lysates of E. coli BL21(DE3)(pLysS, pLP261) as the source of NpdA2, as described in Materials and Methods. The transformation substrates were 2,4,5-trichlorophenol (A) and 4-chlorocatechol (B). Following incubation for 30 min at 25°C, reaction mixtures were acetylated with acetic anhydride and analyzed by GC-MS.
E. coli lysate containing NpdA2 transforms 2,4,6-trichlorophenol into four products having retention times of 15.4, 17.2, 17.9, and 18.5 min. The mass spectral characteristics of the acetylated derivative of the product with a retention time of 15.4 min indicate that it is a dichlorinated compound with two hydroxyl groups and a molecular weight of 178 (Fig. 5A). The compound can theoretically be identified as either 3,5-dichlorocatechol or 2,6-dichlorohydroquinone. However, the transformation of PNP and 4-chlorophenol to hydroquinone, but not to substituted catechols, suggests that the 2,4,6-trichlorophenol transformation product is most likely 2,6-dichlorohydroquinone. The mass spectrum of the acetylated derivative of the product with a retention time of 17.2 min indicates that it is a monochlorinated compound with three hydroxyl groups and a molecular weight of 160 (Fig. 5B). The most likely identity of this transformation product is 6-chlorohydroxyquinol. The mass spectrum of the acetylated derivative of the 2,4,6-trichlorophenol transformation product with a retention time of 18.5 min indicates that the transformation product is a monochlorinated compound with three hydroxyl groups and a molecular weight of 174 (Fig. 5D). The derivative of the remaining transformation product has a nearly identical mass spectrum except for the absence of a detectable 200-mass unit molecular ion peak (Fig. 5C), which is probably present but below the detection threshold. The molecular weights of the two transformation products suggest that they could be methylated 6-chlorohydroxyquinol derivatives, but how such compounds could result from transformation of 2,4,6-trichlorophenol by NpdA2 is unclear.
FIG. 5.
Mass spectra of the acetylated derivatives of 2,4,6-trichlorophenol transformation products. (A) Product identified as 2,6-dichlorohydroquinone. (B) Product identified as 6-chlorohydroxyquinol. (C and D) Unidentified transformation products. Transformations were carried out using cleared lysates of E. coli BL21(DE3)(pLysS, pLP261) as the source of NpdA2, as described in Materials and Methods. Following incubation for 30 min at 25°C, reaction mixtures were acetylated with acetic anhydride and analyzed by GC-MS.
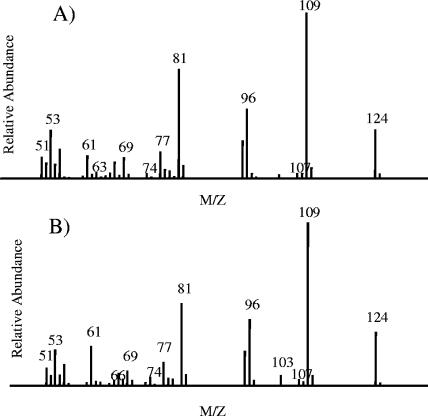
In order to determine the effect of the presence of a non-electron-withdrawing substituent para to a hydroxyl group, the transformation of p-cresol by NpdA2 was tested. This transformation yielded a metabolite which could be extracted with ethyl acetate from an unacetylated transformation reaction mixture. The transformation product's mass spectrum and GC retention time are identical to the mass spectrum and GC retention time of an authentic sample of HMCH (Fig. 6).
FIG. 6.
Mass spectra of the p-cresol transformation product identified as HMCH (A) and authentic HMCH (B). Transformations were carried out using cleared lysates of E. coli BL21(DE3)(pLysS, pLP261) as the source of NpdA2, as described in Materials and Methods.
Transformation of meta-substituted phenols by NpdA2.
PNP-induced JS443 cells transform m-nitrophenol to nitrohydroquinone (20). If NpdA2 is responsible for this activity, this implies that the location of the first NpdA2-mediated hydroxylation is determined by the location of the existing hydroxyl group rather than by the location of the nitro group. It also implies that a second hydroxylation by NpdA2 depends upon the presence of an electron-withdrawing group para to the existing hydroxyl group, as shown previously for TcpA (41). Testing confirmed that the in vitro reaction system containing NpdA2 transforms m-nitrophenol stoichiometrically to nitrohydroquinone without producing any detectable hydroxyquinol. The transformation rate was relatively low compared to those of other substrates for which a rate was determined (Table 3). m-Chlorophenol is transformed to chlorohydroquinone, also with no production of hydroxyquinol, even though NpdA2 is able to transform chlorohydroquinone to hydroxyquinol when chlorohydroquinone is provided as the only substrate. A transformation rate for m-chlorophenol was not determined because transformation was nonlinear (data not shown). To determine the effect of a non-electron-withdrawing substituent in the meta position, m-cresol was tested as a substrate and was found to be transformed to methylhydroquinone. Nitrohydroquinone, chlorohydroquinone, and methylhydroquinone were all identified by HPLC and GC-MS by comparison to authentic standards.
Transformation of dihydroxylated substrates.
The PNP monooxygenase NpcA, which, like NpdA2, is a member of the TC-FDM 4-monooxygenase group, is able to transform 4-nitrocatechol to hydroxyquinol (24). 4-Nitrocatechol, 4-chlorocatechol, and 4-nitroresorsinol are all transformed to hydroxyquinol by PNP-induced cells of JS443 (20). To determine whether NpdA2 can carry out these transformations, 4-nitrocatechol and 4-chlorocatechol were tested as substrates. 4-Nitroresorsinol was not available commercially, so 4-chlororesorcinol was tested instead. 4-Nitrocatechol, 4-chlororesorcinol, and 4-chlorocatechol were all good substrates for NpdA2 (Table 3). 4-Nitrocatechol and 4-chlororesorcinol were transformed stoichiometrically to hydroxyquinol by the in vitro reaction system containing NpdA2. 4-Chlorocatechol was transformed into two products, one of which was hydroxyquinol. The acetylated derivative of the second product had a mass spectrum and GC retention time indistinguishable from those of the acetylated derivative of the 2,4,5-trichlorophenol transformation product identified as 5-chlorohydroxyquinol (Fig. 4). Therefore, the second 4-chlorocatechol transformation product was probably 5-chlorohydroxyquinol. 4-Methylcatechol was transformed to a compound whose acetylated derivative had mass spectral characteristics consistent with those of the acetylated derivative of 5-methylhydroxyquinol (data not shown).
Hydroquinone, resorcinol, and catechol are not substrates for NpdA2. Chlorohydroquinone is a poor substrate but is transformed to hydroxyquinol at a very low rate.
DISCUSSION
Substrate range of NpdA2.
The present study involved a more extensive examination of the substrate range and transformation products than has previously been done for a single TC-FDM belonging to the 4-monooxygenase sequence similarity group. Our results show that NpdA2 has a remarkably broad substrate range, including phenols, catechols, resorcinols, and (hypothetically) 1,4-benzoquinones, with and without chlorine, nitro, or methyl substituents.
Range of substrates that are hydroxylated twice by NpdA2.
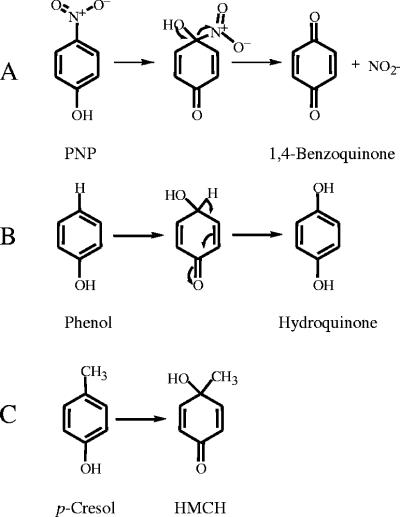
NpdA2 is capable of carrying out para hydroxylation of a broad range of phenolic substrates, but a second hydroxylation is carried out only on phenols that have a para electron-withdrawing substituent, including PNP, p-chlorophenol, 2,4-dichlorophenol, 2,4,5-trichlorophenol, and 2,4,6-trichlorophenol. It is well known that a monooxygenase attack on an aromatic ring at a position occupied by an electron-withdrawing group produces a quinone (nonaromatic ring, often referred to as a benzoquinone) (Fig. 7A), while an attack at an unsubstituted position produces a quinol, such as hydroquinone (Fig. 7B) (6, 15, 16, 33). Therefore, a possible explanation for the observed properties of NpdA2 is that 1,4-benzoquinones are substrates for NpdA2 but hydroquinones are not (with the exception of chlorohydroquinone, which is a very poor substrate). This hypothesis has been proposed previously (41) to explain the differential activity of the NpdA2 homolog TcpA against 2,4,6-trichlorophenol (hydroxylated twice) and 2,6-dichlorophenol (hydroxylated once). 1,4-Benzoquinones and hydroxy-1,4-benzoquinones are not detected as in vitro transformation products because they are readily reduced to hydroquinones and hydroxyquinols by reducing agents and/or reductases used in experimental procedures. Our monooxygenase assay mixtures contained E. coli lysate, which is known to contain enzymes capable of reducing 1,4-benzoquinone to hydroquinone (43). Furthermore, monooxygenase assay samples were treated with the reducing agent sodium dithionite prior to HPLC analysis. This was done for the purpose of reducing benzoquinones, which could otherwise spontaneously form insoluble polymers before HPLC could be performed.
FIG. 7.
Fate of hypothetical monooxygenase reaction intermediates. (A) Monooxygenase attack at a site occupied by an electron-withdrawing substituent. (B) Monooxygenase attack at a site occupied by an electron-donating substituent. (C) Transformation of p-cresol to HMCH by NpdA2.
Effects of substituents on the ability of NpdA2 to transform substrates.
Unsubstituted aromatic compounds make relatively poor substrates. Phenol is transformed slowly, while catechol and hydroquinone are not transformed at all. Interestingly, 4-methylcatechol is a substrate. This suggests that the positive effect of a substituent on transformation by NpdA2 is at least partly steric, since a methyl group would not be expected to play a role in the reaction mechanism. Even substituted hydroquinones are poor substrates. Chlorohydroquinone is transformed extremely slowly, and nitrohydroquinone is not transformed at all. Hydroxylated 1,4-benzoquinones are apparently not substrates, since no compound with more than three hydroxyl groups was detected as a transformation product. The presence of nitrohydroquinone but not hydroxyquinol in the 2,4-dinitrophenol transformation indicates that nitro-1,4-benzoquinone is also not a substrate.
Transformation of p-cresol by NpdA2.
The ability of NpdA2 to transform p-cresol to HMCH is evidence of the unusual promiscuity of NpdA2. It is likely that compounds analogous to HMCH are formed as intermediates during monooxygenase attack on other substrates (Fig. 7A and B). Due to its lack of polarity, the methyl group of HMCH is not eliminated. To our knowledge, this is the first report of the transformation of p-cresol to HMCH by a monooxygenase.
Positional specificity of NpdA2 for catechols and resorcinols.
It is clear from the data in Table 3 that hydroxylation of phenols, catechols, and resorcinols by NpdA2 occurs exclusively at a position para to a hydroxyl group. Catechols and resorcinols therefore have two potential sites for hydroxylation by NpdA2. In the cases of two of the dihydroxylated substrates, 4-nitrocatechol and 4-chlororesorcinol, NpdA2 attacks the substituted position exclusively, yielding hydroxyquinol as the only product. However, NpdA2 has a relaxed positional specificity for 4-chlorocatechol, which can be attacked para to either hydroxyl group, yielding both hydroxyquinol and 5-chlorohydroxyquinol as detectable products. The relaxed positional specificity of NpdA2 for 4-chlorocatechol could be a function of the smaller size of the chlorine substituent relative to the nitro group, resulting in less steric constraint on the position of the substrate in the active site. This would also explain why 4-methylcatechol can be hydroxylated at the unsubstituted position.
Like NpdA2, intact chlorophenol-induced cells of A. chlorophenolicus A6, which carries cphC-I, transform 4-chlorocatechol to 5-chlorohydroxyquinol (27). It is likely, given the sequence similarity between CphC-I and NpdA2, that CphC-I shares with NpdA2 the ability to hydroxylate 4-chlorocatechol para to either hydroxyl group. However, this has not been confirmed with purified or heterologously expressed CphC-I.
Positional specificity of NpdA2 for 1,4-benzoquinones.
Proposed hydroxylations of 1,4-benzoquinones by NpdA2 appear to occur preferentially at chlorinated positions. Only two transformation products each were detected from 2,4-dichlorophenol and 2,4,5-trichlorophenol. In each case, one product was a hydroquinone, presumably reduced from the benzoquinone transformation product, and the other product was a hydroxyquinol having one less chlorine than the hydroquinone. The same was true for 2,4,6-trichlorophenol except that two unknown compounds were also detected. These unidentified compounds were both monochlorinated and could not have resulted from hydroxylation of 2,6-dichloro-1,4-benzoquinone at an unsubstituted position. Because stoichiometric conversion from substrates to products could not be demonstrated for any of the three phenols mentioned above, the possibility of undetected transformation of chlorinated benzoquinones at unsubstituted positions cannot be ruled out. Stoichiometric conversion of 2,4,6-trichlorophenol to 2,6-dichloro-1,4-benzoquinone and 6-chlorohydroxyquinol has been demonstrated for TcpA (26), as would be expected for an enzyme adapted to the catabolism of 2,4,6-trichlorophenol. Likewise, 2,5-dichlorohydroquinone and 5-chlorohydoxyquinol are the only reported products of 2,4,5-trichlorophenol oxidation by TftD (13).
Reevaluation of the proposed PNP catabolic pathway of strain JS443.
Based on the evidence currently available, PNP is degraded by Arthrobacter sp. strain JS443 according to the pathway shown in Fig. 1. The role of hydroquinone in PNP catabolism in JS443 is still unclear. The results of a metabolic trapping experiment indicate that hydroquinone is a minor PNP catabolic intermediate. In the metabolic trapping experiment, 8% of 14C from radiolabeled PNP was recovered as hydroquinone, compared to 88% recovery for hydroxyquinol (20). This is in contrast to the results of an oxygen consumption experiment, in which hydroquinone addition failed to produce an increase in oxygen consumption by PNP-grown JS443 cell lysate (20). Accumulation of hydroquinone by the in vitro system used in this study is not sufficient evidence to conclude that hydroquinone is a PNP metabolite in vivo. In the in vitro system, hydroquinone accumulation is likely due to enzymatic or nonenzymatic reduction of 1,4-benzoquinone. It cannot be assumed that this also occurs under the conditions found in the intact JS443 cell.
Reduction of 2-hydroxy-1,4-benzoquinone to hydroxyquinol by intact JS443 cells is probably an enzymatic process (2, 6, 42). The pnp gene cluster, which encodes a PNP catabolic pathway in Pseudomonas sp. strain ENV2030 (3) and Pseudomonas putida JS444 (44), contains a putative 1,4-benzoquinone reductase gene whose hypothetical product is related to the so-called trp repressor binding protein of E. coli, which was recently shown to be a reductase (28), as well as to fungal benzoquinone reductases (3). No putative 2-hydroxy-1,4-benzoquinone reductase gene has been identified in the npd gene cluster. It is possible that one of the open reading frames with unknown function in the npd gene cluster encodes a 2-hydroxy-1,4-benzoquinone reductase. Alternatively, reduction of 2-hydroxy-1,4-benzoquinone could be carried out by a broad-specificity reductase similar to NfsA (43) of E. coli.
In contrast to data obtained from work with JS443 (20), we failed to detect accumulation of 4-nitrocatechol during oxidation of PNP by E. coli lysate containing NpdA2. The observation that there was transient accumulation of 4-nitrocatechol in JS443 cultures but not in E. coli cell lysate containing NpdA2 suggests that a second PNP monooxygenase is present in strain JS443 but not in E. coli. There is evidence that R. opacus SAO101 and Rhodococcus sp. strain PN1 (24, 35) each possess both a PNP 4-monooxygenase and a PNP 2-monooxygenase. Similarly, both hydroquinone and 4-chlorocatechol are found in 4-chlorophenol-degrading A. chlorophenolicus A6 (27). If a PNP 2-monooxygenase is present in JS443, it is clearly not necessary for conversion of PNP to hydroxyquinol and may or may not play a significant role in PNP degradation. Because neither NpdA2 nor PNP 2-monooxygenase activity has been quantified in JS443 cells, the relative importance of each enzyme for PNP transformation is not known. It is also not known whether 4-nitrocatechol is degraded exclusively by NpdA2 or if a second 4-nitrocatechol pathway might exist, perhaps genetically linked to the PNP 2-monooxygenase. Mutagenesis of NpdA2 will be necessary to determine whether significant PNP catabolism occurs independent of NpdA2.
Acknowledgments
We thank L. Newman, M. Logan, M. Häggblom, L. Launen, H. Fisher, J. Bick, and B. Applegate for helpful discussions. We thank J. Spain for the generous gift of Arthrobacter sp. strain JS443 and both J. Spain and B. Applegate for reviewing the manuscript. We acknowledge M. Murillo, J. Simone, and R. Jacobi for technical assistance with nucleotide sequencing and D. Brune for technical assistance with amino acid sequencing. Finally, we acknowledge the late Carl T. Wigal and his research group at Lebanon Valley College of Pennsylvania for synthesis of the HMCH standard.
Footnotes
Published ahead of print on 24 August 2007.
REFERENCES
- 1.Aponick, A., J. D. McKinley, J. C. Raber, and C. T. Wigal. 1998. Quinone alkylation using organocadmium reagents: a general synthesis of quinols. J. Org. Chem. 63: 2676-2678. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, S., T. R. Patel, and M. Whalen. 1993. Detoxification mechanisms for 1,2,4-benzenetriol employed by a Rhodococcus sp. BPG-8. Arch. Microbiol. 159: 136-140. [Google Scholar]
- 3.Bang, S.-W. 1997. Molecular analysis of p-nitrophenol degradation by Pseudomonas sp. strain ENV2030. Ph.D. thesis. Rutgers, The State University of New Jersey, New Brunswick, NJ.
- 4.Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41: 459-472. [DOI] [PubMed] [Google Scholar]
- 5.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, S. A. Kulam, D. M. McGarrell, G. M. Garrity, and J. M. Tiedje. 2005. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33: D294-D296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai, M., J. B. Rogers, J. R. Warner, and S. D. Copley. 2003. A previously unrecognized step in pentachlorophenol degradation in Sphingobium chlorophenolicum is catalyzed by tetrachlorobenzoquinone reductase (PcpD). J. Bacteriol. 185: 302-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorn, E., and H.-J. Knackmuss. 1978. Chemical structure and biodegradability of halogenated aromatic compounds: substituent effects on 1,2-dioxygenation of catechol. Biochem. J. 174: 85-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffner, F. M., and R. Müller. 1998. A novel phenol hydroxylase and catechol 2,3-dioxygenase from the thermophilic Bacillus thermoleovorans strain A2: nucleotide sequence and analysis of the genes. FEMS Microbiol. Lett. 161: 37-45. [DOI] [PubMed] [Google Scholar]
- 9.Eaton, R. W. 2001. Plasmid-encoded phthalate catabolic pathway in Arthrobacter keyseri 12B. J. Bacteriol. 183: 3689-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Entsch, B., Y. Nan, K. Weaich, and K. F. Scott. 1988. Sequence and organization of pobA, the gene coding for p-hydroxybenzoate hydroxylase, an inducible enzyme from Pseudomonas aeruginosa. Gene 71: 279-291. [DOI] [PubMed] [Google Scholar]
- 11.Galán, B., E. Díaz, M. A. Prieto, and J. L. García. 2000. Functional analysis of the small component of the 4-hydroxyphenylacetate 3-monooxygenase of Escherichia coli W: a prototype of a new flavin:NAD(P)H reductase subfamily. J. Bacteriol. 182: 627-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson, D. T. 1971. Assay of enzymes of aromatic metabolism. Methods Microbiol. 6A: 463-478. [Google Scholar]
- 13.Gisi, M. R., and L. Xun. 2003. Characterization of chlorophenol 4-monooxygenase (TftD) and NADH:flavin adenine dinucleotide oxidoreductase (TftC) of Burkholderia cepacia AC1100. J. Bacteriol. 185: 2786-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Häggblom, M. M., L. J. Nohynek, and M. S. Salkinoja-Salonen. 1988. Degradation and O-methylation of chlorinated phenolic compounds by Rhodococcus and Mycobacterium strains. Appl. Environ. Microbiol. 54: 3043-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haigler, B. E., W. C. Suen, and J. C. Spain. 1996. Purification and sequence analysis of 4-methyl-5-nitrocatechol oxygenase from Burkholderia sp. strain DNT. J. Bacteriol. 178: 6019-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haigler, B. E., S. F. Nishino, and J. C. Spain. 1994. Biodegradation of 4-methyl-5-nitrocatechol by Pseudomonas sp. strain DNT. J. Bacteriol. 176: 3433-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohn, B., and J. Collins. 1980. A small cosmid for efficient cloning of large DNA fragments. Gene 11: 291-298. [DOI] [PubMed] [Google Scholar]
- 18.Hübner, A., C. E. Danganan, L. Xun, A. M. Chakrabarty, and W. Hendrickson. 1998. Genes for 2,4,5-trichlorophenoxyacetic acid metabolism in Burkholderia cepacia AC1100: characterization of the tftC and tftD genes and locations of the tft operons on multiple replicons. Appl. Environ. Microbiol. 64: 2086-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inouye, S. 1994. NAD(P)H-flavin oxidoreductase from the bioluminescent bacterium, Vibrio fischeri ATCC 7744, is a flavoprotein. FEBS Lett. 347: 163-168. [DOI] [PubMed] [Google Scholar]
- 20.Jain, R. K., J. H. Dreisbach, and J. C. Spain. 1994. Biodegradation of p-nitrophenol via 1,2,4-benzenetriol by an Arthrobacter sp. Appl. Environ. Microbiol. 60: 3030-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadiyala, V., and J. C. Spain. 1998. A two-component monooxygenase catalyzes both the hydroxylation of p-nitrophenol and the oxidative release of nitrite from 4-nitrocatechol in Bacillus sphaericus JS905. Appl. Environ. Microbiol. 64: 2479-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamiya, M., and K. Kameyama. 1998. Photochemical effects of humic substances on the degradation of organophosphorus pesticides. Chemosphere 36: 2337-2344. [Google Scholar]
- 23.Kendrew, S. G., S. E. Harding, D. A. Hopwood, and E. N. G. Marsh. 1995. Identification of a flavin-NADH oxidoreductase involved in the biosynthesis of actinorhodin: purification and characterization of the recombinant enzyme. J. Biol. Chem. 270: 17339-17343. [DOI] [PubMed] [Google Scholar]
- 24.Kitagawa, W., N. Kimura, and Y. Kamagata. 2004. A novel p-nitrophenol degradation gene cluster from a gram-positive bacterium, Rhodococcus opacus SAO101. J. Bacteriol. 186: 4894-4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch, C., F. A. Rainey, and E. Stackebrandt. 1994. 16S rDNA studies on members of Arthrobacter and Micrococcus: an aid for their future taxonomic restructuring. FEMS Microbiol. Lett. 123: 167-172. [Google Scholar]
- 26.Louie, T. M., C. M. Webster, and L. Xun. 2002. Genetic and biochemical characterization of a 2,4,6-trichlorophenol degradation pathway in Ralstonia eutropha JMP134. J. Bacteriol. 184: 3492-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordin, K., M. Unell, and J. K. Jansson. 2005. Novel 4-chlorophenol degradation gene cluster and degradation route via hydroxyquinol in Arthrobacter chlorophenolicus A6. Appl. Environ. Microbiol. 71: 6538-6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patridge, E. V., and J. G. Ferry. 2006. WrbA from Escherichia coli and Archaeoglobus fulgidus is an NAD(P)H:quinone oxidoreductase. J. Bacteriol. 188: 3498-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rani, N. L., and D. Lalithakumari. 1994. Degradation of methyl parathion by Pseudomonas putida. Can. J. Microbiol. 40: 1000-1006. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Spain, J. C., P. A. Van Veld, C. A. Monti, P. H. Pritchard, and C. R. Cripe. 1984. Comparison of p-nitrophenol biodegradation in field and laboratory test systems. Appl. Environ. Microbiol. 48: 944-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spain, J. C., and D. T. Gibson. 1991. Pathway for biodegradation of p-nitrophenol in a Moraxella sp. Appl. Environ. Microbiol. 57: 812-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spain, J. C., O. Wyss, and D. T. Gibson. 1979. Enzymatic oxidation of p-nitrophenol. Biochem. Biophys. Res. Commun. 88: 634-641. [DOI] [PubMed] [Google Scholar]
- 34.Stanier, R. Y., Palleroni N. J., and M. Doudoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43: 159-271. [DOI] [PubMed] [Google Scholar]
- 35.Takeo, M., T. Yasukawa, Y. Abe, S. Niihara, Y. Maeda, and S. Negoro. 2003. Cloning and characterization of a 4-nitrophenol hydroxylase gene cluster from Rhodococcus sp. PN1. J. Biosci. Bioeng. 95: 139-145. [PubMed] [Google Scholar]
- 36.Takizawa, N., H. Yokoyama, K. Yanagihara, T. Hatta, and H. Kiyohara. 1995. A locus of Pseudomonas pickettii DTP0602, had, that encodes 2,4,6-trichlorophenol-4-dechlorinase with hydroxylase activity, and hydroxylation of various chlorophenols by the enzyme. J. Ferm. Bioeng. 80: 318-326. [Google Scholar]
- 37.Ueda, K., A. Yamashita, J. Ishikawa, M. Shimada, T. Watsuji, K. Morimura, H. Ikeda, M. Hattori, and T. Beppu. 2004. Genome sequence of Symbiobacterium thermophilum, an uncultivable bacterium that depends on microbial commensalism. Nucleic Acids Res. 32: 4937-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Department of Health and Human Services Agency for Toxic Substances and Disease Registry and U.S. Environmental Protection Agency. 1991. The revised priority list of hazardous substances that will be the subject of toxicological profiles; notice. Fed. Regist. 56: 52166-52175. [Google Scholar]
- 39.Woodrow, J. E., J. N. Seiber, D. G. Crosby, K. W. Moilanen, C. J. Soderquist, and C. Mourer. 1977. Airborne and surface residues of parathion and its conversion products in a treated plum orchard environment. Arch. Environ. Contam. Toxicol. 6: 175-191. [DOI] [PubMed] [Google Scholar]
- 40.Xun, L., and E. R. Sandvik. 2000. Characterization of 4-hydroxyphenylacetate 3-hydroxylase (HpaB) of Escherichia coli as a reduced flavin adenine dinucleotide-utilizing monooxygenase. Appl. Environ. Microbiol. 66: 481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xun, L., and C. M. Webster. 2004. A monooxygenase catalyzes sequential dechlorinations of 2,4,6-trichlorophenol by oxidative and hydrolytic reactions. J. Biol. Chem. 279: 6696-6700. [DOI] [PubMed] [Google Scholar]
- 42.Zaborina, O., D. L. Daubaras, A. Zago, L. Xun, K. Saido, T. Klem, D. Nikolic, and A. M. Chakrabarty. 1998. Novel pathway for conversion of chlorohydroxyquinol to maleylacetate in Burkholderia cepacia AC1100. J. Bacteriol. 180: 4667-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zenno, S., H. Koike, A. Kumar, R. Jayaraman, M. Tanokura, and K. Saigo. 1996. Biochemical characterization of NfsA, the Escherichia coli major nitroreductase exhibiting a high amino acid sequence homology to Frp, a Vibrio harveyi flavin oxidoreductase. J. Bacteriol. 178: 4508-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zylstra, G. J., S.-W. Bang, L. M. Newman, and L. L. Perry. 2000. Microbial degradation of mononitrophenols and mononitrobenzoates, p. 145-160. In J. C. Spain, J. B. Hughes, and H.-J. Knackmuss (ed.), Biodegradation of nitroaromatic compounds and explosives. Lewis Publishers, Boca Raton, FL.